Abstract
BACKGROUND:
Neonatal hypoxic-ischemic encephalopathy disproportionately affects low- and middle-income countries, where ≈96% of affected infants reside. The current standard of care, therapeutic hypothermia, is frequently ineffective in this setting, likely because injury may be occurring earlier during labor. Here, we studied the pharmacokinetics, safety, and efficacy of perinatal caffeine administration in near-term lambs following global ischemic injury to support the development of earlier treatment strategies targeting the fetus in utero as well as the infant postnatally.
METHODS:
Ewes were randomly assigned to receive either 1 g IV caffeine citrate or placebo before delivery and placental transport assessed. Near-term lambs (141–143 days) of both sexes were subjected to severe global hypoxia-ischemia utilizing an acute umbilical cord occlusion model. Lambs that received caffeine in utero also received 20 mg/kg IV caffeine citrate following resuscitation and 10 mg/(kg·d) IV for 2 days. An additional cohort received 60 mg/kg followed by 30 mg/(kg·d) (low dose versus high dose) postnatally. Biochemical, histological, and neurological outcome measures in lambs were assessed over a 6-day period.
RESULTS:
Perinatal caffeine administration demonstrated excellent placental transport kinetics and was well tolerated with lamb plasma levels comparable to those targeted in neonates with apnea of prematurity. Caffeine administration resulted in a systemic immunomodulatory effect, evidenced by significant reductions in proinflammatory IP-10 levels. Treated lambs demonstrated improved neurodevelopmental outcomes, while histological analysis revealed that caffeine reduced gray matter injury and attenuated inflammation in the cingulate and parasagittal cortex. This neuroprotective effect was greater and via a different mode of action than we previously reported for azithromycin. A higher caffeine dosing regimen demonstrated significant toxicity.
CONCLUSIONS:
Perinatal caffeine administration is well tolerated, attenuates systemic and brain inflammation, and contributes to improvements in histological and neurological outcomes in an ovine model of neonatal hypoxic-ischemic encephalopathy.
Keywords: caffeine; developing countries; gray matter; hypoxia-ischemia, brain; sheep
More than 95% of global cases of cerebral palsy1 can be found in low- and middle-income countries (LMIC) and are frequently caused by neonatal brain injury/hypoxic-ischemic encephalopathy (HIE). Multiple factors contribute to a higher incidence of HIE in LMIC, including chronic placental insufficiency.2 The current standard of care, therapeutic hypothermia, is only modestly effective in high-income countries, while its use in LMIC is frequently ineffective or contraindicated due to significantly increased adverse events.3 The search for alternate neuroprotective therapies has been extensive,4,5 yet many, including caffeine, have not been tested in clinical trials without therapeutic hypothermia. Additionally, some are limited by the requirement for early therapy or the lack of pharmaceutical-grade commercial preparations, as well as safety concerns in the postnatal setting, such as with magnesium.6 Importantly, the likely earlier timing of brain injury associated with HIE in LMIC7 is most likely due to chronic placental insufficiency resulting in greater growth restriction and intolerance of labor, triggering repetitive hypoxic insults in utero.7,8 This suggests that antenatal administration of agents with favorable placental transfer kinetics may be necessary to ensure maximal efficacy.
Caffeine citrate (caffeine) has demonstrated an excellent safety profile in preterm neonates over the past 40 years and is the standard of care treatment for apnea of prematurity.9 The Caffeine for Apnea of Prematurity trial demonstrated reduced risks of cerebral palsy and cognitive delay at 18 months with a daily treatment regimen consisting of a 20 mg/kg loading dose followed by daily dosing of up to 10 mg/kg. The effects extended long-term, as benefits were noted on motor development at 5 years of age10 and visuomotor, visuoperceptual, and visuospatial abilities at 11 years.11 The neuroprotective properties of caffeine have also been observed in vitro as well as in various animal models of HIE.9,12
Caffeine and other methylxanthines, such as theophylline, are primarily used to block A1 and A2A adenosine receptors to help stimulate breathing, with predominant blockade of A2A noted during brain ischemia.13 Adenosine receptors play a dual role in brain ischemia, and antagonism may be beneficial in the brain during later stages, where prolonged activation in neurons may be detrimental.14 Additionally, caffeine acts as a potent antioxidant15 and can support integrity of the blood-brain barrier16 while influencing inflammation locally as well as systemically.17 At lower doses, caffeine is a competitive inhibitor of adenosine A1, A2A, and A2B receptors, while at higher concentrations it can inhibit phosphodiesterase activity, which is responsible for many of its reported adverse effects.18
Preclinical models in sheep have been instrumental for advancing some of the most impactful therapies in maternal-fetal medicine and neonatology today. These include antenatal steroids, resuscitation approaches, surfactants, and therapeutic hypothermia.19,20 Due to the potential need for in utero treatment approaches for HIE in the LMIC setting, the ability to define placental transport kinetics, fetal pharmacology, safety, and postnatal efficacy of potential therapeutics requires physiologically relevant large animal models such as sheep to aid translational efforts. In this context, we evaluated the safety, pharmacokinetics, mode of action, and neuroprotective efficacy of perinatal caffeine administration in our model of HIE in near-term lambs designed to develop therapies for LMIC. We additionally evaluated whether a higher dosing regimen than used clinically would be feasible.
METHODS
Data Availability
The data presented in this study are available upon reasonable request. Full descriptions of methods are available in the Supplemental Material.
Animals
All animal research was approved by the University of California Davis Institutional Animal Care and Use Committee and was performed in accordance with the Guide for the Care and Use of Laboratory Animals and follows Animal Research: Reporting of In Vivo Experiments 2.0 guidelines.21
Neonatal Hypoxia-Ischemia
HIE was induced via umbilical cord occlusion (UCO) in near-term lambs at 141 to 143 days gestation (term ≈147–150 days) as previously described.2,22,23 Physiological parameters, such as blood pressure, heart rate, the onset of return of spontaneous circulation (ROSC), and the epinephrine doses required for ROSC, were assessed.
Drug Treatment
Two caffeine dosing regimens were tested. In the first, ewes were randomized to receive either 1 g IV caffeine citrate or placebo total before cesarean section. Following delivery, lambs born to ewes that received caffeine were administered 20 mg/kg IV caffeine citrate over 10 minutes starting 10 minutes following resuscitation, along with 2 additional doses of 10 mg/kg IV caffeine citrate each at 24 and 48 hours of life (low dose [LD]). An additional high-dose (HD) caffeine arm was also investigated where no caffeine was administered to the ewe, but each lamb was randomized to receive either placebo or 60 mg/kg IV caffeine citrate following resuscitation and 30 mg/kg IV at 24 and 48 hours of life. For details, please see Supplemental Material.
Pharmacokinetic Analysis
Plasma samples for pharmacokinetic analyses were collected from the ewe at end of infusion and each lamb before UCO (baseline, before caffeine infusion, and pre-UCO), as well as at the end of drug infusion at 1, 2, 4, 8, 24, 48, 72, 96, 120, and 144 hours following caffeine treatment. Pharmacokinetic analysis is described in detail in Supplemental Material.
Biochemical Markers
Systemic inflammation was measured by cytokine levels 6 days after UCO, as described previously.2,22,23 We also assessed for differences in peripheral blood cells and their inflammatory ratios before UCO (baseline) at 8 hours and on days 1, 2, 3, 5, and 6. Toxicity was assessed by measuring liver and kidney function markers (see Supplemental Material).
Immunohistochemistry and Image Analysis
Histological changes in the brain were assessed 6 days after the UCO by measuring qualitative and morphological changes in gray and white matter structures, as described previously.22 We examined 3 regions of white matter: periventricular white matter (PVWM) and subcortical white matter located in the cingulate and first parasagittal gyrus (SCWM1 and SCWM2). Additionally, we assessed 6 regions of gray matter: cortex of the cingulate and first parasagittal gyrus (Ctx1 and Ctx2), caudate (Caud), putamen (Put), and areas within the hippocampus (Ca1/2 and Ca3).
Neurological Outcomes Assessments
Our neurobehavioral assessment followed observations in sheep post-birth daily for 6 days and included evaluation of motor function, feeding, and activity at rest and their sum as the composite score (Table S1).2,22,23 Lambs without impairment achieved a maximum score of 6, while severely impaired animals with spastic paralysis, encephalopathy, and an inability feed scored 0. See Supplemental Material for additional details.
Statistical Analysis
A comprehensive description of our statistical methods22,24,25 is provided in Supplemental Material. We utilized an adaptive preclinical trial design, where HD caffeine reached futility after only n=8 lambs. All data are shown as mean±SEM. Differences were considered significant at P<0.05.
RESULTS
Caffeine Pharmacokinetics
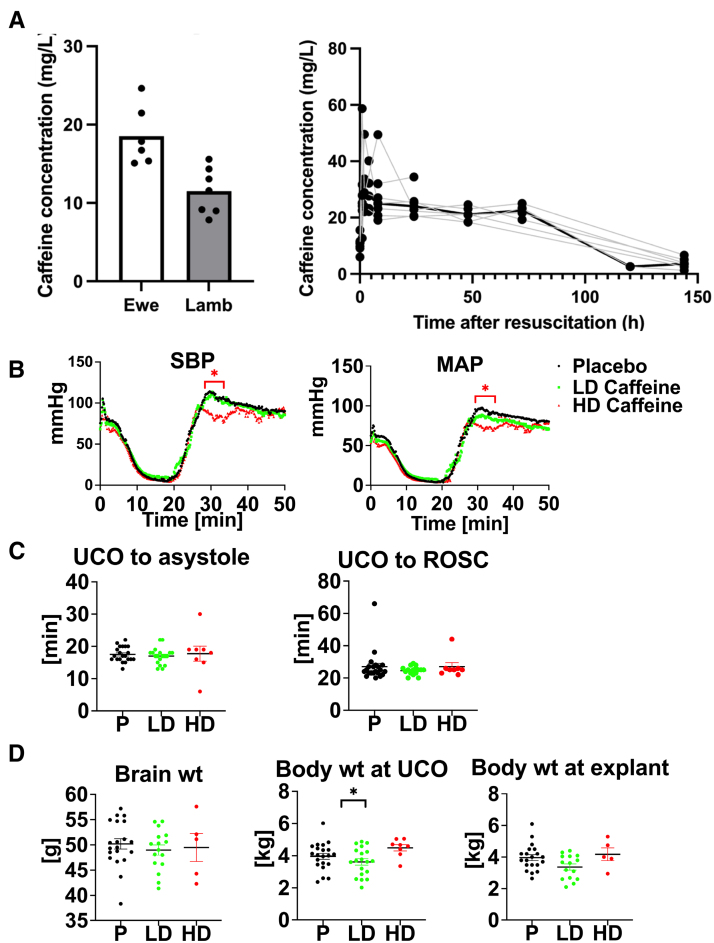
There was highly efficient and rapid (within 1 hour) placental transfer of caffeine from ewe to lamb with a mean percent transfer of 65.5% (range, 36.5%–84.9%). Maximal plasma caffeine concentration (Cmax) of 34.8±14.0 mg/L occurred after the loading dose administered to the lamb (time to maximal concentration, Tmax, 0.33±0.51 hours after infusion) and was slowly eliminated over the 6-day observation period with a mean plasma half-life of 96.3±58.8 hours and an area under the curve of 2507±382 mg×h/L (Figure 1A).
Figure 1.
Caffeine pharmacokinetics and effects on resuscitation. A, Caffeine concentration in ewe (n=6) and lamb (n=7) plasma at the time of umbilical cord occlusion and caffeine concentration in lamb (n=7) plasma over time. B, Hemodynamic changes in response to umbilical cord occlusion (UCO) with drop in systolic blood pressure (SBP) and mean arterial pressure (MAP) followed by increase reflecting return of spontaneous circulation (ROSC). Hemodynamic data were analyzed using grouped analysis of the individual group’s means for a specific time point. Placebo (P): n=19; low-dose (LD) caffeine: n=14; and high-dose (HD) caffeine: n=7. C, Time to asystole and time to ROSC were similar among the compared groups. Groups were compared using the Kruskal-Wallis test. Placebo: n=21; LD caffeine: n=18–19; and HD caffeine: n=8. D, Selected anthropometric parameters between the studied groups. Brain and body weight differences were assessed using ANOVA. Data in graph B are shown as mean and graph C and D as mean±SEM. Placebo: n=20–21; LD caffeine: n=16–19; and HD caffeine: n=5–8. LD-caffeine–treated group is presented in green, HD-caffeine–treated group is presented in red, and placebo is presented in black. *P<0.05. wt indicates weight.
Physiological Outcomes
Hemodynamic parameters exhibited a similar decline following UCO in all groups (Figure 1B). The onset of ROSC following cardiopulmonary resuscitation was also similar between all groups (Figure 1C). HD-caffeine–treated animals exhibited lower blood pressure compared with placebo during the loading dose caffeine infusion after ROSC (P<0.01–0.03; Figure 1B). Epinephrine was administered to all animals in both groups with most animals requiring only 1 dose (P=0.42; Figure S1A). HD lambs had lower glucose versus placebo (P=0.01), but did not differ from the LD lambs (P=0.20; Figure S1A). HD-caffeine group experienced increased mortality (P=0.05; risk ratio, 4.594 [95% CI, 1.255–14.86]; Figure S1B). Caffeine administration did not lead to changes in brain or body weight compared with placebo (LD caffeine: P=0.09; HD caffeine: P=0.86; Figure 1D). Based on interim analyses of outcomes data, HD caffeine was discontinued early as an arm in the trial.
Adverse Events
HD-caffeine lambs experienced higher mortality within 2 days after UCO (4/8; Figure S1B). No other adverse events were noted. The early mortality prevented us from harvesting the brains for histological assessment, collecting samples, and assessing neurological outcomes past day of life 2, thus these data were excluded from final analyses.
Biochemical Parameters
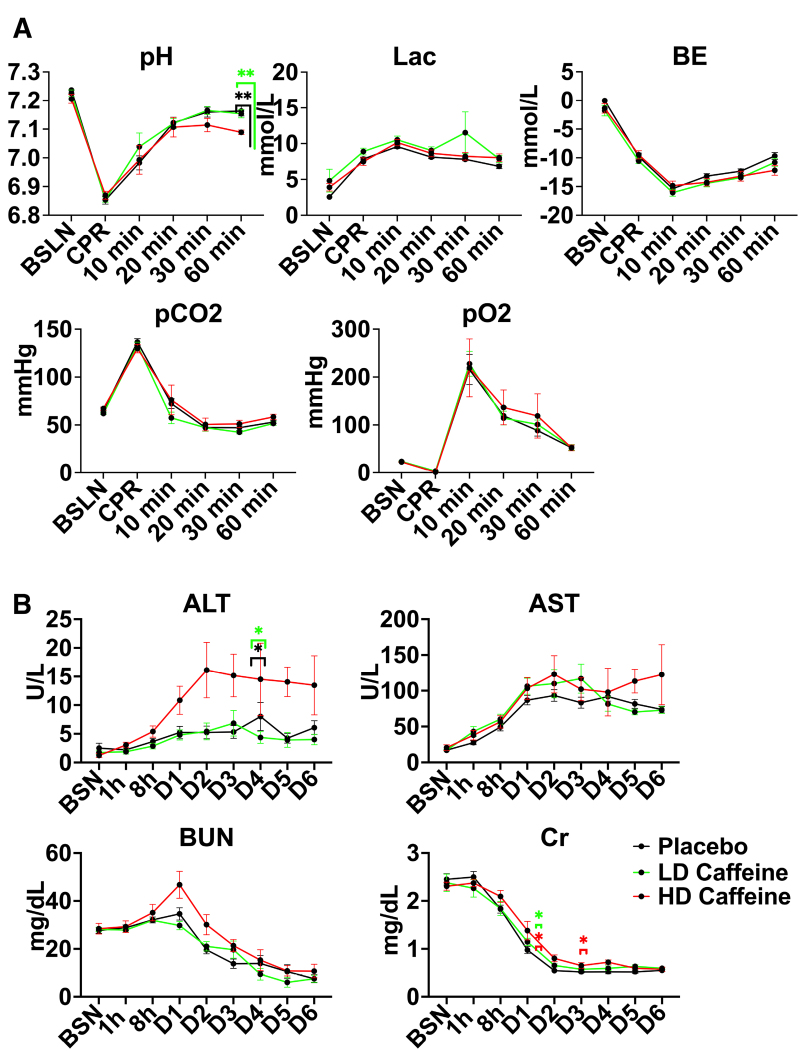
Consistent with our prior studies,2,22,23 the UCO protocol produced a clinically significant combined metabolic and respiratory acidosis (Figure 2A). The HD-caffeine–treated lambs were more acidemic 60 minutes after cardiopulmonary resuscitation compared with placebo (P=0.0014) as well as LD-caffeine treatment groups (P=0.0015), as evidenced by lower pH values. There were no significant changes in oxygenation and ventilation among the studied groups (P>0.05). LD caffeine did not exhibit noticeable toxicity, as reflected by similar chemistry panels relative to placebo-treated animals (Figure 2B). LD animals exhibited slightly higher creatinine levels versus placebo on day 2 (P=0.03). HD-caffeine–treated lambs demonstrated elevated alanine transaminase (ALT) levels compared with the LD-caffeine treatment group and placebo that reached significance on day 5 (P=0.02 and P=0.03; Figure 2B) and creatinine levels on days 2 and 4 compared with placebo (P=0.04 and P=0.04; Figure 2B).
Figure 2.
Biochemical parameters in treated vs placebo groups. A, Umbilical cord occlusion (UCO) leads to profound acidosis in all groups. High-dose (HD)-caffeine–treated lambs demonstrated greater acidosis at 60 minutes compared with the low-dose (LD)-caffeine and placebo groups. All studied groups exhibited similar hyperlactatemia, base excess, carbon dioxide, and oxygen levels. B, HD caffeine demonstrated significant toxicity evidenced by elevated alanine transaminase (ALT) levels on day 5, as well as creatinine on days 2 and 4. No differences in aspartate aminotransferase (AST) and blood urea nitrogen (BUN) levels were noted between the studied groups. The data were analyzed using mixed-effects analysis with Tukey correction for multiple comparisons. Data in the graphs represent mean±SEM. For A, HD caffeine, n=5–8; LD caffeine, n=19–20; and placebo, n=21. For B, HD caffeine, n=4–5; LD caffeine, n=5–15; and placebo, n=7–20. LD-caffeine–treated group is presented in green, HD-caffeine–treated group is presented in red, and placebo is presented in black. *P<0.05, **P<0.01. BE indicates base excess; BSN/BSLN, pre-UCO baseline; CPR, cardiopulmonary resuscitation; and Lac, lactate.
Inflammation
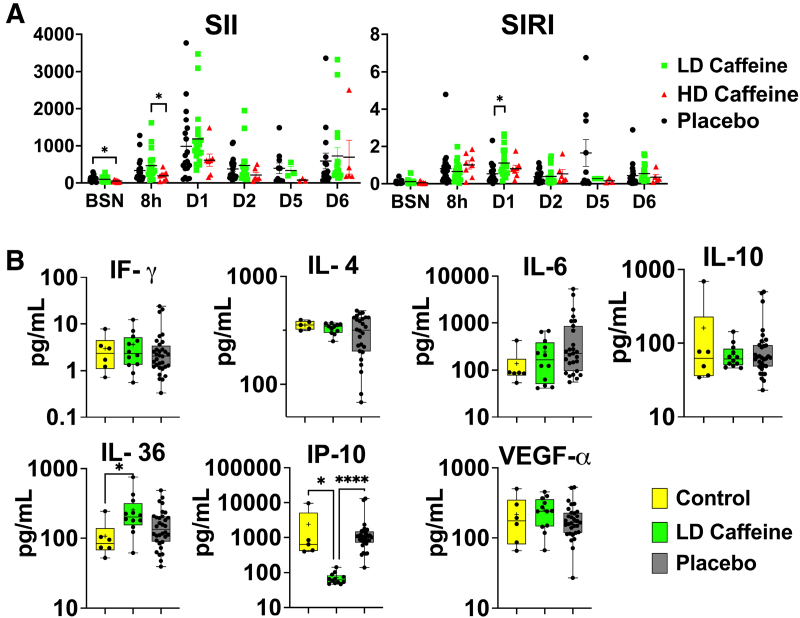
We assessed basic markers of inflammation, including white blood cell count with differential and select cytokine levels. HD-caffeine treatment resulted in a lower platelet count compared with the LD-caffeine group 8 hours after UCO (P=0.004) that resolved over time (Figure S2). The HD-caffeine group had a slightly decreased systemic immune-inflammation index (SII) score at baseline compared with the placebo (P=0.04) and at 8 hours compared with the LD-caffeine group (P=0.03; Figure 3A). The system inflammation response index (SIRI) score was elevated on day 1 in the LD-caffeine group (P=0.01; see Supplemental Material). We did not observe changes in other subgroups of peripheral blood cells (Figure S2). Interestingly, we detected significantly lower levels of IP-10 in LD-caffeine–treated lambs versus placebo (P=0.0001) and controls (P=0.02). The levels of IL-36 were slightly elevated in the LD-caffeine group compared with controls (P=0.03) but not compared with placebo-treated lambs (Figure 3B).
Figure 3.
Peripheral markers of inflammation. A, The peripheral blood cell index systemic immune-inflammation index (SII=ANC×PLT/ALC) was suppressed in high-dose (HD) caffeine at baseline (BSN) compared with placebo, and at 8 hours after umbilical cord occlusion (UCO) compared with low-dose (LD)-caffeine group. Systemic inflammation response index (SIRI=ANC×Mono/ALC) was elevated in the LD-caffeine group compared with the placebo on day 1. The SII and SIRI scores were evaluated by mixed-effect analysis with Tukey correction for multiple comparisons. The summary column graphs are showing means±SEM. HD caffeine: n=3–8; LD caffeine: n=3–19, and placebo: n=10–21. B, At 6 days after the UCO, we measured changes in IP-10 in the LD-caffeine group compared with the placebo, as well as in age-matched control and in IL-36 that was higher in the LD-caffeine group compared with control. No changes were observed in IL (interleukin)-4, IL-6, IF (interferon)-γ, IP (interferon gamma-induced protein 10)-10, or VEGF (vascular endothelial growth factor A)-α. We used Mann-Whitney U test. LD caffeine: n=11–12; and placebo: n=26–32. The box graphs show mean by plus sign and median with interquartile range. *P<0.05, ****P<0.0001. LD-caffeine–treated group is presented in green, control is presented in yellow, and placebo is presented in black. ALC indicates absolute lymphocyte count; ANC, absolute neutrophil count; Mono, monocytes; and PLT, platelets.
Neurohistopathological Outcomes
Gray Matter Injury
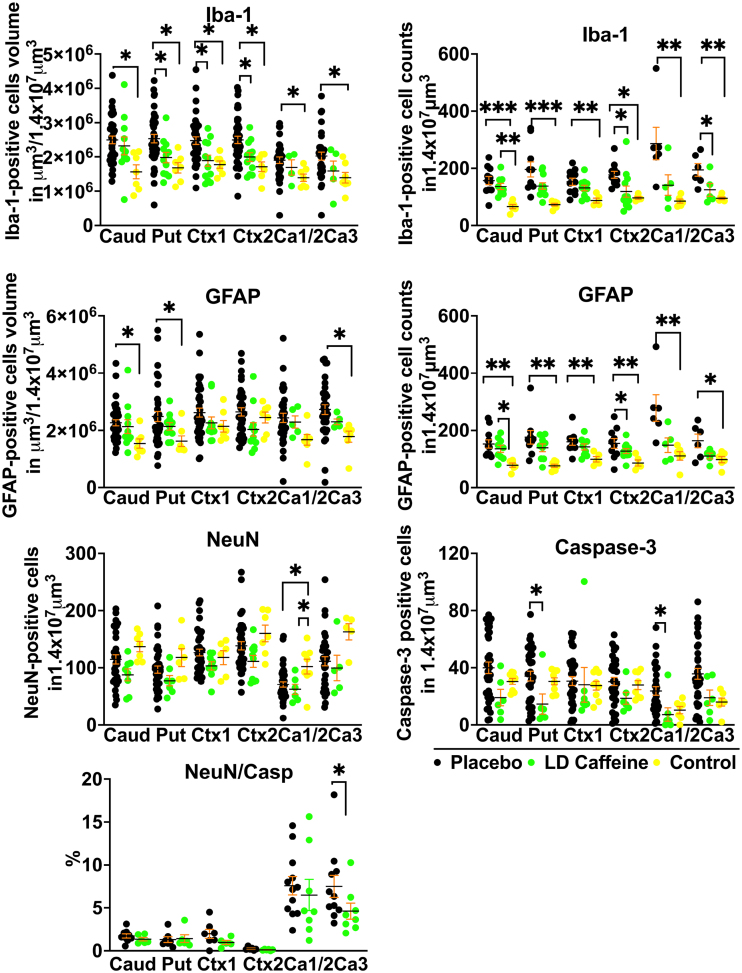
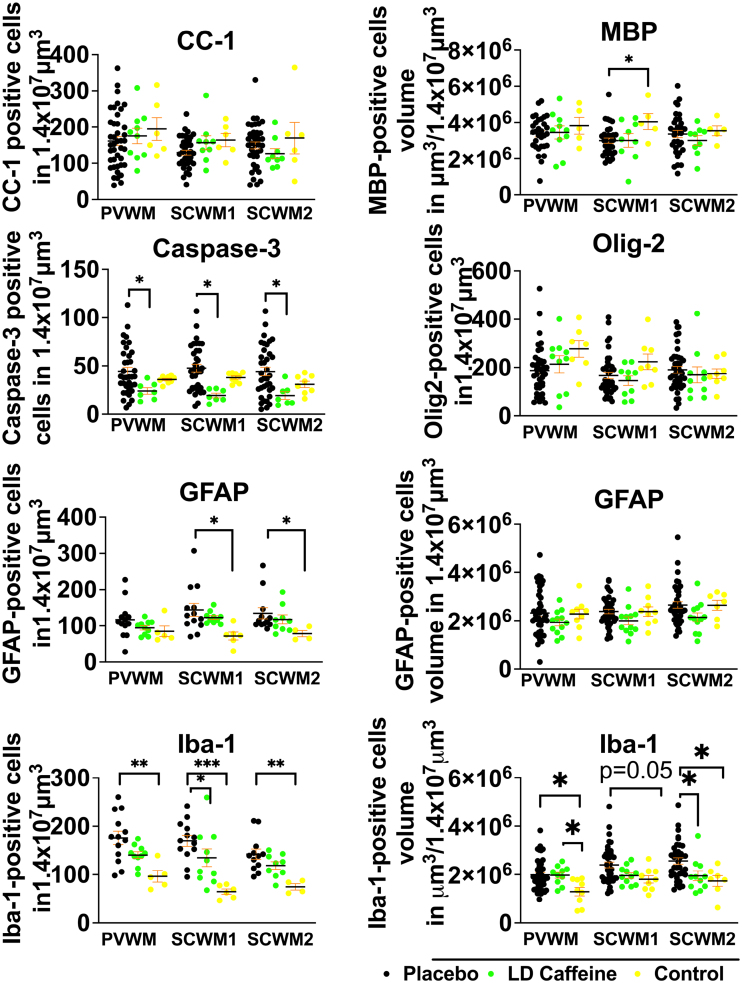
We identified significant modification of gray matter injury patterns in LD-caffeine–treated lambs via histological analysis at day 6. GFAP (glial fibrillary acidic protein)-positive astrocyte volumes increased following UCO in caudate, putamen, and Ca3 region of the hippocampus that was not affected by LD-caffeine treatment (P=0.82 ≥0.99, and 0.58). GFAP cell counts were elevated in all regions in placebo versus control (Caud: P=0.0016; Put: P=0.001; Ctx1: P=0.008; Ctx2: P=0.01; Ca1/2: P=0.007; and Ca3: P=0.03) and in Caud and Put (P=0.01 and 0.02, respectively) in the LD-caffeine group. In contrast, UCO resulted in significant Iba-1-positive microglial accumulation in all gray matter regions evaluated, which demonstrated marked region-specific reductions following LD-caffeine treatment in Put, Ctx1, and Ctx2 (P=0.047, 0.018, and 0.04, respectively). Similarly, Iba-1 cell counts were elevated in placebo in all regions, while LD caffeine had elevated Iba-1 counts in caudate compared with controls (P=0.004) and LD-caffeine treatment decreased the Iba-1 counts in Ctx2 and Ca3 area of the hippocampus compared with placebo (P=0.01 and 0.04). Total neuronal numbers were equally reduced in Ca1/2 region in both LD-caffeine–treated as well as placebo-treated UCO lambs when compared with uninjured controls (P=0.03 and 0.02). The percentage of NeuN cells that were caspase-positive was decreased in LD-caffeine group in Ca3 region of the hippocampus (P=0.04). Total caspase-3–positive apoptotic cell numbers were reduced with LD-caffeine treatment following UCO in Ca1/2 (P=0.03) and putamen (P=0.04), suggesting wide scale inhibition of cell death pathway activation (Figure 4; Figure S3).
Figure 4.
Histological changes in gray matter. We compared quantitative changes in inflammatory markers of gliosis (GFAP [glial fibrillary acidic protein]), microglial accumulation (Iba-1 [ionized calcium-binding adapter molecule 1]), neuronal counts (NeuN), cellular death markers (Casp-3) in cingulate gyrus Ctx (Ctx1), first parasagittal gyrus Ctx (Ctx2), caudate (Caud), putamen (Put), and Ca1/2 (Ca1/2) and Ca3 (Ca3) of the hippocampus. Placebo animals (n=33–41) were compared with the low-dose (LD)-caffeine–treated animals (n=7–11) and controls (n=4–9) using ANOVA or Kruskal-Wallis test as appropriate. Data are presented as mean±SEM. Brackets show significance as follows: *P<0.05. LD-caffeine–treated group is presented in green, control is presented in yellow, and placebo is presented in black.
White Matter Injury
LD-caffeine treatment demonstrated anti-inflammatory and antiapoptotic effects upon histological assessment in white matter regions. No effect of UCO on GFAP-positive astrocyte volume was noted in any of the groups (placebo versus LD caffeine: PVWM, P=0.54; SCWM1, P=0.12; and SCWM2, P=0.13). GFAP-positive cell counts were elevated in SCWM1 and SCWM2 in the placebo group compared with control (P=0.01 and 0.01). The placebo treatment group demonstrated diminished MBP-stained sheets in SCWM1 following UCO when compared with controls (P=0.04) that were not affected by LD-caffeine administration (P=0.99; Figure 5). No change was noted in oligodendrocyte precursor cells or mature oligodendrocytes in any of the groups (placebo versus LD caffeine: PVWM, P=0.75; SCWM1, P>0.99; and SCWM2, P=0.99; Figure 5A). However, we observed a higher density of microglia indicating increased inflammation in PVWM and SCWM2 following UCO compared with controls (PVWM: placebo, P=0.006; LD caffeine, P=0.04; and SCWM2: placebo, P=0.02), with improvement in SCWM2 following LD caffeine treatment (P=0.03). Iba-1 counts were elevated in the placebo group in all regions compared with controls (PVWM: P=0.0018; SCWM1: P=0.0002; and SCWM2: P=0.002) and LD caffeine in SCWM1 (P=0.01). Similar to gray matter regions, LD-caffeine treatment demonstrated antiapoptotic effects, with placebo-treated animals demonstrating greater evidence of apoptotic cell death as assessed by anti-cleaved caspase-3 immunoreactivity in all areas studied (PVWM: P=0.01; SCWM1: P=0.002; and SCWM2: P=0.01; Figure 5; Figure S4).
Figure 5.
Quantitative analysis of white matter markers and markers of inflammation: while overall white matter structure was not significantly altered by umbilical cord occlusion (UCO), there is more cell death in the placebo group compared with low-dose (LD) caffeine, reflected by a higher number of cells labeled with cleaved caspase-3. More inflammation was noted in the SCWM2 in the placebo group, reflected by the accumulation of microglia and higher microglial volumes. Placebo lamb histologies (n=31–41) were compared with the LD-caffeine–treated animals (n=7–12) and controls (n=5–9) using ANOVA or Kruskal-Wallis test as appropriate. Data are presented as mean±SEM. Brackets show significance as follows: *P<0.05. LD-caffeine–treated group is presented in green, control is presented in yellow, and placebo is presented in black. CC-1 indicates adenomatous polyposis coli protein; GFAP, glial fibrillary acidic protein; Iba-1, ionized calcium binding adaptor molecule 1; MBP, myelin basic protein; PVWM, periventricular white matter; and SCWM, subcortical white matter.
Neurobehavioral Milestones
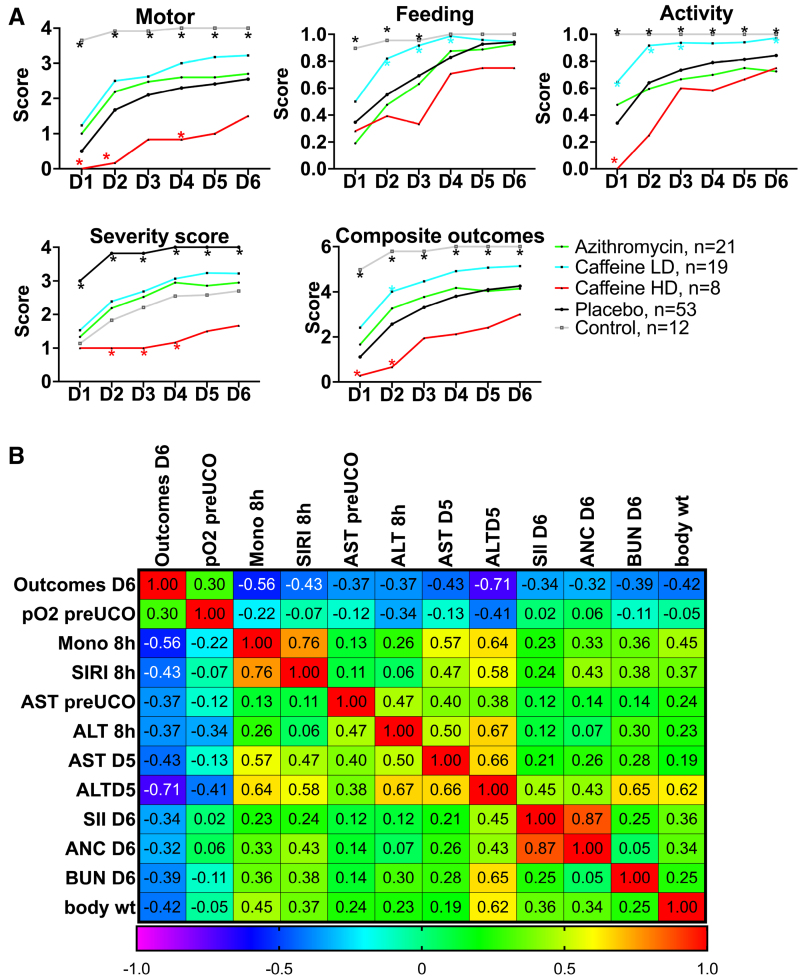
We compared the effects of LD-caffeine and HD-caffeine versus placebo-treated animals, along with naive controls, and the animals we previously treated with azithromycin in a separate study using the identical model and end points.22 LD-caffeine–treated lambs scored significantly higher with respect to feeding scores on days 1 to 3 (P=0.002, 0.006, and 0.003) along with activity (P=0.03, 0.002, and 0.02) that was also improved on day 5 (P=0.03) compared with placebo-treated animals. LD caffeine resulted in improved composite outcomes on day 2 compared with placebo controls (P=0.02; Figure 6A; Figure S5). HD caffeine treatment led to worse motor outcomes compared with placebo on days 1, 2, and 4 (P=0.007, <0.0001, and 0.04), along with worsening overall activity on day 1 (P<0.0001), a worse severity score on days 2 to 4 (P<0.0001, <0.0001, and <0.0001), and lower composite outcomes on days 1 to 2 (P=0.002, P=0.0003) when compared with placebo. Adjusting for maternal randomization and mortality, the LD-caffeine group improved motor scores on day 2 (P=0.03), total feeds+activity score on day 3 (P=0.02), and severity score on day 2 after the UCO (P=0.04) compared with placebo (Figure 6A). These effects were more pronounced than observed following Azithromycin administration.
Figure 6.
Neurodevelopmental outcomes following caffeine or azithromycin treatment. A, We assessed composite and individual outcomes consisting of motor function, feeding, and activity and assigned a severity score. The summary graphs picture the relationship of low-dose (LD) and high-dose (HD) caffeine to placebo animals. LD-caffeine–treated group is presented in blue (n=19), HD-caffeine–treated group is presented in red (n=8), placebo is presented in black (n=53), azithromycin is presented in green (n=21), and control is presented in purple (n=12). B, The correlation matrix depicts Spearman correlation coefficients of selected study parameters with combined neurological outcomes scores on day 6 after umbilical cord occlusion (UCO) that reached statistical significance (P<0.05). ALT, alanine transaminase; ANC, absolute neutrophil count; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Mono, monocytes; SII, systemic immune-inflammation index; SIRI, system inflammation response index; and wt, weight.
Markers Associated With Neurological Outcomes
Finally, we assessed whether any of the parameters investigated in this study were associated with poor neurological outcomes on day 6 after UCO. From the earliest parameters, worse outcomes on day 6 correlated with monocyte and SIRI levels and liver enzymes. Parameters measured at later time points that correlated with poor neurological outcomes included liver enzymes, SII scores, absolute neutrophil counts, and blood urea nitrogen (Figure 6B).
DISCUSSION
Our results suggest that perinatal caffeine administration in a dose-limited manner is safe and may improve certain outcomes for neonates born with HIE in LMIC. The effect of caffeine on HIE in our ovine model may provide compelling evidence for its use clinically in LMIC. Caffeine is used in neonates to treat apnea of prematurity, with a typical dosing of 20 mg/kg IV or PO, followed by a daily maintenance dose of 5 to 10 mg/kg. The reported therapeutic range for this indication is 8 to 40 mg/L, and toxicity is usually observed at levels exceeding >50 mg/L.26 The half-life of caffeine in neonates decreases as gestational age increases and is ≈3 to 4 days in neonates.27 Due to its lipophilic properties, caffeine easily crosses the blood-brain barrier.27 Our results confirmed that the pharmacokinetic parameters of caffeine are similar between neonatal lambs and humans and resulted in improvement in select neurological outcomes at plasma levels within the upper end of the therapeutic range for apnea of prematurity.28 Consistent with prior studies, we observed dose-dependent toxicity in our study.28 Toxic effects in the HD-caffeine group were observed immediately following infusion initiation, as demonstrated by reductions in blood pressure values that resolved thereafter. A bradycardic effect of caffeine has been attributed to a paradoxical induction of cholinergic activation through the inhibition of acetylcholinesterase or the hERG (human ether-a-go-go-related gene) potassium channel responsible for cardiac repolarization.29 At higher doses, caffeine also enhances sympathomimetic effects mediated by phosphodiesterase inhibition. A caffeine-induced rise in norepinephrine and epinephrine30 levels could trigger a hyperadrenergic state with potential detrimental effects, including increased myocardial oxygen consumption, impaired microcirculation, and heart failure after ROSC.31 The suboptimal cardiac output associated with the HD-caffeine administration was reflected by persistent acidosis with a lower pH at 60 minutes and compromised end-organ function. Although the exact cause remains unknown, the administration of HD caffeine has been discouraged due to the increased incidence of cerebellar hemorrhage.32 Unfortunately, the limited survival of the HD-caffeine group beyond 3 days of life prevented us from gathering an adequate number of samples to measure cytokine levels on day 6 and for histological outcome analyses.
Immune dysregulation observed during the first week after a perinatal HIE insult can be predictive of the severity of neurological outcomes.33 In our study, we detected early alterations in the inflammatory indices SIRI and SII following HIE. Elevated SIRI and SII indices are generally associated with worse outcomes after HIE.34 Similarly, we observed a correlation of SIRI at 8 hours and SII at 6 days with worse neurological outcomes. Interestingly, we found an elevated SIRI index in the LD-caffeine group on day 1 after injury and suppressed SII indices in the HD-caffeine group at baseline and 8 hours after UCO. We speculate that some degree of immune response activation is necessary for the clearance of damaged tissue and in the ensuing processes of angiogenesis, tissue remodeling, and regeneration.35
In our analysis of cytokine levels, we found that LD-caffeine administration dramatically suppressed CXCL10 (IP-10). CXCL10 is a chemokine secreted by various immune and nonimmune cells in response to inflammatory stimuli, such as IFN (interferon)-γ.36 Following focal stroke, CXCL10 resulted in prolonged leukocyte recruitment, astrocyte activation, and neuron sprouting.37 CXCL10 also exacerbated blood-brain barrier injury and promoted the recruitment of natural killer cells to the injury site through the CXCR3 receptor and neuronal necrosis mediated by IFN-γ.36 CXCL10 is upregulated early after brain ischemia and remains elevated for an extended period up to 10 days.38 While in our study CXCL10 was suppressed in the caffeine group, IFN-γ levels were unchanged. This direct inhibitory effect of caffeine on the CXCL10 chemokine aligns with findings from other studies.39 CXCL10 suppression may represent one of the potential mechanisms by which caffeine modulates neuroinflammation in the setting of neonatal HIE. We further observed increased IL-36 in the LD-caffeine group. IL-36 is both a homeostatic and inflammatory cytokine. While IL-36 is associated with neutrophilic inflammation,40 and increased neutrophils with adverse outcomes in neonates with HIE,41 neutrophil counts were unchanged in our studied groups. The specific role of IL-36 in neonatal HIE has not been clearly defined. Further research is needed to explore the underlying mechanisms and potential therapeutic interventions targeting immune dysregulation in the context of neonatal HIE.
Histologically, the effects of caffeine were predominantly observed in gray matter. During hypoxia-ischemia, the breakdown of extracellular ATP and the release of adenosine from ischemic cells can lead to a significant increase in extracellular adenosine levels, contributing to neuronal death.42 Similar to findings in rodent models, our study demonstrated that LD-caffeine prevented apoptosis,43 mostly in the Ca3 region of the hippocampus. Our observations align with the distribution of adenosine receptors, where high densities of A1 receptors are expressed in the cortex, hippocampus, and cerebellum, while A2A receptors are more abundant in the striatum and olfactory bulb.13 Caffeine’s nonselective antagonism of A2A receptors can inhibit microglial activation and reduce cytokine release in rodent models.44 In our study, caffeine decreased the density of activated microglial cells in the cortex of the cingulate gyrus, first parasagittal gyrus and striatum, and reduced Iba-1 counts in Ca3 of the hippocampus, which are areas of the brain consistently prone to injury in our model.2 While in preterm models of neonatal HIE caffeine exhibited more positive effects in white matter,45 our study utilizing near-term lambs found less of an effect in white matter areas, although antiapoptotic effects of LD caffeine were consistently noted throughout all brain regions.
Caffeine has previously demonstrated beneficial effects on neurological outcomes in various animal models of neonatal HIE9 as well as in premature neonates.45 Our study is the first demonstrating the feasibility, safety, and efficacy of a perinatal treatment strategy designed to treat HIE in the LMIC setting, where large-scale studies suggest injury mechanisms occurring significantly earlier during labor and that are resistant to the beneficial effects of therapeutic hypothermia.3,7 The therapeutic efficacy of caffeine was dose-dependent, with HD caffeine being toxic. LD-caffeine administration, however, coupled with antenatal dosing of the mother, represents a potentially promising neurotherapeutic approach that enhanced motor performance, improved feeding behavior, increased activity levels, and reduced overall severity outcomes. LD-caffeine treatment, when compared with azithromycin treatment, showed improvement in almost all neurological outcomes by day 6.
Our study has several limitations. First, the data collected in our study represent only selected time points after UCO. Additional time points may be needed to improve our understanding of the dynamics of the neuroimmune response following UCO, as the immune response to UCO is robust, with onset after initiation of cardiopulmonary resuscitation46 and alterations in systemic inflammation being detectable even in older children with cerebral palsy.47 Thus, we would suggest early time points, such as 1 and 4 hours following resuscitation, as well as later time points, including up to 1 year. Second, due to relatively small sample sizes, we were unable to assess sex differences, which are known to be risk factors for neurological outcomes. To detect sex differences effectively, we would need a much larger sample size of ≈ 64 animals (see Supplemental Material), which is not achievable in our large animal model due to cost and ethical considerations. Finally, we cannot exclude the possibility of subclinical seizures occurring, as we monitored seizures solely based on clinical observations.
In conclusion, our study highlights the dose-dependent neuroprotective effects of perinatal caffeine administration and its positive impact on select biochemical, physiological, and histological outcomes in near-term lambs following UCO. Importantly, this treatment strategy demonstrated an excellent safety profile, making it a promising option for perinatal use in LMIC.
ARTICLE INFORMATION
Acknowledgments
The authors thank University of California San Francisco Pediatric Critical Care Division.
Sources of Funding
This study was supported by Bill & Melinda Gates Foundation (Dr Maltepe) and National Institutes of Health grants R35 5R35NS097299 (Dr Ferriero), R01 HD072455 (Dr Maltepe), and K08NS125042 (Dr Mike).
Disclosures
None.
Supplemental Material
Supplemental Methods
Table S1
Figures S1–S5
ARRIVE Guidelines Checklist
Supplementary Material
Nonstandard Abbreviations and Acronyms
- GFAP
- glial fibrillary acidic protein
- hERG
- human ether-a-go-go–related gene
- HD
- high dose
- HIE
- hypoxic-ischemic encephalopathy
- IFN
- interferon
- LD
- low dose
- LMIC
- low- and middle-income countries
- PVWM
- periventricular white matter
- ROSC
- return of spontaneous circulation
- SII
- systemic immune-inflammation index
- SIRI
- system inflammation response index
- UCO
- umbilical cord occlusion
For Sources of Funding and Disclosures, see page 2714.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.124.048264.
Contributor Information
Yasmine White, Email: yasminewhite17@gmail.com.
Janica Ha, Email: janica.ha@gmail.com.
Ariana Iranmahboub, Email: arianaim66@gmail.com.
Cheryl Hawkins, Email: cheryl.hawkins@ucsf.edu.
Rachel S. Hutchings, Email: rachel.hutchings@ucsf.edu.
Hadiya Manzoor, Email: hadiya.manzoor@ucsf.edu.
Aijun Wang, Email: aawang@ucdavis.edu.
Brian D. Goudy, Email: bgoudy@ucdavis.edu.
Payam Vali, Email: payam.vali@gmail.com.
Satyan Lakshminrusimha, Email: slakshmi@ucdavis.edu.
Janel Long-Boyle, Email: janel.long-boyle@ucsf.edu.
Jeffrey R. Fineman, Email: jeff.fineman@ucsf.edu.
Donna M. Ferriero, Email: donna.ferriero@ucsf.edu.
REFERENCES
- 1.Olusanya BO, Nair MKC, Wright SM, Hadders-Algra M; Global Research on Developmental Disabilities Collaborators (GRDDC). Global progress towards early childhood development for children with disabilities, 2013-2023. BMJ Paediatr Open. 2023;7:e002046. doi: 10.1136/bmjpo-2023-002046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mike JK, Wu KY, White Y, Pathipati P, Ndjamen B, Hutchings RS, Losser C, Vento C, Arellano K, Vanhatalo O, et al. Defining longer-term outcomes in an ovine model of moderate perinatal hypoxia-ischemia. Dev Neurosci. 2022;44:277–294. doi: 10.1159/000525150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thayyil S, Pant S, Montaldo P, Shukla D, Oliveira V, Ivain P, Bassett P, Swamy R, Mendoza J, Moreno-Morales M, et al. ; HELIX Consortium. Hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries (HELIX): a randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob Health. 2021;9:e1273–e1285. doi: 10.1016/S2214-109X(21)00264-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranjan AK, Gulati A. Advances in therapies to treat neonatal hypoxic-ischemic encephalopathy. J Clin Med. 2023;12:6653. doi: 10.3390/jcm12206653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Victor S, Rocha-Ferreira E, Rahim A, Hagberg H, Edwards D. New possibilities for neuroprotection in neonatal hypoxic-ischemic encephalopathy. Eur J Pediatr. 2022;181:875–887. doi: 10.1007/s00431-021-04320-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Mara K, McPherson C. Neuroprotective agents for neonates with hypoxic-ischemic encephalopathy. Neonatal Network. 2021;40:406–413. doi: 10.1891/11-T-755 [DOI] [PubMed] [Google Scholar]
- 7.Davidson JO, Battin MR, Gunn AJ. Implications of the HELIX trial for treating infants with hypoxic-ischaemic encephalopathy in low-to-middle-income countries. Arch Dis Child Fetal Neonatal Ed. 2023;108:83–84. doi: 10.1136/archdischild-2021-323743 [DOI] [PubMed] [Google Scholar]
- 8.Montaldo P, Burgod C, Herberg JA, Kaforou M, Cunnington AJ, Mejias A, Cirillo G, Miraglia Del Giudice E, Capristo C, Bandiya P, et al. Whole-blood gene expression profile after hypoxic-ischemic encephalopathy. JAMA Netw Open. 2024;7:e2354433. doi: 10.1001/jamanetworkopen.2023.54433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruschettini M, Moreira A, Pizarro AB, Mustafa S, Romantsik O. The effects of caffeine following hypoxic-ischemic encephalopathy: a systematic review of animal studies. Brain Res. 2022;1790:147990. doi: 10.1016/j.brainres.2022.147990 [DOI] [PubMed] [Google Scholar]
- 10.Marcus CL, Meltzer LJ, Roberts RS, Traylor J, Dix J, D’Ilario J, Asztalos E, Opie G, Doyle LW, Biggs SN, et al. ; Caffeine for Apnea of Prematurity–Sleep Study. Long-term effects of caffeine therapy for apnea of prematurity on sleep at school age. Am J Respir Crit Care Med. 2014;190:791–799. doi: 10.1164/rccm.201406-1092OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mürner-Lavanchy IM, Doyle LW, Schmidt B, Roberts RS, Asztalos EV, Costantini L, Davis PG, Dewey D, D’Ilario J, Grunau RE, et al. ; Caffeine for Apnea of Prematurity (CAP) Trial Group. Neurobehavioral outcomes 11 years after neonatal caffeine therapy for apnea of prematurity. Pediatrics. 2018;141:e20174047. doi: 10.1542/peds.2017-4047 [DOI] [PubMed] [Google Scholar]
- 12.Sabir H, Maes E, Zweyer M, Schleehuber Y, Imam FB, Silverman J, White Y, Pang R, Pasca AM, Robertson NJ, et al. Comparing the efficacy in reducing brain injury of different neuroprotective agents following neonatal hypoxia-ischemia in newborn rats: a multi-drug randomized controlled screening trial. Sci Rep. 2023;13:9467. doi: 10.1038/s41598-023-36653-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira-Figueiredo D, Nascimento AA, Cunha-Rodrigues MC, Brito R, Calaza KC. Caffeine and its neuroprotective role in ischemic events: a mechanism dependent on adenosine receptors. Cell Mol Neurobiol. 2022;42:1693–1725. doi: 10.1007/s10571-021-01077-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan H, Kaur P, Singh TG, Grewal AK, Sood S. Adenosine as a key mediator of neuronal survival in cerebral ischemic injury. Neurochem Res. 2022;47:3543–3555. doi: 10.1007/s11064-022-03737-3 [DOI] [PubMed] [Google Scholar]
- 15.Schepici G, Silvestro S, Bramanti P, Mazzon E. Caffeine: an overview of its beneficial effects in experimental models and clinical trials of Parkinson’s disease. Int J Mol Sci. 2020;21:4766. doi: 10.3390/ijms21134766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Ghribi O, Geiger JD. Caffeine protects against disruptions of the blood-brain barrier in animal models of Alzheimer’s and Parkinson’s diseases. J Alzheimers Dis. 2010;20:S127–S141. doi: 10.3233/JAD-2010-1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paiva C, Beserra B, Reis C, Dorea JG, Da Costa T, Amato AA. Consumption of coffee or caffeine and serum concentration of inflammatory markers: a systematic review. Crit Rev Food Sci Nutr. 2019;59:652–663. doi: 10.1080/10408398.2017.1386159 [DOI] [PubMed] [Google Scholar]
- 18.Winerdal M, Urmaliya V, Winerdal ME, Fredholm BB, Winqvist O, Ådén U. Single dose caffeine protects the neonatal mouse brain against hypoxia ischemia. PLoS One. 2017;12:e0170545. doi: 10.1371/journal.pone.0170545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alsweiler JM, Bloomfield FH, Harding JE. Early-stage translational research in perinatal medicine. Neonatology. 2019;115:182–188. doi: 10.1159/000493004 [DOI] [PubMed] [Google Scholar]
- 20.Hooper SB, Te Pas AB, Polglase GR, Wyckoff M. Animal models in neonatal resuscitation research: what can they teach us? Semin Fetal Neonatal Med. 2018;23:300–305. doi: 10.1016/j.siny.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 21.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. doi: 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mike JK, White Y, Hutchings RS, Vento C, Ha J, Manzoor H, Lee D, Losser C, Arellano K, Vanhatalo O, et al. Perinatal azithromycin provides limited neuroprotection in an ovine model of neonatal hypoxic-ischemic encephalopathy. Stroke. 2023;54:2864–2874. doi: 10.1161/STROKEAHA.123.043040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mike JK, White Y, Hutchings RS, Vento C, Ha J, Iranmahboub A, Manzoor H, Gunewardena A, Cheah C, Wang A, et al. Effect of clemastine on neurophysiological outcomes in an ovine model of neonatal hypoxic-ischemic encephalopathy. Children (Basel). 2023;10:1728. doi: 10.3390/children10111728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCullagh P. Regression models for ordinal data. Journal of the Royal Statistical Society: Series B (Methodological). 1980;42:109–127. doi: 10.1111/j.2517-6161.1980.tb01109.x [Google Scholar]
- 25.Efron B. Bootstrap methods: another look at the jackknife. Ann Stat. 1979;7:1–26. doi: 10.1214/aos/1176344552 [Google Scholar]
- 26.Alexander M, Smith AL, Rosenkrantz TS, Fitch RH. Therapeutic effect of caffeine treatment immediately following neonatal hypoxic-ischemic injury on spatial memory in male rats. Brain Sci. 2013;3:177–190. doi: 10.3390/brainsci3010177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.New Drug Application (NDA): 020793, CAFCIT®. Drugs@FDA: FDA-approved drugs. Accessed September 18, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020793s019lbl.pdf.
- 28.Vesoulis ZA, McPherson C, Neil JJ, Mathur AM, Inder TE. Early high-dose caffeine increases seizure burden in extremely preterm neonates: a preliminary study. J Caffeine Res. 2016;6:101–107. doi: 10.1089/jcr.2016.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda H, Hasumi A, Yoshida KI. Caffeine-induced bradycardia, death, and anxiety-like behavior in zebrafish larvae. Forensic Toxicol. 2021;39:427–436. doi: 10.1007/s11419-021-00577-8 [Google Scholar]
- 30.Voskoboinik A, Kalman JM, Kistler PM. Caffeine and arrhythmias: time to grind the data. JACC Clin Electrophysiol. 2018;4:425–432. doi: 10.1016/j.jacep.2018.01.012 [DOI] [PubMed] [Google Scholar]
- 31.Papastylianou A, Mentzelopoulos S. Current pharmacological advances in the treatment of cardiac arrest. Emerg Med Int. 2012;2012:815857. doi: 10.1155/2012/815857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McPherson C, Neil JJ, Tjoeng TH, Pineda R, Inder TE. A pilot randomized trial of high-dose caffeine therapy in preterm infants. Pediatr Res. 2015;78:198–204. doi: 10.1038/pr.2015.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao X, Zhu Q, Xia X, Yao B, Liang S, Chen Z, Wu M. The correlation between novel peripheral blood cell ratios and 90-day mortality in patients with acute ischemic stroke. PLoS One. 2020;15:e0238312. doi: 10.1371/journal.pone.0238312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ceran B, Alyamaç Dizdar E, Beşer E, Karaçağlar NB, Sari FN. Diagnostic role of systemic inflammatory indices in infants with moderate-to-severe hypoxic ischemic encephalopathy. Am J Perinatol. 2021;248:41. doi: 10.1055/a-1673-1616 [DOI] [PubMed] [Google Scholar]
- 35.Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T, Prass K, Meisel A. Stroke-induced immunodepression. Stroke. 2007;38:770–773. doi: 10.1161/01.STR.0000251441.89665.bc [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Gao Z, Wang D, Zhang T, Sun B, Mu L, Wang J, Liu Y, Kong Q, Liu X, et al. Accumulation of natural killer cells in ischemic brain tissues and the chemotactic effect of IP-10. J Neuroinflammation. 2014;11:79. doi: 10.1186/1742-2094-11-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amin M, Vakilian A, Mahmoodi MH, Hassanshahi G, Falahati-Pour SK, Dolatabadi MR, Nadimi AE. Circulatory levels of C-X-C motif chemokine ligands 1, 9, and 10 are elevated in patients with ischemic stroke. Eurasian J Med. 2017;49:92–96. doi: 10.5152/eurasianjmed.2017.17022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teo EJ, Chand KK, Miller SM, Wixey JA, Colditz PB, Bjorkman ST. Early evolution of glial morphology and inflammatory cytokines following hypoxic-ischemic injury in the newborn piglet brain. Sci Rep. 2023;13:282. doi: 10.1038/s41598-022-27034-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabolacci C, Cordella M, Rossi S, Bonaccio M, Eramo A, Mischiati C, Beninati S, Iacoviello L, Facchiano A, Facchiano F. Targeting melanoma-initiating cells by caffeine: in silico and in vitro approaches. Molecules. 2021;26:3619. doi: 10.3390/molecules26123619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koss CK, Wohnhaas CT, Baker JR, Tilp C, Przibilla M, Lerner C, Frey S, Keck M, Williams CMM, Peter D, et al. IL36 is a critical upstream amplifier of neutrophilic lung inflammation in mice. Commun Biol. 2021;4:172. doi: 10.1038/s42003-021-01703-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernis ME, Zweyer M, Maes E, Schleehuber Y, Sabir H. Neutrophil extracellular traps release following hypoxic-ischemic brain injury in newborn rats treated with therapeutic hypothermia. Int J Mol Sci. 2023;24:3598. doi: 10.3390/ijms24043598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stockwell J, Chen Z, Niazi M, Nosib S, Cayabyab FS. Protein phosphatase role in adenosine A1 receptor-induced AMPA receptor trafficking and rat hippocampal neuronal damage in hypoxia/reperfusion injury. Neuropharmacology. 2016;102:254–265. doi: 10.1016/j.neuropharm.2015.11.018 [DOI] [PubMed] [Google Scholar]
- 43.Li HL, Zaghloul N, Ahmed I, Omelchenko A, Firestein BL, Huang H, Collins L. Caffeine inhibits hypoxia-induced nuclear accumulation in HIF-1α and promotes neonatal neuronal survival. Exp Neurol. 2019;317:66–77. doi: 10.1016/j.expneurol.2019.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang L, Yu X, Zhang Y, Liu N, Xue X, Fu J. Caffeine treatment started before injury reduces hypoxic–ischemic white-matter damage in neonatal rats by regulating phenotypic microglia polarization. Pediatr Res. 2022;92:1543–1554. doi: 10.1038/s41390-021-01924-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S, Zhang X, Liu Y, Yuan X, Yang L, Zhang R, Zhang X, Wang X, Xu F, Zhu C. Early application of caffeine improves white matter development in very preterm infants. Respir Physiol Neurobiol. 2020;281:103495. doi: 10.1016/j.resp.2020.103495 [DOI] [PubMed] [Google Scholar]
- 46.Braunstein M, Williamson M, Kusmenkov T, Landes J, Biberthaler P, Kanz KG, Böcker W, Bogner V. Significant cytokine mRNA expression changes immediately after initiation of cardiopulmonary resuscitation. Mediators Inflamm. 2017;2017:8473171. doi: 10.1155/2017/8473171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pingel J, Barber L, Andersen IT, Walden FV, Wong C, Døssing S, Nielsen JB. Systemic inflammatory markers in individuals with cerebral palsy. Eur J Inflamm. 2019;17:205873921882347. doi: 10.1177/2058739218823474 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon reasonable request. Full descriptions of methods are available in the Supplemental Material.