Abstract
Background:
Female patients using indwelling urinary catheters (IUCs) are disproportionately at risk for developing catheter-associated urinary tract infections (CAUTIs) compared to males. Female external urine wicking devices (FEUWDs) have emerged as potential alternatives to IUCs for incontinence management.
Objectives:
To assess the clinical risks and benefits of FEUWDs as alternatives to IUCs.
Methods:
Ovid MEDLINE, Embase, Scopus, Web of Science Core Collection, CINAHL Complete, and ClinicalTrials.gov were searched from inception to July 10, 2023. Included studies used FEUWDs as an intervention and reported measures of urinary tract infections and secondary outcomes related to incontinence management.
Results:
Of 2,580 returned records, 50 were systematically reviewed. Meta-analyses assessed rates of indwelling CAUTIs and IUC utilization. Following FEUWD implementation, IUC utilization rates decreased 14% (RR = 0.86, 95% CI = [0.76, 0.97]) and indwelling CAUTI rates nonsignificantly decreased up to 32% (IRR = 0.68, 95% CI = [0.39, 1.17]). Limited only to studies that described protocols for implementation, the incidence rate of indwelling CAUTIs decreased significantly up to 54% (IRR = 0.46, 95% CI = [0.32, 0.66]). Secondary outcomes were reported less routinely.
Conclusions:
Overall, FEUWDs nonsignificantly reduced indwelling CAUTI rates, though reductions were significant among studies describing FEUWD implementation protocols. We recommend developing standard definitions for consistent reporting of non-indwelling CAUTI complications such as FEUWD-associated UTIs, skin injuries, and mobility-related complications.
Introduction
Catheter-associated urinary tract infections (CAUTIs) are a leading source of hospital-acquired infections and associated with complications that increase patient morbidity 1,2 . Many hospital-acquired urinary tract infections develop in patients using indwelling urinary catheters (IUCs) (i.e., Foley catheters) 2 . IUCs disrupt innate immune defenses, causing biofilm formation on catheter surfaces and subsequent bacteriuria 1 . The risk of bacteriuria increases each day an IUC is in place, making it imperative to limit the duration of catheterization or avoid catheterization altogether 3 .
IUC alternatives for male patients have existed for quite some time 4–6 . Yet, until recently, a viable alternative for female patients has been lacking. In 2016, a noninvasive collection device designed for female genitalia was developed and trademarked as PureWick 7 . Utilizing a soft and flexible wicking material to absorb urine, which is gently drawn away from the body using low, continuous suction, it is placed between the labia majora, with the wicking material facing the body, the top of the device aligned with the pubic bone, and the bottom tucked into the gluteal cleft 8–10 . These devices have the potential to reduce indwelling CAUTI incidence in female patients, who are particularly susceptible to CAUTIs given the short length of the female urethra and its proximity to the perineum. Current research has examined two similar female external urine wicking devices (henceforth, FEUWDs) that differ only slightly in shape and options for securing the device to the patient: PureWick (Becton, Dickinson and Co.) and PrimaFit (Sage Products LLC) 11,12 . Here, we perform a systematic review and meta-analysis of the existing literature to assess the clinical risks and benefits of FEUWDs, hypothesizing that FEUWD use would result in reduced CAUTI rates (without increasing non-indwelling catheter-associated UTIs) due to their less invasive technology than traditional indwelling catheters. Because non-indwelling catheter-associated UTI data were insufficient, our analysis was only able to evaluate the effect of FEUWD use on CAUTI rates.
Methods
We are compliant with Meta-Analysis of Observation Studies in Epidemiology (MOOSE) reporting guidelines. This systematic review was registered in PROSPERO (ID: CRD42022340503). Protocol updates are provided in Supplement Methods 1.
Data sources and searches
The following databases were searched from inception to July 12, 2022 to identify relevant articles, trials, or meeting abstracts describing alternatives to indwelling catheters: Ovid Medline (Ovid Medline, Embase.com, Scopus, Web of Science Core Collection, CINAHL Complete, and ClinicalTrials.gov. Search updates were performed on March 24, 2023 and July 10, 2023. Each search utilized controlled vocabulary in combination with relevant keywords, and no limits were applied. Reference tracking was performed on highly relevant articles. Original search strategies were developed in Ovid Medline and translated to other databases using the Systematic Review Accelerator Polyglot tool 13 . Complete search terms and strategies are available in Supplement Methods 2.
Study selection
Eligibility criteria were left broad to capture all possible relevant data. Studies of female and male patients were eligible for inclusion based on reports that certain male patients (such as those with penile retraction) may use FEUWDs despite not being designed for male use. Furthermore, both randomized and non-randomized studies, published or unpublished, were eligible for inclusion, given that they implemented an appropriate intervention (i.e., FEUWD) and reported at least one outcome of interest.
Although FEUWDs are the intervention of interest, no formal definition of female external urinary device-associated UTIs (FEUWD-UTIs) currently exists (Supplement Methods 3). Because FEUWD use is hypothesized to reduce IUC use and, consequently, indwelling catheter-associated UTI (CAUTI) rates, indwelling CAUTIs are evaluated as the primary outcome. Secondary outcomes included IUC use, antibiotic use, skin breakdown, skin pressure injury, vaginal/vulvar dryness or chaffing, cost effectiveness, and mobility-related complications.
Citations were deduplicated by librarian WT using a modified version of the Bramer Endnote Deduplication Technique 14 . Within Rayyan, each record was screened by two independent, trained investigators: NP (Records 1-1,256), JW (1–592), JY (593–1,256) 15 . Records were selected if they made sufficient reference to the intervention and outcome(s) of interest or if reference to “external urinary catheters” was vague and needed further clarification.
Having obtained full-text records, citations were discarded if they contained no additional clarification or the wrong catheter type was identified. Records published prior to 2015 were excluded as FEUWDs were brought to market in 2016. Still, experimental use of the devices might have occurred earlier; thus, we restricted included studies to only those dated 2015 to present. At each step, discrepancies in reviewer decisions were resolved through deliberation.
Data extraction and quality assessment
Remaining records underwent dual independent review: NP (Records 1–50), JW (1–20), JY (21–50). Outcome data was collected in identical electronic data extraction forms. Two independent reviewers used the Risk of Bias in Non-Randomized Studies—of Interventions (ROBINS-I) tool to assess the methodological quality of included records, excluding abstracts, which provide limited information on methods used, and non-interventional studies (NP (Records 1–14), JA (1–3), JW (4–6), JY (7–14)) (16).
Data synthesis and analysis
Studies included in meta-analyses provided sufficient data to calculate or infer the rate ratios of CAUTIs per 1,000 patient-days (pd), CAUTIs per 1,000 device-days (dd), or IUC utilization (device-days per patient-days) from pre- to post-FEUWD implementation periods. Random-effects meta-analysis models then determined the pooled effect of FEUWD implementation on the rate of CAUTIs per 1,000 pd, CAUTIs per 1,000 dd, and IUC utilization. Although IUC use is a secondary outcome, it was frequently reported and therefore included in meta-analysis. Other secondary outcomes, although reported inconsistently, were systematically evaluated and summarized. We planned to assess publication bias using funnel plot visualization and Egger’s regression tests.
Some studies included in the meta-analyses explicitly described the use of protocols for FEUWD implementation. However, other studies either did not follow a protocol or failed to specify. Because the nature of implementation could be unclear, studies were categorized post hoc according to their overall risk of bias score (ROB), which reflects the strength of implementation protocols. Measures of heterogeneity were calculated for subgroup and overall random-effects models (Cochran’s Q, I 2, and τ2). Statistical analyses were performed using R Statistical Software, version 4.1.2 (metafor package) 17 .
Role of the funding source
The funder had no role in any part of the design or conduct of the study.
Results
Fifty records were included for review (Supplement Fig. 1). Two randomized control trials were reviewed for eligibility: (1) a pilot in collaboration with Becton, Dickinson and Co. studying the use of PureWick to manage nocturia and nighttime falls associated with going to the bathroom and (2) a C.R. Bard-sponsored trial evaluating risk of skin injury and the urine capture rate of PureWick in incontinent women requiring diapers 18,19 . Because these are ongoing, no results were available for consideration. Consequently, all 50 records were non-randomized intervention studies. Only 14 studies, providing sufficient methodological information and interventional in design, were evaluated for quality. Of these, 7 studies provided adequate outcomes data required for inclusion in meta-analyses. All 7 studies included in meta-analyses demonstrated a moderate or serious risk of bias overall (Supplement Fig. 2).
Table 1 summarizes the outcomes reported in each record. Most reported some measure of UTI and IUC utilization, though only two reported FEUWD-UTIs. With few exceptions, all abstracts reported decreased indwelling CAUTI incidence upon FEUWD implementation, although reporting of statistical significance, confidence intervals, and sample sizes varied. Study characteristics and outcomes of records included in meta-analyses are given in Table 2, with more detailed outcomes provided in Supplement Table 1. Of the 7 studies included in meta-analyses, studies either implemented FEUWDs amid other interventions designed to reduce IUC utilization and CAUTI rates, which could have influenced outcomes, or did not specify (Table 2). Study characteristics and outcomes of all other records are provided in Supplement Table 2. Table 3 provides a summary of secondary outcomes.
Table 1.
Summary of outcomes reported in included records*
| 1st Author, Year | Type of Outcome Reported | |||||
|---|---|---|---|---|---|---|
| UTI, CAUTI, or FEUWD-UTI | IUC Utilization | Urine Cultures Ordered | Bacteriuria | Skin Complications | Other | |
| Peer Reviewed Published Manuscripts | ||||||
| Beeson, 2023 20 | CAUTI | X | UC | |||
| Cilluffo, 2022 21 | CAUTI | X | X | CS | ||
| Eckert, 2020 22 | CAUTI | X | ||||
| Jasperse, 2022 23 | UTI, CAUTI, FEUWD-UTI | X | X | LOH, ABX, MRC | ||
| Khosla, 2022 24 | CS | |||||
| Lem, 2022 25 | UTI, CAUTI, FEUWD-UTI | X | X | X | LOH, ABX, MRC | |
| Noval, 2022 26 | CAUTI | X | X | ABX | ||
| Rearigh, 2021 27 | CAUTI | X | X | |||
| Root, 2021 28 | X | CS | ||||
| Rose, 2021 29 | UC | |||||
| Tran, 2023 30 | CAUTI | X | X | CE, CS | ||
| Van Decker, 2021 31 | CAUTI | |||||
| Warren, 2020 32 | CAUTI | X | ||||
| Whitaker, 2023 33 | CAUTI | X | ||||
| Won, 2023 34 | CAUTI | X | X | |||
| Zavodnick, 2020 35 | CAUTI | X | CS | |||
| Peer Reviewed Abstracts | ||||||
| Auten, 2021 36 | CAUTI | |||||
| Beeson, 2018 37 | CAUTI | X | ||||
| Behrend, 2018 38 | CAUTI | X | UC, CE, CS | |||
| Cassone, 2022 39 | UC | |||||
| Chirca, 2018 40 | CAUTI | X | ||||
| Dublynn, 2019 41 | CAUTI | X | ||||
| Ecklund, 2020 42 | CAUTI | X | X | MRC, UC | ||
| Fields, 2019 43 | CAUTI | X | ||||
| Figueredo, 2020 44 | CAUTI | X | ||||
| Fritsch, 2019 45 | CAUTI | X | ||||
| Gentile, 2020 46 | CAUTI | X | ||||
| Goris, 2020 47 | CAUTI | X | ||||
| Gutzmirtl, 2019 48 | CAUTI | X | ||||
| Henry, 2019 49 | CAUTI | CE | ||||
| Hughes, 2020 50 | CAUTI | X | ||||
| Kelly, 2018 51 | CE | |||||
| Kelly, 2022 52 | CE | |||||
| Kuzow, 2019 53 | CAUTI | X | ||||
| Maydick-Youngberg, 2020 54 | X | |||||
| Mayes, 2020 55 | CAUTI | X | X | |||
| McRae, 2023 56 | UC, CS | |||||
| Mena Lora, 2020 57 | CAUTI | X | ||||
| Minor, 2022 58 | CAUTI | |||||
| Mueller, 2019 59 | CAUTI | X | ||||
| Nalbandian, 2022 60 | CAUTI | X | ||||
| Ohanian, 2022 61 | CAUTI | X | ||||
| Pavlovsky, 2020 62 | X | |||||
| Peters, 2021 63 | UTI | X | ||||
| Reeths, 2020 64 | X | |||||
| Riemenschneider, 2020 65 | X | |||||
| Srisatidnarakul, 2021 66 | CAUTI | X | ||||
| Sundhu, 2022 67 | LOH | |||||
| Taneja, 2023 68 | CS | |||||
| Wilkerson, 2020 69 | CAUTI | X | ||||
All studies are based in the United States except for Cilluffo et al. (Italy).
UTI, urinary tract infection not associated with an indwelling urinary catheter.
CAUTI, indwelling catheter-associated urinary tract infection.
FEUWD-UTI, female external urine wicking device-associated urinary tract infection.
IUC Utilization, indwelling urinary catheter utilization.
Urine Cultures Ordered, a test ordered by a clinician to determine the presence of infectious microorganisms in urine samples.
Bacteriuria, a positive culture of bacteria in urine (with or without symptoms of UTI, treated or untreated with antibiotics).
Skin Complications, inclusive of skin dermatitis, dermatologic allergic reaction, skin breakdown, pressure injuries.
Other: LOH, length of hospitalization; ABX, antibiotic use; MRC, mobility-related complications (e.g., deep vein thrombosis, falls); UC, urine collection; CE, cost-effectiveness; CS, comfort or patient/provider satisfaction.
Table 2.
Characteristics and outcomes of peer-reviewed published manuscripts included in meta-analyses
| 1st Author, Year | Design | Setting | Study Population | No. of Patients | FEUWD Used | Other CAUTI Prevention Strategies | Indwelling CAUTI Trend |
|---|---|---|---|---|---|---|---|
| Beeson, 2023 20 | Pre-post | Critical, progressive care units | Female inpatients requiring UIM, ≥ 18 | NR | PrimaFit | Nurse-empowered IUC removal | Decreased |
| Eckert, 2020 22 | Pre-post | ICU, telemetry, med-surg, orthopedic-neurology, acute rehabilitation inpatient units | Female inpatients requiring UIM | NR | PureWick | CAUTI reduction initiative, auditing bundle adherence* | Decreased |
| Jasperse, 2022 23 | Pre-post | Internal medicine, family medicine, neurology units | Female patients, ≥ 18 | 848 (292 received intervention) | PureWick | NS | Increased |
| Lem, 2022 25 | Pre-post | General surgery or another surgical subspecialty | Female patients, ≥ 18 | 906 (127 received intervention) | PureWick | IUC reduction initiative † | Increased |
| Noval, 2022 26 | Pre-post | ICU (medical, surgical, neurocritical, cardiac surgery) | Female ICU patients | 4,640 patient encounters (∼771 received intervention) | PureWick | NS | Decreased |
| Rearigh, 2021 27 | Pre-post | Hospital-wide (medical and surgical services) | Female inpatients | 2,347 received intervention | PureWick | CAUTI reduction initiative* | Decreased‡ |
| Zavodnick, 2020 35 | Pre-post | ICU | Female ICU patients, ≥ 18 | NR | PureWick | Nurse-empowered IUC removal | Decreased‡ |
Author indicated the use of simultaneous CAUTI reduction initiatives, such as CAUTI bundles or QI projects, but did not provide a specific explanation of included components.
Author indicated the use of simultaneous IUC reduction initiatives but did not provide a specific explanation of included components (e.g., IUC indication restrictions, reminders, or stop orders).
Incidence rate ratios were calculated using only female patient-days. Many studies did not stratify patient-days by sex and were only able to calculate rate ratios using total (male and female) patient-days.
FEUWD, female external urine wicking device; UIM, urinary management; NR, not reported; NS, not specified.
Table 3.
Summary of secondary outcomes
| Outcome | No. of Studies | Findings | Conclusion |
|---|---|---|---|
| Length of Hospitalization | 2 23,25 | FEUWD implementation increased median LOH.* | More data needed |
| Antibiotic Use | 3 23,25,26 | 2 studies found E. coli was the primary causative agent of UTIs in patients with IUCs and FEUWDs.*Another found no change in antibiotic prescribing patterns before and after FEUWD implementation. | More data needed |
| Skin Complications | 8 20,23,25,30,34,38,42,65 | 3 studies reported erythema and skin breakdown due to device firmness, high suction, or allergic reaction to device material. 1 study found that hospital-associated pressure injuries (HAPIs) nonsignificantly increased after FEUWD implementation.† Still, 3 other studies reported HAPIs were avoided or reduced following FEUWD implementation. Several studies reported no or minimal skin breakdown and reductions in incontinence-associated dermatitis. | FEUWDs slightly favored |
| Mobility-Related Complications | 2 23,25 | Cases of deep vein thrombosis and pulmonary embolism were reported following FEUWD implementation.* | More data needed |
| Patient and Nurse Satisfaction | 8 22,24,26,28,30,35,38,68 | Satisfaction was high. Studies indicated good patient and caregiver satisfaction with home, outpatient community, and hospice care use of PureWick. | FEUWDs are favored to IUCs |
| Urine Specimen Collection | 5 20,29,38,39,42 | There were several reports of successful urine diversion and input/output measurements. Validation studies confirmed good agreement between urine samples from normal voiding and PureWick collection for several analytes, including urine protein concentrations. Test strip analysis and automated analysis are recommended, but microscopy is not due to filtration of certain analytes. Because bacteria may grow in the collection chamber, FEUWDs are not recommended for urinalysis. | Little difference between FEUWDs and IUCs (depends on method) |
| Cost-Effectiveness | 6 30,38,49,51,52,57 | Analyses have shown potential savings associated with FEUWD use through the avoidance of toilet-related falls and reduction in time required for incontinence care. Individual institutions have also generated their own estimates of cost savings, ranging from $13,786 on a per-patient basis to $1 million per year at a large academic hospital due to reduced CAUTIs and Medicare fines. | Cost-neutral to cost-saving |
These studies failed to track temporal changes in IUC and FEUWD status, making it difficult to determine if outcomes were associated with IUC or FEUWD use.
HAPIs are multifactorial and may not be associated with urinary catheter use.
Meta-analyses
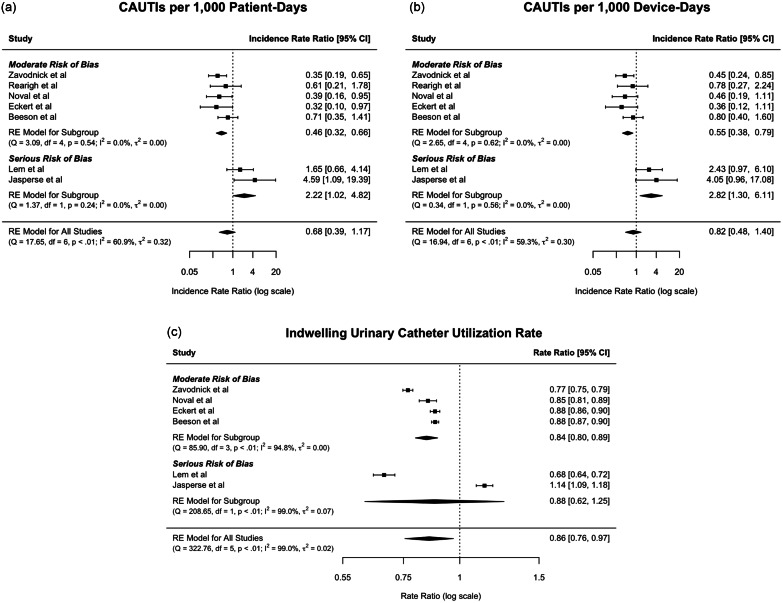
Due to limited reported data, only 7 studies contributed to the pooled estimates of CAUTIs per 1,000 pd and CAUTIs per 1,000 dd, while 6 studies contributed to the pooled estimate of IUC utilization. Overall, there was no significant difference in indwelling CAUTI rates before and after FEUWD implementation (Fig. 1). That is, while CAUTIs per 1,000 pd decreased 32% following implementation of FEUWDs (IRR = 0.68, 95% CI = [0.39, 1.17], p = .1658, I 2 = 60.9%) and CAUTIs per 1,000 dd decreased 18% (IRR = 0.82, 95% CI = [0.48, 1.40], p = .4564, I 2 = 59.3%), neither reduction was significant. The pooled rate of IUC utilization indicates that IUC use decreased significantly by 14% after implementation of FEUWDs (RR = 0.86, 95% CI = [0.76, 0.97], p = .0136, I 2 = 99.0%).
Figure 1.
Meta-analyses of the effect of FEUWDs on CAUTI rates and IUC utilization.
Note that Fig. 1c has a different scale to facilitate the visualization of very narrow confidence intervals.
FEUWD, female external urine wicking device; RE, random effects.
Heterogeneity across studies was high. However, according to post hoc analyses, there were significant reductions and less heterogeneity in studies with moderate ROBs. Specifically, CAUTIs per 1,000 pd decreased 54% (IRR = 0.46, 95% CI = [0.32, 0.66], p < .001, I 2 = 0.0%) and CAUTIs per 1,000 dd decreased 45% (IRR = 0.55, 95% CI = [0.38, 0.79], p = .0014, I 2 = 0.0%). In this subgroup, there was a reduction of 16% for the IUC utilization rate (RR = 0.84, 95% CI = [0.80, 0.89], p < .001, I 2 = 94.8%), though heterogeneity remained high.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis of clinical outcomes associated with female external urine wicking devices (FEUWDs), which have the potential to supplement existing CAUTI prevention initiatives. The results of this meta-analysis suggest that rigorously protocolized implementations of FEUWDs can significantly reduce indwelling urinary catheter (IUC) utilization and indwelling CAUTI rates in hospital settings, though the inconsistent reporting of outcomes such as FEUWD-associated UTIs limits our ability to fully assess the original hypothesis. Secondary outcomes like skin injury and mobility-related complications were reported rarely and with varied or unspecified definitions.
Meta-analyses demonstrated that studies with moderate ROBs were associated with significant reductions in IUC utilization and indwelling CAUTI rates. Because these studies typically indicated the use of protocols to guide their introductions of FEUWDs, it appears that the process by which FEUWDs are implemented is an important determinant of their effect on IUC utilization and indwelling CAUTI rates. Implementation of FEUWDs may reduce CAUTIs through two paths: (1) by reducing IUC utilization (i.e., preventing the onset of the “lifecycle of the catheter”)—patients never catheterized cannot, by definition, have a CAUTI, while patients with fewer catheter-days are at lower risk of CAUTI 71 —and (2) by reducing CAUTI rates among those catheterized (CAUTI per dd) if FEUWD roll-out was part of a “bladder bundle” that optimizes CAUTI prevention practices 72 . Based on the pooled estimates, IUC utilization was reduced by 14% while CAUTI rates among those catheterized were reduced by 45%, suggesting that pathway (2) is very important. This is consistent with protocols that direct FEUWD use toward patients at higher risk of CAUTI but could also reflect bias from other efforts to reduce CAUTIs. FEUWDs also appear to be a promising means of accurate urine collection, associated with high user satisfaction, and a potential cost-cutting intervention, though the need for frequent replacement (i.e., every 8–12 hours or when soiled by blood or stool) might countervail potential savings.
Limitations
All studies included in meta-analyses demonstrated a moderate to serious risk of bias. Because several studies were conducted as QI projects rather than controlled interventions, implementation protocols varied across sites, which contributed to heterogenous effects. This also typically meant that the introduction of FEUWDs occurred alongside existing interventions to reduce CAUTIs. Studies with moderate ROBs failed to adequately account for confounding interventions, which undermined the internal validity of their findings and may be attributable to weak implementation protocols. Studies with serious ROBs demonstrated additional methodological problems. For example, both studies with serious ROBs, which shared language to describe the absence of an implementation protocol, failed to account for temporal changes to intervention status, making it difficult to ascertain which device type (FEUWD or IUC) had the effect of increasing their indwelling CAUTI and FEUWD-associated UTI rates 23,25 .
In the meta-analyses, confidence intervals for IUC utilization rates are exceedingly narrow because included studies treated each patient-day independently rather than nested within patients and we lack the information needed to adjust for this properly. Another limitation is that some studies calculated rates based on total (male and female) patient-days 20,35 . Because interest lies in the comparison of rate ratios as opposed to rates themselves, the rate ratios for these studies can still be compared to others. However, this relies on the assumption that the fraction of female patient-days to total patient-days is relatively stable between pre- and post-intervention periods.
Although publication bias is a potential limitation (Supplement Fig. 3), the low number of studies included in meta-analyses and considerable inter-study heterogeneity make this difficult to assess 70 . The risk of publication bias should be reassessed when more studies are available to be included in meta-analysis.
Recommendations
Because the quality of existing data regarding the clinical outcomes of FEUWDs is limited, the results of our systematic review and meta-analysis should be interpreted judiciously. Nevertheless, we feel that it is important to share these findings to improve the quality of future research on FEUWDs, and we have identified areas for improvement to help researchers achieve this aim.
First, we recommend developing a standardized definition of female external urine wicking device-associated urinary tract infections (FEUWD-UTIs) to facilitate comparison across future studies. In addition to the joint reporting of IUC utilization and indwelling CAUTI rates, FEUWD utilization and FEUWD-UTI rates should be reported, as well as skin- and mobility-related complications associated with FEUWD use. Although some studies have reported measures of FEUWD use, they vary greatly and are often not expressed as device-days per patient-days 22,23,25,27,30,32,33,46,63 . It would also be useful for researchers to report IUC utilization and indwelling CAUTI rates among female patients only so that evaluations of the relationship between FEUWD and IUC use are specific to the patient population of interest.
Ultimately, more rigorously protocolized intervention studies reporting these outcomes are needed to provide sufficient data to measure the correlation between device utilization and UTI outcomes, which could corroborate the hypothesized mechanism by which FEUWDs reduce UTI rates. Ideal FEUWD implementation protocols should include: (1) standardized guidelines for FEUWD use rather than clinician discretion only, (2) consistent and standardized charting, (3) maintained attention to local hygiene as would be expected for IUCs, and (4) documentation of all potential FEUWD-associated complications.
There is also variation in the types of units in which FEUWDs are implemented, which may be an important factor in gauging FEUWD utility. We recommend that FEUWDs first be introduced in the ICU. Warren et al. (2021) recommended first implementing FEUWDs in ICUs given their observation that PureWick implementation had the greatest effect on IUC utilization and indwelling CAUTI rates in ICU patients 32 . Similarly, Gentile et al. (2020) observed significant reductions in IUC utilization and indwelling CAUTI rates after introduction of PureWick in ICUs only 46 . Because ICUs tend to have the greatest number of patients using IUCs, CAUTI incidence is likely to be higher than on other hospital units. These findings suggest that where the preponderance of CAUTI is greatest, so too will be the protective effect of FEUWDs. Further research is needed to ascertain FEUWD effectiveness in environments with traditionally lower IUC use. Still, even in settings with low baseline IUC utilization, Gentile et al. (2020) found that successful FEUWD implementation can lead to further, if modest, reductions in IUC utilization and indwelling CAUTI rates 46 .
Conclusions
FEUWDs have the potential to significantly reduce IUC utilization and indwelling CAUTI rates in hospital settings when introduced with a protocol to guide use. Although secondary outcomes of FEUWD use were reported less routinely, there is evidence that their benefit extends beyond reducing CAUTI incidence. Inconsistent implementation protocols contribute to significant heterogeneity and studies likely do not control for important confounders, limiting our understanding of the true effect of FEUWDs. Improved implementation protocols and a standardized definition of FEUWD-UTIs should help harmonize interventions and comparisons of effects across future studies.
Supporting information
Pryor et al. supplementary material
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/ice.2024.73.
Financial support
This project was funded under grant number R01HS026912 from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services (HHS). The authors are solely responsible for this document’s contents, findings, and conclusions, which do not necessarily represent the views of AHRQ. Jennifer Meddings is an employee of Michigan Medicine with the University of Michigan and a Federal Employee of the VA Ann Arbor Healthcare System. She reports grant funding from the AHRQ, CDC, Ralph E. Wilson Foundation, and the VA HSR&D.
Competing interests
Jennifer Meddings works as an Associate Editor for the Annals of Internal Medicine: Clinical Cases journal.
References
- 1. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections [published correction appears in N Engl J Med. 2022 Jun 16;386(24):2348]. N Engl J Med 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf 2014;23:277–289. doi: 10.1136/bmjqs-2012-001774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010;50:625–663. doi: 10.1086/650482 [DOI] [PubMed] [Google Scholar]
- 4. Gray M, Skinner C, Kaler W. External collection devices as an alternative to the indwelling urinary catheter: Evidence-based review and expert clinical panel deliberations. J Wound Ostomy Continence Nurs 2016;43:301–307. doi: 10.1097/WON.0000000000000220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc 1999;47:1453–1457. doi: 10.1111/j.1532-5415.1999.tb01567.x [DOI] [PubMed] [Google Scholar]
- 6. Saint S, Kaufman SR, Rogers MA, et al. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc 2006;54:1055–1061. doi: 10.1111/j.1532-5415.2006.00785.x [DOI] [PubMed] [Google Scholar]
- 7. Newton C, Call E, Chan KSL. Measuring safety, effectiveness and ease of use of PureWick in the management of urinary incontinence in bedbound women: case studies. 2016. https://docplayer.net/54084269-Measuring-safety-effectiveness-and-ease-of-use-of-purewick-in-the-management-of-urinary-incontinence-in-bedbound-women-case-studies.html. Accessed January 18, 2024.
- 8. Uhr A, Glick L, Barron S, et al. How I do it: PureWick female external catheter: a non-invasive urine management system for incontinent women. Can J Urol 2021;28:10669–10672. [PubMed] [Google Scholar]
- 9. Bagley K, Severud L. Preventing catheter-associated urinary tract infections with incontinence management alternatives: PureWick and condom catheter. Nurs Clin North Am 2021;56:413–425. doi: 10.1016/j.cnur.2021.05.002 [DOI] [PubMed] [Google Scholar]
- 10. Beeson T, Davis C. Urinary management with an external female collection device. J Wound Ostomy Continence Nurs 2018;45:187–189. doi: 10.1097/WON.0000000000000417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The PureWickTM Urine Collection System for Women: Purewick at Home. https://www.purewickathome.com/. Accessed January 18, 2024. [Google Scholar]
- 12. PrimaFit® External Urine Management System for Females – Sage Products LLC. Sageproducts.com. Published 2019. https://sageproducts.com/primafit-external-urine-management-system-for-females/. Accessed January 18, 2024.
- 13. Clark JM, Sanders S, Carter M, et al. Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial. J Med Libr Assoc 2020;108:195–207. doi: 10.5195/jmla.2020.834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bramer WM, Giustini D, de Jonge GB, et al. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc 2016;104:240–243. doi: 10.3163/1536-5050.104.3.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan – a web and mobile app for systematic reviews. Syst Rev 2016;5. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1–48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 18. Chughtai B. A pilot study to evaluate PureWick for nocturia. ClinicalTrials.gov identifier: NCT05090722. Updated October 16, 2023. https://clinicaltrials.gov/ct2/show/NCT05090722. Accessed January 18, 2024.
- 19. Bard CR. A comparative crossover study on the safety, efficacy, and patient quality of life comparing PureWickTM system with an established comparator in the home setting for incontinence overnight. Clinicaltrials.gov identifier: NCT05710718. Updated November 2, 2023. https://clinicaltrials.gov/ct2/show/NCT05710718. Accessed January 18, 2024.
- 20. Beeson T, Pittman J, Davis CR. Effectiveness of an external urinary device for female anatomy and trends in catheter-associated urinary tract infections. J Wound Ostomy Continence Nurs 2023;50:137–141. doi: 10.1097/WON.0000000000000951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cilluffo S, Terzoni S, Destrebecq A, Lusignani M. Efficacy, effectiveness, usability and acceptability of devices for female urinary incontinence: a scoping review. J Clin Nurs Published online July 18, 2022. doi: 10.1111/jocn.16457 [DOI] [PubMed] [Google Scholar]
- 22. Eckert L, Mattia L, Patel S, et al. Reducing the risk of indwelling catheter-associated urinary tract infection in female patients by implementing an alternative female external urinary collection device: a quality improvement project. J Wound Ostomy Continence Nurs 2020;47:50–53. doi: 10.1097/WON.0000000000000601 [DOI] [PubMed] [Google Scholar]
- 23. Jasperse N, Hernandez-Dominguez O, Deyell JS, et al. A single institution pre-/post-comparison after introduction of an external urinary collection device for female medical patients. J Infect Prev 2022;23:149–154. doi: 10.1093/ofid/ofaa439.995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khosla L, Sani JM, Chughtai B. Patient and caretaker satisfaction with the PureWick system. Can J Urol 2022;29:11216–11223. [PubMed] [Google Scholar]
- 25. Lem M, Jasperse N, Grigorian A, et al. Effect of external urinary collection device implementation on female surgical patients. Infect Dis Health 2022;23:23. doi: 10.1016/j.idh.2022.05.005 [DOI] [PubMed] [Google Scholar]
- 26. Noval MM, Leekha S, Armahizer M, et al. Evaluating the incidence of bacteriuria in female patients before and after implementation of external urinary collection devices. Antimicrob Steward Healthc Epidemiol 2022;2. doi: 10.1017/ash.2022.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rearigh L, Gillett G, Sy A, et al. Effect of an external urinary collection device for women on institutional catheter utilization and catheter-associated urinary tract infections. Infect Control Hosp Epidemiol 2021;42:619–621. doi: 10.1017/ice.2020.1259 [DOI] [PubMed] [Google Scholar]
- 28. Root N, Horigan AE, Lough ME. External female urinary catheter: Implementation in the emergency department. J Emerg Nurs 2021;47:131–138. doi: 10.1016/j.jen.2020.09.008 [DOI] [PubMed] [Google Scholar]
- 29. Rose G, Pyle-Eilola AL. The effect of urine collection with a novel external catheter device on common urine chemistry and urinalysis results. J Appl Lab Med 2021;6:1618–1622. doi: 10.1093/jalm/jfab054 [DOI] [PubMed] [Google Scholar]
- 30. Tran C, Rodrigue D, Jones T, Bell N. Addressing CAUTIs with an external female catheter. Am J Nurs 2023;123:50–55. doi: 10.1097/01.NAJ.0000911540.75111.b4 [DOI] [PubMed] [Google Scholar]
- 31. Van Decker SG, Bosch N, Murphy J. Catheter-associated urinary tract infection reduction in critical care units: a bundled care model. BMJ Open Qual 2021;10:12. doi: 10.1136/bmjoq-2021-001534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Warren C, Fosnacht JD, Tremblay EE. Implementation of an external female urinary catheter as an alternative to an indwelling urinary catheter. Am J Infect Control 2021;49:764–768. doi: 10.1016/j.ajic.2020.10.023 [DOI] [PubMed] [Google Scholar]
- 33. Whitaker A, Colgrove G, Scheutzow M, Ramic M, Monaco K, Hill JL. Decreasing catheter-associated urinary tract infection (CAUTI) at a community academic medical center using a multidisciplinary team employing a multi-pronged approach during the COVID-19 pandemic. Am J Infect Control 2023;51:319–323. doi: 10.1016/j.ajic.2022.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Won P, Craig J, Nevarez C, Gillenwater TJ, Yenikomshian HA. Use of female external urinary catheters in a burn intensive care unit: benefits and challenges. Crit Care Nurs 2023;43:38–43. doi: 10.4037/ccn2023317 [DOI] [PubMed] [Google Scholar]
- 35. Zavodnick J, Harley C, Zabriskie K, et al. Effect of a female external urinary catheter on incidence of catheter-associated urinary tract infection. Cureus 2020;12:e11113. doi: 10.7759/cureus.11113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Auten K. Intentional leadership rounds: a proactive approach to CAUTI reduction…Association for Professionals in Infection Control and Epidemiology, Annual Conference (virtual), 28–30 June, 2021. Am J Infect Control 2021;49:S9. [Google Scholar]
- 37. Beeson T, Davis C, Vollman KM. Chasing zero catheter associated urinary tract infections (CAUTIs) through implementing a novel female external urine collection device in a tertiary academic surgical intensive care unit (SICU). Am J Infect Control 2018;46:S14. [Google Scholar]
- 38. Behrend V, French C, Didwania A. Filling the void-an external female catheter to improve patient care and reduce complications in hospitals. J Gen Intern Med 2018;33:786.29549498 [Google Scholar]
- 39. Cassone M, Ameling J, Mody L, et al. Impact of external female urinary catheter use on routine urine test results. J Am Geriatr Soc 2020;70:S220. [DOI] [PubMed] [Google Scholar]
- 40. Chirca I, Henry K, Faircloth C, et al. A successful bundled approach to decrease catheter-associated urinary tract infections in a community hospital. Open Forum Infect Dis 2018;5:S621. [Google Scholar]
- 41. Dublynn T, Episcopia B. Female external catheter use: a new bundle element to reduce CAUTI. Am J Infect Control 2019;47:S39–S40. [Google Scholar]
- 42. Ecklund M. Catch the “P”: External urinary management system: an innovative approach to CAUTI reduction…scientific and clinical abstracts from WOCNext 2020 Reimagined, June 5-7, 2020. J Wound Ostomy Continence Nurs 2020;47:S47. [Google Scholar]
- 43. Fields EM, Fleenor L, Martinez N, et al. Implementation of intermittent catheterization in neurosciences intensive care unit resulting in decreased CAUTI rates – a quality improvement initiative. Neurocrit Care 2019;31:S339. [Google Scholar]
- 44. Figueredo J. Reduction in catheter associated urinary tract infections using unit based champions. Am J Infect Control 2020;48:S48. [Google Scholar]
- 45. Fritsch PF, Sutton J, Roche E, et al. Reinforcing a catheter-associated urinary tract infection (CAUTI) bundle compliance decreases overall catheter days and CAUTIs. Am J Infect Control 2019;47:S22. [Google Scholar]
- 46. Gentile P, Jacob J, Ashraf S. Implementation of a female external urinary catheter reduces indwelling urinary catheter use and catheter-associated urinary tract infections. Infect Control Hosp Epidemiol 2020;41:S482–S483. [Google Scholar]
- 47. Goris AJ, McMullen KM, Dunn GM, et al. Quick to Wick: external female catheters and urinary catheter utilization. Am J Infect Control 2020;48:S7. [Google Scholar]
- 48. Gutzmirtl C. Use of a female external urinary collection device in the intensive care unit. Crit Care Nurs 2019;39:E38. [Google Scholar]
- 49. Henry S, Arocha D, Brown D, et al. CAUTI path to zero: a triple-pronged approach to minding our pees and cues. Open Forum Infect Dis 2019;6:S412–S413. [Google Scholar]
- 50. Hughes AA, Thomas DR. Fact or Foley: Reducing catheter related urinary tract infections by connecting evidence to drive a multidisciplinary urinary program. Am J Infect Control 2020;48:S43–S44. [Google Scholar]
- 51. Kelly TJ, Skelton S, Dawson D. Budget impact analysis (BIA) of employing a female external catheter (FEC) to assist with urine output management (UOM) in the hospital setting. Value Health 2018;21:S248. [Google Scholar]
- 52. Kelly T, Krome H. Economic impact of utilizing a female external catheter (FEC) to reduce the incidence of catheter-associated urinary tract infection (CAUTI): systematic review, analysis, and model. Value Health 2022;25:S537. [Google Scholar]
- 53. Kuzow H, Mansour M, Vaccarello S, et al. MICU reduction of CAUTI’s with Purewick. Crit Care Med 2019;47:1277. [Google Scholar]
- 54. Maydick-Youngberg D, Francis K, Liao J. Lessons learned: management of female urinary incontinence with an external catheter scientific and clinical abstracts from WOCNext 2020 Reimagined. J Wound Ostomy Continence Nurs 2020;47:S36–S37. [Google Scholar]
- 55. Mayes M. Goal of zero harm: incontinence management protocol…scientific and clinical abstracts from WOCNext 2020 Reimagined, June 5–7, 2020. J Wound Ostomy Continence Nurs 2020;47:S48–S49. [Google Scholar]
- 56. McRae AD, Kennelly MJ. Outpatient PureWickTM female external catheter system performance: healthy volunteer study. Neurourol and Urodyn 2023;42:100712. [Google Scholar]
- 57. Mena Lora AJ, Ali M, Krill C, et al. Impact of a daily hospital-wide huddle on urinary catheter device utilization and catheter associated urinary tract infection rates. Am J Infect Control 2020;48:S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Minor L, VanDerSlik AL, Lapsley CE, et al. Implementation of a nurse-driven Foley removal protocol and urine specimen algorithm in a CCU at a Level 1 trauma center. Am J Infect Control 2022;50:S33–S34. [Google Scholar]
- 59. Mueller C. Finally! an external female catheter device for women!…46th Annual Conference, APIC 2019, Philadelphia, PA. Am J Infect Control 2019;47:S13. [Google Scholar]
- 60. Nalbandian M, Reddy H, Conway R. A multidisciplinary team approach for the prevention of catheter-associated urinary tract infections in the ICU. Chest 2022;162:A867. [Google Scholar]
- 61. Ohanian S, Dizon A, Atabaki A, et al. Reducing indwelling urinary catheter utilization results in reduction of associated urinary tract infections. Am J Infect Control 2022;50:S36. [Google Scholar]
- 62. Pavlovsky LL, Schmitt B. Using the NHSN TAP Report and standardized utilization ratio to decrease urinary catheter utilization. Am J Infect Control 2020;48:S50–S51. [Google Scholar]
- 63. Peters JN, Grote AC, Spera LJ, et al. 626 Female external urinary collection device utilization in a female burn ICU patients: a quality improvement project. J Burn Care Res 2021;42:S168–S169. [Google Scholar]
- 64. Reeths A, Merkatoris R. Implementation of external urine collection devices to decrease the standardized utilization ratio of indwelling urinary catheters. Am J Infect Control 2020;48:S33. [Google Scholar]
- 65. Riemenschneider K, Scardillo J, Sheehan L, et al. Female external urinary management systems, incontinence-associated dermatitis, and pressure injuries…one facility’s experience. J Wound Ostomy Continence Nurs 2020;47:S42. [Google Scholar]
- 66. Srisatidnarakul S, Tarver C, Luna F, et al. Reducing catheter-associated urinary-tract infections in cancer patients: a nurse and infection preventionist-led interprofessional program…Association for Professionals in Infection Control and Epidemiology, Annual Conference (Virtual), 28–30 June, 2021. Am J Infect Control 2021;49:S13. [Google Scholar]
- 67. Sundhu M, Reggio C, Nasir U, et al. Switching indwelling Foley catheter to external catheter for outpatient atrial fibrillation ablation: a quality improvement project. J Cardiovasc Electrophysiol 2022;33:793–794. [Google Scholar]
- 68. Taneja A, Weiss M, Kelly T. EE323 Implications for utilizing a female external catheter (FEC) in end-of-life patients – model of the potential impact upon informal, unpaid caregiver (IUC) burden in the community setting. Value Health 2023;26:S118–S119. [Google Scholar]
- 69. Wilkerson SD, Overbay D, Haake H. Multi-pronged CAUTI reduction strategy. Am J Infect Control 2020;48:S46. [Google Scholar]
- 70. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 71. Patel PK, Gupta A, Vaughn VM, et al. Review of strategies to reduce central line-associated bloodstream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI) in adult ICUs. J Hosp Med 2017;13. doi: 10.12788/jhm.2856 [DOI] [PubMed] [Google Scholar]
- 72. Saint S, Olmsted RN, Fakih MG, et al. Translating health care-associated urinary tract infection prevention research into practice via the bladder bundle. J Comm J Qual Patient Saf 2009;35:449–455. doi: 10.1016/s1553-7250(09)35062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pryor et al. supplementary material