Abstract
The Swedish population of Bordetella pertussis strains was characterized from 1,247 isolates covering a whole-cell vaccine program up to 1979, a 17-year period without vaccination (1979 to 1996), and a period after the introduction of general vaccination among newborns with acellular pertussis vaccines (1997 to 2003). Strains were characterized by serotyping and genotyping of pertactin and ptxA and by means of pulsed-field gel electrophoresis (PFGE). With emphasis on vaccine-related markers, the vast majority of circulating strains were of nonvaccine type. There were shifts of serotype connected with shifts of vaccination program. Serotype Fim3 was most frequent during the periods with general vaccination schedules, whereas serotype Fim2 was predominant during the 17-year vaccine-free period. Pertactin 1 was predominant during the pertussis whole-cell (Pw) vaccine period but was thereafter replaced by prn2 and has not reappeared after the introduction of acellular pertussis (Pa) vaccines. ptxA (1) was predominant over all three decades. There was a significant difference in the distribution of serotypes between vaccinated and unvaccinated individuals, but not for pertactin. A few PFGE profiles were predominant over the years: BpSR25 (serotype Fim3 prn1/7) and BpSR18 (serotype Fim3 prn2) during the Pw period, BpSR1 (serotype Fim2 prn2) during the 17 years without general vaccination, and BpSR11 (serotype Fim3 prn2) after the reintroduction of general vaccination in 1996. Despite differences between the pertactin and toxin types of Pa vaccines and circulating strains, there is no evidence that there is a threat, i.e., the vaccination program so far has been effective against whooping cough, and there seems to be no impact on the effectiveness of the vaccination program from the bacterial polymorphism.
Despite general vaccination programs, pertussis has remained an endemic disease, with incidence peaks occurring every 3 to 5 years, even in countries with high vaccine coverage (2, 3, 8, 9, 15, 18, 27, 28, 38-40). A resurgence of pertussis may occur in infants and is often explained by waning immunity after vaccination or disease, causing adults and adolescents to serve as a reservoir for infections in unvaccinated young infants (15, 16, 43). It should, however, be kept in mind that human population immunity is multifactorial and that lack of it is not only due to a decline of antibody concentrations or cell-mediated immune responses. Natural boosting, i.e., contact with the bacteria, will also influence immunity. Thus, a shift of antigenic properties of Bordetella pertussis virulence factors (24, 36) may be essential for the susceptibility of the human population to infection with B. pertussis.
Four virulence factors of a protein nature have been described as suspected antigens responsible for inducing immunity to B. pertussis: pertussis toxin (PT), filamentous hemagglutinin (FHA), pertactin (PRN), and fimbriae (Fim). The purified and detoxified components are used in acellular pertussis (Pa) vaccines. Significant serum antibody concentrations against PRN, Fim, and PT have been shown to correlate with protection against pertussis disease (6, 34), and randomized trials with different diphtheria-tetanus-acellular pertussis (DTPa) vaccines indicate that the protection against pertussis disease increases with the number of components of B. pertussis included in the Pa vaccine (1, 7, 17, 29, 35).
Detoxified PT is present in all Pa vaccines. Four subtypes of PT ptxA (subtypes 1 to 4) (10) have been described. Most vaccines contain toxoids of ptxA (2) or ptxA (3), but the vast majority of clinical isolates are ptxA (1) (2, 5, 19, 26, 27, 41).
FHA is present in most Pa vaccines and has been shown to have some additional effect on vaccine efficacy (35).
PRN is a protein located at the cell surface. At least 12 subtypes have been described, three of which (prn1, prn2, and prn3) are predominant (25). Detoxified PRN is present in three-, four-, or five-component Pa vaccines as prn1.
Fim belong to a class of extracellular filamentous proteins functioning as adhesins. They induce agglutinating antibodies, which have been correlated with immunity to pertussis after vaccination with pertussis whole-cell (Pw) vaccines (23). There are three serotypes of B. pertussis related to Fim, designated serotype Fim2, serotype Fim3, and serotype Fim2 and -3 (Fim2, 3). Fim2 and Fim3 are also present in five-component DTPa (DTPa5) vaccine. There is epidemiological evidence that serotype Fim2 strains have the capability to cause a more severe disease than serotype Fim3 strains (37).
As indicated above, an isolate can be characterized by its subtype of ptxA, prn, and Fim. A more discriminatory approach to study the B. pertussis population, for example on a temporal basis, is pulsed-field gel electrophoresis (PFGE) (2, 20, 22, 25, 31, 41). The association between a PFGE profile and a prn type is consistent. On the other hand, the PFGE profile is more loosely associated with the serotype (2).
There is documented evidence from many countries using Pw vaccine that circulating variants of B. pertussis are essentially different from those strains used for production of vaccines. It has also been shown that the bacterial population has changed over time since the introduction of vaccination about 50 years ago (2, 5, 10, 19, 20, 22, 26, 27, 31, 40, 41).
Serotype Fim3 strains seem to be found at higher frequency in countries with a Pw vaccination program (32). In 1979, the World Health Organization recommended that Pw vaccines should contain strains expressing both serotype Fim2 and serotype Fim3 (42).
The Pa vaccines have replaced Pw vaccines in many countries, and it is of general interest to study what has happened with the bacterial population since. Sweden provides a unique opportunity to compare circulating strains of B. pertussis under different vaccination strategies. In that country, a Pw vaccine was introduced in the 1950s and used with high vaccine coverage until 1979, when it was withdrawn due to concerns about safety and efficacy. Thereafter, there was no vaccination against pertussis in the national childhood immunization schedule until 1996. A total of approximately 90,000 children were vaccinated during their participation in vaccine trials held during the vaccine-free period. These trials are summarized in Table 1. In 1996, general vaccination by use of a DTPa vaccine was reintroduced after randomized placebo-controlled pertussis vaccine trials of DTPa and DTPw vaccines (29). Vaccination against pertussis was subsequently reintroduced in the national childhood immunization schedule after a 17-year period without general vaccination, during which pertussis had become endemic, with an overall incidence of more than 100 cases/100,000 person years of follow-up and up to 1,000 cases/100,000 person years for infants (33).
TABLE 1.
Vaccines in use during periods with different vaccination programs
| Vaccination program | Yr | Trial | Reference | Vaccine typea | Vaccine producerb |
|---|---|---|---|---|---|
| Whole-cell vaccine (1953-1979) | Pw | SBL | |||
| Vaccine-free period (1979-1996) | 1986-1987 | Biken | 1 | Placebo (DT) | SBL |
| 1 comp (PT) | Biken | ||||
| 2 comp (PT, FHA) | Biken | ||||
| 1992-1995 | Stockholm I | 17 | Placebo (DT) | SBL | |
| 2 comp (PT, FHA) | SKB | ||||
| 5 comp (PT, FHA, PRN, Fim2;3) | CL | ||||
| Pw | Connaught, PA | ||||
| 1993-1996 | Stockholm II | 30 | 2 comp (PT, FHA) | SKB | |
| 3 comp (PT, FHA, PRN) | Chiron | ||||
| 5 comp (PT, FHA, PRN, Fim2;3) | CL | ||||
| Pw | Evans | ||||
| Acellular vaccines (1996-2003) | 1996-1997 | 29 | 3 comp (Infanrix) | GSK | |
| 1998-2000 | 2 or 3 comp (Pentavac, Infanrix) | AvP, GSK | |||
| 2000- | 2 or 3 comp (Pentavac, Infanrix-polio+Hib) | AvP, GSK |
Comp, component; DT, diphtheria-tetanus; FHA, filamentous hemagglutinin; PRN, pertactin; PT, pertussis toxin. All pertussis vaccines also included DT.
Avp, Aventis Pasteur MSD; Biken, Biken, Osaka, Japan; Chiron, Chiron Vaccines, Siena, Italy; CL, Connaught Laboratories, Toronto, Canada; Connaught, PA, Connaught Laboratories, Swiftwater, PA; Evans, Evans Medical, United Kingdom; GSK, GlaxoSmithKline UK; SBL, SBL Vaccine AB, Swedish National Bacteriological Laboratory, Stockholm, Sweden; SKB, SmithKline Beecham, Rixensart, Belgium.
An ongoing clinical-epidemiological surveillance program and a laboratory surveillance program of culture-positive pertussis in Sweden was initiated in October 1997 (29), based on identification of culture-confirmed cases in the regular passive notification system in children born in 1992 or later. The main aim of the clinical part of the surveillance program was to follow the effectiveness of the new pertussis vaccination program over time. Clinical, as well as vaccination, data from the culture-confirmed cases were collected through telephone contacts with the households. Children from two previous trial cohorts (17, 30) are also still followed (29) in the same surveillance program.
The objective of the laboratory part of the program was to collect information on B. pertussis isolates and to study the bacterial population during periods with different vaccination programs. Within the surveillance program, there is a nationwide agreement with the clinical bacteriological laboratories to deposit at the Swedish Institute for Infectious Disease Control isolates from children born in 1992 or later with any kind of culture-confirmed whooping cough occurring from September 1997 on.
The primary aim of the present laboratory study was to compare isolates over time by means of the reference methods for epidemiological typing of B. pertussis (25), serotyping and genotyping of pertactin and pertussis toxin, and to compare DNA fingerprinting by means of PFGE. A secondary aim was to compare circulating strains with strains used for production of vaccines when applicable. A third aim was to compare isolates from vaccinated and unvaccinated children. Information on the vaccination status of the children was obtained from the clinical part of the surveillance program (16).
MATERIALS AND METHODS
Isolates. (i) Vaccine strains. (a) Whole-cell vaccination (Pw period; 1953 to 1979).
Strain 44122 was used to produce the Pw vaccine. It showed ptxA (4), prn10, and serotype Fim2 or serotype Fim2,3. SBL Vaccine AB (Solna, Sweden) kindly provided lyophilized subcultures of this strain from 1956 to 1964.
(b) Acellular vaccination (Pa period; 1996 to 2003).
Tohama I (25) has been used for the production of DTPa3 (Infanrix). Nicole Guiso at Institut Pasteur, Paris, France, kindly provided it. Tohama I is of ptxA (2), prn1 type and expresses Fim2.
(ii) Clinical isolates.
In this study 1,247 isolates collected from 1970 to 2003 (Table 2) were characterized by use of the reference methods for serotyping and genotyping of prn and ptxA and DNA fingerprinting by means of PFGE. The means of collection of isolates during the three periods with different vaccination programs are described below.
TABLE 2.
Clinical isolates from vaccinated and unvaccinated Swedish children collected during three periods with different pertussis vaccination schedules
| Period | Yr of collection | No. of isolates from:
|
Total | ||
|---|---|---|---|---|---|
| Vaccination status unknown | Vaccinated | Unvaccinated | |||
| Whole-cell vaccination (Pw) (1953-1979) | 1970-1977 | 71 | 71 | ||
| Without general vaccination (1979-1996) | 1986-1987 | 26 | 48 | 74 | |
| 1992-1995 | 42 | 110 | 152 | ||
| Acellular vaccination (Pa) (1996-2003)a | 1997-1998 | 42 | 134 | 176 | |
| 1998-1999 | 90 | 150 | 240 | ||
| 1999-2000 | 147 | 204 | 351 | ||
| 2000-2001 | 56 | 56 | |||
| 2001-2002 | 82 | 82 | |||
| 2002-2003 | 45 | 45 | |||
| Total | 71 | 530 | 646 | 1247 | |
Annually 1 October to 30 September.
(a) The Pw period (1953 to 1979).
In Sweden, isolates were regularly collected during the 1970s. For this study, a total of 71 isolates were available from the years 1970 to 1977 (Table 2). For this material, there is no information concerning the individual vaccination status, but the coverage was at least 90%.
(b) The vaccine-free period (1979 to 1996).
In this study, we characterized 74 stored isolates from the two-component and the placebo groups from the Biken trial, 1986 to 1987 (1), and 152 stored isolates from the five-component and placebo groups from the Stockholm trial I of 1992 to 1995 (17).
(c) The Pa period (1996 and later).
A total of 950 isolates were continuously characterized from 1 October 1997 through 30 September 2003. Isolates (n = 462) from fully vaccinated children (three doses before or two doses after 2 years of age, before illness) and a random subset of every fifth isolate (n = 488) from unvaccinated children were characterized.
(d) Historical material from the Pw and Pa periods.
For comparison of serotype results, we also referred to 5,648 isolates from 1970 to 1994 previously serotyped and presented in a symposium report (36). These isolates were collected continuously during those years, with about 100 collected annually from 1970 to 1978 and the remainder from 1979 to 1994.
Characterization of isolates.
At the Swedish Institute for Infectious Disease Control, the isolates of B. pertussis were recultured on charcoal horse-blood agar at 36°C for up to 5 days. The isolates were frozen at −70°C according to the reference method (25).
A recommended standard methodology (25) with some modifications (2) was used, based on serotyping by means of monoclonal agglutination in microplates and genotyping of prn and ptxA. For the material collected from 1970 to 1994, serotyping was performed by means of slide agglutination with serotype-specific polyclonal sera (36). The correlation between the two methods was good, though not complete (14). Available isolates from the 1970-to-1994 period were retested with monoclonoal sera in microplates. DNA fingerprinting by means of PFGE was performed after digestion of the chromosomal DNA with the XbaI restriction endonuclease enzyme. A selection of PFGE reference strains was used for identification and designation of isolates as described earlier (2).
All isolates in Table 2 were analyzed for prn, serotype, and PFGE pattern. The ptxA types were documented in only 1,006 of them. The reasons for the constraint in characterization were the facts that ptxA (1) was such a constant finding throughout the three periods and that the resources were limited. Twenty-four strains typed prn1/7 were further examined for the single point mutation in region 2 of the prn gene. Identification of this point mutation distinguished prn1 from prn7. All 24 cases were prn1.
Statistics.
Chi-square analysis was used for statistical comparison of the distributions of strain characteristics within vaccination periods among vaccinated and unvaccinated individuals.
RESULTS
Suitable subgroups of the available isolates were selected to study each of the formulated aims (comparison of strain characteristics over time, comparison of vaccine strains and circulating strains, and comparison of vaccinated and unvaccinated children).
Comparison of strain characteristics over time.
For the analysis of strain characteristics over time, we used all 530 isolates from vaccinated individuals, as well as the earlier 71 isolates from individuals with unknown vaccination status (Table 2).
To strengthen the study of serotype distribution over time, covering the Pw and the vaccine-free periods, we also used the results of the 5,648 isolates from the years 1970 to 1994 (Fig. 1).
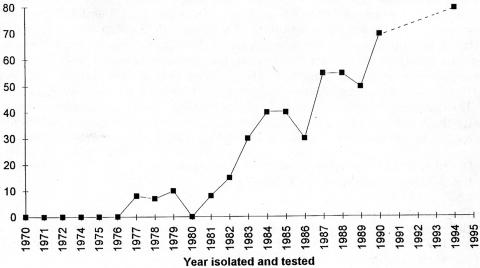
FIG. 1.
Frequency of B. pertussis serotype Fim2 in clinical isolates collected from 1970 to 1994. The proportion is based on the total number of isolates for each year. The dashed line represents all isolates from the Stockholm vaccine trial 1 (17). Reprinted from reference 36 with permission of the publisher.
(i) Fimbrial serotype.
Figure 2 demonstrates changes of serotype over the three periods.
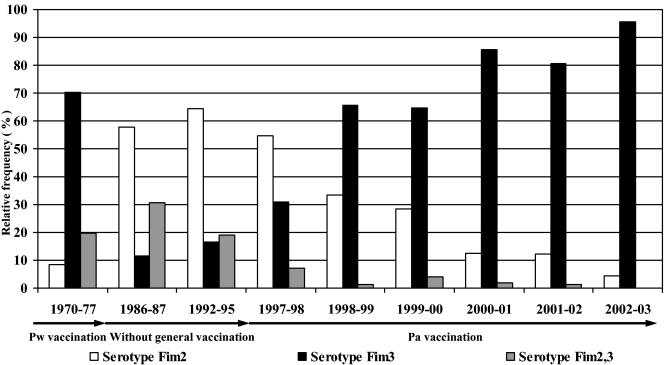
FIG. 2.
Serotype distributions for the Pw period (up to 1979), the vaccine-free period (1979 to 1996), and the Pa period (from 1996 on) as indicated by arrows below the years. The bars show the proportions of serotype Fim2, serotype Fim3, and serotype Fim2,3. The figures are given as proportions (percent) of typed isolates from vaccinees during the respective periods or years.
(a) The Pw period.
Before 1979, when the whole-cell vaccine was used, serotype Fim3 and serotype Fim2,3 predominated and were observed in 70% (50/71) and 20% (14/71) of available isolates, respectively. Serotype Fim2 isolates constituted 8% of the 71 isolates available in this study.
(b) The vaccine-free period.
Serotype Fim2 appeared only at low frequency before 1977 and then continuously increased after cessation of the general vaccination program (Fig. 1 and 2), and in the Biken trial (1986 to 1987) it reached 58% (15/26). This is in agreement with other isolates from the same period (36). The data show a prevalence peak in the 1992-to-1995 trial, with 64% (27/42) among vaccinated individuals.
(c) The Pa period.
After general vaccination was reintroduced in 1996, the prevalence of serotype Fim2 declined, and it was rapidly replaced by serotype Fim3. In 2002 and 2003, serotype Fim3 constituted 96% (43/45) of serotypes among fully vaccinated individuals.
Phenotypic expression levels of serotype Fim2,3) among vaccinated individuals were 31% (8/26) during the Biken study in the 1980s and 19% (8/42) during the Stockholm trial I in the 1990s, further decreasing to 0% in 2002 and 2003 (0/45).
(ii) Genotyping of prn.
Figure 3 demonstrates changes of pertactin type over the three periods.
FIG. 3.
prn type distributions for the Pw period (up to 1979), the vaccine-free period (1979 to 1996), and the Pa period (from 1996 on), as indicated by arrows below the years. The bars show the proportions of prn1/7, prn2, and prn3. The figures are given as proportions (percent) of typed isolates from vaccinees during the respective periods or years.
(a) The Pw period.
Before 1979, when the whole-cell vaccine was used, prn1/7 was the most common pertactin, with a proportion of 68% (48/71) among isolates available for this study.
(b) The vaccine-free period.
In the Biken study, the proportion of prn1/7 decreased to 35% (9/26) of the isolates from the vaccine recipients. It was replaced by prn2, which was seen in 65% (17/26) of isolates. In the Stockholm trial I from 1992 to 1995, prn2 had increased to 74% (31/42).
(c) The Pa period.
A continuous increase of prn2 was seen in 1996 and later, after the introduction of the first Pa vaccine in 1996, with the percentage in 2002 and 2003 being 96% (43/45).
prn3, which was only sporadically seen before 1979 (3%; 2/71), was found in 7% (3/42) of isolates within the 1992-to-1995 trial. After 1996, it increased to reach a peak of 24% (10/42) of the isolates from those vaccinated in 1997 and 1998.
Four unusual pertactin types were found in addition to the three common ones: one prn4, two isolates with prn9, one prn11, and finally, one prn12.
(iii) Genotyping of ptxA.
There were no changes in ptxA during the periods with different vaccination programs. A total of 1,006 isolates from the three periods were examined to determine the ptxA genotype. Type ptxA (1) was found in more than 99% of these isolates. Type ptxA (2) was found in eight isolates, all from the surveillance period.
(iv) DNA fingerprinting by means of PFGE.
A total of 143 individual profiles could be identified by PFGE. The four most frequent profiles were selected for the years of collection, and the presence of each of these selected profiles was also followed and documented during the other years, from 1970 to 2003 (Tables 3 and 4). Due to overlapping, the profiles were reduced to 21 among those vaccinated and 14 among those unvaccinated, as presented in Tables 3 and 4, also showing the relationship between pertactin and serotype and the profiles. The eight most predominant profiles are also shown in Fig. 4.
TABLE 3.
Frequencies during different vaccination programs of the most common PFGE profiles among vaccinated children
| PFGE profile (serotype-prn type) | Frequency of profile (%) during yr of collection
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pw period | Vaccine-free period
|
Pa period
|
|||||||
| 1970-77a | 1986-87 | 1992-95 | 1997-98 | 1998-99 | 1999-00 | 2000-01 | 2001-02 | 2002-03 | |
| BpSR1 (2-2) | 15 | 36 | 10 | 7 | 4 | 4 | |||
| BpSR5 (3-2) | 8 | 7 | 4 | 10 | 2 | ||||
| BpSR6 (3-1/7) | 13 | ||||||||
| BpSR7 (2-2) | 1 | 4 | 5 | 2 | 1 | ||||
| BpSR9 (3-2) | 3 | 2 | 1 | 11 | |||||
| BpSR10 (3-2) | 2 | 1 | 4 | 13 | 7 | 13 | |||
| BpSR11 (3-2) | 12 | 33 | 31 | 55 | 46 | 40 | |||
| BpSR12 (3-2) | 3 | 6 | 2 | ||||||
| BpSR13 (3-2) | 2 | 1 | 13 | ||||||
| BpSR16 (2-2) | 10 | 7 | 14 | 5 | 6 | ||||
| BpSR18 (2/3-2) | 25 | 23 | 5 | 2 | |||||
| BpSR23 (2/3-2) | 8 | 2 | 1 | ||||||
| BpSR25 (3-1/7) | 39 | 4 | 2 | ||||||
| BpSR27 (2/3/2,3-2) | 12 | ||||||||
| BpSR31 (2/3-3) | 5 | 12 | 13 | 5 | 5 | ||||
| BpSR46 (2-2) | 12 | ||||||||
| BpSR63 (2-2) | 7 | 2 | 1 | ||||||
| BpSR100 (2,3-2) | 4 | 5 | 1 | ||||||
| BpSR106 (2-2) | 5 | 1 | |||||||
| BpSR109 (2-2) | 5 | ||||||||
| BpSR160 (2-3) | 7 | ||||||||
| Total (%) | 86 | 74 | 72 | 57 | 78 | 69 | 91 | 81 | 81 |
| Total no. of isolates | 71 | 26 | 42 | 42 | 90 | 147 | 56 | 82 | 45 |
| Total no. of profiles | 11 | 14 | 19 | 22 | 23 | 32 | 12 | 19 | 12 |
Individual vaccination status unknown.
TABLE 4.
Frequencies during different vaccination programs of the most common PFGE profiles among unvaccinated children
| PFGE profile (serotype-prn type) | Frequency of profile (%) during yr of collection
|
|||||
|---|---|---|---|---|---|---|
| Pw period | Vaccine-free period
|
Pa period
|
||||
| 1970-1977 | 1986-1987 | 1992-1995 | 1997-1998 | 1998-1999 | 1999-2000 | |
| BpSR1 (2-2) | 33 | 44 | 14 | 11 | 4 | |
| BpSR5 (3-2) | 2 | 3 | ||||
| BpSR7 (2-2) | 4 | 3 | 1 | 2 | ||
| BpSR10 (3-2) | 1 | 5 | 2 | |||
| BpSR11 (3-2) | 10 | 21 | 32 | |||
| BpSR16 (2-2) | 1 | 4 | 15 | 17 | ||
| BpSR17 (2-2) | 5 | |||||
| BpSR18 (2/3-2) | 19 | 6 | 4 | 2 | ||
| BpSR19 (2-2) | 10 | 1 | ||||
| BpSR22 (2-2) | 5 | |||||
| BpSR27 (2/3/2,3-1/7) | 6 | |||||
| BpSR28 (3-2) | 1 | 5 | 4 | |||
| BpSR31 (2/3-2) | 6 | 2 | 2 | 7 | 2 | |
| BpSR63 (2-2) | 3 | 5 | 1 | 2 | ||
| Total (%) | 68 | 69 | 52 | 69 | 69 | |
| Total no. of isolates | 48 | 110 | 134 | 150 | 204 | |
| Total no. of profiles | 17 | 32 | 41 | 35 | 37 | |
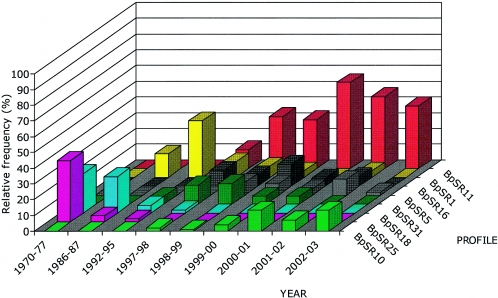
FIG. 4.
Distribution over time of eight predominant PFGE profiles (percent) from vaccinated persons (n = 385). The proportion is based on the total number for each profile and year.
(a) The Pw period.
The period 1970 to 1979 was dominated by the profiles BpSR25, BpSR6, and BpSR18 among those isolates available. BpSR25 and BpSR6 were characterized by prn1/7 and BpSR18 by prn2. All show serotype Fim3 or serotypes Fim2 and -3 during this period. In the 1980s, the phenotypic expression of BpSR18 changed to serotype Fim2.
(b) The vaccine-free period.
After the cessation of general vaccination in 1979, the BpSR25 and BpSR6 profiles disappeared and were replaced by BpSR1, showing prn2 and serotype Fim2, as the predominant profile.
BpSR18 disappeared more slowly and still represented 19 to 23% in the 1986-to-1987 trial, disappearing in 1998.
(c) The Pa period.
After general pertussis vaccination was reintroduced, a new profile, BpSR11, appeared in 1997, characterized by prn2 and serotype Fim3. Simultaneously with an incidence peak in 1999 and 2000, it replaced BpSR1 as the predominant profile and has continued to be dominant during the 6-year surveillance period from 1997 to 2003.
By means of profile analysis, it has also been possible to identify strains with time-related appearance. One example is BpSR31, with serotype Fim3 and prn3, which was seen mainly in the 1990s and declined thereafter. Other recently seen variants of serotype Fim3 and prn2, such as BpSR9, BpSR10, and BpSR13, will be of interest for further study.
Comparison of vaccine strains and circulating strains.
For this analysis, the characteristics of the vaccine strains, 44122 and Tohama I, were compared with those of the circulating strains during the Pw and Pa periods, respectively. Such analysis is not applicable to the vaccine-free period, when pertussis was not included in the general vaccination program.
(i) Fimbriae. (a) The Pw period.
The subcultures of the 44122 strain, used for the production of Pw vaccine in Sweden, expressed serotype Fim2,3 or only serotype Fim2, but some were not typeable because of autoagglutination. On the other hand, 70% of the circulating strains were of serotype Fim3.
(b) The Pa period.
A comparison between circulating strains and vaccine strains during the Pa period may seem to be irrelevant, since fimbrial antigens are not present in the acellular vaccines used in Sweden. However, it is still interesting to notice that a change from serotype Fim2 to serotype Fim3 took place when Pa vaccination was introduced.
(ii) Genotyping of prn. (a) The Pw period.
Strains expressing prn10, as in the vaccine strain 44122, have not been found among circulating strains at all. prn1/7 was predominant during this period and was seen in 68% (48/71) of tested isolates.
(b) The Pa period.
Before and after the introduction of acellular vaccines in Sweden, the circulating variants were increasingly dominated by prn2 subtypes, which differ from Tohama I, used for the production of some acellular pertussis vaccines, with prn1 characteristics.
(iii) Genotyping of ptxA.
Strain 44122 showed ptxA (4), which has not been found among circulating strains at all. The Pa vaccines contain either toxin ptxA (2) or ptxA (3). Type ptxA (2) was found in eight isolates, representing approximately 1% of the isolates, all from the surveillance period after 1997. The whole-cell vaccines, as well as the acellular vaccines, contained toxin types other than those in the circulating strains showing ptxA (1).
(iv) DNA fingerprinting by means of PFGE.
The PFGE profiles of the vaccine strains 44122 and Tohama I were not represented at all among profiles from Swedish clinical isolates. Dendrogram analyses performed on the 35 most prevalent Swedish profiles showed a minimum of 70% relatedness (2). Strains 44122 and Tohama I demonstrated 42% and 56% relatedness, respectively (data not shown).
Comparison of vaccinated and unvaccinated children.
When possible, isolates from vaccinated and unvaccinated children were compared. Comparisons were made within the vaccine-free and the Pa periods. From the vaccine-free period, the group of 152 isolates, collected in the Stockholm trial I, was used for statistical comparisons of serotype and prn distributions between vaccinated (n = 42) and placebo (DT)-vaccinated (n = 110) children. We chose the DTPa5 vaccine group used in this trial, since it was a Fim-containing vaccine.
All isolates from fully vaccinated children (n = 279) were compared with a random subset of every fifth isolate from unvaccinated children (n = 488). All of the latter group of isolates were obtained between October 1997 and September 2000 (Table 2).
(i) Fimbriae.
Table 5 compares serotypes in isolates collected from vaccinated and unvaccinated children during the Stockholm trial I, as well as for the first 3 years of the surveillance period (1997 to 2000). There is a significant difference in the distributions of serotype between vaccinated and unvaccinated children within each of the two materials (P < 0.01). In the vaccine trial from 1992 to 1995, serotype Fim2 was predominant but occurred in a lower proportion among those vaccinated with DTPa5. During the surveillance period, the proportion of serotype Fim3 isolates was 60% among vaccinated and 47% among unvaccinated children in comparison with 34% and 49%, respectively, for serotype Fim2.
TABLE 5.
Fimbrial serotype comparison within periods between isolates from vaccinated and unvaccinated individuals during trial I, as well as for surveillance years (1997-2000)
| Serotype | Trial I (1992-1995)a
|
Surveillance (1997-2000)a
|
||
|---|---|---|---|---|
| Vaccinated | Placebo | Vaccinated | Unvaccinated | |
| 2 | 27 (64%) | 97 (88%) | 95 (34%) | 240 (49%) |
| 3 | 7 (17%) | 7 (6%) | 167 (60%) | 228 (47%) |
| 2,3 | 8 (19%) | 6 (6%) | 10 (4%) | 17 (3%) |
| Autoagglutination | 7 (2%) | 3 (1%) | ||
| Total | 42 (100%) | 110 (100%) | 279 (100%) | 488 (100%) |
P < 0.01 within period.
In the Biken trial (1986 to 1987), isolates from the DTPa2 vaccine group and the DT group showed identical proportions of serotype Fim2, serotype Fim3, and serotype Fim2,3, namely, 58%, 11%, and 31%, respectively.
(ii) Genotyping of prn.
The three dominant pertactin types from vaccinated and unvaccinated children are presented in Table 6. No significant difference was found between isolates from vaccinated and unvaccinated children from the same period in either of the two materials. prn2 dominated in all groups.
TABLE 6.
Pertactin type comparison within periods between isolates from vaccinated and unvaccinated individuals during trial I, as well as for the surveillance years (1997-2000)
| Pertactin type | Trial I (1992-1995)a
|
Surveillance (1997-2000)b
|
||
|---|---|---|---|---|
| Vaccinated | Placebo | Vaccinated | Unvaccinated | |
| 1/7 | 8 (19%) | 8 (7%) | 15 (5%) | 20 (4%) |
| 2 | 31 (74%) | 93 (85%) | 218 (78%) | 406 (83%) |
| 3 | 3 (7%) | 6 (5%) | 46 (16%) | 61 (13%) |
| Other prn types | 3 (3%) | 1 (0%) | ||
| Total | 42 (100%) | 110 (100%) | 279 (100%) | 488 (100%) |
P = 0.08 within period.
P = 0.15 within period.
In the Biken study, the proportions of prn1/7, prn2, and prn3 were 35% (9/26), 65% (17/26), and 0%, respectively, in vaccinated children compared with 15% (7/48), 77% (37/48), and 8% (4/48) in unvaccinated children.
(iii) Genotyping of ptxA.
The ptxA (1) type and the ptxA (2) type were evenly distributed between vaccinated and unvaccinated children.
(iv) DNA fingerprinting by means of PFGE.
Available isolates from vaccinated and unvaccinated children (Table 2) were characterized and compared between the two groups.
The 21 most prevalent PFGE profiles from vaccinated children and the 14 most prevalent from unvaccinated children were selected and followed over time (Tables 3 and 4). The same predominant profiles were seen among both vaccinated and unvaccinated children. In the placebo-controlled trial (1992 to 1995), BpSR1 was seen in 36% of vaccinated children (Table 3) and in 44% of unvaccinated children (Table 4). BpSR11, which was predominant after 1996, was seen in 12%, 33%, and 31% of vaccinated children during the first 3 years (Table 3) and in 10%, 21%, and 32% of unvaccinated children during the same years (Table 4).
DISCUSSION
The results of the strain characterization by means of serotype, prn type, and PFGE type indicate that there have been shifts in the B. pertussis population over the years. Similar observations of polymorphism and variation in virulence factors and strains have been made in many other countries using Pw vaccines and in which nonvaccine strains are circulating (2, 5, 10, 19, 20, 22, 26, 27, 40, 41).
Detoxified fimbriae, pertactin, and toxin are used as components in acellular vaccines. It is therefore of particular interest to study these single markers separately over time in relation to the vaccination program used.
We have previously documented that serotype Fim2 strains were only seldom seen among our isolates before 1977 but increased continuously thereafter during the vaccine-free era (36). In the Stockolm trial I, the proportion of serotype Fim2 was 88% for the placebo group (Table 5). Earlier studies also showed serotype Fim2 to be most prevalent in unvaccinated populations, whereas serotype Fim3 and serotype Fim2,3 seem to be most common in vaccinated populations, leaving better conditions for expressing serotype Fim3 (32, 42). It may be that Fim2 is a superior antigen to Fim3 and that the Swedish whole-cell vaccine used up to 1979 was efficient against serotype Fim2 strains. The highly developed fimbriae 2 may also confer a colonization advantage, explaining their predominance during the period without vaccination.
An immunity-driven selection is further supported by the significant differences in serotypes between those vaccinated with vaccines containing fimbriae and unvaccinated children observed in the Stockholm trial I (1992 to 1995) (17, 36). In that vaccine trial, it was shown that there was a lower rate of the serotype Fim2 type in both the DTPw and the DTPa5 vaccine groups (both containing fimbriae), 72.4% and 72.5%, respectively, compared to the rate of Fim2 in the DT and DTPa2 groups (neither containing fimbriae), 84.2% and 83.3%, respectively. This difference was also confirmed in the present study when isolates from two of the groups from the Stockholm trial I were reanalyzed using microplate agglutination with monoclonal antibodies (Table 5). There was no such difference when the DTPa2 group was compared with the placebo group, either in the 1992-to-1995 study or in the 1986-to-1987 study (36).
Interestingly, this difference in serotype Fim2 expression between vaccinated and unvaccinated individuals was also present during the first years after the introduction of the Pa vaccination program, indicating an effect of vaccination, although the mechanism is unclear. The selection of Fim3 may be indirectly due to an effect of the immune system on the expression of genes, or possibly gene combinations, not all of which are known.
More remarkable is the fact that serotype Fim3 gradually replaced serotype Fim2 after the renewal of vaccination in 1996, with the use of three- or two-component acellular vaccines. None of the vaccines contain fimbriae; the two-component vaccine does not even contain pertactin. It may be that impurities are present in the vaccines. In fact, at least one two-component vaccine similar to that used in parts of Sweden since 1998 was shown to induce a booster response to agglutinins in a Dutch study, indicating that a small amount of fimbriae may be present in the vaccine (4).
In some studies, there are indications that vaccines containing fimbriae are more efficient in the serotype Fim2 environment. In the Stockholm trial I from 1992 to 1995 (17), the efficacy of a DTPa5 vaccine was 85% and that of a DTPw vaccine was 48%. In a parallel Italian study (11), the efficacy of the same DTPw vaccine was only 36% with a different, and perhaps less favorable, bacterial population. In Sweden, the proportion of serotype Fim2 strains was above 80%, and in Italy, 16%. The corresponding figures for serotype Fim3 were 13% and 76%, respectively (12). During the Stockholm trial II from 1993 to 1996 (30), the incidences per 100,000 person years for another DTPw, a DTPa3, and a DTPa5 vaccine were 61, 155, and 85, respectively. In a follow-up study after 6 years, the incidences were 25, 40, and 42 (16). The earlier advantage of the DTPa5 over the DTPa3 vaccine was leveled out, perhaps due to the shift from serotype Fim2 to serotype Fim3 strains.
From the host perspective, it would be of great interest to follow anti-Fim2 and anti-Fim3 antibody profiles over the years. Unfortunately however, it has not been possible to obtain separate Fim2 and Fim3 antigens for this purpose. In the Swedish vaccine trials (17, 30), antibody response against fimbriae was measured using a combined Fim2-Fim3 antigen. Alternatively, an inhibition antibody enzyme-linked immunosorbent assay could be used if anti-Fim2 and anti-Fim3 monoclonal antibodies were available in sufficient amounts.
As for pertactin, the circulating population of B. pertussis has regularly presented pertactins of nonvaccine type. Based on the polymorphism of pertactin and toxin, Mooi et al. in 1998 (27) suggested a vaccine-driven evolution of new subtypes in The Netherlands, referring to temporal trends. This seems to hold true for Sweden also. The Swedish whole-cell vaccine contained prn10. During the Pw period, prn1/7 was most frequent and predominated over prn2. After 1979, there has been an expansion of B. pertussis strains with prn2 that did not change with the introduction of DTPa2 and DTPa3 after 1996, a finding corresponding to the lack of prn2 in the DTPa vaccines. However, the DTPa3 vaccine contains prn1. After 1996, there was a decrease in prn1/7 until after 2001 to 2003, when this pertactin type was no longer seen. For pertactin, no significant difference was seen between vaccinated and unvaccinated individuals, supporting the Italian results (24). Isolates showing prn3 appeared more often shortly after 1996 and reached a peak in 1999, a trend that was discontinued in 2001. It may be that pertactin 1-based vaccines give some protection against pertactin 3. Results by He et al. (21) suggest that the specificities of the immune responses induced by strains harboring prn2 do not induce antibodies against the conformational epitopes that are induced by vaccine strains expressing prn1 and prn3. This might explain the emergence of B. pertussis strains with prn2.
Besides the three common prn types, we found only four other prn types in single isolates; one of them was prn4, which was seen in 9% of Finnish isolates from the 1990s (26) and has also been reported from Poland (19).
For toxin, there has been no selective pressure from vaccines. Most acellular vaccines contain ptxA (2) or ptxA (3), but the vast majority of circulating clones now produce ptxA (1) (25). Also, in our material over the three decades, almost all strains were of ptxA (1) type from the beginning of the study in the 1970s, when a whole-cell vaccine expressing ptxA (4) was used. However, this divergence between the vaccine toxin type and the type of toxin produced by clinical isolates was not seen in the United Kingdom. All of the most recently circulating 105 strains (from 1998 and 1999) were of the same pertussis toxin type as those present in the United Kingdom DTPw, i.e., ptxA (1) and ptxA (2) (10).
Chromosomal fingerprinting by means of PFGE has been shown to be a very discriminating way to discover changes in the circulating bacterial population (2, 20, 22, 25, 31, 41). Most changes seen in the DNA structure may be due to gain or loss of restriction sites or rearrangement of the fluid genome. These changes may also reflect an antigenic shift most evident for pertactin (2). This may happen independently, but new variants may be selected by the immune status of the population, due to natural infections, together with vaccination. It is therefore of specific interest to study how the PFGE profiles, along with vaccine-related markers, change over time.
Our study demonstrates that as time has gone by the strain variants have come in waves, gradually peaking over time, disappearing, and being replaced by new profiles (Fig. 4). The total number of PFGE profiles seen in Sweden during the years 1970 to 2003 may be large, but there have been only a few predominant ones. A general observation was that the main patterns changed with the period of vaccination policy. During the Pw period (1970 to 1977), BpSR25 (serotype Fim3 prn1/7) and BpSR18 (serotype Fim3 prn2) were predominant. After the cessation of general vaccination in 1979, the PFGE profile BpSR1 (serotype Fim2 prn2) took over, to be replaced in turn by BpSR11 (serotype Fim3 prn2) during an incidence peak after the reintroduction of general vaccination in 1996. The BpSR11 PFGE profile had already appeared in Europe (IVβ) before 1996 (41).
It has been argued that the changes over time may well be due to natural variation rather than to immunity-driven selection. In Japan, the first country to introduce acellular pertussis vaccines in 1981, it was suggested that if acellular pertussis vaccine-induced antigenic divergence exists, it is likely to be a slow or rare process, but the number of studied isolates were few (13).
On the other hand, immunity-driven selection may well explain changes in the Swedish B. pertussis populations over time, as characterized by serotype, prn, and PFGE profiles. Adapted B. pertussis variants may appear due to vaccination and/or boosting of immunity by natural infection. At least, this seems very obvious when the serotypes during the Pw- and the vaccine-free periods are compared, in addition to the observations recorded during the vaccine trials during the vaccine-free period.
Swedish experience so far does not indicate that the divergence between toxin and pertactin subtypes used in acellular vaccines and those found in clinical isolates has had significant influence on the effectiveness of the acellular vaccines against disease. Other parts of the antigen molecules probably contribute to the human immune response. The reported incidence of pertussis has dropped from 120 to 150 cases per 100,000 person years of follow-up during the years 1993 through 1995 to 11, 15, and 7/100,000 in 2001, 2002, and 2003, respectively.
In conclusion, shifts in the B. pertussis population coincide with changes in vaccination policy, from the use of DTPw vaccine in the 1970s to a 17-year period without general vaccination starting in 1979 and once more after 1996, when vaccination with Pa-containing vaccines was introduced.
It is too early to predict whether bacterial polymorphism will have a negative effect on the vaccination program against pertussis in the long run and how or if there will be an immunity-driven selection of new clones or adapted mutants. It may even be that the selected variants are less virulent. Interestingly, serotype Fim2 strains seem to cause more severe illness in terms of cough duration and hospital admission, as reported in the United Kingdom (37). It would be interesting to compare isolates from regions using DTPa2 with isolates from regions using DTPa3 and to relate profiles to clinical outcome (studies are ongoing). For the future, it is important to follow the bacterial population and study the impact of changes in relation to vaccination policy and incidence of disease. In Sweden, it is of particular interest to follow a possible reappearance of variants expressing serotype Fim2 due to waning immunity, as serotype Fim2 is not present in the acellular vaccines used after 1996 or prevalent among circulating strains.
Acknowledgments
This work was supported by GlaxoSmithKline and Aventis Pasteur and by the European Commission, contract no. QLK2-CT-2001-01819, EUpertstrain.
We thank all of the Swedish clinical bacteriology laboratories for their generous contribution of B. pertussis isolates.
REFERENCES
- 1.Ad Hoc Group for the Study of Pertussis Vaccines. 1988. Placebo-controlled trial of two acellular pertussis vaccines in Sweden—protective efficacy and adverse events. Lancet i:955-960. [PubMed] [Google Scholar]
- 2.Advani, A., D. Donnelly, and H. Hallander. 2004. Reference system for characterization of Bordetella pertussis pulsed-field gel electrophoresis profiles. J. Clin. Microbiol. 42:2890-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron, S., E. Njamkepo, E. Grimprel, P. Begue, J. C. Desenclos, J. Drucker, and N. Guiso. 1998. Epidemiology of pertussis in French hospitals in 1993 and 1994: thirty years after a routine use of vaccination. Pediatr. Infect. Dis. J. 17:412-418. [DOI] [PubMed] [Google Scholar]
- 4.Berbers, G. A. M., A. B. Lafeber, J. Labadie, P. E. Vermeer-de Bondt, D. J. A. Bolscher, and A. D. Plantinga. 1999. A randomised controlled study with whole-cell or acellular pertussis vaccines in combination with regular DT-IPV vaccine and a new poliomyelitis (IPV-vero) component in children 4 years of age in The Netherlands. RIVM report 105000 001. RIVM, Bilthoven, The Netherlands.
- 5.Cassiday, P., G. Sanden, K. Heuvelman, F. Mooi, K. M. Bisgard, and T. Popovic. 2000. Polymorphism in Bordetella pertussis pertactin and pertussis toxin virulence factors in the United States, 1935-1999. J. Infect. Dis. 182:1402-1408. [DOI] [PubMed] [Google Scholar]
- 6.Cherry, J. D., J. Gornbein, U. Heininger, and K. Stehr. 1998. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine 16:1901-1906. [DOI] [PubMed] [Google Scholar]
- 7.Cherry, J. D., and P. Olin. 1999. The science and fiction of pertussis vaccines. Pediatrics 104:1381-1383. [DOI] [PubMed] [Google Scholar]
- 8.Cordova, S. P., M. T. Gilles, and M. Y. Beers. 2000. The outbreak that had to happen: Bordetella pertussis in north-west Western Australia in 1999. Commun. Dis. Intell. 24:375-379. [DOI] [PubMed] [Google Scholar]
- 9.de Moissac, Y. R., S. L. Ronald, and M. S. Peppler. 1994. Use of pulsed-field gel electrophoresis for epidemiological study of Bordetella pertussis in a whooping cough outbreak. J. Clin. Microbiol. 32:398-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fry, N. K., S. Neal, T. G. Harrison, E. Miller, R. Matthews, and R. C. George. 2001. Genotypic variation in the Bordetella pertussis virulence factors pertactin and pertussis toxin in historical and recent clinical isolates in the United Kingdom. Infect. Immun. 69:5520-5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greco, D., S. Salmaso, P. Mastrantonio, M. Giuliano, A. E. Tozzi, A. Anemona, M. L. Ciofi degli Atti, A. Giammanco, P. Panei, W. C. Blackwelder, D. L. Klein, S. G. Wassilak, et al. 1996. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. N. Engl. J. Med. 334:341-348. [DOI] [PubMed] [Google Scholar]
- 12.Guiso, N. 1997. Isolation, identification and characterization of Bordetella pertussis. Dev. Biol. Stand. 89:233-238. [PubMed] [Google Scholar]
- 13.Guiso, N., C. Boursaux-Eude, C. Weber, S. Z. Hausman, H. Sato, M. Iwaki, K. Kamachi, T. Konda, and D. L. Burns. 2001. Analysis of Bordetella pertussis isolates collected in Japan before and after introduction of acellular pertussis vaccines. Vaccine 19:3248-3252. [DOI] [PubMed] [Google Scholar]
- 14.Guiso, N., C. H. von Konig, C. Becker, and H. Hallander. 2001. Fimbrial typing of Bordetella pertussis isolates: agglutination with polyclonal and monoclonal antisera. J. Clin. Microbiol. 39:1684-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guris, D., P. M. Strebel, B. Bardenheier, M. Brennan, R. Tachdjian, E. Finch, M. Wharton, and J. R. Livengood. 1999. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990-1996. Clin. Infect. Dis. 28:1230-1237. [DOI] [PubMed] [Google Scholar]
- 16.Gustafsson, L., H. O. Hallander, A. Advani, R.-M. Carlsson, and P. Olin. 6 July 2004, posting date. Six year report. Pertussis surveillance in Sweden. Progress Report October 1997-September 2003. Smittskyddsinstitutets rapportserie2. [Online.] http://www.smittskyddsinstitutet.se/upload/6985/SMI-rapport-2004-2(2).pdf.
- 17.Gustafsson, L., H. O. Hallander, P. Olin, E. Reizenstein, and J. Storsaeter. 1996. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N. Engl. J. Med. 334:349-355. [DOI] [PubMed] [Google Scholar]
- 18.Gzyl, A., E. Augustynowicz, D. Rabczenko, G. Gniadek, and J. Slusarczyk. 2004. Pertussis in Poland. Int. J. Epidemiol. 33:358-365. [DOI] [PubMed] [Google Scholar]
- 19.Gzyl, A., E. Augustynowicz, I. van Loo, and J. Slusarczyk. 2001. Temporal nucleotide changes in pertactin and pertussis toxin genes in Bordetella pertussis strains isolated from clinical cases in Poland. Vaccine 20:299-303. [DOI] [PubMed] [Google Scholar]
- 20.Hardwick, T. H., P. Cassiday, R. S. Weyant, K. M. Bisgard, and G. N. Sanden. 2002. Changes in predominance and diversity of genomic subtypes of Bordetella pertussis isolated in the United States, 1935 to 1999. Emerg. Infect. Dis. 8:44-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He, Q., J. Makinen, G. Berbers, F. R. Mooi, M. K. Viljanen, H. Arvilommi, and J. Mertsola. 2003. Bordetella pertussis protein pertactin induces type-specific antibodies: one possible explanation for the emergence of antigenic variants? J. Infect. Dis. 187:1200-1205. [DOI] [PubMed] [Google Scholar]
- 22.Lee, Y. S., C. Y. Yang, C. H. Lu, and Y. H. Tseng. 2003. Molecular epidemiology of Bordetella pertussis isolated in Taiwan, 1992-1997. Microbiol. Immunol. 47:903-909. [DOI] [PubMed] [Google Scholar]
- 23.Li, Z. M., M. J. Brennan, J. L. David, P. H. Carter, J. L. Cowell, and C. R. Manclark. 1988. Comparison of type 2 and type 6 fimbriae of Bordetella pertussis by using agglutinating monoclonal antibodies. Infect. Immun. 56:3184-3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mastrantonio, P., P. Spigaglia, H. van Oirschot, H. G. van der Heide, K. Heuvelman, P. Stefanelli, and F. R. Mooi. 1999. Antigenic variants in Bordetella pertussis strains isolated from vaccinated and unvaccinated children. Microbiology 145:2069-2075. [DOI] [PubMed] [Google Scholar]
- 25.Mooi, F. R., H. Hallander, C. H. Wirsing von Konig, B. Hoet, and N. Guiso. 2000. Epidemiological typing of Bordetella pertussis isolates: recommendations for a standard methodology. Eur. J. Clin. Microbiol. Infect. Dis. 19:174-181. [DOI] [PubMed] [Google Scholar]
- 26.Mooi, F. R., Q. He, H. van Oirschot, and J. Mertsola. 1999. Variation in the Bordetella pertussis virulence factors pertussis toxin and pertactin in vaccine strains and clinical isolates in Finland. Infect. Immun. 67:3133-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mooi, F. R., H. van Oirschot, K. Heuvelman, H. G. van der Heide, W. Gaastra, and R. J. Willems. 1998. Polymorphism in the Bordetella pertussis virulence factors P.69/pertactin and pertussis toxin in The Netherlands: temporal trends and evidence for vaccine-driven evolution. Infect. Immun. 66:670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen, A., and S. O. Larsen. 1994. Epidemiology of pertussis in Denmark: the impact of herd immunity. Int. J. Epidemiol. 23:1300-1308. [DOI] [PubMed] [Google Scholar]
- 29.Olin, P., L. Gustafsson, L. Barreto, L. Hessel, T. C. Mast, A. V. Rie, H. Bogaerts, and J. Storsaeter. 2003. Declining pertussis incidence in Sweden following the introduction of acellular pertussis vaccine. Vaccine 21:2015-2021. [DOI] [PubMed] [Google Scholar]
- 30.Olin, P., F. Rasmussen, L. Gustafsson, H. O. Hallander, and H. Heijbel, et al. 1997. Randomised controlled trial of two-component, three-component, and five-component acellular pertussis vaccines compared with whole-cell pertussis vaccine. Lancet 350:1569-1577. [DOI] [PubMed] [Google Scholar]
- 31.Peppler, M. S., S. Kuny, A. Nevesinjac, C. Rogers, Y. R. de Moissac, K. Knowles, M. Lorange, G. De Serres, and J. Talbot. 2003. Strain variation among Bordetella pertussis isolates from Quebec and Alberta provinces of Canada from 1985 to 1994. J. Clin. Microbiol. 41:3344-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preston, N. W. 1985. Essential immunogens in human pertussis: the role of fimbriae. Dev. Biol. Stand. 61:137-141. [PubMed] [Google Scholar]
- 33.Romanus, V., R. Jonsell, and S. O. Bergquist. 1987. Pertussis in Sweden after the cessation of general immunization in 1979. Pediatr. Infect. Dis. J. 6:364-371. [DOI] [PubMed] [Google Scholar]
- 34.Storsaeter, J., H. O. Hallander, L. Gustafsson, and P. Olin. 1998. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 16:1907-1916. [DOI] [PubMed] [Google Scholar]
- 35.Storsaeter, J., and P. Olin. 1992. Relative efficacy of two acellular pertussis vaccines during three years of passive surveillance. Vaccine 10:142-144. [DOI] [PubMed] [Google Scholar]
- 36.Tiru, M., P. Askelof, M. Granstrom, and H. Hallander. 1997. Bordetella pertussis serotype of clinical isolates in Sweden during 1970-1995 and influence of vaccine efficacy studies. Dev. Biol. Stand. 89:239-245. [PubMed] [Google Scholar]
- 37.Van Buynder, P. G., D. Owen, J. E. Vurdien, N. J. Andrews, R. C. Matthews, and E. Miller. 1999. Bordetella pertussis surveillance in England and Wales: 1995-7. Epidemiol. Infect. 123:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Zee, A., S. Vernooij, M. Peeters, J. van Embden, and F. R. Mooi. 1996. Dynamics of the population structure of Bordetella pertussis as measured by IS1002-associated RFLP: comparison of pre- and post-vaccination strains and global distribution. Microbiology 142:3479-3485. [DOI] [PubMed] [Google Scholar]
- 39.Van Loo, I. H., and F. R. Mooi. 2002. Changes in the Dutch Bordetella pertussis population in the first 20 years after the introduction of whole-cell vaccines. Microbiology 148:2011-2018. [DOI] [PubMed] [Google Scholar]
- 40.van Loo, I. H., H. G. van der Heide, N. J. Nagelkerke, J. Verhoef, and F. R. Mooi. 1999. Temporal trends in the population structure of Bordetella pertussis during 1949-1996 in a highly vaccinated population. J. Infect. Dis. 179:915-923. [DOI] [PubMed] [Google Scholar]
- 41.Weber, C., C. Boursaux-Eude, G. Coralie, V. Caro, and N. Guiso. 2001. Polymorphism of Bordetella pertussis isolates circulating for the last 10 years in France, where a single effective whole-cell vaccine has been used for more than 30 years. J. Clin. Microbiol. 39:4396-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. 1979. W.H.O. Expert Committee on Biological Standardization, 30th report. Tech. Rep. Ser. 638:61-65. [PubMed] [Google Scholar]
- 43.von Konig, C. H., S. Halperin, M. Riffelmann, and N. Guiso. 2002. Pertussis of adults and infants. Lancet Infect. Dis. 2:744-750. [DOI] [PubMed] [Google Scholar]