Abstract
Serotyping Streptococcus pneumoniae is a technique generally confined to reference laboratories, as purchasing pneumococcal antisera is a huge investment. Many attempts have been made to modify serological agglutination techniques to make them more accessible, and more recently developments in serotyping have focused on molecular techniques. This paper describes a PCR assay which amplifies the entire capsulation locus between dexB and aliA. Amplicons are digested to produce serotype-specific patterns. We have shown, using 81 epidemiologically unrelated strains representing 46 different serotypes, that the patterns correlate with a 90 to 100% similarity range for the same serotype or serogroup. Prospective testing of 73 isolates of unknown serotype confirmed reliable serotype attribution, and serotype profiles are reproducible on repeated testing. Once our database contains all 90 serotypes, this technique should be fully portable, cost-effective, and useful in any laboratory with sufficient molecular experience.
The species Streptococcus pneumoniae possesses more than 90 serotypes defined by their polysaccharide capsule (1). Immunologically similar serotypes, such as 19F, 19A, 19B, and 19C, are grouped together in serogroups. The immune response to capsular polysaccharide is critical for recovery from infection (17), and the first effective treatment for pneumococcal infection, serum therapy, depended on identifying the infecting serotype rapidly so that type-specific horse serum could be administered (9). The capsule is the focus for the development of an effective vaccine, initially with the 23-valent polysaccharide and more recently the protein-polysaccharide conjugate preparations that are undergoing clinical evaluation and have entered clinical use (1, 35). Some reports on the implementation of the conjugate vaccine in different communities show that there is an increase in the carriage of serotypes that are not included in the vaccine (6, 7). Additionally, serotype surveillance in developing countries and special populations, such as Aboriginal Australians, suggests that vaccine serotype coverage may be lower than for the United States and Europe, from which surveillance data were considered in vaccine formulations (2, 4, 25). Thus, there is a continuing need to study the epidemiology of S. pneumoniae as defined by capsular polysaccharide in monitoring the effect of conjugate vaccines and to aide in the development of new vaccine formulations for developing countries and special populations (12, 13, 16).
Effective serotyping depends on a full set of group- and type-specific antisera prepared by the Statens Serum Institut (14), an investment that is beyond the resources of many laboratories. Therefore, most laboratories confine typing to the serotypes and serogroups found in the 23-valent vaccine, which represent most of the invasive types found in industrialized countries (22). As prevailing serotypes change, a wider selection of sera will be required to cover common types, thus increasing the cost. In addition to the cost, equivocal results due to autoagglutination are common, and discrepancies are reported in the typing of other organisms, such as Haemophilus influenzae, between PCR and standard slide agglutination. In one such study, this discrepancy is thought to have been due to laboratory error, implying that PCR-based typing is more reliable (21).
Alternative typing methods not based on the capsular polysaccharide are described for S. pneumoniae using various molecular methods and phenotypic methods. These include pulsed-field gel electrophoresis, restriction fragment end labeling, BOX PCR, arbitrarily primed PCR, and multilocus sequence typing (MLST) (8, 15a, 34, 36). Although these methods prove valuable for distinguishing strains, studying epidemiology, and monitoring transmission, the importance of the capsule in pathogenesis and immunity emphasizes the fact that serotyping will remain in demand.
The genes encoding the S. pneumoniae capsule are contained within a gene cassette which is flanked by two genes, dexB and aliA, in all serotypes that have been sequenced to date (28, 29, 32). Within this locus, each serotype has a set of homologous genes and a set which define serotype and thus are variable (30). This combination of homology and diversity makes the capsulation gene cassette an attractive target for a DNA amplification-based method of defining serotype. Furthermore, with the capsulation loci for the majority of serotypes having been sequenced, confirmation of serotype-specific patterns is possible by in silico digestions.
We report a PCR capable of amplifying the entire capsule locus for all serotypes tested to date, and digestion of the amplicons with HinfI yields serotype-unique patterns that distinguish them from other serotypes, most of which are confirmed in silico.
MATERIALS AND METHODS
Bacteria.
Isolates of S. pneumoniae used in this study were obtained from clinical specimens submitted to the diagnostic microbiology laboratory of the Royal Free Hospital (RFH), a carriage survey of children collected in the Rombo district of Tanzania (Tz), and the Health Protection Agency, Colindale, United Kingdom (HPA). Table 1 contains strains used to generate the Bionumerics database of serotype-specific patterns.
TABLE 1.
Isolates used to establish a Bionumerics database of serotype-specific patterns
| Serotype | Strain | Clinical sourcea |
|---|---|---|
| 1 | RFH 116, RFH 206 | BC |
| 3 | Tz 8810-1, Tz 2604-1b, Tz 2604-1a, Tz 5809.2 | TS |
| 4 | Tz 1104-2, Tz 3682-2, Tz 2706-1 | TS |
| 5 | Tz 7310-1 | TS |
| 6A | RFH 260, RFH 6 | BC |
| Tz 4613-3, Tz 4202-1, Tz 1003-2, Tz 1003-3 | TS | |
| HPA 6A | C | |
| 6B | Tz 5303-2, Tz 7712-2b | TS |
| RFH 200, RFH 221 | BC | |
| HPA 6B | C | |
| 7F | HPA 7F | C |
| 7A | HPA 7A | C |
| 7B | HPA 7B | C |
| 7C | Tz 2514-1, Tz 4711-1, Tz 5310-2 | TS |
| 8 | Tz 4603-3 | TS |
| 9A | HPA 9A | C |
| 9L | HPA 9L | C |
| 9N | RFH 18, RFH 48, RFH 93 | BC |
| 9V | RFH 78, RFH 98, RFH 120 | BC |
| 10B | HPA 10B | C |
| 10C | HPA 10C | C |
| 11A | HPA 11A | C |
| 11B | HPA 11B | C |
| 11 (subtype not known) | Tz 1101-2, Tz 3912-1, Tz 1102-1, Tz 5907-1 | TS |
| 12F/B | Tz 3407-1, Tz 8103-2 | TS |
| 13 | HPA 13 | TS |
| 14 | RFH 16, RFH 34, RFH 40 | BC |
| 15A | HPA 15A | C |
| 15C | HPA 15C | C |
| 17F | HPA 17F | C |
| 18F | HPA 18F | C |
| 18A | HPA 18A | C |
| 18B/C | Tz 8715-1 | TS |
| 19A | RFH 25 | BC |
| HPA 19A | C | |
| 19B | HPA 19B | C |
| 19F | RFH 5, RFH 32 | BC |
| Tz 2701-2, Tz 7910-1 | TS | |
| 20 | Tz 1103-1 | TS |
| 21 | HPA 21 | C |
| 22 | RFH 33 | BC |
| 23A | HPA 23A | C |
| 23F | RFH 13, RFH 329, RFH 6, RFH 7 | BC |
| 24F | HPA 24F | C |
| 24A | HPA 24A | C |
| 24B | HPA 24B | C |
| 25F | HPA 25F | |
| 25A | HPA 25A | C |
| 29 | HPA 29 | C |
| 32F | HPA 32F | C |
| 35A | HPA 35A | C |
| 38 | HPA 38 | C |
C, control strains from a reference laboratory; TS, carriage isolates from throat swabs; BC, isolates from blood culture.
Preparation of genomic DNA.
Bacterial cells were harvested from a fresh overnight culture on blood agar, and DNA was extracted using the Wizard Genomic DNA purification kit (Promega, Southampton, United Kingdom) following the manufacturer's instructions. Briefly, a dense suspension of cells was made in 480 μl 0.05 M EDTA (Sigma, Poole, United Kingdom). One hundred twenty microliters of cell lysis solution and 60 μl lysozyme (Sigma) were added, mixed by gentle inversion, and incubated at 37°C for 30 to 60 min. The tubes were centrifuged at 13,000 × g for 2 min, and the supernatant was discarded. The cells were resuspended in 600 μl nuclei lysis solution and mixed by gentle pipetting. The tubes were incubated at 80°C for 5 min and cooled to room temperature. Three microliters of RNase solution was added, and the tubes were incubated at 37°C for 15 to 30 min and cooled to room temperature. Protein precipitation solution (200 μl) was added, and the tubes were vortexed for 20 seconds and then incubated on ice for 5 min. Cell debris was precipitated by centrifuging at 13,000 × g for 3 min and the supernatant transferred to a fresh tube containing 0.6 ml room-temperature isopropanol. The suspension was mixed by gentle inversion until a precipitate could be seen. Tubes were then centrifuged for 10 min, and the pellet was washed in 70% ethanol (vol/vol) and dried for 15 min at 37°C. The pellet was then redissolved in 100 μl DNA rehydration solution and incubated at 65°C in a water bath for 60 min, with mixing every 20 min. The isolated DNA was used immediately. Experiments using DNA which had been stored at 4°C for more than 24 h showed multiple PCR amplicons (data not shown).
PCR conditions.
The primers used were high-performance liquid chromatography purified (Sigma Genosys, Poole, United Kingdom) and had the following sequences: for AliA2, 5′-ATG CAG CTA AAG TAG TCG CC-3′; for DexB2, 5′-GAC CGT CGC TTC CTA GTT GT-3′.
Amplification was achieved using REDAccuTaq LA DNA polymerase (Sigma). The optimal reaction tube contained 1.5 μl of genomic fresh DNA extract, 2 μl Taq (2 U), 5 μl buffer, deoxynucleoside triphosphates at 0.5 mM, and 0.6 mM primers, made up to 50 μl final reaction volume with PCR-grade water.
The optimal reaction cycle consisted of one cycle of 93°C for 2 min and 30 cycles of 93°C for 15 seconds, 50°C for 30 seconds, and 68°C for 20 min, with a hold temperature of 4°C. All reactions were performed on a Progene (Techne, United Kingdom) or GeneAmp 9700 (Applied Biosystems, Warrington, United Kingdom) machine using 0.2-ml thin-walled tubes (Alpha Laboratories, Hampshire, United Kingdom or Applied Biosystems). Amplimers were kept at 4°C until digests were performed and are known to be stable at this temperature for up to 1 month.
RFLP.
Amplicons from the PCRs were digested using HinfI as follows. A 20-μl aliquot from the PCR was incubated with 1 μl enzyme (10 U), 5 μl digestion buffer, and 0.2 μl bovine serum albumin (Promega) for 4 h at 37°C according to the manufacturer's instructions. The digests were subjected immediately to gel electrophoresis using a 30-ml tank (Horizon 58; Life Technologies) 3% (wt/vol) agarose gel (Bioline, London, United Kingdom) with the voltage set at 100 V (Power pack model 4001P; Life Technologies). No pretreatment of restriction fragment length polymorphism (RFLP) digests was performed before loading, and the RFLP digests are stable for storage at 4°C for at least a few days. Electrophoresis was continued until the loading dye was at the bottom of the gel. An image of the gel was taken using a stand-mounted digital camera DC 120 and Kodak Digital Science 2.0 imaging software, and the image was stored as a Tiff file.
Analysis of data.
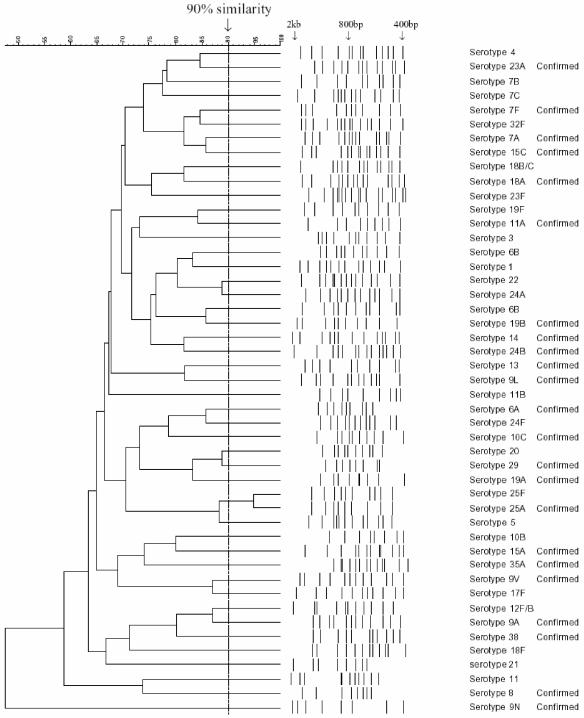
The Tiff images were opened in Bionumerics software 3.5 (Applied Maths, Kortrjk, Belgium) as an RFLP experiment. The RFLP patterns were entered by a single investigator using Bionumerics software and saved into a single database. The gels were all normalized by assigning band sizes to the bands of hyperladder I (Bioline). All bands that could be seen on the image of the gel down to the 400-bp marker were selected (Fig. 1). Double bands were selected only when two distinct peaks could be seen on the gel image and the densitometric curves (the densitometric curve is seen in the analysis window of Bionumerics). These criteria were applied to every gel.
FIG. 1.
Dendrogram showing one isolate for each of the 46 representative RFLP patterns. Serotypes 1, 3, 4, 5, 6A, 6B, 7F, 7A, 7B, 7C, 8, 9A, 9N, 9V, 9L, 10B, 10C, 11A, 11B, 11 (subtype not known), 12F/B, 13, 14, 15A, 15C, 17F, 18F, 18A, 18B/C, 19F, 19A, 19B, 20, 21, 22, 23A, 23F, 24F, 24A, 24B, 25F, 25A, 29, 32F, 35A, and 38 are included. The 12F and B and 18B and C patterns could not be discriminated using in silico data, since digests with HinfI were very similar. No in silico data were available for serotype 25F. “Confirmed” identifies patterns confirmed by in silico data (where available). Alignments were produced using Bionumerics software (Applied Maths).
Cluster analysis.
Cluster analysis was performed using the Dice coefficient. Similarity was calculated using parameter settings at 2% band position tolerance and optimization at 1%. The program computed a dendrogram using the unweighted-pair group method using average linkages clustering algorithm. This algorithm links the serotype at the level of the branch so the percentage shown by clicking on the level of the branch shows the percentage similarity of the two serotypes. To set the definition of serotype and serogroup, all (n = 81) strains for which a serotype or serogroup was known were clustered together on a single dendrogram using Bionumerics.
Identifying the serotype of an unknown sample.
To identify the serotype of an unknown sample, cluster analysis was performed blind for 73 isolates. For an unknown isolate, the normalization process described above was followed. A comparison was created with the unknown strain using one example of every type and group in the database. If a match with similarity greater than 90% was detected, this was defined as the serotype of the strain. If the match was less than 90%, this indicated a serotype not currently in our database (Fig. 1).
In silico digestions.
The sequences from the capsulation locus were downloaded from the Sanger website (http://www.sanger.ac.uk/Projects/S_pneumoniae/CPS/). Digestions were performed using Restrict from the UK HGMP Resource Centre (http://www.hgmp.mrc.ac.uk/) and band sizes compared to those on agarose gels and used to confirm experimental digestions where possible.
RESULTS
Amplifiable serotypes.
The most important requirement of a molecular serotyping method is the capacity to type all of the possible serotypes of S. pneumoniae. To test this, the PCR was performed on genomic DNA obtained from multiple isolates. PCR amplicons were obtained from all isolates, and the size range was around 14 to 23 kbp.
Serotype and serogroup identification.
Isolates chosen for study were those that were likely to be epidemiologically unrelated. This was achieved by selecting examples from our U.K. laboratory, isolates from field studies in Tanzania, and isolates of unusual serotypes from the Health Protection Agency. This procedure was followed to reduce the risk of false positive matches among common serotypes that were actually identical strains. Where multiple isolates of the same serotype occurred, as defined by agglutination tests, they had RFLP patterns that were at least 90% similar.
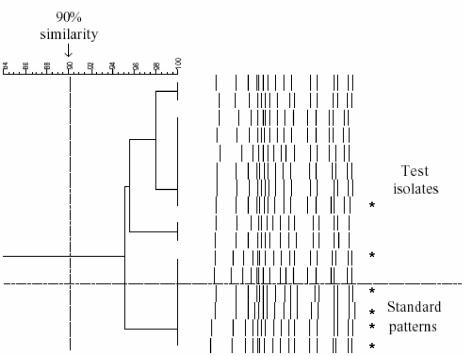
Figure 1 shows 46 serotypes and serogroups aligned using the Bionumerics software. Isolates of the same serotype showed greater than 90% similarity, and isolates of different types showed less than 90% similarity. Examples of the same serotype were tested repeatedly and shown to have an identical pattern, for example, 16 times for serotype 23F (including standard patterns) (Fig. 2). Multiple isolates from the same patient were shown to have identical patterns.
FIG. 2.
Dendrograms of multiple S. pneumoniae serotype 23F isolates. Four standard patterns showed greater than 94% similarity with 12 throat swab test isolates. Asterisks represent the strains that were typed using MLST.
Exceptions to the rule that only members of the same serotype are >90% similar were found on four occasions. A pair of isolates was identified as serogroup 18, but the subtype could not be determined. Similar results were obtained for two strains, identified as serogroup 12 and serogroup 25, with the subtype not being distinguishable. Additionally, serotype 6B has two representative patterns.
Identification of the serotype for an unknown sample.
A total of 73 unknown strains serotyped conventionally were examined in a blinded fashion. The following serotypes were correctly identified: 3 (n = 5), 6A (n = 5), 6B (n = 3), 9A (n = 1), 11 (n = 1), 12B/C (n = 11), 19A (n = 2), 19F (n = 1), 22 (n = 1), 23F (n = 12), and 29 (n = 1). Additionally, 30 isolates representing 14 patterns not currently in the database were identified.
DISCUSSION
Bacterial agglutination is, arguably, one of the simplest procedures performed in microbiology laboratories, yet it is one where there are many pitfalls. In some cases, the agglutination may be weak, making the result difficult to see. Alternatively, some strains may autoagglutinate, making it impossible to obtain a typing result. Difficulties in the performance of serotyping are perhaps demonstrated by the numerous modifications made to the original capsule swelling technique by different investigators, which include attempts to harness latex agglutination or counterimmunoelectrophoresis (14, 15, 20, 26, 27, 33).
A method using PCR amplification of a portion within the locus between cpsA and cpsB (located immediately upstream of dexB) followed by restriction fragment polymorphism was described previously (23). However, the patterns produced permit discrimination only to the level of serogroup rather than serotype. This is because the PCR primers amplify a section of the capsulation locus between cpsA and cpsB. cpsA-cpsD genes are conserved, and cpsA is >90% identical in all of the gene clusters sequenced fully (11). In addition, for some serotypes there are significant sequence differences in the cpsB homologue (11). Another drawback is that two or three enzymes are required to generate patterns capable of discrimination and add to the time taken to evaluate the strain. More recently, two groups described molecular methods that enable the identification of serotypes and serogroups using multiplex PCR (3, 24) and sequencing of cpsA-B genes with additional PCRs for serotype-specific sequences (19). Lawrence et al.'s method requires the use of three multiplex PCRs and recognizes a limited set of serotypes (24). To include more serotypes would require additional multiplex PCRs, which would not be feasible for all 90 serotypes, and currently the method is feasible only where a capillary sequencer is available. Brito et al.'s method uses two successive rounds of PCR, and the choice of PCR used in the second round requires the amplicons from the first round to be electrophoresed and resolved into groups (3). The drawback of these methods is that they become more complicated as the number of serotypes to be identified increases.
Although we made many attempts, it was not possible to design a simple PCR system based on portions of the capsulation locus because of the diversity of gene arrangement in the capsulation cassettes of different serotypes. It is for this reason that we chose to develop a set of primers based on the aliA and dexB genes, as the method reported here has been designed to be used in routine microbiology practice. dexB and aliA genes are found in all serotypes, and it is this homology that makes capsule switching possible for S. pneumoniae (5, 10, 11). Others used a similar PCR protocol to amplify the capsulation locus for sequencing (18). We demonstrate that the digestion of amplicons of the whole locus is capable of discriminating at least 46 types of S. pneumoniae to serogroup or serotype level and that the band profiles observed from the restriction digest protocol reported are robust and reproducible (Fig. 2). For serogroups 12, 18, and 25, subtypes 12F and B, 18B and C, and 25F and A could not be distinguished. The inability to distinguish these serotypes is confirmed by the in silico digestion, as the sequences are very closely related (http://www.sanger.ac.uk/Projects/S_pneumoniae/CPS/). Types 12F and 12B can be distinguished using a second enzyme, AvaI, which cuts serotype 12B but not serotype 12F into bands of 17,836 and 5,327 bp, as demonstrated in silico. Types 18B and 18C have whole locus sequences that differ at only two base pairs; a distinction could be made using a second PCR around one of the base pair locations and digestion with TspDTI, which will cut only 18B, as the restriction site for this enzyme is lost for the other base pair. There is no sequence available for serotype 25F, and thus it is not yet possible to suggest an enzyme to differentiate these types.
For all serotype patterns where sequences are available, the digests we obtained are matched by the in silico experiments, during which the predicted band sizes are compared to those on the gel pattern of the corresponding type. This analysis is limited to only the sequences which are currently available.
To produce a simple and robust method, we have chosen to use a single set of primers and a single restriction enzyme. The choice of Taq appears critical, as experiments using other enzyme systems did not provide such reliable results (data not shown). Only the purification of high-quality DNA provided reliable amplification products. Although this is an important drawback, the technique requires only modest competence in molecular biological techniques. This is more than compensated for by the fact that all of the subsequent steps are identical irrespective of serotype. The agglutination technique requires multiple reiterative steps, so that operators can process only a limited number of isolates daily. The PCR technique described here would permit a large number of isolates to be examined on a single day, the scale being limited by the equipment available.
Out of the 12 isolates identified as serotype 23F using this PCR method, 10 were multiple-colony picks taken from throat swabs of two children, demonstrating the reproducibility of the same and different strains. A total of six isolates of serotype 23F, including MLST types 242, 124, and 81 (two examples) and two new unique types, were tested and aligned together (Fig. 2). This indicates the robustness of the technique for strains of different genotypes but with identical serotypes. Similarly, the method was successfully applied to each of the different PCR machines available in our laboratory, which suggests that the method is likely to be reproducible in other laboratories.
The application of this technique in our laboratory has improved the number of serotypes that can be recognized from 23 to 46. A significant number of the isolates obtained in our Tanzanian survey are nontypeable using the pneumotest 23-valent antisera kit (2). Our method was able to produce amplicons from all of these nontypeable strains, which suggests that our PCR method will be more flexible in serotype coverage in areas where nonvaccine types are common. In addition, it opens up the possibility that serotypes that have previously not been recognized will be identified and described more quickly, provided they share the aliA-dexB cassette arrangement. Once a comprehensive set of RFLP patterns is established for this PCR-RFLP protocol, an isolate that does not align with one of these can be sequenced to indicate whether it represents a new serotype or serogroup.
Due to the relative simplicity and flexibility of this technique, we believe that it will be of value to reference centers and to researchers investigating serotype replacement and the population biology of S. pneumoniae and that it has the potential to be a robust alternative to serological serotyping.
Acknowledgments
The work for this paper was funded by Pfizer Inc.
REFERENCES
- 1.Austrian, R. 1989. Pneumococcal polysaccharide vaccines. Rev. Infect. Dis. 11(Suppl. 3):S598-S602. [DOI] [PubMed] [Google Scholar]
- 2.Batt, S. L., B. M. Charalambous, A. W. Solomon, C. Knirsch, P. A. Massae, S. Safari, N. E. Sam, D. Everett, D. C. Mabey, and S. H. Gillespie. 2003. Impact of azithromycin administration for trachoma control on the carriage of antibiotic-resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 47:2765-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brito, D. A., M. Ramirez, and H. de Lencastre. 2003. Serotyping Streptococcus pneumoniae by multiplex PCR. J. Clin. Microbiol. 41:2378-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler, J. C., S. Crengle, J. E. Cheek, A. J. Leach, D. Lennon, K. L. O'Brien, and M. Santosham. 2001. Emerging infectious diseases among indigenous peoples. Emerg. Infect. Dis. 7:554-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coffey, T. J., M. C. Enright, M. Daniels, J. K. Morona, R. Morona, W. Hryniewicz, J. C. Paton, and B. G. Spratt. 1998. Recombinational exchanges at the capsular polysaccharide biosynthetic locus lead to frequent serotype changes among natural isolates of Streptococcus pneumoniae. Mol. Microbiol. 27:73-83. [DOI] [PubMed] [Google Scholar]
- 6.Dagan, R., N. Givon-Lavi, O. Zamir, and D. Fraser. 2003. Effect of a nonavalent conjugate vaccine on carriage of antibiotic-resistant Streptococcus pneumoniae in day-care centers. Pediatr. Infect. Dis. J. 22:532-540. [DOI] [PubMed] [Google Scholar]
- 7.Dagan, R., N. Givon-Lavi, O. Zamir, M. Sikuler-Cohen, L. Guy, J. Janco, P. Yagupsky, and D. Fraser. 2002. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J. Infect. Dis. 185:927-936. [DOI] [PubMed] [Google Scholar]
- 8.Enright, M. C., and B. G. Spratt. 1999. Multilocus sequence typing. Trends Microbiol. 7:482-487. [DOI] [PubMed] [Google Scholar]
- 9.Finland, M., and W. D. Sutliff. 1931. Specific cutaneous reactions and circulating antibodies in the course of lobar pneumonia: II. Cases treated with antipneumococcic sera. J. Exp. Med. 54:653-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia, E., D. Llull, and R. Lopez. 1999. Functional organization of the gene cluster involved in the synthesis of the pneumococcal capsule. Int. Microbiol. 2:169-176. [PubMed] [Google Scholar]
- 11.Garcia, E., D. Llull, R. Munoz, M. Mollerach, and R. Lopez. 2000. Current trends in capsular polysaccharide biosynthesis of Streptococcus pneumoniae. Res. Microbiol. 151:429-435. [DOI] [PubMed] [Google Scholar]
- 12.Hausdorff, W. P., J. Bryant, C. Kloek, P. R. Paradiso, and G. R. Siber. 2000. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin. Infect. Dis. 30:122-140. [DOI] [PubMed] [Google Scholar]
- 13.Hausdorff, W. P., J. Bryant, P. R. Paradiso, and G. R. Siber. 2000. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin. Infect. Dis. 30:100-121. [DOI] [PubMed] [Google Scholar]
- 14.Henrichsen, J. 1979. The pneumococcal typing system. J. Infect. 1(Suppl. 2):31-37. [Google Scholar]
- 15.Henrichsen, J., E. Berntsson, and B. Kaijser. 1980. Comparison of counterimmunoelectrophoresis and the capsular reaction test for typing of pneumococci. J. Clin. Microbiol. 11:589-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Hermans, P. W., M. Sluijter, T. Hoogenboezem, H. Heersma, A. van Belkum, and R. de Groot.1995. Comparative study of five different DNA fingerprint techniques for molecular typing of Streptococcus pneumoniae strains. J. Clin. Microbiol. 33:1606-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaffar, S., A. Leach, A. J. Hall, S. Obaro, K. P. McAdam, P. G. Smith, and B. M. Greenwood. 1999. Preparation for a pneumococcal vaccine trial in The Gambia: individual or community randomisation? Vaccine 18:633-640. [DOI] [PubMed] [Google Scholar]
- 17.Janoff, E. N., C. Fasching, J. M. Orenstein, J. B. Rubins, N. L. Opstad, and A. P. Dalmasso. 1999. Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes. J. Clin. Investig. 104:1139-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang, S.-M., L. Wang, and P. R. Reeves. 2001. Molecular characterization of Streptococcus pneumoniae type 4, 6B, 8, and 18C capsular polysaccharide gene clusters. Infect. Immun. 69:1244-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong, F., and G. L. Gilbert. 2003. Using cpsA-cpsB sequence polymorphisms and serotype-/group-specific PCR to predict 51 Streptococcus pneumoniae capsular serotypes. J. Med. Microbiol. 52:1047-1058. [DOI] [PubMed] [Google Scholar]
- 20.Kronvall, G. 1973. A rapid slide-agglutination method for typing pneumococci by means of specific antibody adsorbed to protein A-containing staphylococci. J. Med. Microbiol. 6:187-190. [DOI] [PubMed] [Google Scholar]
- 21.LaClaire, L. L., M. L. Tondella, D. S. Beall, C. A. Noble, P. L. Raghunathan, N. E. Rosenstein, and T. Popovic. 2003. Identification of Haemophilus influenzae serotypes by standard slide agglutination serotyping and PCR-based capsule typing. J. Clin. Microbiol. 41:393-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lalitha, M. K., K. Thomas, R. S. Kumar, and M. C. Steinhoff. 1999. Serotyping of Streptococcus pneumoniae by coagglutination with 12 pooled antisera. J. Clin. Microbiol. 37:263-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawrence, E. R., C. A. Arias, B. Duke, D. Beste, K. Broughton, A. Efstratiou, R. C. George, and L. M. Hall. 2000. Evaluation of serotype prediction by cpsA-cpsB gene polymorphism in Streptococcus pneumoniae. J. Clin. Microbiol. 38:1319-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawrence, E. R., D. B. Griffiths, S. A. Martin, R. C. George, and L. M. Hall. 2003. Evaluation of semiautomated multiplex PCR assay for determination of Streptococcus pneumoniae serotypes and serogroups. J. Clin. Microbiol. 41:601-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leach, A. J., T. M. Shelby-James, M. Mayo, M. Gratten, A. C. Laming, B. J. Currie, and J. D. Mathews. 1997. A prospective study of the impact of community-based azithromycin treatment of trachoma on carriage and resistance of Streptococcus pneumoniae. Clin. Infect. Dis. 24:356-362. [DOI] [PubMed] [Google Scholar]
- 26.Lund, E., and J. Henrichsen. 1978. Laboratory diagnosis, serology and epidemiology of Streptococcus pneumoniae, p. 241-262. In T. Bergan and J. R. Norris (ed.), Methods in microbiology. Academic Press, London, United Kingdom.
- 27.Lund, E., and P. Rasmussen. 1966. Omni-serum. A diagnostic Pneumococcus serum, reacting with the 82 known types of Pneumococcus. Acta Pathol. Microbiol. Scand. 68:458-460. [DOI] [PubMed] [Google Scholar]
- 28.Morona, J. K., D. C. Miller, T. J. Coffey, C. J. Vindurampulle, B. G. Spratt, R. Morona, and J. C. Paton. 1999. Molecular and genetic characterization of the capsule biosynthesis locus of Streptococcus pneumoniae type 23F. Microbiology 145:781-789. [DOI] [PubMed] [Google Scholar]
- 29.Morona, J. K., R. Morona, and J. C. Paton. 1997. Molecular and genetic characterization of the capsule biosynthesis locus of Streptococcus pneumoniae type 19B. J. Bacteriol. 179:4953-4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morona, J. K., R. Morona, and J. C. Paton. 1999. Comparative genetics of capsular polysaccharide biosynthesis in Streptococcus pneumoniae types belonging to serogroup 19. J. Bacteriol. 181:5355-5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reference deleted.
- 32.Ramirez, M., and A. Tomasz. 1999. Acquisition of new capsular genes among clinical isolates of antibiotic-resistant Streptococcus pneumoniae. Microb. Drug Resist. 5:241-246. [DOI] [PubMed] [Google Scholar]
- 33.Slotved, H.-C., M. Kaltoft, I. C. Skovsted, M. B. Kerrn, and F. Espersen. 2004. Simple, rapid latex agglutination test for serotyping of pneumococci (Pneumotest-Latex). J. Clin. Microbiol. 42:2518-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sluijter, M., H. Faden, R. de Groot, N. Lemmens, W. H. Goessens, A. van Belkum, and P. W. Hermans. 1998. Molecular characterization of pneumococcal nasopharynx isolates collected from children during their first 2 years of life. J. Clin. Microbiol. 36:2248-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. 1999. Pneumococcal vaccines: World Health Organization position paper. Can. Commun. Dis. Rep. 25:150-151. [PubMed] [Google Scholar]
- 36.Yano, H., M. Suetake, A. Kuga, K. Irinoda, R. Okamoto, T. Kobayashi, and M. Inoue. 2000. Pulsed-field gel electrophoresis analysis of nasopharyngeal flora in children attending a day care center. J. Clin. Microbiol. 38:625-629. [DOI] [PMC free article] [PubMed] [Google Scholar]