Abstract
Canine DNA samples from South Africa were found to contain 16S rRNA gene nucleotide and citrate synthase gene nucleotide and deduced amino acid sequences that were most similar to Anaplasma phagocytophilum: 98%, 66%, and 69% similarity, respectively. This suggests that a new Anaplasma species closely related to A. phagocytophilum occurs in Africa.
Ehrlichioses are tick-borne diseases that cause significant morbidity and mortality in dogs and people worldwide. In South Africa, canine ehrlichiosis is encountered commonly in veterinary practice (15, 17) and there is evidence of human ehrlichiosis (13). Currently, there are eight species in the family Anaplasmataceae that are pathogens in dogs: Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia ewingii, Ehrlichia ruminantium, Anaplasma platys, Anaplasma phagocytophilum, Neorickettsia helminthoeca, and Neorickettsia risticii. Although canine ehrlichiosis was first recognized in South Africa in 1938 (9), the organisms causing infections in the country have yet to be isolated and characterized. Serological studies, however, have shown that many dogs (up to 75%) have significant antibody titers against E. canis and E. chaffeensis (12). Also, DNA of E. canis and DNA of a novel Ehrlichia sp. closely related to E. ruminantium have been found in the blood of dogs in South Africa (1). There is also little definitive data on infections in dogs in other parts of Africa. The initial description of E. canis was made in dogs in Algeria in 1935 (5), and DNA of A. platys has been found in blood from a dog in the Democratic Republic of Congo (16).
Unfortunately, detecting members of the family Anaplasmataceae is not easy as they are fastidious intracellular bacteria, and their isolation and characterization require sophisticated laboratory facilities and substantive funding which are not generally available in Africa. While serological studies can be performed in less equipped laboratories, their results are hindered by antigenic cross-reactivity between the etiological agents. Molecular techniques, however, have been shown to be sensitive and specific in detecting infections with members of the family Anaplasmataceae (10, 14), and in this report we describe our use of PCR and sequence analysis to investigate the presence of these organisms in dogs in South Africa.
In 2000, morulae were observed in stained (Diff-QuicK; Harleco) central blood smears from three dogs (SA1108, SA1076, and SA1245) which presented at the Veterinary Teaching Hospital of the Medical University of South Africa. A QIAamp tissue kit (QIAGEN GmbH, Hilden) was used to extract DNA from whole blood collected in EDTA from each dog. The DNA samples were stored at −20°C in 200 μl of Tris-EDTA buffer until screened with a PCR using primers EHR16SD and EHR16SR, which amplify a 345-bp fragment of the 16S rRNA gene found in the genera Anaplasma, Ehrlichia, Neorickettsia, and Wolbachia of the family Anaplasmataceae (11). The three samples revealed appropriate amplicons, and for further phylogenetic studies, we carried out PCRs with primer sets fD1/EHR16SR and EHR16SD/Rp2, which amplify a longer sequence of the 16S rRNA gene (11), and with primers ANA-CS646F (5′ TGCATGCAGATCATGAAC 3′) and ANA-CS1076R [5′ GAGTAAAA(A or G)TCAACATT(G or C or T)GG 3′], which amplify a 431-bp partial sequence of the Anaplasma citrate synthase gene (gltA). Direct sequencing of the PCR products and analysis of the sequences obtained were performed as described previously (8). The GenBank accession numbers of the 16S rRNA gene sequences used to construct phylogenetic trees and to analyze percent identities were as follows: A. phagocytophilum, M73220, M73223, M73224, U02521, U10873, AF036645 to -036647, AF093788, AF093789, AF172164 to -172167, AF189153, AF241532, AJ242784, AY527213, and AY527214; A. platys, M82801, AF286699, AF287153, AF303467, AF536828, and AY530806; A. bovis, U03775, AF294789, AF470698, and AY144729; A. ovis, AF309865, AF318945, and AF414870; Anaplasma sp. found in a sheep in South Africa (Omatjenne), U54806; Anaplasma sp. found in a goat in Mozambique (Bom Pastor), AF318023; A. marginale, AF414871; A. centrale, AF414869; E. canis, M73221; Ehrlichia ruminantium, AF325175; Wolbachia pipientis, AF179630; and N. risticii, M21290. The GenBank accession numbers of the gltA nucleotide sequences used in our study were as follows: A. phagocytophilum, AF304136 to -304138, and AY464132 to -464138; A. platys, AB058782, AF478130, AY077620, and AY530807; A. marginale, AF304140; A. centrale, AF304141; E. canis, AF304143; E. ruminantium, AF304146; W. pipientis, AF332584; and N. risticii, AF304147.
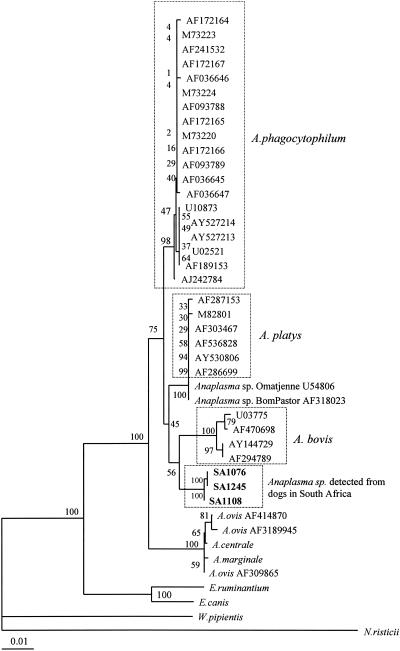
The 1,389-bp sequences of the 16S rRNA gene we obtained with the DNA extracted from the blood of the three dogs were identical (for dogs SA1076 and SA1245) or very similar (99.30%) (Table 1). They had the highest levels of similarity to sequences reported for other Anaplasma spp. and lower levels of similarity to E. canis, W. pipientis, and N. risticii (Table 1). Among the Anaplasma spp., the greatest levels of similarity were with strains of A. phagocytophilum, but these were always less than 99%. The percentages of similarity between the sequences derived from the South African dogs and Anaplasma from southern Africa (Omatjenne from a sheep [2] and Bom Pastor from a goat [4]) were also relatively low, ranging from 97.69 to 97.84%. In the phylogenetic tree inferred from the 16S rRNA gene data, the sequences obtained from the South Africa dogs grouped together to form a distinct cluster which was most closely related to sequences reported for A. phagocytophilum, A. platys, and Anaplasma, identified in ruminants in South Africa, and A. bovis (Fig. 1). The bootstrap values we determined, however, were low and were only poorly supportive of these relationships. The above data indicates that the dogs we studied were infected with an Anaplasma species that is different from other reported Anaplasma spp. but most closely related to A. phagocytophilum.
TABLE 1.
Percent similarities between the 16S rRNA gene sequences detected in dogs SA1108, SA1076, and SA1245 in South Africa and those reported for Anaplasma and other related species
| Species or dog (GenBank accession numbers) | % 16S rRNA gene similarity
|
|
|---|---|---|
| SA1108 | SA1076 and SA1245 | |
| SA1108 | 100 | 99.93 |
| SA1076 and SA1245 | 99.93 | 100 |
| A. phagocytophilum Webster (U02521) | 98.56 | 98.49 |
| A. platys (AF303467) | 98.13 | 98.06 |
| Anaplasma sp. from goat in Mozambique (AF318023) | 97.84 | 97.77 |
| Anaplasma sp. from sheep in South Africa (U54806) | 97.77 | 97.69 |
| A. bovis (U03775) | 96.83 | 96.90 |
| A. marginale (AF414871) | 96.18 | 96.11 |
| A. ovis (AF414870) | 95.89 | 95.82 |
| A. centrale (AF414869) | 95.97 | 95.89 |
| E. canis (M73221) | 91.85 | 91.77 |
| W. pipientis (AF179630) | 88.11 | 88.04 |
| N. risticii (M21290) | 85.37 | 85.30 |
FIG. 1.
Phylogenetic relationships between the new Anaplasma sp. detected in this study and Anaplasma, Ehrlichia, Wolbachia, and Neorickettsia based on partial nucleotide sequences of the 16S rRNA gene. The numbers at nodes are the proportions of 100 bootstrap resamplings that support the topology shown. The scale bar represents 10% divergence.
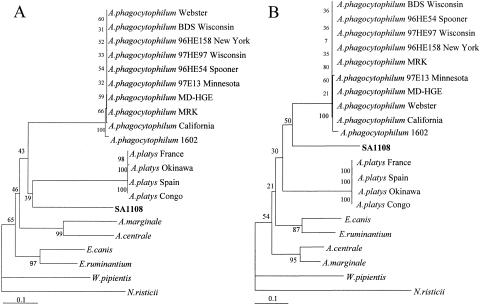
Analyses of similarity and phylogenetic relationships using partial nucleotide and deduced amino acid sequences of the gltA were also performed for a representative of the organisms identified in the South African dogs (SA1108) (Table 2). Although bootstrap values were again low, in the phylogenetic trees based on the gltA data, the Anaplasma sp. in the South African dog was distinct from A. phagocytophilum and A. platys (Fig. 2). The percent similarities between SA1108 and A. phagocytophilum for the gltA nucleotide and deduced amino acid sequences were only 66.44 and 69.47%, respectively (Table 2). These results are consistent with our findings using analyses of the 16S rRNA gene sequences.
TABLE 2.
Percent similarity of nucleotide and deduced amino acid sequence of the citrate synthase gene detected in dog SA1108 and those reported in Anaplasma and other species
| Species (GenBank accession no.) | % Identity to SA1108 citrate synthase gene
|
|
|---|---|---|
| Nucleotide | Amino acid | |
| A. phagocytophilum Webster (AF304136, AAL01656) | 66.44 | 69.47 |
| A. centrale (AF304141, AAL01659) | 63.67 | 68.42 |
| A. marginale (AF304140, AAL01658) | 63.32 | 66.32 |
| A. platys (AB058782, AAB87841) | 62.98 | 65.26 |
| E. canis (AF304143, AAL01661) | 62.98 | 66.32 |
| E. ruminantium (AF304146, AAL01664) | 64.01 | 63.16 |
| W. pipientis (AF332584, AAL57051) | 59.52 | 60.00 |
| N. risticii (AF304147, AAL01665) | 57.80 | 55.91 |
FIG. 2.
Phylogenetic relationships between the new Anaplasma sp. detected in this study and Anaplasma, Ehrlichia, Wolbachia, and Neorickettsia based on partial nucleotide sequences of gltA (A) and deduced amino acid sequences of gltA (B). The numbers at nodes are the proportions of 100 bootstrap resamplings that support the topology shown. The scale bar represents 10% divergence.
Our report is the first describing an Anaplasma sp. occurring in dogs in South Africa. The new Anaplasma sp. we detected is most closely related to A. phagocytophilum, which is an agent of canine (6, 7) and human (3) granulocytic anaplasmosis in the United States and Europe. Also, it is closely related to A. platys, which may cause cyclic thrombocytopenia in dogs and is known to occur in Africa (10). Organisms closely related to A. platys and to the new Anaplasma sp. have been detected in the blood of a goat in Mozambique that died with clinical signs suggesting heartwater due to E. ruminantium (4). They have also been found in a sheep in South Africa which also had signs of heartwater and was concurrently infected with E. ruminantium (2). Our finding of an Anaplasma sp. in dogs in South Africa adds to the scant information on the Anaplasmataceae that occur in Africa. Unfortunately, records containing the laboratory and clinical data on the dogs in which we identified the novel Anaplasma sp. have been lost. Additional studies are indicated to further characterize the organism and to determine its pathogenicity in dogs and perhaps other species, its distribution, vectors, and epidemiology.
Nucleotide sequence accession number.
The 16S rRNA gene sequences obtained from dogs SA1076, SA1108, and SA1245 have been deposited in the GenBank database under accession numbers AY570539, AY570538, and AY570540, respectively. The gltA sequence obtained from dog SA1108 has been registered under accession number AY570541.
Acknowledgments
The authors thank Didier Raoult for providing samples submitted to his laboratory and the technical expertise of the DNA Core facility of the Center for Gene Research, Yamaguchi University.
Our study was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan, the Institut National de la Sante et de la Recherche Medicale, and the Japan Society for the Promotion of Science.
REFERENCES
- 1.Allsopp, M. T. E. P., and B. A. Allsopp. 2001. Novel Ehrlichia genotype detected in dogs in South Africa. J. Clin. Microbiol. 39:4204-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allsopp, M. T. E. P., E. S. Visser, J. L. du Plessis, S. W. Vogel, and B. A. Allsopp. 1997. Different organisms associated with heartwater as shown by analysis of 16S ribosomal RNA gene sequences. Vet. Parasitol. 71:283-300. [DOI] [PubMed] [Google Scholar]
- 3.Bakken, J. S., and J. S. Dumler. 2000. Human granulocytic ehrlichiosis. Clin. Infect. Dis. 31:554-560. [DOI] [PubMed] [Google Scholar]
- 4.Bekker, C. P. J., D. Vink, C. M. Lopes Pereira, W. Wapenaar, A. Langa, and F. Jongejan. 2001. Heartwater (Cowdria ruminantium infection) as a cause of postrestocking mortality of goats in Mozambique. Clin. Diagn. Lab. Immunol. 8:843-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donatien, A., and A. Lestoquard. 1935. Existance en Algerie d'une rickettsia du chien. Bull. Soc. Pathol. Exot. 28:418-419. [Google Scholar]
- 6.Egenvall, A., A. Bjoersdorff, I. Lilliehook, O. Engvall, E. Karlstam, K. Artursson, A. Hedhammar, and A. Gunnarsson. 1998. Early manifestations of granulocytic ehrlichiosis in dogs inoculated experimentally with a Swedish Ehrlichia species isolate. Vet. Rec. 143:412-417. [DOI] [PubMed] [Google Scholar]
- 7.Egenvall, A. E., A. A. Hedhammar, and A. I. Bjoersdorff. 1997. Clinical features and serology of 14 dogs affected by granulocytic ehrlichiosis in Sweden. Vet. Rec. 140:222-226. [DOI] [PubMed] [Google Scholar]
- 8.Inokuma, H., K. Fujii, M. Okuda, T. Onishi, J.-P. Beaufils, D. Raoult, and P. Brouqui. 2002. Determination of the nucleotide sequences of heat shock operon groESL and the citrate synthase gene (gltA) of Anaplasma (Ehrlichia) platys for phylogenetic studies. Clin. Diagn. Lab. Immunol. 9:1132-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neitz, W. O., and A. D. Thomas. 1938. Rickettsiosis in the dog. J. S. Afr. Vet. Assoc. 9:166-169. [Google Scholar]
- 10.Parola, P., H. Inokuma, J.-L. Camicas, P. Brouqui, and D. Raoult. 2001. Detection and identification of spotted fever group Rickettsiae and Ehrlichiae in African ticks. Emerg. Infect. Dis. 7:1014-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parola, P., V. Roux, J.-L. Camicas, I. Baradji, P. Brouqui, and D. Raoult. 2000. Detection of ehrlichiae in African ticks by PCR. Trans. R. Soc. Trop. Med. Hyg. 94:707-708. [DOI] [PubMed] [Google Scholar]
- 12.Pretorius, A. M., and P. J. Kelly. 1998. Serological survey for antibodies reactive with Ehrlichia canis and E. chaffeensis in dogs from the Bloemfontein area, South Africa. J. S. Afr. Vet. Assoc. 69:126-128. [DOI] [PubMed] [Google Scholar]
- 13.Pretorius, A. M., T. P. Venter, E. Van der Ryst, and P. J. Kelly. 1999. A case report of possible human ehrlichiosis in the Free State Province of South Africa. S. Afr. Med. J. 89:961. [PubMed] [Google Scholar]
- 14.Pusterla, N., J. Huder, C. Wolfensberger, B. Litschi, A. Parvis, and H. Lutz. 1997. Granulocytic ehrlichiosis in two dogs in Switzerland. J. Clin. Microbiol. 35:2307-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rautenbach, G. H., J. Boomker, and I. L. De Villiers. 1991. A descriptive of the canine population in a rural town in South Africa. J. S. Afr. Vet. Assoc. 62:158-162. [PubMed] [Google Scholar]
- 16.Sanogo, Y. O., B. Davoust, H. Inokuma, J.-L. Camicas, P. Parola, and P. Brouqui. 2003. First evidence of Anaplasma platys in Rhipicephalus sanguineus (Acari: Ixodida) collected from dogs in Africa. Onderstepoort J. Vet. Res. 70:205-212. [PubMed] [Google Scholar]
- 17.Van Heerden, J. 1982. A retrospective study on 120 natural cases of canine ehrlichiosis. J. S. Afr. Vet. Assoc. 53:17-22. [PubMed] [Google Scholar]