Abstract
Background
Patients with inflammatory bowel disease (IBD) are at higher risk for severe COVID-19 infection. However, most studies are single-center, and nationwide data in the United States are lacking. This study aimed to investigate hospital-related outcomes and predictors of these outcomes in patients with IBD and COVID-19 infection.
Methods
The National Inpatient Sample and National Readmission database were queried for all the patient hospitalizations with IBD with concurrent COVID-19 in the study group and non-COVID-19 related hospitalizations in the control group. For patients under 18 years, elective and trauma-related hospitalizations were excluded. Primary outcomes included mortality, septic shock, mechanical ventilation, and intensive care utilization. Secondary outcomes included length of stay and total hospitalization costs.
Results
From this query, 8865 adult patients with IBD and COVID-19 were identified. These patients were relatively older (62.8 vs 57.7 years, P < .01), and the majority were females (52.1% with COVID-19 vs 55.2% without COVID-19). Patients with IBD and COVID-19 had higher mortality (12.24% vs 2.55%; P < .01), increased incidence of septic shock (7.9% vs 4.4%; P < .01), mechanical ventilation (11.5% vs 3.7%; P < .01), and intensive care utilization (12% vs 4.6%; P < .01). These patients also had higher mean length of stay (8.28 days vs 5.47 days; P < .01) and total hospitalization costs ($21 390 vs $16 468; P < .01) than those without COVID-19 infection.
Conclusions
Patients with IBD and COVID-19 have worse outcomes, with a higher incidence of severe COVID-19 disease, leading to higher mortality rates, longer lengths of stay, and increased total hospitalization costs. Encouraging preventive health measures and treating promptly with advanced COVID-19 therapies may improve outcomes and decrease the healthcare burden.
Keywords: inflammatory bowel disease, resource utilization, severity of COVID-19 infection, mortality, mean length of stay
Key Messages.
What is already known?
Patients with inflammatory bowel disease (IBD) are at higher risk for severe COVID-19 infection.
What is new here?
This study found that patients with IBD and COVID-19 had worse outcomes, including higher mortality rates, longer lengths of stay, and increased total hospitalization costs.
How can this study help patient care?
The study highlights that encouraging preventive health measures and treating promptly with advanced COVID-19 therapies may improve outcomes and decrease the healthcare burden for patients with IBD and COVID-19.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic is considered one of the major catastrophes of this century that has placed an unparalleled burden on the healthcare systems. As of December 2022, the World Health Organization (WHO) has reported greater than 640 million confirmed cases and 6 million deaths due to the pandemic.1 Although the implementation of strict preventive health measures including infection control, social distancing, movement restrictions, and vaccination campaigns, have partially mitigated the risk of contraction, the infection risk has lingered due to the emergence of newer mutant strains of the virus. Patients who are elderly or with certain comorbid conditions such as diabetes, obesity, chronic lung disease or asthma, sickle cell disease, or are immunocompromised are considered vulnerable to the severe outcomes of COVID-19 infection.2 Inflammatory bowel disease (IBD) is an immune-mediated disease with a chronic relapsing-remitting pattern that exhibits immunological dysregulation. The advanced therapeutic modalities for IBD include immunomodulators, biologics, and small molecules, which may trigger a transient or persistent immunocompromised state, making patients susceptible to opportunistic and nosocomial infections. Theoretically, individuals with IBD are more susceptible to contracting COVID-19 and having poor outcomes due to immune dysregulation, especially in patients with active disease and on advanced therapies.3 In the early stages of the pandemic, initial studies reported an overall incidence rate of COVID-19 infection between 3.9 to 6.2 cases per 1000 patients, and a recent meta-analysis reported a prevalence of 1.01% of COVID-19 infection in patients with IBD.4 The Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) is a global database created to investigate the effects of COVID-19 infection on individuals with IBD reported a hospitalization rate of 31% and a mortality rate of 3.4%.5 Another multicenter study focusing on IBD patients on biological therapy reported a hospitalization rate of 57%, with a case fatality rate of 29%.6 The published meta-analyses reported results that had a high degree of variability due to the differences in the pandemic stage, availability of the data, sample sizes, study designs, and healthcare settings.7-9 Given a lot of variation in the reported outcomes in the hospitalization rate and mortality existing in the literature, we intended to explore and objectively report on the hospital-related outcomes of COVID-19 infection and its predictors in IBD patients utilizing the 2 largest national-level inpatient US databases.
Methods
Data Source
We utilized the National Inpatient Sample (NIS) and the National Readmission Database (NRD) for the year 2020, included within the Healthcare Cost and Utilization Project (HCUP), administered by the Agency for Healthcare Research and Quality (AHRQ).10 The AHRQ is a federal institution founded with the purpose of providing essential knowledge, tools, and data aimed at enhancing the safety and quality of healthcare services within the United States. These publicly available and de-identified databases contain a 20% stratified subset of hospitalizations from community hospitals across the United States. Notably, these databases exclude rehabilitation and long-term acute care hospitals. The NIS database for the year 2020 covers more than 97%, and the NRD of 2020 database represents 62.2% of the total US resident population respectively.11,12 The variables included in the databases represent patient characteristics, including patient demographics (except for race which is not available in the NRD), principal and secondary diagnoses at discharge, length of stay, procedures performed, and hospitalization charges. Similarly, the hospital-related variables include ownership, number of beds, teaching status, rural/urban location, and geographical location. However, the NRD differs from NIS in that each patient is assigned a verified linkage number that identifies discharges belonging to the same patient, which allows the determination of the readmission rate in one calendar year. The diagnoses and procedures conducted throughout the hospital stay are determined using the International Classification of Diseases, Tenth Revision, and Clinical Modification (ICD-10-CM). The details regarding the NRD data are available at https://www.hcup-us.ahrq.gov/nrdoverview.jsp.11,12
Study Population
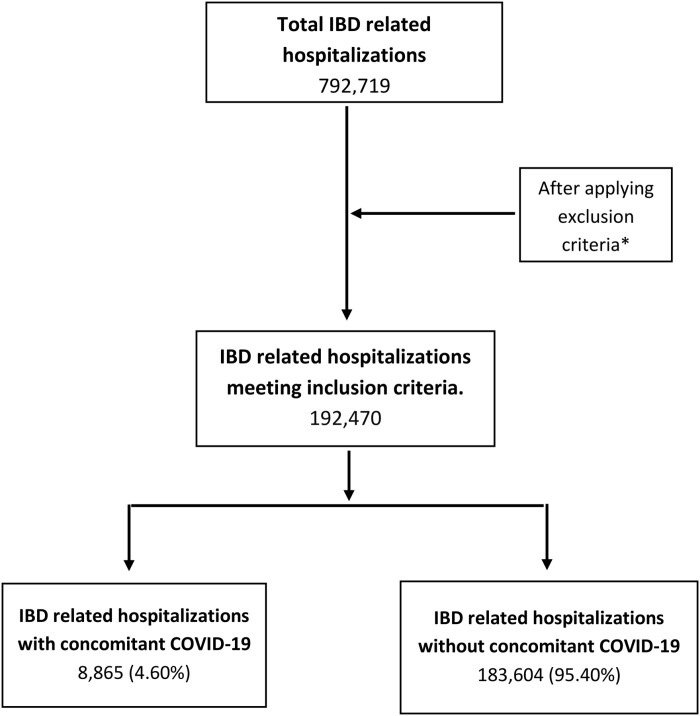
The study population comprised hospitalized patients aged 18 or older admitted with COVID-19 infection who also had a concurrent diagnosis of IBD in the study group. The control group included non-COVID-19 related hospitalizations in the IBD group (Figure 1). The IBD was further classified into Crohn’s disease (CD) and ulcerative colitis (UC) in the subgroup analysis. We excluded all the elective or trauma-related admissions and hospitalizations with overlapping diagnosis codes of both UC and CD to correctly identify and perform subgroup analysis. The diagnosis codes to identify these patients are provided in the Supplementary Material.
Figure 1.
Patient selection flow diagram.
Measures of Outcome
The primary outcome of the study was to examine all-cause inpatient mortality and the severity of COVID-19, as determined by the need for mechanical ventilation, septic shock, and ICU utilization. Secondary outcomes of interest were all-cause 30-day readmission and resource utilization, including the mean length of stay (LOS) and total hospitalization cost. We also performed both univariate and multivariate regression analyses to find the independent predictors of mortality, LOS, and hospitalization cost.
Study Variables
Most of the study variables are readily available in the databases themselves, such as age, gender, race (not available in the NRD), median income based on the zip code, primary insurance payer for the hospitalization, hospital bed size, urban/rural location, teaching status, and geographical location (not available in the NRD). We used Deyo’s modification of the Charlson comorbidity index (CCI) to identify individual comorbidities and calculate the overall comorbidity burden.13 The CCI serves as a validated tool for evaluating the comorbidity burden and estimating the 1-year risk of death due to comorbid conditions in studies using administrative databases. In the NRD, the LOS and total hospitalization charges were documented as reported, where total hospitalization charges represent the billed amount by hospitals and may not necessarily reflect the actual cost of care. The actual cost per hospital visit was calculated by combining the Cost-to-Charge Ratio files provided by HCUP with the hospitalization charges for each hospitalization from both databases.14
Statistical Analysis
We conducted our analysis using STATA version 16.0, a statistical analysis software developed by StataCorp in College Station, Texas. We employed the χ2 test to compare the proportions of patient demographics and hospital characteristics, whereas the Student t test was utilized to compare continuous variables. We considered a P value less than 0.05 to be statistically significant. We conducted a univariate regression analysis to compute unadjusted odds ratios or coefficients for both the primary and secondary outcomes. We utilized a P value cutoff of 0.2 in the univariate analyses as a threshold to construct multivariate regression models, enabling us to account for potential confounding factors. To determine the 30-day readmission rate, we defined readmission as a nonelective return hospitalization for a principal diagnosis (excluding trauma) within 30 days of the initial admission. We counted only the initial readmission if a patient experienced multiple readmissions within 30 days of being discharged from the index hospitalization. Patients who died during the initial admission were not included in the denominator.
Results
Baseline Characteristics
A total of 192 470 IBD-related hospitalizations were identified after excluding nonelective and pediatric patients for the year 2020. Of these, 8865 (4.6%) had a concurrent diagnosis of COVID-19, with 4630 (2.4%) patients with CD and 4235 (2.2%) patients with UC. The mean age of IBD patients in the COVID-19 group was significantly higher compared with those without COVID-19 (62.8 years vs 57.7 years; P < .01), and the majority in both groups were female (52.1% and 55.2% for the COVID-19 and non-COVID-19 groups, respectively). The mean age in the COVID-19 group was higher for both CD (61. 5 years vs 55.9 years; P < .01) and UC (64.2 years vs 60.6 years; P < .01) than the non-COVID group. The most common ethnicity among IBD patients in both groups was Caucasian (74.7% and 79.8%; P < .01), respectively. On subgroup analysis, Caucasians were also the most common race among CD patients (73.3%) in the COVID-19 group and (80.4%) in the non-COVID group (P < .01), as well as among UC patients (76.2%) in the COVID-19 group and (78.8%) in the non-COVID group (P < .01).
Regarding comorbidity burden, the non-COVID-19 group had a higher CCI >3 than the COVID-19 group in IBD patients. However, no significant difference was observed in both groups regarding individual comorbid conditions except for COPD, which was higher in the COVID-19 group (28.2% vs 25.1%; P < .01) than in the non-COVID group. Medicare was the highest payer, and most patients were admitted to large teaching hospitals in all groups. The remaining demographics were comparable among the groups (see Tables 1 and 2).
Table 1.
Baseline patient and hospital characteristics for hospitalized cases of IBD with and without COVID-19 infection.
| Patient Characteristics | IBD with COVID-19 | IBD without COVID-19 | P |
|---|---|---|---|
| No. of patients | 8865 | 183 604 | |
| Female N (%) | 4618 (52.1%) | 101 716.6 (55.4%) | <.01 |
| Mean age (years) | 62.78 yrs. | 57.68 yrs. | |
| Race N (%) | <.01 | ||
| White | 6621(74.7%) | 146 516 (79.8%) | |
| African American | 1081 (12.2%) | 20 013 (10.9%) | |
| Hispanic | 833 (9.4%) | 10 282 (5.6%) | |
| Other | 328 (3.7%) | 6793 (3.7%) | |
| Charlson Comorbidity Index N (%) | <.01 | ||
| 0 | 2606 (29.4%) | 58 202 (31.7%) | |
| 1 | 2038 (23%) | 37 455 (20.4%) | |
| 2 | 1533 (17.3%) | 28 458 (15.5%) | |
| 3 or more | 2686(30.3%) | 59 488 (32.4%) | |
| Chronic Comorbid Conditions N (%) | |||
| Hypertension | 178 (0.2%) | 551 (0.3%) | .63 |
| Diabetes mellitus | 62 (0.7%) | 1469 (0.8%) | .55 |
| Chronic kidney disease | <.01 | ||
| Stage 2 | 80 (0.9%) | 1836 (1%) | |
| Stage 3 | 328 (3.7%) | 12 485 (6.8%) | |
| Stage 4 | 257 (2.9%) | 4590 (2.5%) | |
| Stage 5 | 267 (0.3%) | 551 (0.3%) | |
| Unspecified | 346 (3.9%) | 6059 (3.3%) | |
| End-stage renal disease | 239(2.7%) | 5875 (3.2%) | .19 |
| Chronic pulmonary disease | 2500 (28.2%) | 46 085 (25.1%) | <.01 |
| Congestive heart failure | 1516 (17.1%) | 31 580 (17.2%) | .98 |
| Median Income Based on Zip Codes | .15 | ||
| $1-$38 999 | 2429 (27.4%) | 45 717 (24.9%) | |
| $39 000-$47 999 | 2367 (26.7%) | 49 573(27%) | |
| $48 000-$62 999 | 2065 (23.3%) | 44 799 (24.4%) | |
| >$63 000 | 2003 (22.6%) | 43 330 (23.6%) | |
| Insurance Provider N (%) | <.01 | ||
| Medicare | 5061(57.1%) | 94 556 (51.5%) | |
| Medicaid | 833 (9.4%) | 27 173 (14.8%) | |
| Private | 2810 (31.7%) | 55 632(30.3%) | |
| Self-Pay | 159 (1.8%) | 6242(3.4%) | |
| Hospital Characteristics | |||
| Hospital Teaching Status N (%) | .03 | ||
| Nonteaching | 2366 (26.7%) | 44 616 (24.3%) | |
| Teaching | 6497 (73.3%) | 138 988 (75.7%) | |
| Hospital Bed Size N (%) | .33 | ||
| Small | 2154 (22.9%) | 40 576 (22.1%) | |
| Medium | 2455(26.1%) | 50 491 (27.5%) | |
| Large | 4798(51%) | 92 720 (50.5%) | |
| Hospital Region N (%) | <.01 | ||
| Large metropolitan | 18 256(20.6%) | 39 842 (21.7%) | |
| Small metropolitan | 2464 (27.8%) | 43 514 (23.7%) | |
| Micropolitan areas | 3253 (36.7%) | 69 035 (37.6%) | |
| Others | 13 112 (14.8%) | 31 396 (17.1%) |
Table 2.
Baseline patient and hospital characteristics for hospitalized cases of CD and UC with and without COVID-19 infection.
| Patient Characteristics | CD with COVID-19 | CD without COVID-19 | P | UC with COVID-19 | UC without COVID-19 | P |
|---|---|---|---|---|---|---|
| No. patients | 4630 | 113 505 | 4235 | 70 100 | ||
| Female N (%) | 2537 (54.8%) | 64 925 (57.2%) | .13 | 2215 (49.1%) | 36 662 (52.3%) | .06 |
| Mean age (years) | 61.45 yrs. | 55.89 yrs. | <.01 | 64.24 yrs. | 60.58 yrs. | <.01 |
| Race N (%) | <.01 | .06 | ||||
| White | 3394 (73.3%) | 91 258 (80.4%) | 3337 (76.2%) | 55 239 (78.8%) | ||
| African American | 676 (14.6%) | 13 734 (12.1%) | 385 (9.5%) | 6379 (9.1%) | ||
| Hispanic | 407 (8.8%) | 4994 (4.4%) | 322 (10%) | 5328 (7.6%) | ||
| Other | 153 (3.3%) | 3519 (3.1%) | 191 (4.2%) | 3155 (4.5%) | ||
| Charlson Comorbidity Index N (%) | <.01 | <.01 | ||||
| 0 | 1301 (28.1%) | 38 138 (33.6%) | 1207 (30.8%) | 19 979 (28.5%) | ||
| 1 | 1046 (22.6%) | 22 814 (20.1%) | 885 (23.5%) | 14 651 (20.9%) | ||
| 2 | 852 (18.4%) | 17 480 (15.4%) | 656 (16.1%) | 10 866 (15.5%) | ||
| 3 or more | 1435 (31%) | 34 959 (30.8%) | 1486 (29.6%) | 24 605 (35.1%) | ||
| Chronic Comorbid Conditions N (%) | ||||||
| Hypertension | 9 (0.2%) | 340 (0.3%) | .83 | 13 (0.2%) | 210 (0.3%) | .59 |
| Diabetes Mellitus | 37 (0.8%) | 908 (0.8%) | .93 | 34 (0.6%) | 561 (0.8%) | .43 |
| Chronic Kidney Disease | <.01 | .02 | ||||
| Stage 2 | 42 (0.9%) | 1135 (1%) | 38 (0.9%) | 631 (0.9%) | ||
| Stage 3 | 167 (3.6%) | 7718 (6.8%) | 284 (3.8%) | 4697 (6.7%) | ||
| Stage 4 | 153 (3.3%) | 3178 (2.8%) | 93 (2.5%) | 1542 (2.2%) | ||
| Stage 5 | 9 (0.2%) | 340 (0.3%) | 8 (0.4%) | 140 (0.2%) | ||
| Unspecified | 208 (4.5%) | 3859 (3.4%) | 136 (3.2%) | 2243 (3.2%) | ||
| End-Stage Renal Disease | 134 (2.9%) | 3859 (3.4%) | .39 | 119 (2.4%) | 1963 (2.8%) | .44 |
| Chronic Pulmonary Disease | 1403 (30.3%) | 29 284 (25.8%) | <.01 | 1016 (25.9%) | 16 824 (24%) | .2 |
| Congestive Heart Failure | 838 (18.1%) | 18 955 (16.7%) | .24 | 762 (16.1%) | 12 618 (18%) | .16 |
| Median Income Based on Zip Codes | .2 | .06 | ||||
| $1-$38 999 | 1361 (29.4%) | 30 192 (26.6%) | 936 (25.2%) | 15 492 (22.1%) | ||
| $39 000-$47 999 | 1227 (26.5%) | 31 214 (27.5%) | 1118 (27%) | 18 506 (26.4%) | ||
| $48 000-$62 999 | 1000 (21.6%) | 27 241 (24%) | 1063 (25.2%) | 17 595 (25.1%) | ||
| >$63 000 | 1042 (22.5%) | 24 857 (21.9%) | 1118 (22.7%) | 18 506 (26.4%) | ||
| Insurance Provider N (%) | <.01 | |||||
| Medicare | 2658 (57.4%) | 56 639 (49.9%) | 2283 (56.7%) | 37 784 (53.9%) | ||
| Medicaid | 468 (10.1%) | 18 387 (16.2%) | 534 (8.6%) | 8833 (12.6%) | ||
| Private | 1421 (30.7%) | 34 278 (30.2%) | 1292 (32.8%) | 21 381 (30.5%) | ||
| Self-Pay | 83 (1.8%) | 4200 (3.7%) | 127 (1.8%) | 2103 (3%) | ||
| Hospital Characteristics | ||||||
| Hospital teaching Status N (%) | .08 | .1 | ||||
| Nonteaching | 1278 (27.6%) | 28 376 (25%) | 983 (25.6%) | 16 263 (23.2%) | ||
| Teaching | 3352 (72.4%) | 85 129 (75%) | 3252 (74.4%) | 53 837 (76.8%) | ||
| Hospital Bed Size N (%) | .53 | .11 | ||||
| Small | 1102 (23.8%) | 25 312 (22.3%) | 919 (22.8%) | 15212 (21.7%) | ||
| Medium | 1278 (27.6%) | 31 100 (27.4%) | 1169 (24.2%) | 19 348 (27.6%) | ||
| Large | 2250 (48.6%) | 57 093 (50.3%) | 2147 (53%) | 35 541 (50.7%) | ||
| Hospital Region N (%) | .03 | <.01 | ||||
| Northeast | 857 (18.5%) | 23 836 (21%) | 966 (23%) | 15 983 (22.8%) | ||
| Midwest | 1287 (27.8%) | 27 922 (24.6%) | 940 (27.9%) | 15 562 (22.2%) | ||
| South | 1889 (40.8%) | 44 494 (39.2%) | 1478 (32.2%) | 24 465 (34.9%) | ||
| West | 602 (13%) | 17 253 (15.2%) | 851 (16.9%) | 14 090 (20.1%) |
Primary Outcomes
Patients with both IBD and concomitant COVID-19 diagnosis had a significantly higher inpatient mortality rate (12.2% vs 2.6%; P < .01), mechanical ventilation (MV) requirement (11.5% vs 3.7%; P < .01), and ICU utilization (12% vs 4.6%; P < .01) than those without COVID-19. The COVID-19 group also had a greater incidence of septic shock (7.9% vs 4.4%; P < .01; Table 3).
Table 3.
Outcomes for hospitalized cases of IBD with and without COVID-19 infection.
| Outcomes | |||
|---|---|---|---|
| Patient Characteristics | IBD with COVID-19 | IBD without COVID-19 | P |
| Mortality, % | 1085 (12.24%) | 4694 (2.55%) | <.01 |
| Mechanical Ventilation | 1019 (11.5%) | 6793 (3.7%) | <.01 |
| Intensive Care Unit | 1064 (12%) | 8446 (4.6%) | <.01 |
| Septic Shock | 700 (7.9%) | 8078 (4.4%) | <.01 |
| Mean Length of Stay (days) | 8.28 days | 5.74 days | <.01 |
| Mean Hospitalization costs | $21 390 | $16 468 | <.01 |
Furthermore, the subgroup analysis showed that the COVID-19 group had higher inpatient mortality rates in both CD (12.41% vs 2.15%; P < .01) and UC (12.04% vs 3.20%; P < .01) compared with the non-COVID group. The COVID-19 group also had higher utilization of ICU (CD, 11% vs 4.1%, P < .01; UC, 13% vs 5.4%, P < .01) and MV requirement (CD, 10.7% vs 3.4%, P < .01; UC, 12.4% vs 4.3%, P < .01) than the non-COVID group (Table 4).
Table 4.
Outcomes for hospitalized cases of CD and UC with and without COVID-19 infection.
| Outcomes | ||||||
|---|---|---|---|---|---|---|
| Patient Characteristics | CD with COVID-19 | CD without COVID-19 | P | UC with COVID-19 | UC without COVID-19 | P |
| Mortality, % | 575 (12.41%) | 2449 (2.15%) | <.01 | 510 (12.04%) | 2245 (3.20%) | <.01 |
| Mechanical Ventilation | 495 (10.7%) | 3859 (3.4%) | <.01 | 182 (12.4%) | 3014 (4.3%) | <.01 |
| Intensive Care Unit | 509 (11%) | 4654 (4.1%) | <.01 | 229 (13%) | 3785 (5.4%) | <.01 |
| Septic Shock | 380 (8.2%) | 44 267 (3.9%) | <.01 | 224 (7.6%) | 3715 (5.3%) | <.01 |
| Mean Length of Stay (days) | 7.56 days | 5.54 days | <.01 | 9.07 days | 6 days | <.01 |
| Mean Hospitalization costs | $19 317 | $15 467 | <.01 | $23 657 | $18 089 | <.01 |
Secondary Outcomes and Resource Utilization
Inflammatory bowel disease patients with COVID-19 had higher mean LOS (8.28 days vs 5.74 days, P ≤ .01) and total hospitalization cost ($21 390 vs $16 468, P < .01) in comparison to patients without COVID-19 infection (Table 3). In addition, on subgroup analysis, the COVID-19 group had higher LOS (CD, 7.56 days vs 5.54 days, P < .01; UC, 9.07 days vs 6 days, P < .01) and hospitalization costs (CD, $19 317 vs $15 467, P < .01; UC, $23 657 vs $18 089, P < .01) compared with the non-COVID groups. Patients with COVID-19 were found to have an all-cause 30-day readmission rate of 5.1% vs 12.2% in the non-COVID group. In the COVID group, the most common causes for readmissions included sepsis and acute kidney injury, followed by pneumonia.
Predictors of Outcomes
Multivariate logistic regression analyses showed that COVID-19 infection (adjusted odds ratio [adj OR] = 3.52; 95% confidence interval [CI], 2.82-4.37; P < .01), age (adj OR, 1.04; 95% CI, 1.03-1.06; P < .01), CCI >3 (adj OR, 3.52; 95% CI, 2.63-4.71; P < .01), ICU admission (adj OR, 3.84; 95% CI, 2.52-5.87; P < .01), MV (adj OR, 2.89; 95% CI, 1.88-4.44; P < .01) and septic shock (adj OR, 4.30; 95% CI, 3.46-5.35; P < .01) were the independent predictors for mortality (Table 5). Additionally, ICU admission, MV, and septic shock were also independent predictors of increased LOS and hospitalization costs in IBD patients with COVID-19 (Tables 6 and 7).
Table 5.
Bivariate and multivariate logistic regression showing predictors for mortality in patients with IBD hospitalized with COVID-19 infection.
| Bivariate logistic regression | Multivariate logistic regression | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | P | Odds Ratio | 95% Confidence Interval | P | |
| COVID-19 Infection | 6.47 | (5.51-7.59) | < .01 | 3.52 | (2.83-4.37) | < .01 |
| Female | 0.8 | (0.71-0.9) | < .01 | 0.85 | (0.73-0.99) | .03 |
| Race | ||||||
| White | Reference | Reference | ||||
| Black | 0.86 | (0.71-1.05) | .13 | 1.06 | (0.8-1.4) | .67 |
| Hispanic | 0.85 | (0.67-1.08) | .19 | 0.99 | (0.72-1.37) | .97 |
| Other | 0.95 | (0.69-1.3) | .73 | 0.65 | (0.43-0.99) | .05 |
| Age (mean) | 1.04 | (1.04-1.05) | < .01 | 1.04 | (1.03-1.06) | < .01 |
| 18-34 | Reference | Reference | ||||
| 35-44 | 2.36 | (1.5-3.73) | < .01 | 0.77 | (0.45-1.32) | .34 |
| 45-64 | 6.8 | (4.66-9.92) | < .01 | 0.7 | (0.41-1.21) | .21 |
| ≥65 | 15.03 | (10.36-21.81) | < .01 | 0.68 | (0.34-1.39) | .3 |
| Charlson comorbidity index | ||||||
| 1 | Reference | Reference | ||||
| 2 | 5.24 | (4.16-6.6) | < .01 | 2.06 | (1.55-2.75) | < .01 |
| 3 or more | 9.58 | (7.78-11.79) | < .01 | 3.52 | (2.63-4.71) | < .01 |
| Median income based on the zip code | ||||||
| $1-$38 999 | Reference | Reference | ||||
| $39 000-$47 999 | 0.95 | (0.81-1.11) | .50 | 0.86 | (0.69-1.07) | .18 |
| $48 000-$62 999 | 0.85 | (0.72-1) | .05 | 0.74 | (0.59-0.92) | .01 |
| >$63 000 | 1.02 | (0.86-1.2) | .85 | 0.81 | (0.65-1.02) | .08 |
| Insurance provider | ||||||
| Medicare | Reference | Reference | ||||
| Medicaid | 0.33 | (0.26-0.4) | < .01 | 1.16 | (0.86-1.58) | .34 |
| Private | 0.35 | (0.3-0.41) | < .01 | 1.04 | (0.82-1.31) | .74 |
| Uninsured | 0.4 | (0.27-0.6) | < .01 | 2.1 | (1.32-3.34) | < .01 |
| Comorbidities | ||||||
| Diabetes mellitus | 1.3 | (0.7-2.44) | .41 | — | — | — |
| Hypertension | 0.7 | (0.18-2.76) | .61 | — | — | — |
| Smoking Status | 0.75 | (0.65-0.87) | < .01 | 0.83 | (0.69-0.98) | .03 |
| Alcohol Disorder | 1.49 | (0.98-2.26) | .06 | 2.04 | (1.17-3.58) | .01 |
| ESRD | 3.37 | (2.71-4.2) | < .01 | 1.33 | (0.96-1.85) | .09 |
| Congestive Heart Failure | 3.27 | (2.89-3.7) | < .01 | 0.89 | (0.74-1.08) | .24 |
| COPD | 1.37 | (1.2-1.56) | < .01 | 0.63 | (0.53-0.76) | < .01 |
| Hospital teaching status | ||||||
| Non-teaching | Reference | Reference | ||||
| Teaching | 1.21 | (1.05-1.4) | < .01 | 1.17 | (0.97-1.42) | .1 |
| Hospital size | ||||||
| Small | Reference | Reference | ||||
| Medium | 1.18 | (0.99-1.42) | .07 | 1.07 | (0.85-1.35) | .58 |
| Large | 1.31 | (1.11-1.54) | .00 | 1.19 | (0.97-1.46) | .1 |
| Hospital region | ||||||
| Northeast | Reference | Reference | ||||
| Midwest | 0.8 | (0.67-0.95) | .01 | 0.75 | (0.6-0.93) | < .01 |
| South | 0.74 | (0.63-0.87) | < .01 | 0.72 | (0.59-0.89) | < .01 |
| West | 0.91 | (0.76-1.09) | .33 | 0.91 | (0.72-1.15) | .43 |
| Complications | ||||||
| Intensive care unit | 47.24 | (41.51-53.76) | < .01 | 3.84 | (2.52-5.87) | < .01 |
| Mechanical ventilation | 52.8 | (46.14-60.42) | < .01 | 2.89 | (1.88-4.44) | < .01 |
| Septic Shock | 27.73 | (24.39-31.53) | < .01 | 4.3 | (3.46-5.35) | < .01 |
Table 6.
Bivariate and multivariate logistic regression showing predictors for LOS in patients with IBD hospitalized with COVID-19 infection.
| Bivariate logistic regression | Multivariate logistic regression | |||||
|---|---|---|---|---|---|---|
| Coefficient | 95% Confidence Interval | P | Coefficient | 95% Confidence Interval | P | |
| COVID-19 Infection | 2.66 | (2.27-3.04) | < .01 | 1.13 | (0.76-1.5) | < .01 |
| Female | −0.19 | (−0.31 to −0.07) | < .01 | −0.14 | (−0.26 to −0.03) | .02 |
| Race | ||||||
| White | Reference | Reference | ||||
| Black | 0.31 | (0.09-0.52) | .01 | 0.17 | (−0.05-0.39) | .14 |
| Hispanic | 0.06 | (−0.25-0.37) | .7 | 0.08 | (−0.23-0.39) | .6 |
| Other | 0.57 | (0.18-0.96) | < .01 | 0.39 | (0.04-0.73) | .03 |
| Age (mean) | 0.03 | (0.02-0.03) | < .01 | −0.01 | (−0.02-0) | < .01 |
| 18-34 | Reference | Reference | ||||
| 35-44 | 0.16 | (−0.06-0.37) | .16 | 0.07 | (−0.18-0.32) | .6 |
| 45-64 | 0.97 | (0.79-1.14) | < .01 | 0.57 | (0.24-0.89) | < .01 |
| ≥65 | 1.32 | (1.15-1.49) | < .01 | 0.76 | (0.24-1.29) | .01 |
| Charlson comorbidity index | ||||||
| 1 | Reference | Reference | ||||
| 2 | 1.19 | (0.99-1.38) | < .01 | 0.6 | (0.37-0.82) | < .01 |
| 3 or more | 2.04 | (1.88-2.19) | < .01 | 1.1 | (0.87-1.34) | < .01 |
| Median income based on the zip code | ||||||
| $1-$38 999 | Reference | Reference | ||||
| $39 000-$47 999 | −0.14 | (−0.3-0.02) | .09 | −0.02 | (−0.18-0.13) | .77 |
| $48 000-$62 999 | −0.16 | (−0.33-0) | .05 | −0.09 | (−0.26-0.07) | .26 |
| >$63 000 | −0.03 | (−0.23-0.17) | .8 | 0.02 | (−0.18-0.22) | .87 |
| Insurance provider | ||||||
| Medicare | Reference | Reference | ||||
| Medicaid | −0.48 | (−0.68 to −0.28) | < .01 | 0.17 | (−0.07-0.41) | .17 |
| Private | −0.93 | (−1.06 to −0.8) | < .01 | −0.18 | (−0.37-0.01) | .07 |
| Uninsured | −1.14 | (−1.44 to −0.85) | < .01 | −0.23 | (−0.55-0.1) | .17 |
| Comorbidities | ||||||
| Diabetes mellitus | 1 | (0.09-1.91) | .03 | −0.16 | (−1.06-0.73) | .72 |
| Hypertension | 1.38 | (−0.1-2.87) | .07 | 0.49 | (−0.94-1.93) | .5 |
| Smoking Status | −0.57 | (−0.7 to −0.44) | < .01 | −0.51 | (−0.64 to −0.38) | < .01 |
| Alcohol Disorder | 0.36 | (−0.17-0.89) | .19 | 0.14 | (−0.41-0.69) | .62 |
| ESRD | 2.87 | (2.27-3.46) | < .01 | 0.72 | (0.16-1.27) | .01 |
| Congestive Heart Failure | 1.87 | (1.66-2.08) | < .01 | 0.51 | (0.27-0.75) | < .01 |
| COPD | 0.23 | (0.09-0.37) | < .01 | −0.55 | (−0.71 to −0.39) | < .01 |
| Hospital teaching status | ||||||
| Non-teaching | Reference | Reference | ||||
| Teaching | 1.06 | (0.92-1.2) | < .01 | 1.07 | (0.94-1.21) | < .01 |
| Hospital size | ||||||
| Small | Reference | Reference | ||||
| Medium | 0.55 | (0.39-0.71) | < .01 | 1.13 | (0.96-1.29) | < .01 |
| Large | 1.09 | (0.93-1.26) | < .01 | 1.13 | (0.96-1.29) | < .01 |
| Hospital region | ||||||
| Northeast | Reference | Reference | ||||
| Midwest | −0.62 | (−0.86 to −0.38) | < .01 | −0.7 | (−0.92 to −0.48) | < .01 |
| South | −0.45 | (−0.66 to −0.25) | < .01 | −0.42 | (−0.61 to −0.23) | < .01 |
| West | −0.49 | (−0.75 to −0.23) | < .01 | −0.59 | (−0.85 to −0.34) | < .01 |
| Complications | ||||||
| Intensive Care Unit | 8.42 | (7.73-9.1) | < .01 | 2.25 | (1.04-3.47) | < .01 |
| Mechanical ventilation | 9.03 | (8.26-9.81) | < .01 | 2.51 | (1.08-3.95) | < .01 |
| Septic Shock | 7.64 | (6.93-8.34) | < .01 | 3.57 | (2.83-4.31) | < .01 |
Table 7.
Bivariate and multivariate logistic regression showing predictors for hospitalization costs in patients with IBD hospitalized with COVID-19 infection.
| Bivariate Logistic Regression | Multivariate Logistic Regression | |||||
|---|---|---|---|---|---|---|
| Coefficient | 95% Confidence Interval | P | Coefficient | 95% Confidence Interval | P | |
| COVID-19 infection | 5703.51 | (4299.56-7107.46) | <.01 | −283.45 | (−1677.12 to 1110.22) | .69 |
| Female | -1796.76 | (−2242.48 to −1351.04) | <.01 | −1188.40 | (−1581.76 to −795.05) | <.01 |
| Race | ||||||
| White | Reference | Reference | ||||
| Black | -439.27 | (−1109.45-230.91) | .2 | 463.34 | (−183.4-1110.08) | .16 |
| Hispanic | 1733.88 | (562.58-2905.18) | <.01 | 1271.77 | (172.54-2371) | .02 |
| Other | 5889.37 | (3919.18-7859.57) | <.01 | 3760.38 | (2139.37-5381.39) | <.01 |
| Age (mean) | 81.91 | (70.98-92.85) | <.01 | −92.26 | (−128.67- -55.87) | <.01 |
| 18-34 | Reference | Reference | ||||
| 35-44 | 815.71 | (59.78-1571.64) | .03 | 1153.17 | (299.51-2006.83) | <.01 |
| 45-64 | 3322.86 | (2696.28-3949.45) | <.01 | 3030.33 | (1934.11-4126.56) | <.01 |
| ≥65 | 4566.78 | (3973.21-5160.35) | <.01 | 4596.22 | (2804.29-6388.16) | <.01 |
| Charlson comorbidity index | ||||||
| 1 | Reference | Reference | ||||
| 2 | 4884.96 | (4188.77-5581.15) | <.01 | 2931.62 | (2163.68-3699.56) | <.01 |
| 3 or more | 7878.7 | (7278.35-8479.05) | <.01 | 4129.53 | (3325.72-4933.35) | <.01 |
| Median income based on the zip code | ||||||
| $1-$38 999 | Reference | Reference | ||||
| $39 000-$47 999 | 843.47 | (334.44-1352.49) | <.01 | 768.09 | (283.1-1253.07) | <.01 |
| $48 000-$62 999 | 1632.28 | (1058.08-2206.49) | <.01 | 1042.45 | (517.77-1567.12) | <.01 |
| >$63 000 | 4133.38 | (3238.21-5028.56) | <.01 | 2913.68 | (2154.55-3672.8) | <.01 |
| Insurance provider | ||||||
| Medicare | Reference | Reference | ||||
| Medicaid | -1794.78 | (−2511.79 to −1077.78) | <.01 | 318.62 | (−446.68-1083.93) | .41 |
| Private | -1887.77 | (−2374.99 to −1400.54) | <.01 | 701.57 | (79.57-1323.57) | .03 |
| Uninsured | -4740.57 | (−5572.84 to −3908.3) | <.01 | −191.54 | (−1084.85-701.77) | .67 |
| Comorbidities | ||||||
| Diabetes mellitus | 3167.52 | (235.06-6099.98) | .03 | −616.68 | (−3454.37-2221) | .67 |
| Hypertension | 2705.67 | (−1032.05-6443.38) | .16 | −74.60 | (−3655.67-3506.47) | .97 |
| Chronic kidney disease | 263.38 | (115.69-411.07) | <.01 | — | - | - |
| ESRD | 11 955.96 | (9443.68-14 468.23) | <.01 | 4631.27 | (2471.84-6790.71) | <.01 |
| Congestive Heart Failure | 6889.98 | (6048.35-7731.62) | <.01 | 1726.18 | (811.87-2640.5) | <.01 |
| COPD | 645.08 | (155.71-1134.44) | .01 | −1928.36 | (−2455.69 to −1401.03) | <.01 |
| Hospital teaching status | ||||||
| Non-teaching | Reference | Reference | ||||
| Teaching | 3428.05 | (2828.57-4027.53) | <.01 | 2853.07 | (2319.05-3387.1) | <.01 |
| Hospital size | ||||||
| Small | Reference | Reference | ||||
| Medium | 1501.38 | (834.13-2168.63) | <.01 | 1198.90 | (586.2-1811.59) | <.01 |
| Large | 3976.9 | (3198.99-4754.81) | <.01 | 3512.88 | (2772.71-4253.06) | <.01 |
| Hospital region | ||||||
| Northeast | Reference | Reference | ||||
| Midwest | -3046.6 | (−4274.49 to −1818.71) | <.01 | −2580.12 | (−3714.52 to −1445.72) | <.01 |
| South | -4167.56 | (−5270.71 to −3064.42) | <.01 | −3232.69 | (−4228.55 to −2236.83) | <.01 |
| West | 3477.34 | (1893.37-5061.31) | <.01 | 3165.74 | (1628.67-4702.81) | <.01 |
| Complications | ||||||
| Intensive Care Unit | 42 604.42 | (39 280.43-45 928.4) | <.01 | 11 696.53 | (6583.16-16 809.91) | <.01 |
| Mechanical ventilation | 46 279.36 | (42 549.44-50 009.29) | <.01 | 20 147.13 | (13 868.29-26 425.97) | <.01 |
| Septic Shock | 34 448.96 | (31 245.76-37 652.16) | <.01 | 15 374.11 | (12 323.34-18 424.88) | <.01 |
Discussion
Our study is among the first in the United States to analyze the outcomes of COVID-19 among inpatient IBD patients on a national level. The results showed that IBD patients who also had COVID-19 had a 5-fold higher risk of inpatient death compared with those without COVID-19. Moreover, COVID-19-positive IBD patients were more likely to require mechanical ventilation and experienced a higher incidence of acute respiratory failure (ARF) and septic shock. After adjusting for multiple factors, COVID-19 was found to be an independent predictor of increased inpatient mortality, prolonged LOS, and higher hospitalization costs. The subgroup analysis showed no statistically significant difference in mortality between CD and UC patients. However, UC patients had a longer LOS and a greater need for ICU care and mechanical ventilation, resulting in increased hospitalization costs compared with CD patients.
Multiple studies have investigated the impact of COVID-19 on IBD patients and found that they do not seem to be at higher risk of contracting COVID-19 infection compared with the general population. However, previous studies have reported varying results regarding COVID-19-related outcomes in IBD patients, with mortality rates ranging from 0% to 20%.7-9,15,16 This variability can be attributed to differences in the design of the study, sample size, comparison groups, population characteristics, pandemic stage, and the healthcare system. The SECURE IBD registry, which compiled data from 525 patients in 33 countries, was one of the first few studies to be published in May 2020. The study reported an overall mortality rate of 3%, and 7% of patients experienced a combination of ICU care, MV use, or death.17 At the beginning of the pandemic, limited data were available, and much of what was known came from small case series and observational studies. However, as the pandemic worsened, larger studies came out, and a clearer picture of the impact of COVID-19 on IBD patients emerged. A recent meta-analysis by Abdulla and colleagues, which included data from 24 studies, reported an overall mortality rate of 1.94% (95% CI, 1.29%-2.59%), with a higher mortality rate for patients with CD (2.79%) compared with those with UC (0.14%).18 However, these findings are limited by significant variability among studies and are based on single-center studies from different healthcare settings. Our study is a population-based study that analyzed the earliest pandemic phase, utilizing the largest national-level US database with a large sample size and a broad demographic representation, providing more generalizability in terms of findings. The results of our study align with those of a multicenter nationwide IBD cohort study from the Netherlands, which reported a similar mortality rate of 13.0% and 10% ICU utilization,19 Nevertheless, this study has limitations attributable to its small sample size, stemming from the initial phase of the pandemic.
There are several potential mechanisms by which IBD patients may be more likely to experience worse outcomes from COVID-19 infection. One possibility is that the immune dysregulation associated with IBD may make patients more susceptible to infections, including COVID-19.20 In addition, medications used to treat IBD, such as immunomodulators and biologics, may decrease the immune response and increase the risk of opportunistic infections.21-23 Furthermore, the presence of IBD may indicate underlying comorbidities, such as obesity or cardiovascular disease, which have been linked to worse outcomes from COVID-19 infection. Finally, IBD patients may be at elevated risk of severe COVID-19 infection due to changes in their access to medical care or usual routines during the pandemic.24
There is a paucity of data reporting the resource utilizations, including hospital LOS and the cost incurred due to COVID-19 infection in IBD patients. The LOS and hospitalization costs associated with COVID-19 infection in IBD patients can vary widely, depending on several factors, such as the severity of the illness, overall health status, the presence of underlying comorbidities, and the requirement of ICU care, including mechanical ventilation. A single-center study based on the Canadian health system suggested a median duration of hospitalization of 10.5 days (interquartile range [IQR], 3.8-29.0); however, these findings were limited to only 7.3% of the IBD patients who required hospitalization.25 In a separate attempt to discern variations in resource utilization before and amid the COVID-19 pandemic, a different Canadian study noted an extended hospitalization period for the prepandemic group, with an average duration of 10.11 ± 17.19 days compared with 7.13 ± 8.95 days for the pandemic group after controlling for covariates such as age, gender, rural setting, and disease type (adjusted P = .015).26 A study published in the Journal of Crohn’s and Colitis in 2020 found that COVID-19 infection in patients with IBD was associated with increased healthcare resource utilization, including higher hospitalization costs and longer lengths of stay in the hospital. The study found that the median length of hospital stay for IBD patients with COVID-19 was 14 days, which was longer than the median length of stay for patients without IBD. The study also found that IBD patients with COVID-19 had significantly higher hospitalization costs compared with patients without IBD.27 It is important to recognize that these findings may not be generalizable to all populations, as the cost and length of stay estimates may vary based on the type of IBD, geographical location, and country. Our study also determined the all-cause 30-day readmission rate in IBD patients hospitalized with COVID-19, which is significantly lower than in the non-COVID group. This can be explained by the higher inpatient mortality in the index hospitalization in the COVID group, which resulted in the lower 30-day readmission group. As far as we know, this is the first study to date to report the all-cause 30-day readmission rate, LOS, and hospitalization costs incurred in the management of COVID-19 infection in IBD patients.
We acknowledge several limitations in our study. First, this database study relies on accurate reporting of ICD codes to identify the patient population, which can lead to over- or under-reporting. However, database studies allow for the examination of large sample sizes, increasing the results’ generalizability. Second, the database does not include information on medications (immunomodulators, biologics, and small molecules), lab values, or radiographic findings, which limits our ability to assess the severity of COVID-19 infection or the overall disease status of the IBD patients. However, we used validated tools such as the Charlson Comorbidity Index and individual comorbidities as surrogates to ascertain the comorbidity burden. Third, the scope of this study is limited to inpatient data and does not include information on the outpatient outcomes of patients with IBD who have nonsevere COVID-19. Similarly, we could not determine those patients who underwent surgical or endoscopic procedures to treat IBD. Finally, we could not capture out-of-state readmissions because NRD is derived from state-based databases and does not use the exact linkage identification between states. We assume, however, that a limited number of patients were readmitted out-of-state due to insurance-related concerns.
Despite some limitations, our study is the first to analyze the national data on the impact of COVID-19-related hospitalizations in IBD patients in the early pandemic phase. The large sample size with a diverse population, including variables from small, medium, and large hospitals, rural and urban locations, and teaching and nonteaching hospitals, makes our results more generalizable. Additionally, the inclusion of the variables in the database allowed us to examine factors including hospitalization cost, patient income, and other hospital characteristics that are not often studied in single-center studies. In conclusion, our study provides valuable insights into the existing and evolving body of evidence on the impact of COVID-19 on IBD patients. We found that COVID-19 is an independent predictor of mortality in hospitalized IBD patients and results in poor outcomes, including increased mortality and resource utilization. To mitigate the impact of COVID-19 on IBD patients, it is critical to promote preventive measures, such as vaccination and booster doses, and to provide timely treatment with advanced COVID-19 therapies. This will help improve outcomes and reduce healthcare costs and utilization. Although these data predate the availability of vaccines and advanced treatments for COVID-19, it would be interesting to see the results of future studies to see if these trends persist for subsequent years of the pandemic.
Supplementary Material
Contributor Information
Khadija Naseem, Cleveland Clinic Foundation, Cleveland, OH, USA.
Abdullah Sohail, The University of Iowa Hospitals and Clinics, Iowa City, IA, USA.
Vu Quang Nguyen, Case Western Reserve University, Cleveland, OH, USA.
Ahmad Khan, Case Western Reserve University, Cleveland, OH, USA.
Gregory Cooper, Case Western Reserve University, Cleveland, OH, USA.
Bret Lashner, Cleveland Clinic Foundation, Cleveland, OH, USA.
Jeffry Katz, Case Western Reserve University, Cleveland, OH, USA.
Fabio Cominelli, Case Western Reserve University, Cleveland, OH, USA.
Miguel Regueiro, Cleveland Clinic Foundation, Cleveland, OH, USA.
Emad Mansoor, Case Western Reserve University, Cleveland, OH, USA.
Author Contributions
K.N., A.K., G.C., and A.S.: Significantly involved in the conceptualization and development of the project, as well as obtaining and comprehending data. Thoroughly reviewed the project for important intellectual content and endorsed the final draft for publication.
V.Q.N., B.L., J.K., F.C., M.R.: Significantly involved in the conceptualization and development of the project. Thoroughly reviewed the project for important intellectual content and endorsed the final version for publication.
E.M.: Significantly involved in the conceptualization and development of the project. Thoroughly reviewed the project for important intellectual content and endorsed the final draft for publication.
Conflicts of Interest
J.K. has been doing research for Janssen Inc., Eli Lilly Inc., and Abivax Inc.
M.R. is on the advisory board and consultant for Abbvie, Janssen, UCB, Takeda, Pfizer, BMS, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, Target Pharma Solutions, Trellis, and Boehringer Ingelheim Pharmaceuticals, Inc (BIPI).
All other authors have nothing to declare.
References
- 1. World Health Organization. WHO Coronavirus (COVID-19) Dashboard. World Health Organization; 2022. https://covid19.who.int/ [Google Scholar]
- 2. Gao YD, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76(2):428-455. doi: 10.1111/all.14657 [DOI] [PubMed] [Google Scholar]
- 3. Taxonera C, Sagastagoitia I, Alba C, et al. intestinal inflammation, COVID-19 and gastrointestinal ACE2-exploring RAS inhibitors. Authors’ reply. Aliment Pharmacol Ther. 2020;52(3):571-572. doi: 10.1111/apt.15893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tripathi K, Godoy Brewer G, Thu Nguyen M, et al. COVID-19 and outcomes in patients with inflammatory bowel disease: systematic review and meta-analysis. Inflamm Bowel Dis. 2022;28(8):1265-1279. doi: 10.1093/ibd/izab236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ungaro RC, Brenner EJ, Gearry RB, et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut. 2021;70(4):725-732. doi: 10.1136/gutjnl-2020-322539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ardizzone S, Ferretti F, Monico MC, et al. Lower incidence of COVID-19 in patients with inflammatory bowel disease treated with non-gut selective biologic therapy. J Gastroenterol Hepatol. 2021;36(11):3050-3055. doi: 10.1111/jgh.15591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee MH, Li HJ, Wasuwanich P, et al. COVID-19 susceptibility and clinical outcomes in inflammatory bowel disease: an updated systematic review and meta-analysis. Rev Med Virol. 2023;33(2):e2414. doi: 10.1002/rmv.2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen L, Hu K, Cheng C, et al. Risk of adverse outcomes in inflammatory bowel disease patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Colorectal Dis. 2022;37(11):2277-2289. doi: 10.1007/s00384-022-04265-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alrashed F, Alasfour H, Shehab M. Impact of biologics and small molecules for inflammatory bowel disease on COVID-19-related hospitalization and mortality: a systematic review and meta-analysis. JGH Open. 2022;6(4):241-250. doi: 10.1002/jgh3.12728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; 2022. Accessed December 6, 2022. www.hcup-us.ahrq.gov/nisoverview.jsp [Google Scholar]
- 11. Agency for Healthcare Research and Quality. Introduction the HCUP Nationwide Inpatient Sample (NIS). Agency for Healthcare Research and Quality; 2020. Accessed December 6, 2022. https://www.hcup-us.ahrq.gov/db/nation/nis/NISIntroduction2020.pdf [Google Scholar]
- 12. Agency for Healthcare Research and Quality. Introduction the HCUP Nationwide Readmissions Database (NRD). Agency for Healthcare Research and Quality; 2020. Accessed December 6, 2022. https://www.hcup-us.ahrq.gov/db/nation/nrd/Introduction_NRD_2020.pdf [Google Scholar]
- 13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 14. HCUP Cost-to-Charge Ratio (CCR) for the National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; 2020. www.hcup-us.ahrq.gov/db/ccr/ip-ccr/ip-ccr.jsp. Accessed January 11, 2023. [Google Scholar]
- 15. Macaluso FS, Orlando A. COVID-19 in patients with inflammatory bowel disease: a systematic review of clinical data. Dig Liver Dis. 2020;52(11):1222-1227. doi: 10.1016/j.dld.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ke Y, Lu W, Liu W, et al. Non-typhoidal salmonella infections among children in a tertiary hospital in Ningbo, Zhejiang, China, 2012-2019. PLoS NeglTrop Dis. 2020;14(10):e0008732. doi: 10.1371/journal.pntd.0008732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159(2):481-491.e3. doi: 10.1053/j.gastro.2020.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abdulla M, Mohammed N, AlQamish J, Mosli M. Inflammatory bowel disease and COVID-19 outcomes: a meta-analysis. Sci Rep. 2022;12(1):21333. doi: 10.1038/s41598-022-25429-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Derikx L, Lantinga MA, de Jong DJ, et al. Clinical outcomes of Covid-19 in patients with inflammatory bowel disease: a nationwide cohort study. J Crohns Colitis. 2021;15(4):529-539. doi: 10.1093/ecco-jcc/jjaa215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dulai PS, Thompson KD, Blunt HB, Dubinsky MC, Siegel CA. Risks of serious infection or lymphoma with anti-tumor necrosis factor therapy for pediatric inflammatory bowel disease: a systematic review. Clin Gastroenterol Hepatol. 2014;12(9):1443-1451; quiz e88. doi: 10.1016/j.cgh.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 21. Li Y, Yang Y, Yin S, et al. Inedible Azo Dyes and their analytical methods in foodstuffs and beverages. J AOAC Int. 2018;101(5):1314-1327. doi: 10.5740/jaoacint.18-0048 [DOI] [PubMed] [Google Scholar]
- 22. Rahier JF, Magro F, Abreu C, et al. ; European Crohn's and Colitis Organisation (ECCO). Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8(6):443-468. doi: 10.1016/j.crohns.2013.12.013 [DOI] [PubMed] [Google Scholar]
- 23. Khalid M, Rahman SU, Huang D. Molecular mechanism underlying Piriformospora indica-mediated plant improvement/protection for sustainable agriculture. Acta Biochim Biophys Sin (Shanghai). 2019;51(3):229-242. doi: 10.1093/abbs/gmz004 [DOI] [PubMed] [Google Scholar]
- 24. Kumric M, Ticinovic Kurir T, Martinovic D, Zivkovic PM, Bozic J. Impact of the COVID-19 pandemic on inflammatory bowel disease patients: a review of the current evidence. World J Gastroenterol. 2021;27(25):3748-3761. doi: 10.3748/wjg.v27.i25.3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wetwittayakhlang P, Albader F, Golovics PA, et al. Clinical outcomes of COVID-19 and impact on disease course in patients with inflammatory bowel disease. Can J Gastroenterol Hepatol. 2021;2021:7591141. doi: 10.1155/2021/7591141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malhi G, Minhas G, Chambers J, et al. Increased hospitalization for IBD patients seen in the ER during the COVID-19 pandemic. J Can Assoc Gastroenterol. 2022;5(6):271-275. doi: 10.1093/jcag/gwac020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ungaro RC, Chou B, Mo J, et al. Impact of COVID-19 on healthcare resource utilisation among patients with inflammatory bowel disease in the USA. J Crohns Colitis. 2022;16(9):1405-1414. doi: 10.1093/ecco-jcc/jjac056 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.