Abstract
Introduction: Early administration of appropriate antibiotics has been shown to be among the most effective interventions to reduce mortality in septic patients. We evaluated the attainment of efficacy and safety targets at 24 h associated with the use of intensive beta-lactam therapy in patients admitted to the intensive care unit for sepsis.
Methods: This was a prospective study with patients who received beta-lactams for sepsis or septic shock between February 2023 and September 2023. The antibiotic dose was unadjusted for renal function and administered by a loading dose followed by extended infusions, according to local practices. Blood samples were taken at the trough 24 h after the start of the beta-lactam to obtain serum levels. These levels were compared to efficacy and innocuity thresholds found in the literature.
Results: Among 36 included patients, all of them achieved serum concentrations above the minimum inhibitory concentration (MIC) for 100% of the therapeutic interval and 75% of them achieved serum concentrations above four times the MIC for 100% of the therapeutic interval. The predefined toxicity thresholds were reached by 8.3% of patients. Renal impairment was the factor most associated with the achievement of higher serum levels.
Conclusion: Nonrenally adjusted doses of beta-lactams administered by extended infusion showed good attainment of effective concentrations and few toxic concentrations in critically ill patients with sepsis or septic shock. Further studies are needed to better define the association between toxic concentrations and toxicity manifestations.
Keywords: beta-lactams, drug monitoring, extended infusion, loading dose, prolonged infusion, sepsis, TDM
1. Introduction
Bacterial infections are an important cause of morbidity and mortality in patients admitted to the intensive care unit (ICU). Early administration of an appropriate antibiotic has been shown to be among the most effective interventions to reduce mortality in these patients, with beta-lactams (BLs) being the most frequently prescribed antibiotics in this situation [1–3]. Since BLs are time-dependent antibiotics, their effectiveness depends on the percentage of time that the unbound drug concentration is maintained above the minimum inhibitory concentration (MIC) of the targeted pathogen (expressed as %fT > MIC) [2–4]. It is also known that the extended infusion of BL optimizes the %fT > MIC and reduces the ICU length of stay, duration of mechanical ventilation, and mortality [3, 5].
Critically ill patients are known to have pharmacokinetic (PK) variability secondary to pathophysiological changes such as modifications in the volume of distribution, altered protein binding, or augmented renal clearance [6, 7]. Moreover, estimation of renal function using the serum creatinine level may be biased in critically ill patients [8]. Because of rapid changes in renal function, chronic kidney disease (CKD) formulas should not be used, and urine sample collection is imprecise. The use of BL doses unadjusted for renal function, chronic or acute, has thus been suggested for the first 24 h of treatment, to ensure rapid attainment of therapeutic targets [9, 10].
Different observational studies have used therapeutic drug monitoring (TDM) to evaluate the BL serum levels associated with different dosing regimens in patients with sepsis or septic shock, with the results indicating that about a third of patients do not reach 100% > 1x MIC following administration by either continuous or intermittent infusion [2, 11]. However, the optimal unbound drug concentration target remains uncertain in this setting, with recommendations ranging from one to eight times the MIC of the suspected pathogen. New recommendations suggest a target of 100% fT > 1x MIC to ensure efficacy in the critically ill, while a target of 100% fT > 4x MIC could be used to minimize the development of bacterial resistance and to maximize antibiotic concentration at the infection site [4, 12–15]. Data concerning toxicity thresholds are also variable, with authors suggesting fixed concentrations for different antibiotics [16, 17].
Considering the high morbidity and mortality associated with suboptimal treatment of sepsis and septic shock in the ICU, dosing regimens combining extended infusion and unadjusted renal doses of BL have been suggested, with preoccupations remaining considering the efficacy and safety of this strategy [13, 18]. The aim of this study was to evaluate the use of nonrenally adjusted doses of BL administered by extended infusion in terms of reaching efficacy and safety targets at 24 h using TDM in patients admitted to the ICU for sepsis or septic shock.
2. Methods
2.1. Study Design and Participants
This observational, prospective study was performed in the three adult ICUs of the Centre Hospitalier Universitaire de Sherbrooke (CHUS). It was funded by the CIUSSS de l'Estrie-CHUS pharmacy department and by the University of Montreal. The trial protocol was approved by the CHUS and University of Montreal's ethics comities. The serum BL assays were performed by the STP2 laboratory at the University of Montreal Faculty of Pharmacy.
Included patients were adults aged 18 and older, admitted to the ICU between February 21, 2023, and September 15, 2023, with a confirmed or suspected bacterial infection and receiving piperacillin–tazobactam (PT) or meropenem according to the local BL dosing regimen for patients in the ICU, as described below. Only patients who required vasopressors for their infection were included. Patients were excluded if their BL was changed or stopped within the first 24 h, if they were transferred outside the ICU within 24 h on study BL, or if the studied BL had already been administered for more than 24 h at the time of recruitment. Consent was obtained from the patients or their representatives.
The current practice in the included ICUs consists of administering BL doses nonadjusted for renal function, including acute kidney injury (AKI), CKD, or CRRT, for the first 24 h to rapidly attain therapeutic serum concentrations. The first dose is administered as a bolus over 30 min and subsequent doses as a 3-h infusion, which is referred to as an extended infusion. The subsequent dosing regimen and the treatment duration were determined by the treating team and depended on patient-specific factors and are therefore not presented.
2.2. Endpoints
The primary outcome was the achievement of a plasma BL concentration greater than 1x the MIC of the target pathogen for 100% of the therapeutic interval (100% fT > 1x MIC).
The first two secondary outcomes were the achievement of a plasma concentration greater than four times the MIC for 100% of the therapeutic interval (100% fT > 4x MIC) and the achievement of the toxic plasma trough concentration.
The third secondary outcome was the association between achievement of these three targets and clinical impact, assessed by hospital length of stay, duration of mechanical ventilation, time to awakening (time between starting the antibiotic and reaching a score greater than 3 on the Sedation Assessment Scale [SAS] in intubated patients), incidence of delirium (a score greater than 3 on the Intensive Care Delirium Screening Checklist [ICSDC]), incidence of seizures, and mortality at 30 days. The fourth secondary outcome was the assessment of patient characteristics associated with a greater risk of having subtherapeutic or supratherapeutic serum levels.
For the primary and the first two secondary outcomes, we compared the obtained serum concentrations to the MIC of the targeted pathogen. When available, we used the MIC from the antibiogram of the pathogen cultured at the primary site of infection. When no antibiogram was available for an identified pathogen, we used the MIC determined by the Clinical & Laboratory Standards Institute (CLSI) for this specific pathogen [19]. If no pathogen was isolated, we used the highest MIC associated with the most common pathogens found in the identified primary site of infection, according to the Sanford Guide to Antimicrobial Therapy [20]. If no site of infection was identified, we used a MIC of 16 mg/L for PT and 4 mg/L for meropenem, corresponding to the highest MIC expected from susceptible pathogens according to the CLSI [19].
We selected a toxic trough unbound concentration of 157 mg/L for PT and 64 mg/L for meropenem [16, 17, 21].
2.3. Post Hoc Analysis
A post hoc analysis was also conducted in which we compared the obtained serum levels to a MIC of 16 mg/L for PT and 4 mg/L for meropenem to allow interpretation of the results in cases where local sensitivities would be less favorable [15].
2.4. Blood Samples
Blood samples were taken at the trough, 24 h after starting the studied BL. BL total concentrations were obtained using a validated ultrahigh-performance liquid chromatography method.
Since the bioanalysis method used to measure the serum levels was developed for total concentrations, conversion factors were used to calculate the corresponding unbound concentration of BL. A factor of 70% was chosen for PT [22, 23] and 98% for meropenem [22, 24].
2.5. Sample Size
Based on the historical ICU admission data, we estimated a recruitment capacity of 35 to 70 patients for the duration of the study. Using the formula n=(Z2 p(1−P))/d2 with an alpha of 0.05 and a proportion of 70% of patients having reached the target of 100% fT > MIC at 24 h as obtained in a trial by De Waele et al. [25], this gives an estimated accuracy of the obtained result between 10% (with 70 patients) and 15% (with 35 patients), which is acceptable for our study.
2.6. Statistical Analysis
Statistical analyses were performed by a statistician affiliated with the CHUS. All statistical tests were bilateral, and we considered a significant threshold at 5%. The results were obtained using IBM SPSS Statistics software Version 28. No imputation method was used for missing data.
We obtained dichotomous data for the primary outcome and the first two secondary outcomes. Data are reported with 95% CIs. For the third secondary outcome, we compared dichotomous data of mortality, delirium, and seizures using the Fisher exact test. Continuous variables of hospital length of stay, duration of ventilation, and time to awakening were compared using a Mann–Whitney U test. For the fourth secondary outcome, we calculated relative risks and 95% CIs using the modified Poisson regression with robust error measurement.
3. Results
3.1. Demographic and Clinical Characteristics
Between February 21, 2023, and September 15, 2023, 69 patients were eligible and 36 were included (Figure 1). No patients were lost to follow-up.
Figure 1.
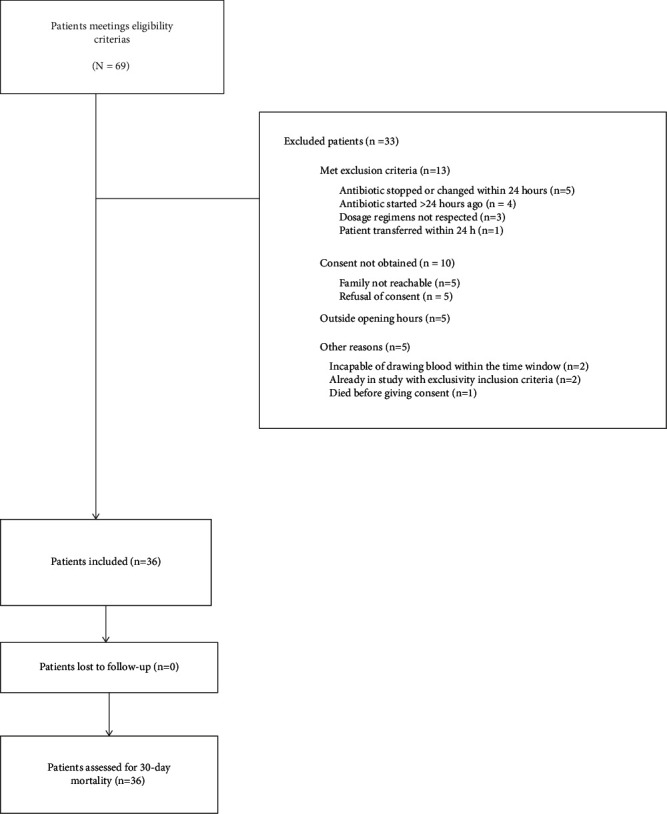
Assessment, exclusions, and follow-up of patients.
Demographic and clinical characteristics are described in Table 1. The mean age was 68.6 years, 27 patients (75%) were men, 29 patients (80.6%) received PT, and 7 (19.4%) received meropenem. The most common infections were intra-abdominal infections (41.7%), urinary tract infections (19.4%), and pulmonary infections (16.7%). Enterobacteriaceae were identified in 38.9% of patients. Eleven patients (30.6%) were mechanically ventilated, 23 patients (69.7%) had CKD (Stages 1–3) [26], 17 patients had AKI (Stages 1–3) [27], and continuous venovenous hemodiafiltration (CVVHDF) was started in the first 24 h in three patients.
Table 1.
Demographic and clinical characteristics.
| Characteristics | N = 36 |
|---|---|
| Age (years)—mean ± σ | 68.6 ± 16.8 |
| Male sex—n (%) | 27 (75) |
| BMI (calculated) (kg/m2)—mean ± σ | 27.5 ± 6.8 |
| Serum albumin (g/L)—mean ± σ | 22.1 ± 4.6 (n = 12/36) |
| Chronic kidney disease—n (%) | |
| Normal kidney function | 10/33 (30.3) |
| Stage 1 | 15/33 (45.5) |
| Stage 2 | 4/33(12.1) |
| Stage 3 | 4/33 (12.1) |
| Stages 4–5 | 0/33 |
| Acute kidney injury—n (%) | |
| No AKI | 17/34 (50.0) |
| Stage 1 | 7/34 (20.6) |
| Stage 2 | 4/34 (11.8) |
| Stage 3 | 6/34 (17.6) |
| APACHE II score—mean ± σ | 23.2 ± 12.1 |
| Antibiotic—n (%) | |
| Piperacillin–tazobactam† | 29 (80.6) |
| Meropenem† | 7 (19.4) |
| Infection sites—n (%) | |
| Intra-abdominal | 15 (41.7) |
| Urinary | 7 (19.4) |
| Pulmonary | 6 (16.7) |
| Osteomyelitis/necrotizing fasciitis | 3 (8.3) |
| Soft tissue | 2 (5.6) |
| Cardiac | 2 (5.6) |
| Effusion/empyema | 1 (2.8) |
| Catheters | 1 (2.8) |
| Not identified | 2 (5.6) |
| Isolated bacteria—n (%) | |
| Enterobacteriaceae | 14 (38.9) |
| Streptococcus | 6 (16.7) |
| Anaerobes | 5 (13.9) |
| Staphylococcus | 2 (5.6) |
| Enterococcus | 1 (2.8) |
| H. influenzae | 1 (2.8) |
| Not isolated | 14 (38.9) |
| MIC of identified pathogens (mg/L)—median (interquartile range) | |
| Piperacillin–tazobactam | 4 (1–8) |
| Meropenem | 1 (0.375–3) |
| Comedication‡-n (%) | |
| Other antibiotics | 12 (33.3) |
| Fluid repletion (mL)‡—mean ± σ | 6322 ± 2720 |
| Fluid balance (mL)‡—mean ± σ | 4333 ± 2553 |
| Renal replacement therapy†—n (%) | |
| Hemodialysis | 0 |
| CVVHDF | 3 (8.3) |
| Mechanical ventilation—n (%) | 11 (30.6) |
| Severity of illness§ | |
| Sepsis | 16/35 (45.7) |
| Septic shock | 19/35 (54.3) |
†PT, meropenem, and imipenem were the three BLs administered by prolonged infusion at the CIUSSSE-CHUS ICU. However, no patients meeting the inclusion criteria received imipenem during the study period.
‡From diagnosis to first BL sampling.
§According to sepsis-4 criteria.
For all patients, the administered BL dose was unadjusted for renal function for the first 24 h of treatment. For PT, a dose of 4 g every 6 h was used in 7/29 patients (24%), while a dose of 3 g every 6 h was used in 18/29 patients (62%). In 4/29 patients (14%), the dose was modified between 3 and 4 g every 6 h during the first 24 h of treatment following the clinical decision. For meropenem, clinicians chose a dose of 2 g every 8 h for 2/7 patients (29%) and a dose of 1 g every 8 h for 5/7 patients (71%).
As presented in Figure 2, the median (interquartile range) trough unbound serum concentration obtained for PT was 57.2 mg/L (25.8–79.1), while that of meropenem was 13.03 mg/L (5.19–32.63).
Figure 2.
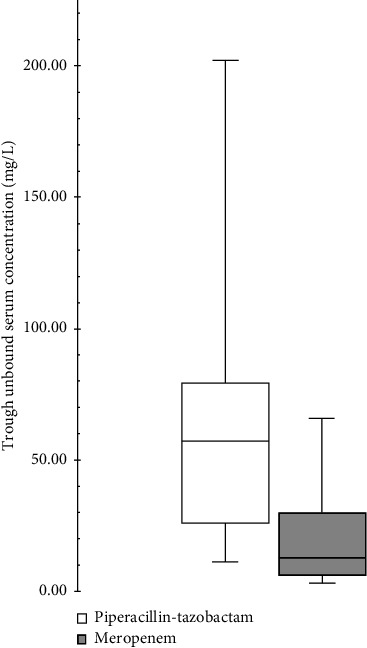
Boxplot of antibiotics through unbound serum concentrations.
3.2. Primary Outcome
All patients (36/36) achieved a plasma BL concentration greater than 1x the MIC of the target pathogen for 100% of the therapeutic interval (100% fT > 1x MIC) at 24 h (95% CI: 0.90–1.0) (Figure 3).
Figure 3.
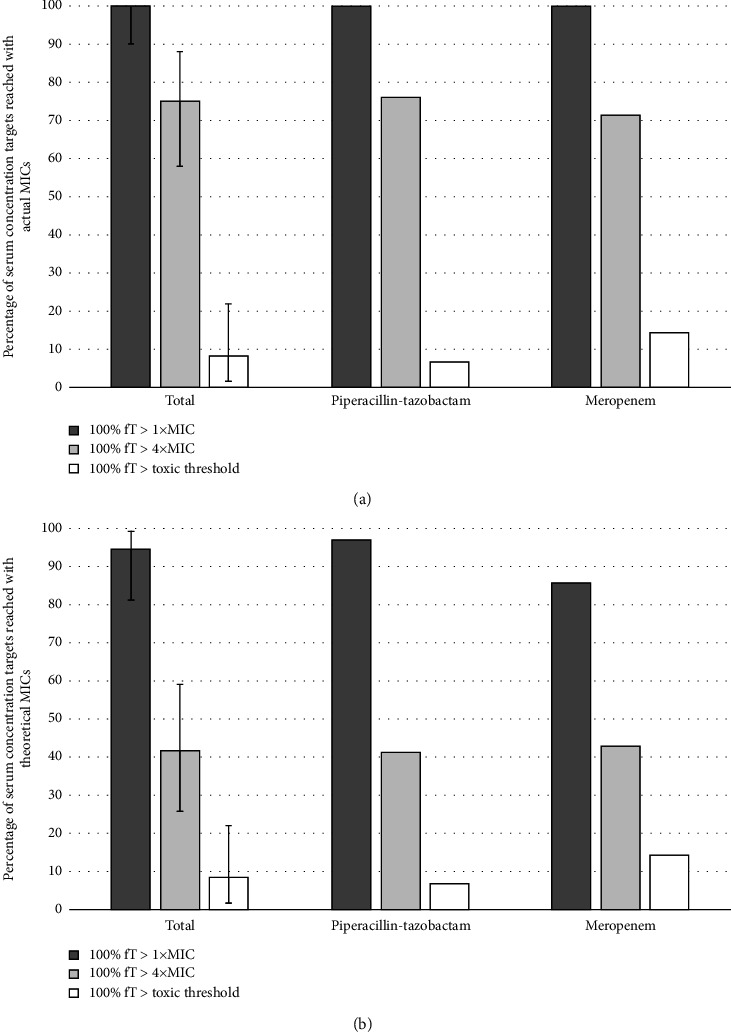
Percentage of serum concentration targets reached (a) with actual MICs and (b) with theoretical MICs.
3.3. Secondary Outcomes
A plasma concentration greater than four times the MIC for 100% of the therapeutic interval (100% fT > 4x MIC) was achieved in 27 patients (75%) (95% CI: 0.58–0.88) (Figure 3). Three patients (8.3%) achieved a toxic plasma trough concentration (95% CI: 0.017–0.22) (Figure 3). Two of them were receiving PT, and one of them was receiving meropenem.
There was no significant difference between patients that achieved 100% fT > 4x MIC and those who did not in terms of clinical impact assessed by mortality at 30 days (p=1), hospital length of stay (p=0.92), duration of mechanical ventilation (p=0.78), time to awakening (p=0.39), and incidence of delirium (p=0.63) (Table 2).
Table 2.
Association between target achievement and clinical impact.
| 4 x MIC | 100% fT < 4 x MIC (n = 9) | 100% fT ≥ 4 x MIC (n = 27) | p value |
|---|---|---|---|
| Mortality at 30 days—n (%) | 1 (11.1) | 4 (14.8) | 1.00 |
| Delirium—n (%) | 2 (22.2) | 4 (14.8) | 0.63 |
| Seizures—n (%) | 0 | 0 | - |
| Hospital length of stay (days)—mean ± σ | 20.1 ± 24.1 | 16.9 ± 19 | 0.92 |
| Duration of mechanical ventilation (days)—mean ± σ | 2.48 ± 2.23 | 3.89 ± 5.27 | 0.78 |
| Time to awakening (hours)—mean ± σ | 43.8 ± 26.5 | 22.9 ± 23.7 | 0.39 |
|
| |||
| Toxic threshold | Toxic trough levels (n = 3) | Nontoxic trough levels (n = 33) | p value |
|
| |||
| Mortality at 30 days—n (%) | 1 (33.3) | 4 (12.1) | 0.37 |
| Delirium—n (%) | 1 (33.3) | 5 (15.1) | 0.43 |
| Seizures—n (%) | 0 | 0 | — |
| Hospital length of stay (days)—mean ± σ | 29.3 ± 36.1 | 16.9 ± 19.4 | 0.33 |
| Duration of ventilation (days)—mean ± σ | 0.92 ± 0.45 | 4.08 ± 5.0 | 0.58 |
| Time to awakening (hours)—mean ± σ | 15.8 ± NA | 32.9 ± 27.1 | 0.75 |
There was also no significant difference in the outcomes between patients that achieved a toxic plasma through concentration and those who did not (Table 2).
A comparison of patient characteristics of those who did not reach 100% fT > 4x MIC to those who did is detailed in Table 3. There was no significant difference in their age (p=0.55), sex (0.22), BMI (p=0.86), fluid repletion (p=0.66), or fluid balance (p=0.46). However, patients with AKI had increased chances of reaching 100% fT > 4x MIC (RR = 1.60 [95% CI: 1.06–2.42], p=0.027). The same association was observed with renal replacement therapy (RR = 1.37 [95% CI: 1.12–1.69] p=0.003). No significant difference was observed when comparing patients with normal kidney function and patients with all stages of CKD (p=0.25). However, it is possible to note that patients with Stage 2 CKD seem to have higher chances of reaching 100% ft > 4x MIC when compared to patients without kidney disease (RR = 1.67 [95% CI: 1.00–2.76] p=0.05).
Table 3.
Comparison of patient characteristics who had subtherapeutic or supratherapeutic serum levels to those who had reached target levels.
| Characteristics | 100% fT ≥ 4x MIC not reached (n = 9) | 100% fT ≥ 4x MIC reached (n = 27) | RR [95% CI] | p value |
|---|---|---|---|---|
| Age (years)—mean ± σ | 66.0 ± 15.9 | 69.5 ± 17.1 | 1.00 [0.99–1.02] | 0.55 |
| Male sex—n. (%) | 5 (55.6) | 22 (81.5) | 1.47 [0.80–2.70] | 0.22 |
| BMI (kg/m2)—mean ± σ | 27.2 ± 5.7 | 27.6 ± 7.29 | 1.00 [0.97–1.03] | 0.86 |
| Fluid repletion (mL)†—mean ± σ | 6667 ± 3564 | 6207 ± 2468 | 1.00 [1.00–1.00] | 0.66 |
| Fluid balance (mL)†—mean ± σ | 4887 ± 3332 | 4148 ± 2325 | 1.00 [1.00–1.00] | 0.46 |
| Chronic kidney disease—n. (%) | ||||
| Normal kidney function | 4/8 (50) | 6/25 (24) | ||
| Stage 1 | 3/8 (37.5) | 12/25 (48) | 1.33 [0.76–2.35] | 0.32 |
| Stage 2 | 1/8(12.5) | 4/25 (16) | 1.67 [1.00–2.76] | 0.05 |
| Stage 3 | 0/8 | 3/25 (12) | 1.25 [0.58–2.67] | 0.56 |
| Missing data | 1 (11) | 2 (7) | — | — |
| Acute kidney injury—n (%) | ||||
| No AKI | 7/8 (87.5) | 10/26 (38.5) | ||
| Stage 1 | 0/8 | 7/26 (26.9) | 1.70 [1.14–2.53] | 0.009 |
| Stage 2 | 1/8 (12.5) | 3/26 (11.5) | 1.27 [0.64–2.55] | 0.49 |
| Stage 3 | 0/8 | 6/26 (23.1) | 1.70 [1.14–2.53] | 0.009 |
| Missing data | 1 (11) | 1 (4) | ||
| Renal replacement therapy†—n (%) | 0 | 3 (11.1) | 1.37 [1.12–1.69] | 0.003 |
| Antibiotic—n (%) | ||||
| Piperacillin–tazobactam | 7 (77.8) | 22 (81.5) | ||
| Meropenem | 2 (22.2) | 5 (18.5) | ||
|
| ||||
| Characteristics | Toxic trough levels (n = 3) | Nontoxic trough levels (n = 33) | R R [95% CI]‡ | p value ‡ |
|
| ||||
| Age (years)—mean ± σ | 77 ± 6.67 | 67.8 ± 17.5 | — | — |
| Male sex—n (%) | 2 (66.7) | 25 (75.8) | — | — |
| BMI (kg/m2)—mean ± σ | 26.1 ± 2.2 | 27.6 ± 7.14 | — | — |
| Fluid repletion (mL)†—mean ± σ | 8704 ± 2802 | 6105 ± 2612 | — | — |
| Fluid balance (mL)†—mean ± σ | 7828 ± 3099 | 4015 ± 2263 | — | — |
| Chronic kidney disease—n (%) | ||||
| Normal kidney function | 0 | 10/30 (33.3) | — | — |
| Stage 1 | 2 (66.7) | 13/30 (43.3) | — | — |
| Stage 2 | 1 (33.3) | 3/30 (10) | — | — |
| Stage 3 | 0 | 4/30 (13.3) | — | — |
| Missing data | 0 | 3 (9) | — | — |
| Acute kidney injury—n (%) | ||||
| No AKI | 0 | 17/31 (54.8) | — | — |
| Stage 1 | 0 | 7/31 (22.6) | — | — |
| Stage 2 | 1 (33.3) | 3/31 (9.7) | — | — |
| Stage 3 | 2 (66.7) | 4/31 (12.9) | — | — |
| Missing data | 2 (6) | — | — | |
| Renal replacement therapy†—n (%) | 0 | 3 (9.1) | — | — |
| Antibiotic—n (%) | ||||
| Piperacillin–tazobactam | 2 (66.7) | 27 (81.8) | — | — |
| Meropenem | 1 (33.3) | 6 (18.2) | — | - |
†From diagnosis to first BL sampling.
‡Because of the small number of patients with toxic trough levels, accurate RR and p value could not be extracted.
A comparison of patients who reached toxic plasma trough concentration to patients that did not is also detailed in Table 3. These results are based on data from three patients reaching the toxic threshold and their interpretation must only be exploratory.
3.4. Post Hoc Analysis
When considering a theoretical MIC of 16 mg/L for PT and 4 mg/L for meropenem, 94.4% of patients (34/36) reached 100% fT > 1x MIC (95% CI: 0.81–0.99) (Figure 3). Of the patients who did not reach 1x MIC, one received PT and the other one meropenem.
A plasma concentration greater than four times the MIC for 100% of the therapeutic interval (100% fT > 4x MIC) was achieved in 15 patients (41.7%) (95% CI: 0.26–0.59) (Figure 3). Among those who did not reach 4x MIC, 17 of them received PT and four of them received meropenem.
There was no significant difference between patients who achieved 100% fT > 1x MIC and those who did not and between patients who achieved 100% fT > 4x MIC and those who did not in terms of clinical impact, as defined above (Table 4).
Table 4.
Association between targets achievement and clinical impact—post hoc analysis.
| 1x MIC | 100% fT ≥ MIC reached (n = 34) | 100% fT ≥ MIC not reached (n = 2) | p value † |
|---|---|---|---|
| Mortality at 30 days—nb. (%) | 5 (14.7) | 0 | — |
| Delirium—nb. (%) | 6 (17.6) | 0 | — |
| Seizures—nb. (%) | 0 | 0 | — |
| Hospital length of stay (days)—mean ± σ | 17.7 ± 20 | 18.6 (30.2) | — |
| Duration of mechanical ventilation (days)—mean ± σ | 1.81 ± 4.39 | 0 | — |
| Time to awakening (hours)—mean ± σ | 23.05 ± 22.06 | 0 | — |
|
| |||
| 100% fT < 4 x MIC (n = 21) | 100% fT ≥ 4 x MIC (n = 15) | p value | |
|
| |||
| Mortality at 30 days—n (%) | 2 (9.5) | 3 (20) | 0.63 |
| Delirium—n (%) | 3 (14.3) | 3 (20) | 0.68 |
| Seizures—n (%) | 0 | 0 | — |
| Hospital length of stay (days)—mean ± σ | 17.1 ± 18.2 | 18.5 ± 23.6 | 0.76 |
| Duration of mechanical ventilation (days)—mean ± σ | 2.09 ± 1.87 | 4.69 ± 6.31 | 0.93 |
| Time to awakening (hours)—mean ± σ | 34.6 ± 31.6 | 26.9 ± 26 | 0.89 |
†Because of the small number of patients with 100% fT >MIC not reached, accurate p value could not be extracted.
A comparison of patients who reached 100% fT > 4x MIC to patients who did not is also detailed in the appendix (Table 5). Patients with AKI had higher chances of reaching 100% fT > 4x MIC (RR = 3.67 [95% CI: 1.24–10.85], p=0.02). There was no significant difference in all other characteristics.
Table 5.
Comparison of patient characteristics who had subtherapeutic or supratherapeutic serum levels to those who had reached target levels—post hoc analysis.
| Characteristics | 100% fT ≥ 4x MIC not reached (n = 21) | 100% fT ≥ 4x MIC reached (n = 15) | RR [95% CI] | p value |
|---|---|---|---|---|
| Age (years)—mean ± σ | 67.3 ± 18.3 | 70.5 ± 14.8 | 1.00 [0.97–1.04] | 0.57 |
| Male sex—n. (%) | 14 (66.7) | 13 (86.7) | 2.17 [0.60–7.82] | 0.24 |
| BMI (kg/m2)—mean ± σ | 28.00 ± 6.99 | 26.6 ± 6.81 | 0.97 [0.91–1.05] | 0.50 |
| Fluid repletion (mL)†—mean ± σ | 6452 ± 2790 | 6140 ± 2729 | 1.00 [1.00–1.00] | 0.69 |
| Fluid balance† (mL)—mean ± σ | 4229 ± 2404 | 4478 ± 2883 | 1.00 [1.00–1.00] | 0.74 |
| Chronic kidney disease—n. (%) | ||||
| Normal kidney function | 9/20 (45) | 1/13 (7.7.) | — | — |
| Stage 1 | 10/20 (50) | 5/13 (38.5) | 3.33 [0.46–24.44] | 0.24 |
| Stage 2 | 0/20 | 4/13 (30.8) | 10.00 [1.56–64.2] | 0.02 |
| Stage 3 | 1/20 (5) | 3/13(23.1) | 7.50 [1.07–52.38] | 0.04 |
| Missing data | 1 (5) | 2 (13) | — | — |
| Acute kidney injury—n (%) | ||||
| No AKI | 14/20 (70) | 3/14 (21.4) | — | — |
| Stage 1 | 4/20 (20) | 3/14 (21.4) | 2.43 [0.64–9.24] | 0.19 |
| Stage 2 | 1/20 (5) | 3/14 (21.4) | 4.25 [1.32–13.73] | 0.02 |
| Stage 3 | 1/20 (5) | 5/14 (35.7) | 4.72 [1.59–14.01] | 0.005 |
| Missing data | 1 (5) | 1 (7) | — | — |
| Renal replacement therapy†—n (%) | 1 (4.8) | 2 (13.3) | 1.69 [0.68–4.18] | 0.25 |
| Antibiotic—n (%) | ||||
| Piperacillin–tazobactam | 17 (81) | 12 (80) | — | — |
| Meropenem | 4 (19) | 3 (20) | — | — |
†From diagnosis to first BL sampling.
4. Discussion
In sepsis, early administration of an appropriate antibiotic reduces mortality. This prospective study showed that the short-term use of a nonrenally adjusted dose of BL administered by bolus followed by prolonged infusion in the critically ill allowed prompt attainment of efficacity targets in most patients while causing toxic levels in only a few patients.
Considering that the MICs for the pathogens identified for included patients were somewhat low, we added a post hoc analysis comparing the obtained serum levels to the CLSI breakpoints for Pseudomonas aeruginosa, to allow interpretation of our results in other populations with higher MIC [15, 19, 28].
Our results suggest that the studied dosing regimen allows achievement of 1x MIC in all patients and 4x MIC in most patients, contrary to what is often reported in the literature [2, 29]. A study published in 2014 showed that approximately one-third of patients do not achieve 100% > 1x MIC with PT and meropenem [2]. They also reported that only 30.3% of patients for PT and 41.6% of patients for meropenem reached 4x MIC [2]. The difference in the proportion of patients who achieved 100% > 1x MIC can partially be explained by the fact that 67% of their patients received BL by intermittent infusion. Moreover, they included patients who did not meet criteria for sepsis or septic shock and received BL for prophylaxis indications. It is also important to note that Pseudomonas aeruginosa also represented 16% of their identified pathogens but none of ours.
In the post hoc analysis using higher theoretical MIC, two patients did not reach 1x MIC. This could indicate that the studied dosing regimen might not be adequate for more resistant pathogens. The proportion of patients reaching 1x MIC is still superior to those reported in the literature, as discussed above [2, 29].
When considering patient characteristics associated with the achievement of 4x MIC, the occurrence of AKI and the use of renal replacement therapy presented a statistically significant association. However, it should be noted that the three patients who underwent CVVHDF were patients who initially presented with significant AKI and had CVVHDF initiated after receiving doses of BL. This association was not observed with CKD as a dichotomous outcome, which was expected considering we mostly recruited patients with Stage 2 and 3 CKD, whereas the monographs recommend adjusting PT doses based on creatinine clearance less than 40 mL/min. However, the large confidence interval highlights that more patients with CKD would have been necessary to better define this association. These results are consequent to the fact that PT and meropenem are mainly renally excreted [23, 30, 31]. Augmented renal clearance was also identified in the literature as a factor associated with lower serum levels [15], but our data did not show any association, with only three patients with creatinine clearance above 130 mL/min.
The number of patients reaching the toxic threshold was too low to draw any strong conclusion on associated outcomes or patient characteristics. These results are also closely linked to the choice of toxic thresholds. It can be noticed that the suggested threshold for PT represents a concentration around 10 times the MIC, while that of meropenem represents a concentration of 16 times the MIC, leaving more room between effective and toxic concentrations.
We can still note that our results are different from what is seen in the literature regarding the proportion of patients exhibiting toxic manifestations following the attainment of toxic thresholds. Authors have reported the occurrence of neurotoxicity in 10%–15% of ICU patients following BL administration and in 50% of patients who reached toxic thresholds [15, 16]. The observed signs of neurotoxicity reported by different authors included confusion, delirium, and seizures, which occurred between 24 h and 30 days following the initiation of the BL [16, 32, 33]. Even though we only collected serum levels after the first 24 h, we did collect data regarding clinical manifestations of neurotoxicity until 24 h after the discontinuation of the studied BL. In our study, no patients had seizures, and the incidence of delirium was not significantly higher in patients with toxic levels, with an overall incidence of 16.7%, which is lower than the expected frequency in the ICU. However, it is important to note that delirium in the ICU is multifactorial and that our study was not powered to assess direct BL contribution to the incidence of delirium [33].
The main risk factor associated with the neurotoxicity of BL is renal impairment [15, 16]. In our study, we can note that all three patients who reached the toxic threshold had severe acute renal impairment, even if we cannot draw any conclusion regarding neurotoxicity manifestations. Our results are relatively reassuring for patients with Stage 1 and 2 AKI, while also highlighting the need to be cautious in patients with anuric Stage 3 AKI. Indeed, although our data demonstrate that four patients with Stage 3 AKI did not reach the toxic thresholds, we were able to note that CVVHDF was started at some point in the first 24 h in three of them. By excluding patients who received renal replacement therapy, a greater proportion of patients with severe AKI, two out of three, presented toxic levels, possibly highlighting a population in which the regimen used may not be as safe as in the rest of the population.
Other trials assessing the BL levels associated with different dosing regimens have used conversions factors representing unbound fraction ranging from 70% to 100% for PT [22, 23, 28, 34, 35]. For the reasons explained above, we chose a factor of 70%, but still wanted to explore our predefined outcomes with the levels obtained with different unbound fractions. No difference was observed in the number of patients reaching toxic threshold when using an unbound fraction of 81% [28] and only one more patient reached a level above 157 mg/L when using an unbound fraction of 90% [34, 35]. These results are reassuring concerning the conversion factor used for our study.
The important variability observed for serum concentrations also highlights the potential usefulness of TDM in assessing efficacity and innocuity, as suggested by different authors [2, 36, 37]. Our study suggests that patients with altered renal function could potentially benefit from TDM to ensure innocuity, but more studies would be needed to evaluate the impact of dose modifications following subtherapeutic or supratherapeutic results [11].
A strength of this study is that it examines both the clinical impacts and the serum levels associated with prolonged infusion of BL, using a validated method to quantify serum concentrations [38]. No patients were lost to follow-up, and we were able to recruit the desired sample size. Our post hoc analysis improved external validity, which was, however, impaired by the fact that this study was only conducted in one center. The results of this study are also reassuring with regard to the current clinical practice at the CIUSSS de l'Estrie-CHUS, confirming that our standard regimen allowed the achievement of therapeutic targets in most patients with only a minority of patients reaching toxic thresholds. Along with the recently published international guidelines, this study could encourage other centers to adopt the practice of using extended infusion of B-lactams in patients presenting with sepsis or septic shock. The use of doses unadjusted for renal function for the first 24 h of treatment could also be considered for patients at risk of infection with multidrug-resistant organisms to maximize the drug exposure. Despite the relatively low risk of attaining toxic antibiotic levels at 24 h, access to TDM would be ideal in order to quickly identify patients at risk for toxicity.
This study has some limitations. First, although it was adequate for the chosen study design, the sample size did not allow for subgroup analyses, such as separate analyses for meropenem and PT. Moreover, some results, such as characteristics of patients who reached toxic thresholds, were based on too few patients to be reliable, serving only to generate hypotheses on the subject. We also did not include any patients with initial Stage 4 and 5 CKD, which can have an impact on the number of patients reaching toxic thresholds. However, this represents the real-life population that presented at the CHUS with sepsis and is similar to other trials with septic patients [28].
5. Conclusion
Prolonged infusion of a nonadjusted dose of BL for the first 24 h of treatment in patients admitted to the ICU for sepsis or septic shock was associated with attainment of targets of 1x MIC in all patients and of 4x MIC in 75% of patients, with only 8.3% of patients reaching theoretical toxic levels after 24 h, suggesting that this regimen is safe and effective. Uncertainty remains concerning the best way to maintain adequate levels for the rest of the treatment course. No differences in clinical outcomes were observed between the different groups of target attainment. Altered renal function was the most associated factor with the achievement of higher serum levels.
Acknowledgments
We would like to thank Ibrahim El-Haffaf, Marie Gendreau, Élaine Carbonneau, Julie Belisle, and Line Côté for their help.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Theriault E, upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research and publication of this article: This work was supported by the Departement de pharmacie du CHUS, Sherbrooke, Canada, which is included in the Canadian publicly funded health care system.
References
- 1.Evans L., Rhodes A., Alhazzani W., et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Medicine . 2021;47(11):1181–1247. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts J. A., Paul S. K., Akova M., et al. DALI: Defining Antibiotic Levels in Intensive Care Unit Patients: Are Current β-Lactam Antibiotic Doses Sufficient for Critically Ill Patients? Clinical Infectious Diseases . 2014;58(8):1072–1083. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 3.Richter D. C., Dietrich M., Lalev L. D., et al. Prolonged Infusion of Beta-Lactams Decreases Mortality in Patients With Septic Shock: A Retrospective Before-And-After Study. Antibiotics . 2021;10(6) doi: 10.3390/antibiotics10060687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landmesser K. B., Clark J. A., Burgess D. S. Time Above All Else: Pharmacodynamic Analysis of Beta-Lactams in Critically Ill Patients. The Journal of Clinical Pharmacology . 2022;62(4):479–485. doi: 10.1002/jcph.1977. [DOI] [PubMed] [Google Scholar]
- 5.Wu C. C., Su Y. C., Wu K. S., Wu T. H., Yang C. S. Loading Dose and Efficacy of Continuous or Extended Infusion of Beta-Lactams Compared With Intermittent Administration in Patients With Critical Illnesses: A Subgroup Meta-Analysis and Meta-Regression Analysis. Journal of Clinical Pharmacy and Therapeutics . 2021;46(2):424–432. doi: 10.1111/jcpt.13301. [DOI] [PubMed] [Google Scholar]
- 6.Martínez-Casanova J., Esteve-Pitarch E., Colom-Codina H., et al. Predictive Factors of Piperacillin Exposure and the Impact on Target Attainment After Continuous Infusion Administration to Critically Ill Patients. Antibiotics (Basel) . 2023;12(3) doi: 10.3390/antibiotics12030531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passon S. G., Schmidt A. R., Wittmann M., Velten M., Baehner T. Evaluation of Continuous Ampicillin/Sulbactam Infusion in Critically Ill Patients. Life Sciences . 2023;320:p. 121567. doi: 10.1016/j.lfs.2023.121567. [DOI] [PubMed] [Google Scholar]
- 8.Hoste E. A., Damen J., Vanholder R. C., et al. Assessment of Renal Function in Recently Admitted Critically Ill Patients With Normal Serum Creatinine. Nephrology Dialysis Transplantation . 2005;20(4):747–753. doi: 10.1093/ndt/gfh707. [DOI] [PubMed] [Google Scholar]
- 9.Pereira J. G., Fernandes J., Duarte A. R., Fernandes S. M. β-Lactam Dosing in Critical Patients: A Narrative Review of Optimal Efficacy and the Prevention of Resistance and Toxicity. Antibiotics (Basel) . 2022;11(12) doi: 10.3390/antibiotics11121839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes S., Heard K. L., Mughal N., Moore L. S. P. Optimization of Antimicrobial Dosing in Patients With Acute Kidney Injury: A Single-Centre Observational Study. JAC-Antimicrobial Resistance . 2022;4(4):p. dlac080. doi: 10.1093/jacamr/dlac080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoenenberger-Arnaiz J. A., Ahmad-Diaz F., Miralbes-Torner M., Aragones-Eroles A., Cano-Marron M., Palomar-Martinez M. Usefulness of Therapeutic Drug Monitoring of Piperacillin and Meropenem in Routine Clinical Practice: A Prospective Cohort Study in Critically Ill Patients. The European Journal of Hospital Pharmacy: Science and Practice . 2020;27(e1):e30–e35. doi: 10.1136/ejhpharm-2018-001713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdul-Aziz M. H., Alffenaar J. C., Bassetti M., et al. Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: A Position Paper. Intensive Care Medicine . 2020;46(6):1127–1153. doi: 10.1007/s00134-020-06050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guilhaumou R., Benaboud S., Bennis Y., et al. Optimization of the Treatment With Beta-Lactam Antibiotics in Critically Ill Patients-Guidelines From the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation-SFAR) Critical Care . 2019;23(1):p. 104. doi: 10.1186/s13054-019-2378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sumi C. D., Heffernan A. J., Lipman J., Roberts J. A., Sime F. B. What Antibiotic Exposures are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review. Clinical Pharmacokinetics . 2019;58(11):1407–1443. doi: 10.1007/s40262-019-00791-z. [DOI] [PubMed] [Google Scholar]
- 15.Legg A., Carmichael S., Chai M. G., Roberts J. A., Cotta M. O. Beta-Lactam Dose Optimisation in the Intensive Care Unit: Targets, Therapeutic Drug Monitoring and Toxicity. Antibiotics (Basel) . 2023;12(5) doi: 10.3390/antibiotics12050870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roger C., Louart B. Beta-Lactams Toxicity in the Intensive Care Unit: An Underestimated Collateral Damage? Microorganisms . 2021;9(7) doi: 10.3390/microorganisms9071505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhaese S. A. M., Hoste E. A., De Waele J. J. Why We May Need Higher Doses of Beta-Lactam Antibiotics: Introducing the ‘Maximum Tolerable Dose’. Antibiotics (Basel) . 2022;11(7) doi: 10.3390/antibiotics11070889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilanus A., Drusano G. Optimizing the Use of Beta-Lactam Antibiotics in Clinical Practice: A Test of Time. Open Forum Infectious Diseases . 2023;10(7):p. ofad305. doi: 10.1093/ofid/ofad305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CLSI. Peformance Standards for Antimicrobial Susceptibility Testing . 32nd. Wayne, PA: CLSI Supplement M100, Clinical and Laboratory Standards Institute; 2022. [Google Scholar]
- 20.Gilbert D. N., Moellering R. C., Eliopoulos G. M., Chambers H. F., Saag M. S., editors. The Sanford Guide to Antimicrobial Therapy . Sperryville, VA: Antimicrobial Therapy, Inc; 2010. [Google Scholar]
- 21.Quinton M. C., Bodeau S., Kontar L., et al. Neurotoxic Concentration of Piperacillin During Continuous Infusion in Critically Ill Patients. Antimicrobial Agents and Chemotherapy . 2017;61(9) doi: 10.1128/AAC.00654-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong G., Briscoe S., Adnan S., et al. Protein Binding of β-Lactam Antibiotics in Critically Ill Patients: Can We Successfully Predict Unbound Concentrations? Antimicrobial Agents and Chemotherapy . 2013;57(12):6165–6170. doi: 10.1128/AAC.00951-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfizer. Zosyn (Piperacillin and Tazobactam) for Injection . New York: Pfizer; 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/050684s88s89s90_050750s37s38s39lbl.pdf . [Google Scholar]
- 24.Craig W. A. The Pharmacology of Meropenem, A New Carbapenem Antibiotic. Clinical Infectious Diseases . 1997;24(Suppl 2):S266–S275. doi: 10.1093/clinids/24.supplement_2.s266. [DOI] [PubMed] [Google Scholar]
- 25.De Waele J. J., Carrette S., Carlier M., et al. Therapeutic Drug Monitoring-Based Dose Optimisation of Piperacillin and Meropenem: A Randomised Controlled Trial. Intensive Care Medicine . 2014;40(3):380–387. doi: 10.1007/s00134-013-3187-2. [DOI] [PubMed] [Google Scholar]
- 26.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements . 2013;3:1–150. doi: 10.1016/j.kisu.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khwaja A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clinical Practice . 2012;120(4):c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 28.Hagel S., Bach F., Brenner T., et al. Effect of Therapeutic Drug Monitoring-Based Dose Optimization of Piperacillin/Tazobactam on Sepsis-Related Organ Dysfunction in Patients With Sepsis: A Randomized Controlled Trial. Intensive Care Medicine . 2022;48(3):311–321. doi: 10.1007/s00134-021-06609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fournier A., Eggimann P., Pagani J. L., et al. Impact of the Introduction of Real-Time Therapeutic Drug Monitoring on Empirical Doses of Carbapenems in Critically Ill Burn Patients. Burns . 2015;41(5):956–968. doi: 10.1016/j.burns.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Yu Z. Renal Replacement Therapy Does Have Impact on Beta-Lactam Clearance. Annals of Intensive Care . 2023;13(1):p. 48. doi: 10.1186/s13613-023-01148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.AstraZeneca. Product Monograph Merrem (Meropenem for Injection) AstraZeneca. 2013. https://pdf.hres.ca/dpd_pm/00021315.PDF .
- 32.Zerbib Y., Gaulin C., Bodeau S., et al. Neurological Burden and Outcomes of Excessive β-Lactam Serum Concentrations of Critically Ill Septic Patients: A Prospective Cohort Study. Journal of Antimicrobial Chemotherapy . 2023;78(11):2691–2695. doi: 10.1093/jac/dkad284. [DOI] [PubMed] [Google Scholar]
- 33.Mattappalil A., Mergenhagen K. A. Neurotoxicity With Antimicrobials in the Elderly: A Review. Clinical Therapeutics . 2014;36(11):1489–1511.e4. doi: 10.1016/j.clinthera.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Schießer S., Hitzenbichler F., Kees M. G., et al. Measurement of Free Plasma Concentrations of Beta-Lactam Antibiotics: An Applicability Study in Intensive Care Unit Patients. Therapeutic Drug Monitoring . 2021;43(2):264–270. doi: 10.1097/FTD.0000000000000827. [DOI] [PubMed] [Google Scholar]
- 35.Colman S., Stove V., De Waele J. J., Verstraete A. G. Measuring Unbound Versus Total Piperacillin Concentrations in Plasma of Critically Ill Patients: Methodological Issues and Relevance. Therapeutic Drug Monitoring . 2019;41(3):325–330. doi: 10.1097/FTD.0000000000000602. [DOI] [PubMed] [Google Scholar]
- 36.Dilworth T. J., Schulz L. T., Micek S. T., Kollef M. H., Rose W. E. β-Lactam Therapeutic Drug Monitoring in Critically Ill Patients: Weighing the Challenges and Opportunities to Assess Clinical Value. Critical Care Explorations . 2022;4(7):p. e0726. doi: 10.1097/CCE.0000000000000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ausman S. E., Moreland-Head L. N., Abu Saleh O. M., et al. BLOOM Study Group. “How to” Guide for Pharmacist-Led Implementation of Beta-Lactam Therapeutic Drug Monitoring in the Critically Ill. Journal of the American College of Clinical Pharmacy . 2023;6(8):964–975. doi: 10.1002/jac5.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Legrand T., Vodovar D., Tournier N., Khoudour N., Hulin A. Simultaneous Determination of Eight β-Lactam Antibiotics, Amoxicillin, Cefazolin, Cefepime, Cefotaxime, Ceftazidime, Cloxacillin, Oxacillin, and Piperacillin, in Human Plasma by Using Ultra-High-Performance Liquid Chromatography With Ultraviolet Detection. Antimicrobial Agents and Chemotherapy . 2016;60(8):4734–4742. doi: 10.1128/AAC.00176-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Theriault E, upon reasonable request.


