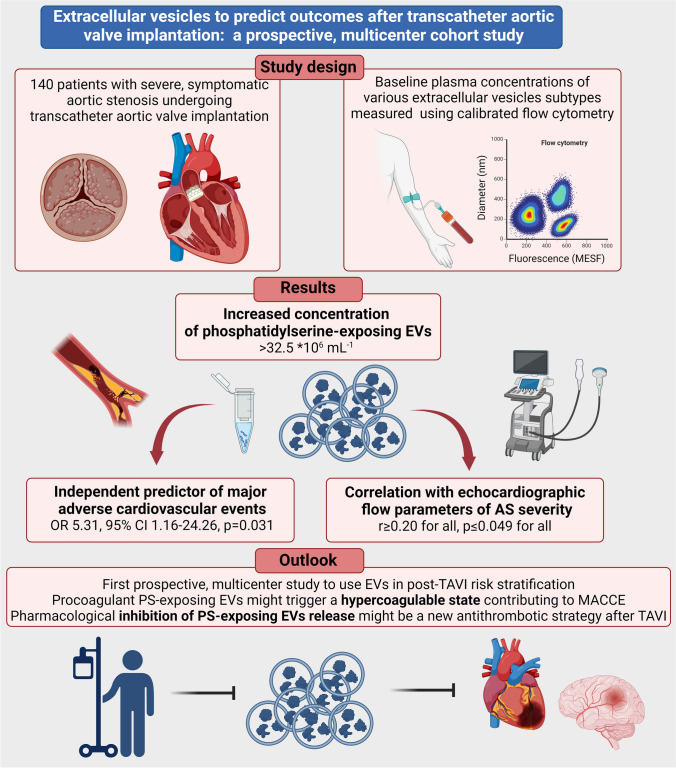
Abstract
Introduction
Transcatheter aortic valve implantation (TAVI) is an established treatment for aortic stenosis (AS) in patients at intermediate and high surgical risk. Circulating extracellular vesicles (EVs) are nanoparticles involved in cardiovascular diseases. We aimed to (i) determine the effect of TAVI on plasma concentrations of five EV subtypes and (ii) evaluate the predictive value of EVs for post-TAVI outcomes.
Methods
Blood samples were collected 1 day before TAVI and at hospital discharge. Concentrations of EVs were evaluated using flow cytometry.
Results
Concentration of leukocytes EVs decreased after TAVI, compared to the measurement before (p = 0.008). Among 123 patients discharged from the hospital, 19.5% experienced MACCE during the median of 10.3 months. Increased pre-TAVI concentration of phosphatidylserine-exposing EVs was an independent predictor of MACCE in multivariable analysis (OR 5.313, 95% CI 1.164–24.258, p = 0.031).
Conclusions
Patients with increased pre-TAVI concentration of procoagulant, PS-exposing EVs have over fivefold higher odds of adverse outcomes.
Graphical Abstract
Keywords: Aortic stenosis, Extracellular vesicles (EVs), Prognosis, Transcatheter aortic valve implantation (TAVI)
Introduction
Aortic stenosis (AS) is the most common valve disease requiring interventional treatment, with the prevalence increasing with age. AS affects less than 1% of patients in their fifties and almost 10% of octogenarians (1, 2). Progressive degeneration of the aortic valve is a result of fibrous remodeling triggered by complex factors including lipoprotein deposition or chronic inflammation [3]. Clinically, severe AS obstructs blood outflow from the left ventricle, with 50% mortality within 2-years from the moment when the first symptoms occur [4]. The recommended interventions in patients with AS treatment include surgical aortic valve replacement (SAVR) or transcatheter aortic valve implantation (TAVI).
Introduction of TAVI led to a paradigm shift towards minimally invasive procedure and has revolutionized clinical outcomes in AS, especially in inoperable patients [5]. Despite recent advancements in TAVI techniques and careful selection of patients undergoing this procedure, over 15% of patients experience major adverse cardiac and cerebrovascular events (MACCE) in the first year after the intervention [6, 7]. Many factors associated with post-TAVI MACCE have been identified so far, including diabetes or pre-operative anemia, but have not been applied in clinical practice as reliable risk predictors [8, 9]. The most common risk scale for TAVI patients, which is the Society of Thoracic Surgeons (STS) scale underestimates the risk in long-term perspective, especially among high-risk patients [10]. Importantly, STS does not include significant clinical factors that may affect long-term outcomes after TAVI, such as oncologic disease or frailty status. Introduction of novel prediction models to accurately assess risk of post-TAVI MACCE is therefore crucial for the adequate management of post-TAVI.
Extracellular vesicles (EVs) are nanoparticles released from blood cells and vascular endothelium, which are involved in the development of cardiovascular events, including myocardial infarction, stroke, or heart failure decompensation [11]. EVs are both passive and active players in cardiovascular disease, reflecting the activation of cells of their origin (e.g. platelets or leukocytes) and interacting with other cells, respectively. Recent proteomics study highlighted tissue EVs as important drivers of aortic valve calcification – an important mechanism contributing to AS progression [12]. Circulating EVs increase the adhesion of platelets and deposition of fibrin on human atherosclerotic plaques that increases the risk of thrombus development [13]. Procoagulant properties of EVs are mediated by proteins exposed on their surface, such as PS, tissue factor (TF) or P-selectin, which either directly or indirectly activate the coagulation cascade [14]. Since EVs are established markers of cell activation and involved in vascular homeostasis, concentrations of different subtypes of EVs may serve as a predictor of upcoming cardiovascular events. The objectives of this study were (i) to determine the effect of TAVI on the concentrations of different EVs subtypes and (ii) to evaluate the predictive value of these EVs for MACCE within 1 year after TAVI.
Methods
Study Design
This was a prospective study conducted at 3 academic centers in Poland between November 2018 and June 2020, in collaboration with Amsterdam Vesicle Center, Amsterdam University Medical Centers (UMC), the Netherlands. The study protocol was approved by the Ethics Committee of Medical University of Warsaw (approval number: KB/128/2018, KB/4/A2021).
Study population included patients diagnosed with severe AS and qualified for TAVI based on the Heart Team decision, who provided written informed consent to participate in the study. Severe AS was defined according to the recent Guidelines for the management of valvular heart disease as aortic valve area (AVA) < 1.0 cm2 or indexed AVA < 0.6 cm2/m2 as calculated by the continuity equation on transthoracic echocardiography (TTE) [15]. Exclusion criteria were transcatheter valve-in-valve implantation, chronic kidney disease (glomerular filtration rate < 30 mL/min), autoimmune diseases, active neoplastic disease, pregnancy and breast-feeding.
Transcatheter aortic valve implantation was performed by an interventional cardiologist (B.R., J.K., Z.H.) and a cardiac surgeon (R.W.) in a hybrid operating room. Pharmacotherapy after TAVI included single antiplatelet therapy (acetylsalicylic acid or clopidogrel) in patients with no indication for oral anticoagulation (OAC), or OAC if required [16, 17]. Other drugs were continued at the discretion of the treating physician.
Clinical data were collected during the index hospitalization and follow-up visit at 12 ± 3 months after TAVI, when control TTE was performed and data regarding MACCE (all-cause death, cardiovascular death, myocardial infarction, stroke, transient ischemic attack (TIA), decompensation of heart failure or clinical valve thrombosis) were recorded.
The primary endpoint was the predictive value of different EVs subtypes for the occurrence of MACCE during the follow-up period. The secondary endpoint was the difference in plasma expression of EVs before and after TAVI.
Sample Collection and Handling
Sample collection and handling were done in 3 Polish academic centers by trained professionals (K.P., M.C., O.D., S.J.) according to the to the recent guidelines to study EVs [18]. EV measurements in all samples were done in one block in Vesicle Observation Centre, Amsterdam UMC, following the shipment of all samples on dry ice.
Blood was collected from fasting patients twice into 7.5 mL 0.109 mol/L ethylenediaminetetraacetic acid (EDTA) plastic tubes (S-Monovette, Sarstedt) via antecubital vein puncture: 1 day before TAVI and 5–7 days after TAVI (at hospital discharge). Following preparation of platelet-depleted plasma using double centrifugation (2500 g, 15 min, 20 °C, acceleration speed 1, no brake), samples stored at − 80 °C until analyzed, according to the current guidelines to store biological samples before EV measurements [18]. Prior to analysis, samples were thawed for 1 min in a water bath (37 °C) to avoid cryoprecipitation.
Flow cytometry (A60-Micro, Apogee Flow Systems) was used to determine the concentration of following EV subtypes in platelet-depleted plasma EVs derived from all platelets (CD61 +), activated platelets (CD61 + /CD62p +), leucocytes (CD45 +), erythrocytes (CD235a +) and exposing phosphatidylserine (PS +). To improve the reproducibility of our EV flow cytometry experiments, we (i) applied the framework for standardized reporting of EV flow cytometry experiments (MIFlowCyt-EV) [22] (ii) calibrated all detectors, (iii) determined the EV diameter and refractive index by the flow cytometry scatter ratio (Flow-SR) [20], 20, and (iv) applied custom-built software to fully automate data calibration and processing. All relevant details regarding sample collection and handling, assay controls, instrument calibration, data acquisition, and EV characterization are included in the Supplementary File.
Statistical Analysis
As there is no data regarding the differences in EV concentrations in patients with and without MACCE, the power calculation for the primary end-point was based on the differences in EV concentrations in patients with calcified AS and healthy controls [23]. Patients with calcified AS had on average twofold higher EV concentrations compared to controls. The required sample size was calculated by a two-sided t-test at a significance level of 0.05 with the following assumptions: (i) mean difference between the groups with and without MACCE = 1.0, (ii) standard deviation (SD) ± 1.0, and (iii) nominal test power = 0.8. Hence, at least 17 patients with MACCE should be enrolled in the study to observe a difference in EV concentrations in patients with or without MACCE. Given that 15% rate of MACCE within a year after TAVI, at least 122 patients should be enrolled.
Statistical analyses were conducted using IBM SPSS Statistics, version 27.0 (IBM, New York, USA). Categorical variables were presented as number and percent and compared using χ2 test. The Shapiro–Wilk test was used to assess normal distribution of continuous variables. Continuous variables were presented as mean with standard deviation (SD) or median with interquartile range (IQR). Changes of EVs concentration before and after TAVI procedure were calculated with Wilcoxon signed-rank test or paired t-test depending on data distribution. To assess the difference in variables between patients with and without MACCE, unpaired t-test or U-Mann Whitney test were used to compare data with and without normal distribution, respectively. Chi-square test was used to compare categorical variables. The predictive value of EVs for MACCE and the cut-offs were calculated using a receiver operating characteristic (ROC) curve. Logistic regression model incorporating EVs concentration and clinical characteristics were used to determine the clinically and statistically optimal model for MACCE. A two-sided p-value below 0.05 was considered significant.
Results
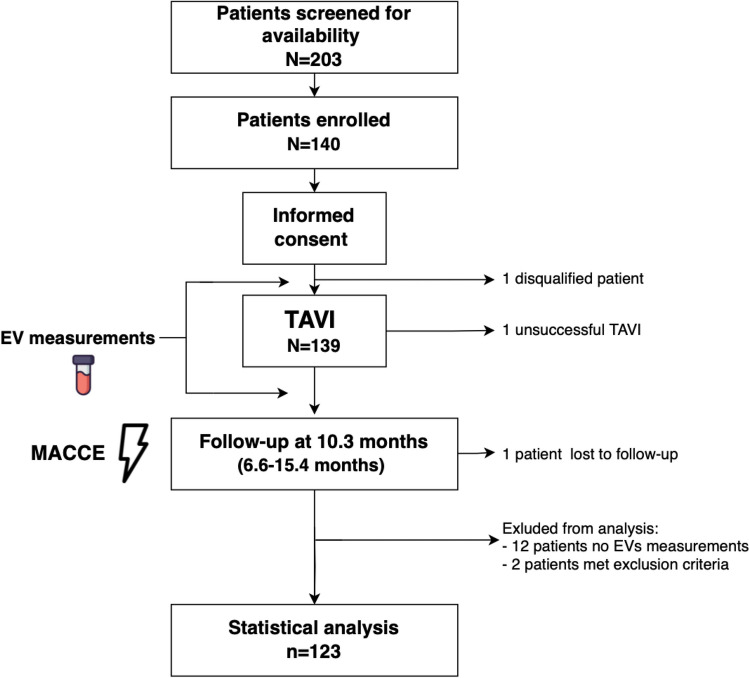
Figure 1 shows the study design and flow chart. Patient characteristics are presented in Table 1. Among 123 patients included in the analysis, 24 (19.5%) patients experienced MACCE during the median follow-up time of 10.3 months (6.6–15.4 months): 5 (20.8%) all-cause deaths, 5 (20.8%) cardiovascular deaths, 1 (4.2%) stroke, 1 (4.2%) TIA, 11 (45.8%) readmissions due to decompensated heart failure and 1 (4.2%) clinical valve thrombosis. Patients who experienced MACCE were older (median age 83.0 vs. 79.0 years, p = 0.006) and more often suffered from COPD (25% vs 8%, p = 0.03). There were no other differences between the groups. The incidence of procedural complications was similar in patients with and without MACCE. At follow-up, the mean LVEF and mean aortic valve gradient were comparable in both groups (60% vs. 55%, p = 0.91 and 8.0 mmHg vs. 9.0 mmHg, p = 0.33, respectively).
Fig. 1.
Study design and flow chart; MACCE — major adverse cardiac and cerebrovascular events; TAVI — transcatheter aortic valve implantation
Table 1.
Comparison of baseline characteristics between patients who experienced MACCE and those who did not during a median follow-up of 10.3 months. Number of patients: 123. Statistical tests used: unpaired t-test or U-Mann Whitney test to compare data with and without normal distribution, respectively; Chi-square test to compare categorical variables
| Total population (N = 123) |
No MACCE (N = 99) |
MACCE (N = 24) |
p-value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age [years] | 80 (74.00–83.00) | 79 (74.00–82.00) | 83 (79.25–84.00) | 0.006 |
| Gender. male | 56 (45.5%) | 44 (44.40%) | 12 (50.0%) | 0.624 |
| BMI [kg/m2] | 26.35 (24.50–29.83) | 26.61 (24.50–29.97) | 25.83 (23.11–29.67) | 0.359 |
| Co-morbidities | ||||
| Hypertension | 102 (82.90%) | 81(79.41%) | 21(20.59%) | 0.763 |
| Diabetes mellitus | 46 (37.70%) | 40(40.82%) | 6 (25.00%) | 0.152 |
| Atrial fibrillation | 42(34.10%) | 34 (34.34%) | 8 (33.33%) | 0.925 |
| Prior stroke/TIA | 12 (9.80%) | 10 (10.10%) | 2 (8.33%) | 1.000 |
| Prior myocardial infarction | 33 (26.80%) | 27 (27.27%) | 6 (25.00%) | 0.822 |
| Prior PCI | 54 (43.90%) | 43 (43.43%) | 11(45.83%) | 0.832 |
| Prior CABG | 10 (8.10%) | 9 (9.09%) | 1 (4.17%) | 0.685 |
| COPD | 14 (11.40%) | 8 (8.08%) | 6 (25.00%) | 0.030 |
| Heart failure (NYHA III/IV) | 56 (45.52%) | 48 (48.48%) | 8 (33.33%) | 0.181 |
| EuroSCORE II [%] | 3.70 (2.45–5.07) | 3.71 (2.45–4.92) | 3.31 (2.51–5.09) | 0.785 |
| CKD > 3a | 25 (20.30%) | 19 (19.19%) | 6 (25.00%) | 0.574 |
| Laboratory data | ||||
| Hemoglobin [g/d] | 11.900 (10.10–13.30) | 11.90(9.30–13.30) | 11.95 (11.05–13.33) | 0.446 |
| Leukocytes (thousand/dl) | 6.96 (5.85–8.56) | 6.96 (5.84–8.56) | 6.97 (6.47–8.55) | 0.592 |
| Platelets count (per microliter) | 177.00 (153.00–220.00) | 179.00 (153.00–223.00) | 168.00 (152.25–214.25) | 0.431 |
| Creatinine [mg/dL] | 1.28 (0.98–1.70) | 1.28 (1.00–1.95) | 1.26 (0.94–1.44) | 0.139 |
| Estimated GFR [mL/min/1.73 m2] | 55.00 (43.00–70.00) | 56.00 (42.00–70.00) | 50.00 (43.00–61.75) | 0.350 |
| NT-proBNP (pg/ml) | 1863.50 (571.75–3715.25) | 2107.50 (564.25–3982.50) | 1729.00 (635.50–3307.25) | 0.787 |
| CRP | 1.20 (0.22–4.65) | 1.20 (0.22–4.25) | 1.23 (0.24–14.28) | 0.557 |
| Echocardiography before TAVI | ||||
| Ejection fraction [%] | 56.00(46.00–61.50) | 55.00 (46.50–60.00) | 60.50 (45.25–65.00) | 0.101 |
| V max [m/s] | 4.2000 (3.80–4.50) | 4.20 (3.80–4.50) | 4.20 (3.73–4.55) | 0.776 |
| Gradient max [mmHg] | 71.00 (61.90–82.00) | 71.00 (64.00–82.50) | 72.00 (45.75–81.75) | 0.369 |
| Gradient mean [mmHg] | 42.22 (33.25–51.00) | 43.00 (34.70–52.50) | 41.00 (27.00–45.00) | 0.077 |
| AVA (VTI) [cm2] | 0.78 (0.64–0.87) | 0.78 (0.62–0.86) | 0.80 (0.64–0.90) | 0.836 |
| AVAi [cm2/m2] | 0.42 (0.35–0.48) | 0.42 (0.35–0.48) | 0.48 (0.35–0.52) | 0.238 |
| Low-flow. low-gradient AS | 28 (22.76%) | 19 (20.65%) | 9 (39.13%) | 0.065 |
| Procedural characteristics | ||||
| Access site: | 0.102 | |||
| Femoral | 119 (96.75%) | 97 (97.98%) | 22 (91.67%) | |
| Subclavian | 1 (0.81%) | 1 (1.01%) | 0 (0.00%) | |
| Carotid | 3 (2.44%) | 1 (1.01%) | 2 (8.33%) | |
| Prosthesis size [mm]: | 0.402 | |||
| 20 | 1 (0.81%) | 1 (1.01%) | 0 (0.00%) | |
| 22 | 2 (1.62%) | 2 (2.02%) | 0 (0.00%) | |
| 23 | 7 (5.7%) | 3 (2.03%) | 4 (16.67%) | |
| 24 | 4 (3.25%) | 4 (4.04%) | 0 (0.00%) | |
| 25 | 27 (21.95%) | 21 (21.21%) | 6 (25.00%) | |
| 26 | 9 (7.32%) | 8 (8.08%) | 1 (4.17%) | |
| 27 | 16 (13.00%) | 11 (11.11%) | 5 (20.83%) | |
| 29 | 28 (22.76%) | 24 (24.24%) | 4 (16.67%) | |
| 34 | 26 (21.14%) | 22 (22.22%) | 4 (16.67%) | |
| Missing data | 3 (2.44%) | 3 (3.03%) | 0 (0.00%) | |
| Valve type: | 0.685 | |||
| EvolutR | 40 (32.52%) | 35 (35.35%) | 5 (20.83%) | |
| EvolutPRO | 12 (9.76%) | 9 (9.09%) | 3 (12.50%) | |
| Portico | 35 (28.46%) | 24 (24.24%) | 11 (45.83%) | |
| Accurate_Neo | 10 (8.13%) | 8 (8.08%) | 2 (8.33%) | |
| Accurate_Neo2 | 24 (19.51%) | 21 (21.21%) | 3 (12.50%) | |
| Hydra | 1 (0.81%) | 1 (1.01%) | 0 (0.00%) | |
| Navitor | 1 (0.81%) | 1 (1.01%) | 0 (0.00%) | |
| Procedure complications | ||||
| Life-threatening or disabling bleeding* | 11 (8.94%) | 8 (8.08%) | 3 (12.50%) | 0.447 |
| Surgical intervention at access site | 8 (6.5%) | 6 (6.06%) | 2 (8.33%) | 0.653 |
| Echocardiography at follow-up | ||||
| Ejection fraction [%] | 60.00(49.50–60.00) | 60.00 (50.00–60.00) | 55.00 (42.50–64.00) | 0.913 |
| V max [m/s] | 2.01 (+ -0.44) | 2.00 (+ -0.45) | 2.05 (+ -0.40) | 0.685 |
| Peak AV gradient [mmHg] | 17.43 (+ -6.98) | 17.30 (+ -7.12) | 18.21 (+ -6.26) | 0.645 |
| Mean AV gradient [mmHg] | 8.00 (6.00–10.00) | 8.00 (6.00–10.25) | 9.00 (7.00–10.50) | 0.334 |
| AVA (VTI) [cm2] | 1.92 (+ -0.41) | 1.93 (+ -0.42) | 1.881875 (+ -0.37) | 0.690 |
| AVAi [cm2/m2] | 1.02 (0.94–1.18) | 1.01 (0.93–1.18) | 1.12 (0.93–1.20) | 0.355 |
| Paravalvular leak ≥ moderate | 9 (7.32%) | 8 (8.08%) | 1 (4.17%) | 0.509 |
| Post-TAVI procedure concomitant medications | ||||
| Beta-blockers | 97 (78.86%) | 81 (84.38%) | 16(76.19%) | 0.353 |
| ACE inhibitors | 74 (60.16%) | 59 (60.20%) | 15(71.43%) | 0.336 |
| MRA | 32 (26.01%) | 29 (30.21%) | 3(15.00%) | 0.166 |
| Diuretics | 101 (82.11%) | 81 (82.65%) | 20 (95.24%) | 0.192 |
| Statins | 102 (82.93%) | 83 (85.57%) | 19 (90.48%) | 0.734 |
| Proton pump inhibitors | 92 (74.80%) | 74 (76.29%) | 18 (85.71%) | 0.561 |
| Acetylsalicylic acid | 81 (65.85%) | 70 (71.43%) | 11(52.38%) | 0.089 |
| P2Y12 inhibitor | 88 (71.54%) | 74 (76.29%) | 14 (66.66%) | 0.359 |
| Anticoagulant | 52 (42.27%) | 42 (42.42%) | 10 (41.67%) | 0.946 |
*According to the Valve Academic Research Consortium (VARC). Bold p value indicates significantly different (< 0.05). Data are shown as
number (percentage). median (interquartile range). mean ± standard deviation; ACE — angiotensin-converting enzyme;
AVA — aortic valve area; AVAi — aortic valve area index; CABG — coronary artery bypass graft surgery; COPD —
chronic obstructive pulmonary disease; CKD — chronic kidney disease; GFR — glomerular filtration rate; CRP- C-reactive protein. MACCE — major adverse cardiac and cerebrovascular events; MRA — mineralocorticoid receptor antagonists; NT-proBNP — N-terminal pro B natriuretic peptide; NYHA — New York Heart Association; PCI — percutaneous coronary intervention; TIA — transient ischemic attack
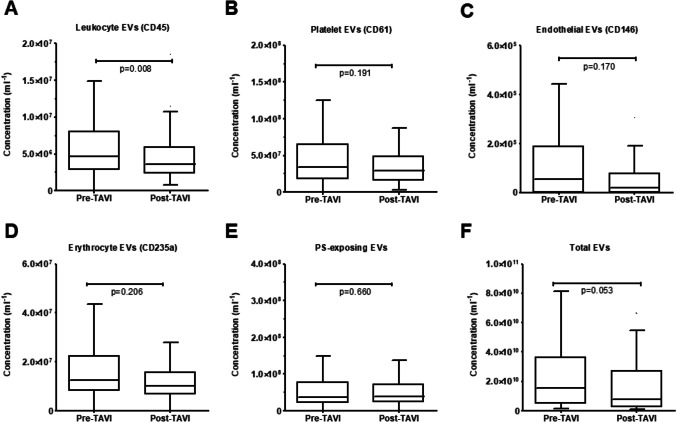
Concentration of EVs from leukocytes (CD45 +) were lower after TAVI compared to bassline (p = 0.008; Fig. 2). There was a trend towards lower concentrations of total EVs after TAVI (p = 0.053; Fig. 2). We found no significant differences in the concentrations of other EV subtypes (p ≥ 0.170 for all) (Fig. 2, Supplementary Table 1). When analyzing the changes in percentage of different EVs subtypes compared to total EV concentrations before and after TAVI, we found no significant differences (Supplementary Fig. 1).
Fig. 2.
Comparison of plasma extracellular vesicles (EVs) concentrations before and after transcatheter aortic valve implantation (TAVI). Concentration of EVs from leucocytes were lower after TAVI, compared to the measurement before (panel A). There was a trend towards lower concentrations of total EVs after TAVI (panel F). There were no significant differences in the concentrations of other EV subtypes. Number of patients: 123. Statistical tests used: paired t-test or Wilcoxon signed-rank test to compare data with and without normal distribution, respectively
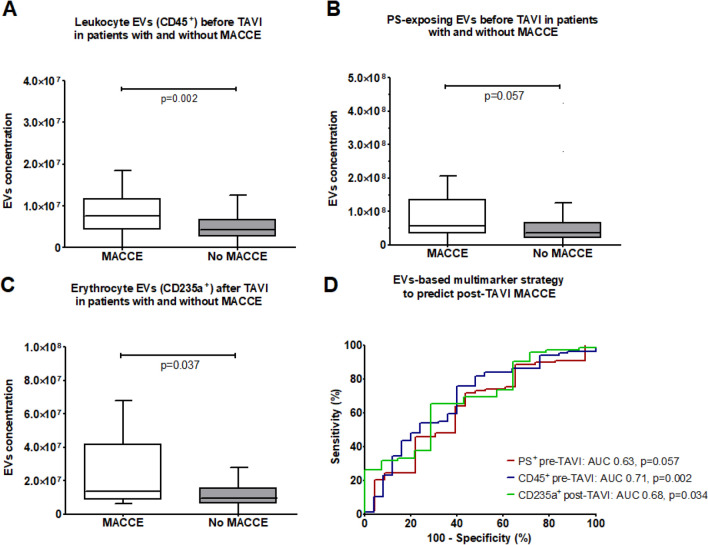
Baseline concentrations of leukocyte EVs (CD45 +) was higher in patients who experienced MACCE, compared to those who did not (p = 0.002; Fig. 3A) and discriminated between these two groups of patients (area under ROC curve (AUC) = 0.707, p = 0.002; Fig. 3B). There was a trend toward higher concentrations of pre-TAVI PS-exposing EVs (PS +), and post-TAVI EVs from erythrocytes (CD235a +) in patients who experienced MACCE (p = 0.057, p = 0.034, respectively; Fig. 3C-F). Concentrations of other analyzed subtypes of EVs did not differ among patients with and without MACCE (Supplementary Table 2).
Fig. 3.
Pre-TAVI concentrations of EVs from leukocytes (A) and EVs exposing PS (B), and post-TAVI concentrations of EVs from erythrocytes (C) in patients who did and did not experience MACCE during follow-up period with ROC curves for prediction of MACCE (D). Number of patients: 123. Statistical tests used: U-Mann Whitney test, receiver operating characteristic (ROC) analysis
Table 2 shows the statistical estimates for the prediction of MACCE by pre-TAVI concentrations of EVs from leukocytes (CD45 +) and EVs exposing PS and post-TAVI concentrations of EVs from erythrocytes (CD235a +), determined based on the ROC curve. In univariable analysis, MACCE were predicted by pre-TAVI concentrations of leukocyte EVs (62.5% sensitivity, 76.1% specificity), pre-TAVI concentrations of PS + EVs (78.3% sensitivity, 46.1% specificity) and post-TAVI concentrations of erythrocyte EVs (71.4% sensitivity and 65.2% specificity). To check whether these three EVs were independent predictors of MACCE, they were incorporated in multivariable Cox regression analyses (Table 3, Supplementary Table 3). Patients with increased pre-TAVI concentration of PS-exposing EVs had over fivefold higher odds of MACCE after TAVI, independent of other clinical variables (odds ratio [OR] 5.313, 95% confidence interval [CI] 1.164–24.258, p = 0.031). High baseline pre-TAVI concentrations of leukocyte EVs or post-TAVI concentrations of erythrocytes EVs did not predict MACCE in multivariable analyses (p = 0.532 and p = 0.391, respectively).
Table 2.
Statistical estimates for prediction of MACCE by subtypes of EVs which significantly differed among patients with and without MACCE. Number of patients: 123. Statistical test used: receiver operating characteristic (ROC) analysis
| EVs | AUC (95% CI) | p-value | Cut-off (particles * × 106 per mL plasma) | Sensitivity | Specificity | PPV | NPV | PLR |
|---|---|---|---|---|---|---|---|---|
| Pre-TAVI CD45 + | 0.71 (0.59–0.82) | 0.002 | 6.73 | 62.5% | 76.1% | 41.7% | 88.2% | 2.62 |
| Pre-TAVI PS + | 0.63 (0.50–0.76) | 0.057 | 32.5 | 78.3% | 46.1% | 27.3% | 89.1% | 1.45 |
| Post-TAVI CD235a + | 0.68 (0.53- 0.83) | 0.034 | 12.7 | 71.4% | 65.3% | 28.6% | 92.2% | 2.06 |
AUC — area under the curve; CI — confidence interval; EVs – extracellular vesicles; MACCE – major adverse cardiac and cerebrovascular events; PPV — positive predictive value; NPV — negative
predictive value; PLR — positive likelihood ratio
Table 3.
Multivariable Cox regression analysis for prediction of MACCE by subtypes of EVs which significantly differed among patients with and without MACCE, after adjustment for sex, age, chronic obstructive pulmonary disease, mean gradient, low-flow and low-gradient aortic stenosis and acetylsalicylic acid use. Number of patients: 123
| EVs | Cox regression | OR | 95% CI | P | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
|
High pre-TAVI CD45 + EVs concentration (cut-off > 6.73*106 mL−1) |
Univariable | 2.545 | 1.080 | 5.995 | 0.033 |
| Multivariable* | 1.409 | 0.480 | 4.134 | 0.532 | |
|
High pre-TAVI PS + EVs concentration (cut-off > 32.54*106 mL−1) |
Univariable | 2.855 | 0.964 | 8.456 | 0.058 |
| Multivariable* | 5.313 | 1.164 | 24.258 | 0.031 | |
|
High post-TAVI CD235a + EVs concentration (cut-off > 12.75 *106 mL−1) |
Univariable | 2.914 | 0.877 | 9.682 | 0.081 |
| Multivariable* | 1.783 | 0.476 | 6.671 | 0.391 | |
EVs – extracellular vesicles; MACCE- major adverse cardiac and cerebrovascular events
Bold p value indicates significantly different (< 0.05)
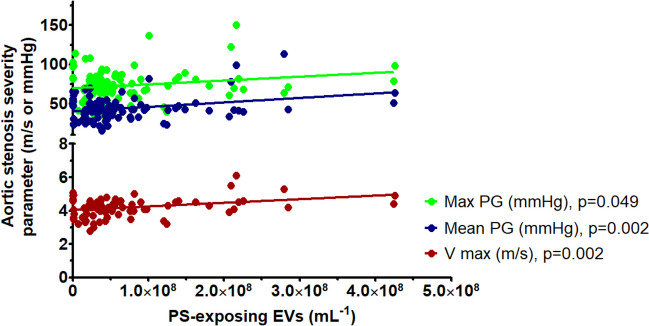
There was a positive correlation between baseline PS-exposing EV concentrations and (i) peak aortic valve velocity, (ii) peak aortic valve gradient and (iii) mean aortic valve gradient (p ≤ 0.049 for all) (Fig. 4). There were no significant correlations between PS-exposing EV concentrations and AVA, AVAi, EF and NT-proBNP (≥ 0.055 for all, Supplementary Table 4).
Fig. 4.
Correlation between plasma PS-exposing EV concentrations and aortic blood flow parameters, assessed in echocardiography before TAVI. Max PG – peak aortic valve gradient, mean PG – mean aortic valve gradient, V max – peak aortic valve velocity. Number of patients: 123. Statistical test used: Spearman's rank correlation coefficient
Kaplan–Meier analysis of event-free survival for MACCE in patients after TAVI (Fig. 5) demonstrated that patients with high concentration of PS-exposing EV concentrations (defined as > 32.54 *106 per mL plasma based on the ROC curve) had a lower chance of event-free survival during follow-up, compared to patients with concentrations of PS-exposing EV below the cut-off concentration (p = 0.048 for the log-rank test). A representative flow cytometry chart showing PS-exposing EVs in plasma is shown in Fig. 6.
Fig. 5.
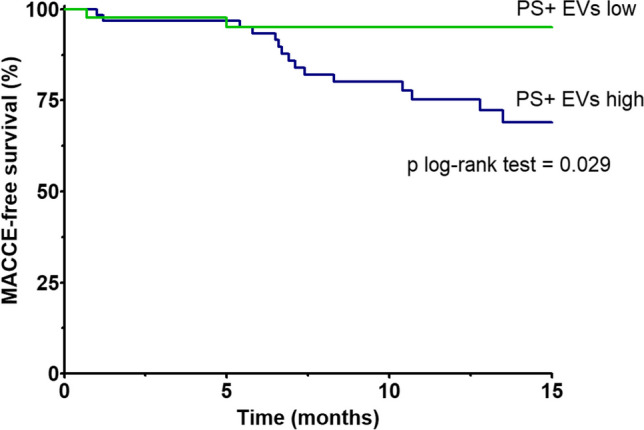
Kaplan–Meier survival analysis for MACCE after TAVI patients with high or low concentration of PS-exposing EVs, which was the only predictor of MACCE in multivariable analysis. Number of patients: 123. Statistical test used: log-rank test
Fig. 6.
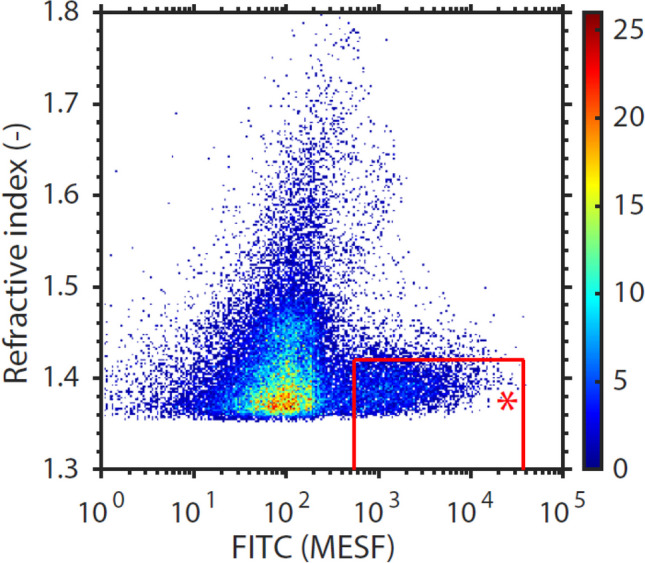
A representative flow cytometry chart (A60-Micro, Apogee Flow Systems) showing phosphatidylserine-exposing EVs positive at the fluorescence detector corresponding to fluorescein isothiocyanate (FITC) EVs in platelet-depleted plasma (red gate with red star). The FITC fluorescence is expressed in standard units of the number of molecules of equivalent soluble fluorochrome (MESF). Particles left to the red gate are negative for FITC and correspond to the background noise level of the FITC detector. Particles above the red gate have a refractive index > 1.42, likely corresponding to positively labeled chylomicrons. The gated events are related to plasma concentration by taking into account the sample dilution, flow rate and measurement time
Discussion
To our best knowledge, this is the first prospective, multicenter study investigating the effect of TAVI on plasma EV concentrations and providing a prediction model for post-TAVI MACCE based on EV concentration analysis. In addition, this is the first multicenter study, where the framework for standardized reporting of EV flow cytometry experiments (MIFlowCyt-EV) has been applied to improve the reproducibility of EV flow cytometry experiments. The main findings of our study are that (i) TAVI leads to a decrease in plasma concentration of EVs originating from leukocytes (CD45 +), and (ii) patients with increased pre-TAVI concentration of PS-exposing EVs have over fivefold higher odds of adverse post-TAVI outcomes, independent of other clinical variables, during the median observation time of over 10 months.
Obstruction of blood outflow leads to pressure overload-induced heart failure with subsequent release of EVs from activated blood cells and endothelium [23–25]. TAVI restores normal hemodynamic conditions, as reflected by decreased activation and suppressed pro-inflammatory and pro-atherogenic properties of monocytes [26]. In our study, we also showed that TAVI decreases leukocytes activation, reflected by decreased EVs release, which supports the previously observed anti-inflammatory effect of TAVI.
Reports regarding the effect of TAVI on the concentrations of other blood cells- and endothelial cell-derived EVs are inconsistent. In two studies including 92 and 9 patients with severe AS, TAVI had no effect on platelet- and endothelial-derived EVs 5–7 days after the procedure [27, 28]. Another study showed an increase in platelet-derived and PS-exposing EV concentrations, along with a decrease in endothelial-derived EV concentrations 7 days after TAVI [29]. Two studies showed a decrease in endothelial EVs concentrations 3–6 months after TAVI [29, 30], which may indicate restoration of endothelial integrity following the correction of vascular hemodynamics in a long-term observation [31]. These inconsistent results might be explained by different timepoints of EV measurements after TAVI, and different antibody subtypes to detect endothelial and platelet-derived EVs, hampering head-to-head comparisons. It could be also speculated that the rapid decrease in leukocyte EV concentration after TAVI is due to the fact that leukocytes (specifically monocytes) (i) are the largest blood cells and thereby most susceptible to shear stress caused by aortic stenosis and (ii) have exceptional adhesive properties that interact with stenotic valve, leading to leukocytes activation and release of EVs. Finally, the post-TAVI decreased in leukocyte EV concentrations might be not due to decreased EV release, but due to increased EV clearance. For example, if more PS is exposed on EVs, which is recognized as an “eat-me signal” [32], the leukocyte EV clearance would be faster. Although we have not seen differences in PS-exposing EV concentrations after TAVI, other authors observed a gradual increase in PS-exposing EVs up to 6 months post-TAVI, which might explain the decrease in the concentration of other EV subtypes [29].
We found that increased pre-TAVI concentration of PS-exposing EVs predict post-TAVI MACCE. PS exposed on the membrane surface binds clotting factors and further propagate thrombin generation [33]33. Hence, higher concentrations of PS-exposing EVs reflect increased ability to promote coagulation, which in our study was reflected by more MACCE. A recent study also showed that the concentration of PS-exposing EVs increases gradually in the first 6 months after TAVI, which was associated with higher serum coagulation activity in vivo [29], supporting our results that the excessive release of circulating PS-exposing EVs contributes to higher incidence of post-TAVI MACCE. We did not observe a higher prevalence of thrombosis-related MACCE, including MI, TIA, stroke or valve thrombosis among patients with high pre-TAVI PS-exposing EVs concentration. Nevertheless, given relatively small number of thrombosis-related MACCE in our study, such association cannot be excluded and should be investigated in a larger group of patients. We showed that the concentrations of PS-exposing EVs are higher in patients with more severe aortic blood flow disturbances, based on echocardiographic parameters – mean and peak aortic gradient pressure and peak aortic gradient velocity. Interestingly, the concentration of these EVs did not correlate with morphological severity of AS, as assessed by AVA and AVAi. It could be speculated that excessive release of PS-exposing EVs results from shear stress, which might be reflecting AS progression more accurately than echocardiographic examination.
Identification of patients at high risk of adverse outcomes after TAVI becomes an increasing clinical challenge, especially given the expansion of TAVI to young patients at intermediate and low perioperative risk [35, 36]. It is important for optimization of antithrombotic and/or anticoagulation strategies. Future possible targeted therapies, such as inhibition of PS-dependent hypercoagulable state might become a milestone of long-term care of patients after TAVI. The associated between higher baseline concentration of procoagulant PS-exposing EVs and MACCE might bring a rationale for administration of antiplatelet and/or anticoagulant therapy before TAVI procedure in a subset of patients. Currently, monotherapy with a single antiplatelet drug (aspirin or clopidogrel) is the standard care in post-TAVI patients without an indication for oral anticoagulation. However, aspirin does not decrease the concentrations of plasma EVs, whereas clopidogrel seems to have only minor effect on EVs [37]. In contrast, a potent P2Y12 receptor inhibitor ticagrelor was shown to decrease the concentrations of PS-exposing EVs more than clopidogrel [37]. From the pathophysiological point of view, ticagrelor might be a viable option after TAVI, especially in the subgroup of patients with high baseline concentrations of PS-exposing EVs. This interesting hypothesis remains to be investigated in future clinical studies to establish evidence-based recommendations.
Limitations
There are limitations of this study, which should be acknowledged. First of all, the number of thrombosis-associated MACCE is our study was too low to reach statistical power in subanalysis regarding the association between PS-exposing EV and individual MACCE events. Secondly, the aim of study was to investigate the effect of TAVI on EVs concentration and an association between EV concentration and MACCE, so no platelet function tests or coagulation activity tests were performed to confirm the functionality of PS-exposing EVs in vivo, demonstrated in other studies [29]. Third, all TAVI procedures were done by the same team, which eliminated the bias due to various expertise levels, but also limited the general results applicability. Fourth, many previous studies showed that PS-exposing EVs are elevated in cardiovascular diseases, limiting the specificity of our finding. However, considering the fact that the biomarkers which are currently most widely established in cardiovascular disease such as D-dimer and cardiac troponin also have low specificity, this limitation does not exclude the diagnostic utility of PS-exposing EVs in patients undergoing TAVI. Finally, PS exposure might be an artifact related to presence of platelets and/or cells fragmented during centrifugation and freeze-thawing. Although we did not study our plasma samples with electron microscopy, the standardized pre-analytical and analytical protocols, partly developed by our research group and applied in this study [18–20, 22, 38], were used to maximize the quality and reliability of the results.
Conclusions
Patients with increased pre-TAVI concentration of procoagulant, PS-exposing EVs have over fivefold higher odds of adverse outcomes after TAVI. The next step is to conduct a multicenter trial specifically focusing on PS-exposing EVs to predict post-TAVI MACCE.
Acknowledgements
We acknowledge the patients for participating in the study.
Abbreviations
- ACE
Angiotensin-converting enzyme
- AS
Aortic stenosis
- AVA
Aortic valve area
- AVAi
Aortic valve area index
- AUC
Area under the curve
- CI
Confidence interval
- CABG
Coronary artery bypass graft surgery
- COPD
Chronic obstructive pulmonary disease
- CKD
Chronic kidney disease
- CRP
C-reactive protein
- GFR
Glomerular filtration rate
- EVs
Extracellular vesicles
- LVEF
Left ventricle ejection fraction
- MACCE
Major adverse cardiac and cerebrovascular events
- MRA
Mineralocorticoid receptor antagonists
- NPV
Negative predictive value
- NT-proBNP
N-terminal pro B natriuretic peptide
- NYHA
New York Heart Association
- PCI
Percutaneous coronary intervention
- PLR
Positive likelihood ratio
- PG
Pressure gradient
- SVI
Stroke volume index
- PS
Phosphatidylserine
- PPV
Positive predictive value
- STS
Society of Thoracic Surgeons
- SAVR
Surgical aortic valve replacement
- SD
Standard deviation
- TF
Tissue factor
- TIA
Transient ischemic attack
- TAVI
Transcatheter aortic valve implantation
- TTE
Transthoracic echocardiography
- VARC
Valve Academic Research Consortium
Funding
The study was funded by the Young Investigator Grant 2020 of the Club “30” of the Polish Society of Cardiology (1WR/DAR13/20) to A. Gąsecka. The funders had no role in the study design; collection, analyses, or interpretation of data; writing of the manuscript; decision to publish the results.
Data Availability
Source data are available upon request to the corresponding author.
Declarations
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Disclosures
The authors report no relationships that could be construed as a conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–11. [DOI] [PubMed] [Google Scholar]
- 2.Iung B, Baron G, Tornos P, Gohlke-Bärwolf C, Butchart EG, Vahanian A. Valvular heart disease in the community: a European experience. Curr Probl Cardiol. 2007;32(11):609–61. [DOI] [PubMed] [Google Scholar]
- 3.Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM, et al. Calcific aortic stenosis. Nat Rev Dis Primers. 2016;2:16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Dor I, Pichard AD, Gonzalez MA, Weissman G, Li Y, Goldstein SA, et al. Correlates and causes of death in patients with severe symptomatic aortic stenosis who are not eligible to participate in a clinical trial of transcatheter aortic valve implantation. Circulation. 2010;122(11 Suppl):S37-42. [DOI] [PubMed] [Google Scholar]
- 5.Howard C, Jullian L, Joshi M, Noshirwani A, Bashir M, Harky A. TAVI and the future of aortic valve replacement. J Card Surg. 2019;34(12):1577–90. [DOI] [PubMed] [Google Scholar]
- 6.Barbanti M, Tamburino C, D’Errigo P, Biancari F, Ranucci M, Rosato S, et al. Five-Year Outcomes of Transfemoral Transcatheter Aortic Valve Replacement or Surgical Aortic Valve Replacement in a Real World Population. Circ Cardiovasc Interv. 2019;12(7):e007825. [DOI] [PubMed] [Google Scholar]
- 7.Walczewski M, Gasecka A, Huczek Z, Rymuza B, Kochman J. Ten-year experience with transcatheter aortic valve implantation in bicuspid aortic valve: lessons learned and future perspectives. Postepy Kardiol Interwencyjnej. 2021;17(3):251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abushouk A, Agrawal A, Hariri E, Dykun I, Kansara T, Saad A, et al. Association between haematological parameters and outcomes following transcatheter aortic valve implantation at mid-term follow-up. Open Heart. 2022;9(2):e002108. [DOI] [PMC free article] [PubMed]
- 9.Jeong YJ, Ahn JM, Kang DY, Park H, Ko E, Kim HJ, et al. Incidence, Predictors, and Prognostic Impact of Immediate Improvement in Left Ventricular Systolic Function After Transcatheter Aortic Valve Implantation. Am J Cardiol. 2021;152:99–105. [DOI] [PubMed] [Google Scholar]
- 10.Ishizu K, Shirai S, Isotani A, Hayashi M, Kawaguchi T, Taniguchi T, et al. Long-Term Prognostic Value of the Society of Thoracic Surgery Risk Score in Patients Undergoing Transcatheter Aortic Valve Implantation (From the OCEAN-TAVI Registry). Am J Cardiol. 2021;149:86–94. [DOI] [PubMed] [Google Scholar]
- 11.Shah R, Patel T, Freedman JE. Circulating Extracellular Vesicles in Human Disease. N Engl J Med. 2018;379(10):958–66. [DOI] [PubMed] [Google Scholar]
- 12.Blaser MC, Buffolo F, Halu A, Turner ME, Schlotter F, Higashi H, et al. Multiomics of Tissue Extracellular Vesicles Identifies Unique Modulators of Atherosclerosis and Calcific Aortic Valve Stenosis. Circulation. 2023;148(8):661–678. [DOI] [PMC free article] [PubMed]
- 13.Suades R, Padró T, Vilahur G, Badimon L. Circulating and platelet-derived microparticles in human blood enhance thrombosis on atherosclerotic plaques. Thromb Haemost. 2012;108(6):1208–19. [DOI] [PubMed] [Google Scholar]
- 14.Zifkos K, Dubois C, Schäfer K. Extracellular Vesicles and Thrombosis: Update on the Clinical and Experimental Evidence. Int J Mol Sci. 2021;22(17):9317. [DOI] [PMC free article] [PubMed]
- 15.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Rev Esp Cardiol (Engl Ed). 2018;71(2):110. [DOI] [PubMed] [Google Scholar]
- 16.Nijenhuis VJ, Brouwer J, Delewi R, Hermanides RS, Holvoet W, Dubois CLF, et al. Anticoagulation with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. N Engl J Med. 2020;382(18):1696–707. [DOI] [PubMed] [Google Scholar]
- 17.Brouwer J, Nijenhuis VJ, Delewi R, Hermanides RS, Holvoet W, Dubois CLF, et al. Aspirin with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. N Engl J Med. 2020;383(15):1447–57. [DOI] [PubMed] [Google Scholar]
- 18.Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, et al. Methodological Guidelines to Study Extracellular Vesicles. Circ Res. 2017;120(10):1632–48. [DOI] [PubMed] [Google Scholar]
- 19.Buntsma NC, Gąsecka A, Roos Y, van Leeuwen TG, van der Pol E, Nieuwland R. EDTA stabilizes the concentration of platelet-derived extracellular vesicles during blood collection and handling. Platelets. 2022;33(5):764–71. [DOI] [PubMed] [Google Scholar]
- 20.van der Pol E, de Rond L, Coumans FAW, Gool EL, Böing AN, Sturk A, et al. Absolute sizing and label-free identification of extracellular vesicles by flow cytometry. Nanomedicine. 2018;14(3):801–10. [DOI] [PubMed] [Google Scholar]
- 21.de Rond L, Libregts S, Rikkert LG, Hau CM, van der Pol E, Nieuwland R, et al. Refractive index to evaluate staining specificity of extracellular vesicles by flow cytometry. J Extracell Vesicles. 2019;8(1):1643671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welsh JA, Arkesteijn GJA, Bremer M, Cimorelli M, Dignat-George F, Giebel B, et al. A compendium of single extracellular vesicle flow cytometry. J Extracell Vesicles. 2023;12(2):e12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diehl P, Nagy F, Sossong V, Helbing T, Beyersdorf F, Olschewski M, et al. Increased levels of circulating microparticles in patients with severe aortic valve stenosis. Thromb Haemost. 2008;99(4):711–9. [DOI] [PubMed] [Google Scholar]
- 24.Pfeifer P, Zietzer A, Hölscher M, Jehle J, Nickenig G, Werner N, et al. Transverse aortic constriction-induced heart failure leads to increased levels of circulating microparticles. Int J Cardiol. 2022;347:54–8. [DOI] [PubMed] [Google Scholar]
- 25.deAlmeida AC, van Oort RJ, Wehrens XH. Transverse aortic constriction in mice. J Vis Exp. 2010(38):1729. [DOI] [PMC free article] [PubMed]
- 26.Baratchi S, Zaldivia MTK, Wallert M, Loseff-Silver J, Al-Aryahi S, Zamani J, et al. Transcatheter Aortic Valve Implantation Represents an Anti-Inflammatory Therapy Via Reduction of Shear Stress-Induced, Piezo-1-Mediated Monocyte Activation. Circulation. 2020;142(11):1092–105. [DOI] [PubMed] [Google Scholar]
- 27.Jung C, Lichtenauer M, Figulla HR, Wernly B, Goebel B, Foerster M, et al. Microparticles in patients undergoing transcatheter aortic valve implantation (TAVI). Heart Vessels. 2017;32(4):458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchini JF, Miyakawa AA, Tarasoutchi F, Krieger JE, Lemos P, Croce K. Endothelial, platelet, and macrophage microparticle levels do not change acutely following transcatheter aortic valve replacement. J Negat Results Biomed. 2016;15:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chi H, Shao Y, Xie F, Zhang J, Zhang G, Jiang G, et al. Procoagulant effect of extracellular vesicles in patients after transcatheter aortic valve replacement or transcatheter aortic valve replacement with percutaneous coronary intervention. J Thromb Thrombolysis. 2023;56(2):264–74. [DOI] [PubMed] [Google Scholar]
- 30.Horn P, Stern D, Veulemans V, Heiss C, Zeus T, Merx MW, et al. Improved endothelial function and decreased levels of endothelium-derived microparticles after transcatheter aortic valve implantation. EuroIntervention. 2015;10(12):1456–63. [DOI] [PubMed] [Google Scholar]
- 31.Lugo-Gavidia LM, Burger D, Matthews VB, Nolde JM, Galindo Kiuchi M, Carnagarin R, et al. Role of Microparticles in Cardiovascular Disease: Implications for Endothelial Dysfunction, Thrombosis, and Inflammation. Hypertension. 2021;77(6):1825–44. [DOI] [PubMed] [Google Scholar]
- 32.Segawa K, Nagata S. An Apoptotic “Eat Me” Signal: Phosphatidylserine Exposure. Trends Cell Biol. 2015;25(11):639–50. [DOI] [PubMed] [Google Scholar]
- 33.Heemskerk JW, Mattheij NJ, Cosemans JM. Platelet-based coagulation: different populations, different functions. J Thromb Haemost. 2013;11(1):2–16. [DOI] [PubMed] [Google Scholar]
- 34.Banner DW, D’Arcy A, Chène C, Winkler FK, Guha A, Konigsberg WH, et al. The crystal structure of the complex of blood coagulation factor VIIa with soluble tissue factor. Nature. 1996;380(6569):41–6. [DOI] [PubMed] [Google Scholar]
- 35.Mack MJ, Leon MB, Thourani VH, Pibarot P, Hahn RT, Genereux P, et al. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N Engl J Med. 2023;389(21):1949–60. [DOI] [PubMed]
- 36.Forrest JK, Deeb GM, Yakubov SJ, Gada H, Mumtaz MA, Ramlawi B, et al. 4-Year Outcomes of Patients With Aortic Stenosis in the Evolut Low Risk Trial. J Am Coll Cardiol. 2023;82(22):2163–65. [DOI] [PubMed]
- 37.Gasecka A, Nieuwland R, van der Pol E, Hajji N, Ćwiek A, Pluta K, et al. P2Y12 antagonist ticagrelor inhibits the release of procoagulant extracellular vesicles from activated platelets. Cardiol J. 2019;26(6):782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bettin B, Gasecka A, Li B, Dhondt B, Hendrix A, Nieuwland R, et al. Removal of platelets from blood plasma to improve the quality of extracellular vesicle research. J Thromb Haemost. 2022;20(11):2679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Source data are available upon request to the corresponding author.