Abstract
Introduction
Intercostal nerve transfer is a surgical technique used to restore function in patients with total brachial plexus injury. Stem cell and secretome therapy has been explored as a potential treatment for brachial plexus injuries. This study aimed to compare the functional and histologic outcome of intercostal nerve transfer to median nerve with local stem cells or secretome injection in total type brachial plexus injuries.
Materials and methods
This was a double-blinded, randomized controlled study (RCT). We included patients with neglected total type brachial plexus injury (BPI) who underwent nerve transfer and local injection of either umbilical cord-derived mesenchymal stem cells (UC-MSC) or secretome into median nerve–flexor digitorum superficialis (FDS) neuromuscular junction (NMJ). We measured preoperative and 8-month postoperative FDS muscle strength, SF-36, DASH score, and histologic assessment. We then analyzed the difference outcome between those two groups.
Result
A total of 15 patients were included in this study. Our study found that after nerve transfer and implantation with either UC-MSC or secretome, significant postoperative improvements were observed in physical functioning, role limitations, energy/fatigue, emotional well-being, social functioning, pain, general health, and DASH scores, particularly in the overall cohort and the secretome group. When we compared the mean difference of clinical outcome from preoperative to postoperative between UC-MSC and secretome groups, the UC-MSC group showed better improvement of health change in SF-36 subgroup compared to secretome group. From the analysis, there was no significant difference in the histologic outcomes (inflammation, regeneration, and fibrosis) in overall cohort between preoperative and postoperative cohort. There was also no significant difference in mean change of the histologic outcomes (inflammation, regeneration, and fibrosis) preoperative and postoperatively between UC-MSC and secretome groups.
Discussion and conclusion
Implantation of either UC-MSC or secretome along with nerve transfer may provide clinical improvement, while to achieve histologic improvement, further conditioning should be performed.
Keywords: Brachial plexus injury, Stem cells, Secretome, Neuromuscular junction, Clinical outcomes
Introduction
A total brachial plexus injury, also known as a pan-brachial plexus injury, involves damage to all the roots of the brachial plexus, a network of nerves that conducts signals from the spinal cord to the shoulder, arm, and hand. This type of injury can lead to significant impairment, affecting not only the shoulder and elbow function but also the hand function. Brachial plexus injuries are often caused by trauma, with road traffic accidents, particularly motorcycle accidents, being the dominant cause. The injury can be quite challenging for surgeons due to the complexity of the brachial plexus and the severity of the damage in total injuries [1].
Reconstructive procedures for total brachial plexus injuries commonly include nerve repair, grafting, neurotization (nerve transfer), tendon transfer, and free functional muscle transfer (FFMT) [2]. A study reported successful results in 79% of cases after direct repair (nerve grafting) and in 56% of cases after end-to-end neurotization. The success of neurotization depended on the type of donor nerve used, with intraplexal nerves (motor branches of the brachial plexus) having a significantly higher success rate than extraplexal nerves (81% compared with 49%, respectively). End-to-side neurorrhaphy had a success rate of 64.3% when using the axillary nerve as a recipient, which was similar to neurotization using intraplexal nerves (68.4%) and better than that achieved using extraplexal nerves (47.4%). A meta-analysis found that nerve transfer was associated with greater shoulder external rotation relative to sural nerve grafting and a lower rate of secondary shoulder surgery in obstetrical brachial plexus injury. Meanwhile, the success rates of FFMT innervated by intercostal nerves and spinal accessory nerves were 64.1% and 65.4%, respectively, with no significant difference in outcomes between the two. [3]
Intercostal nerve transfer is a surgical technique used to restore function in patients with total brachial plexus injury. This procedure involves transferring intercostal nerves to the thoracodorsal nerve, which then innervates the latissimus dorsi muscle, aiding in hand function. This study is aimed to discover the clinical functional outcome and histomorphometry analysis of late onset total type brachial plexus injury treated with intercostal transfer to median nerve and stem cell or secretome injection [4, 5].
Stem cell therapy has been explored as a potential treatment for brachial plexus injuries. For instance, a pilot study found that the injection of autologous bone marrow-derived mononuclear cells into partially denervated biceps of patients with brachial plexus injuries was safe and suggested enhanced muscle reinnervation and regeneration [6, 7]. This study aimed to compare the clinical and functional outcome of intercostal nerve transfer to median nerve with local stem cells or secretome injection in total type brachial plexus injuries (Table 1).
Table 1.
Baseline characteristic
| Variable | N (%) | Intervention group (n = 7) | Control group (n = 8) | p-value |
|---|---|---|---|---|
| Gender | ||||
| Male | 14 (93.3) | 7 (100) | 7 (87.5) | |
| Female | 1 (6.7) | 0 (0) | 1 (12.5) | |
| Age | 30.25 ± 11.54 | 24.67 ± 4.73 | 33.6 ± 13.58 | 0.837 |
| Duration between surgeries | 8 (2–9) | 8 (8–9) | 8 (2–8) | 0.613 |
| Onset | 10.38 ± 7.15 | 11 ± 8.54 | 10 ± 7.25 | 0.865 |
Materials and methods
This investigation used a randomized clinical trial (RCT) design. The study was conducted using consecutive and double-blinded sampling to minimize selection bias and ensure the robustness of the results. We included patients who were allocated into intervention and control groups. Participants were recruited from our institutional hospital, based on predefined inclusion and exclusion criteria. The inclusion criteria were total neglected (6 to less than 18 months) brachial plexus injury in patients with age of more than 18 years who were planned to have neurotization (nerve transfer) procedure, specifically 3rd–5th intercostal nerves to median nerve transfer. The exclusion criteria were previous intervention in median nerve, presence of spontaneous nerve regeneration, destruction of hand flexor muscles, upper motor neuron deficits, and diabetes mellitus. Informed consent was obtained from all eligible participants before their enrollment in the study. Randomization was achieved using computerized block, and the allocation sequence was concealed to both participants and investigators. The double-blinded nature of the study ensured that neither the participants nor the researchers involved in data collection, analysis, and interpretation were aware of the treatment assignments, thereby minimizing potential bias.
Baseline characteristic including age, sex, dominant hand, onset from trauma to surgery, and duration of surgery would be analyzed. Preoperative and postoperative (at final follow-up of 8 months) flexor digitorum superficialis (FDS) muscle strength, Disabilities of Arm, Shoulder, and Hand (DASH) score, and Short Form Survey (SF-36) were measured. Intraoperatively at first surgery, beside performing 3rd–5th intercostal nerves to median nerve transfer, neuromuscular junction (NMJ) biopsy and NMJ injection using UC-MSC or secretome were performed. At the next surgery (at about 8 months after first surgery), NMJ biopsy was performed. The purpose of the next surgery (at about 8 months after first surgery) was to obtain the biopsy sample from the previously UC-MSC or secreteome injected NMJ, to compare it with the preoperative data. FDS muscle strength was measured using motoric strength assessment according to Manual Muscle Testing (MMT) into score of 0–5.
The stem cells used were allograft mesenchymal stem cells from umbilical cord, the umbilical cord mesenchymal stem cells (UC–MSC). The processing of the UC-MSC was performed in the Stem Cell Medical Technology Integrated Service Unit, Cipto Mangunkusumo National Central Public Hospital, Faculty of Medicine Universitas Indonesia. The UC-MSC and secretome processing were in accordance with the established methods described by Kurniawan et al. [8] and Dilogo et al. [9] The UC-MSC and secretome preparations were as follows: umbilical cords were obtained from cesarean section after the mothers signed for informed consent. Ten cm umbilical cord was collected in 50 mL transport medium, which contained alpha minimal essential medium (αMEM [GIBCO 12,000-022 1]), penicillin/streptomycin (final concentration 300 U/mL [Gibco 15,140-122]), and amphotericin B (final concentration 7500 ng/mL [JR Scientific 50701]). The tissue was processed in less than 8 h after collection; explant cultures were placed in xeno-free media and then recultured after the first harvest. This is a multiharvest explant method that yielded far more viable cells than the standard explant method. Secretome is the collection of conditioned medium used to wash the UC-MSC when it reached confluence during the culture process. UC-MSC that was cultured in containing alpha minimal essential medium (α-MEM) was supplemented with serum (10% thrombocyte concentrate lysate (DMEM (Gibco, US)). For secretome collection, the culture medium was replaced in two different time points: The first was replaced with serum containing culture medium when the cells reached 50% confluence, while the other was replaced with culture medium without serum when the cells reached 70% confluence. The secretome before this medium replacement was collected. After the culture medium was replaced, the cells were incubated for 48 h in an incubator at 37 °C, with 5% CO2. The UC-MSCs were then harvested. The collected secretome samples were filtered and placed in a 15-mL tube in − 20 oC once before being transferred to 1.5 ml and 5 ml tubes and stored in − 20 °C.
The nerve transfer procedure included the transfer of 3rd–5th intercostal nerves to median nerve. Before the nerve transfer, the biopsy of median nerve to FDS NMJ was obtained. Sample sized 0.5 × 0.5 × 0.5 was obtained using microscope. After biopsy taking, UC-MSC or secretome, according to the allocated group, were injected into the NMJ. Ten million of stem cells were injected to the site, specifically in 0.5 cm from the insertion of median nerve to FDS muscle. The injected site was then marked using 8.0 prolene needle. Beside intercostal nerves transfer to median nerve, the intervention group received UC-MSC injection while the control group received secretome injection. The administration of treatments was standardized, and participants were monitored for adherence throughout the study period.
The NMJ would be examined semi-quantitatively for its morphological hallmark according to scoring by Wedderburn et al. [10] modified by Kolbel et al. [11] into the evaluation of inflammation, regeneration, and fibrosis. For evaluation of inflammation, the score would be 0, 1, 2, and 3 if the inflammation was absent, focal low, focal moderate (multifocal sporadic), and multifocal abundant, respectively. For evaluation of regeneration, the score would be 0, 1, 2, and 3 if the regeneration was absent, focal small, focal large, and multifocal, respectively. For evaluation of fibrosis, the score would be 0, 1, 2, 3, and 4 if the fibrosis was absent, proliferation of perimysium connective tissue only, additional mild endomysial fibrosis, additional moderate endomysial fibrosis, and additional severe endomysial fibrosis, respectively.
Results
A total of 15 patients were included in this study, with the baseline characteristics of the study participants, categorized into two groups: UC-MSC (n = 7) and secretome (n = 8), are presented in Table 4. The gender distribution shows a predominance of males in both groups, with 100% males in the UC-MSC group and 87.5% in the secretome group. Only one female participant was presented in the secretome group (12.5%). The mean age of participants was slightly higher in the secretome group (33.6 ± 13.58 years) compared to the UC-MSC group (24.67 ± 4.73 years), although this difference was not statistically significant (p = 0.837). All participants were right-hand dominant. The duration between surgery and the study ranged from 2 to 9 months, with median durations of 8 months in both groups, and no significant difference was found between them (p = 0.613). The mean onset of symptoms was also comparable between the groups, with the UC-MSC group at 11 ± 8.54 months and the secretome group at 10 ± 7.25 months (p = 0.865). These baseline characteristics indicate that the two groups were well-matched in terms of key demographic and clinical variables.
Table 4.
Result of histologic analysis in UC-MSC and secretome groups
| Variable | Overall | p-value | UC-MSC | p-value | Secretome | p-value |
|---|---|---|---|---|---|---|
| (n = 15) | (n = 7) | (n = 8) | ||||
| N (%) | N (%) | N (%) | ||||
| Inflammation (mean) | 1.0 | 1.0 | 1.0 | |||
| Preoperative | ||||||
| Absent | 9 (64.3) | 6 (42.9) | 3 (21.4) | |||
| Focal low | 3 (21.4) | 0 | 3 (21.4) | |||
| Focal Moderate | 0 | 0 | 0 | |||
| Multifocal abundant | 2 (14.3) | 1 (7.1) | 1 (7.1) | |||
| Postoperative | ||||||
| Absent | 5 (50) | 4 (40) | 1 (10) | |||
| Focal low | 3 (30) | 1 (10) | 2 (20) | |||
| Focal moderate | 1 (10) | 1 (10) | 0 | |||
| Multifocal abundant | 1 (10) | 0 | 1 (10) | |||
| Regeneration (mean) | 0.564 | 0.317 | 1.00 | |||
| Preoperative | ||||||
| Absent | 6 (42.9) | 3 (21.4) | 3 (21.4) | |||
| Focal small | 6 (42.9) | 3 (21.4) | 3 (21.4) | |||
| Focal large | 2 (14.3) | 1 (7.1) | 1 (7.1) | |||
| Multifocal | 0 | 0 | 0 | |||
| Postoperative | ||||||
| Absent | 3 (30) | 1 (10) | 2 (20) | |||
| Focal small | 4 (40) | 4 (40) | 0 | |||
| Focal large | 3 (30) | 1 (10) | 2 (20) | |||
| Multifocal | 0 | 0 | ||||
| Fibrosis | 0.48 | 0.414 | 1.00 | |||
| Preoperative | ||||||
| Absent | 0 | 0 | 0 | |||
| Proliferation of perimysial connective tissue only | 1 (7.1) | 0 | 1 (7.1) | |||
| Additional mild endomysial fibrosis | 2 (14.3) | 0 | 2 (14.3) | |||
| Additional moderate endomysial fibrosis | 3 (7.1) | 2 (14.3) | 1 (7.1) | |||
| Additional severe endomysial fibrosis | 8 (57.1) | 5 (35.7) | 3 (21.4) | |||
| Postoperative | ||||||
| Absent | ||||||
| Proliferation of perimysial connective tissue only | 1 (10) | 0 | 1 (10) | |||
| Additional mild endomysial fibrosis | 1 (10) | 1 (10) | 0 | |||
| Additional moderate endomysial fibrosis | 4 (40) | 2 (20) | 2 (20) | |||
| Additional severe endomysial fibrosis | 4 (40) | 3 (30) | 1 (10) |
Biopsy of the NMJ were successfully obtained in all patients before stem cell or secretome injection and at the last surgery. As mentioned, sample sized 0.5 × 0.5 × 0.5 was obtained using microscope (Fig. 1) and the nerve transfer procedure included the transfer of 3rd–5th intercostal nerves to median nerve performed in the first surgery (Fig. 2).
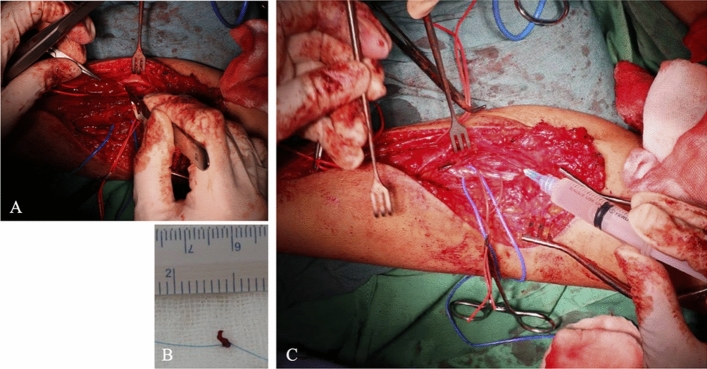
Fig. 1.
NMJ Biopsy and Injection. A Biopsy was performed in median nerve – to FDS muscle NMJ with the sample size of (B) 0.5 × 0.5 × 0.5 cm. (C) After biopsy, injection of either UC-MSC or secretome according to intended group was performed in NMJ in the first surgery. At the last surgery 8 months later, only NMJ biopsy was performed
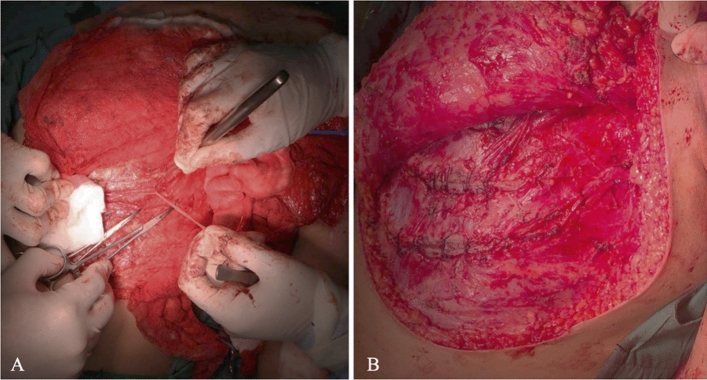
Fig. 2.
3rd–5th intercostal nerves transfer to median nerve. A Harvesting of 3rd to 5th intercostal nerves. B Intercostal nerves transfer to median nerve
Clinical outcomes measured preoperative and postoperative SF-36, DASH score, and FDS muscle strength. SF-36 was sub grouped into nine subgroups, namely physical functioning, role limitations due to physical health, role limitation due to emotional problems, energy/fatigue, emotional well-being, social functioning, pain, general health, and health change. Table 2 illustrates the functional outcomes of participants in both UC-MSC and secretome groups, measured using SF-36 and DASH scores. The overall physical functioning improved significantly postoperatively in the overall cohort (p = 0.028), although the individual group improvements were not statistically significant (UC-MSC p = 0.180, secretome p = 0.068). Role limitations due to physical health decreased postoperatively in the overall cohort (p = 0.038), with no significant change within the groups (UC-MSC p = 0.157, secretome p = 0.102).
Table 2.
Results on functional outcomes in UC-MSC and secretome groups
| Variable | Overall (n = 15) | p-value | UC-MSC (n = 7) | p-value | Secretome (n = 8) | p-value |
|---|---|---|---|---|---|---|
| SF-36 | ||||||
| Physical functioning | ||||||
| Preoperative | 0 | 0 | 0 | |||
| Postoperative | 40 ± 31.05 | 0.028# | 45 ± 40.93 | 0.180 | 37 ± 28.64 | 0.068 |
| Role limitations due to physical health | ||||||
| Preoperative | 0 | 0 | 0 | |||
| Postoperative | 25 (0–100) | 0.038# | 100 (0–100) | 0.157 | 30 ± 41.08 | 0.102 |
| Role limitations due to emotional problems | ||||||
| Preoperative | 0 | 0 | 0 | |||
| Postoperative | 66.7 (0–100) | 0.016# | 75.57 ± 21.42 | 0.109 | 66.68 ± 40.82 | 0.063 |
| Energy/fatigue | ||||||
| Preoperative | 20 (15–60) | 60 (20–60) | 20(15–20) | |||
| Postoperative | 66.75 ± 19.46 | 0.012# | 74.67 ± 17.62 | 0.109 | 62 ± 20.79 | 0.043# |
| Emotional well-being | ||||||
| Preoperative | 29.5 ± 13.17 | 42.67 ± 6.11 | 21.6 ± 8.76 | |||
| Postoperative | 69.44 ± 13.43 | < 0.001* | 74.5 ± 11.32 | 0.009* | 66.4 ± 14.86 | 0.002* |
| Social functioning | ||||||
| Preoperative | 45.31 ± 14.85 | 62.5 (25–62.5) | 50(25–50) | |||
| Postoperative | 68.13 ± 17.92 | 0.006* | 77.5 ± 11.46 | 0.109 | 62.5 ± 19.76 | 0.066 |
| Pain | ||||||
| Preoperative | 22.5 (10–22.5) | 22.5 (10–22.5) | 22.5(10–22.5) | |||
| Postoperative | 52.5(45–100) | 0.012# | 65 ± 30.41 | 0.109 | 62.5 ± 22.91 | 0.043# |
| General health | ||||||
| Preoperative | 36.25 ± 9.54 | 43.3 (40–50) | 32 ± 9.08 | |||
| Postoperative | 50(45–90) | 0.011# | 70 ± 20 | 0.102 | 50(45–85) | 0.042# |
| Health change | ||||||
| Preoperative | 25 (0–25) | 0 (0–25) | 0 (0–25) | |||
| Postoperative | 56.25 ± 17.68 | 0.016# | 75 (50–75) | 0.102 | 50 ± 17.68 | 0.059 |
| DASH | ||||||
| Preoperative | 89.59 ± 5.77 | 95 (90.8–95) | 87.18 ± 6 | |||
| Postoperative | 56.13 ± 14.76 | < 0.001* | 51.1 ± 15.12 | 0.109 | 59.14 ± 15.38 | 0.013* |
| Motoric | ||||||
| Preoperative | 0 | 0 | 0 | |||
| Postoperative | 0 | – | 0 | – | 0 | – |
*paired t-test, #Wilcoxon
Role limitations due to emotional problems showed significant improvement postoperatively across the overall cohort (p = 0.016), and in the UC-MSC group (p = 0.009), but not in the secretome group (p = 0.063). Energy/fatigue levels significantly improved postoperatively in the overall cohort (p = 0.012) and in the secretome group (p = 0.043). Emotional well-being saw substantial postoperative improvement in both the overall cohort (p < 0.001) and within the UC-MSC (p = 0.009) and secretome groups (p = 0.002). Social functioning improved postoperatively in the overall cohort (p = 0.006) and in the UC-MSC group (p = 0.009), but not significantly in the secretome group (p = 0.066). Pain levels improved significantly postoperatively in the overall cohort (p = 0.004), and in the secretome group (p = 0.043), but not in the UC-MSC group (p = 0.055). General health improved significantly in the overall cohort (p = 0.042) and in the secretome group (p = 0.042), with no significant change in the UC-MSC group (p = 0.129). Health change scores showed significant improvement postoperatively in the overall cohort (p = 0.016), but not in either individual group (UC-MSC p = 0.072, secretome p = 0.059).
DASH scores showed significant improvement postoperatively in the overall cohort (p < 0.001), and in the secretome group (p = 0.013), with no significant change in the UC-MSC group (p = 0.109). Motoric scores remained unchanged pre- and postoperatively across all groups. In summary, significant postoperative improvements were observed in physical functioning, role limitations, energy/fatigue, emotional well-being, social functioning, pain, general health, and DASH scores, particularly in the overall cohort and the secretome group. When we compared the mean difference of clinical outcome from preoperative to postoperative between UC-MSC and secretome groups, the UC-MSC group showed better improvement of health change in SF-36 subgroup compared to secretome group (Table 3).
Table 3.
Differences between preoperative and postoperative result on clinical outcomes
| Variable | UC-MSC | Secretome | p-value |
|---|---|---|---|
| SF-36 (mean change) | |||
| Physical functioning | 45 ± 40.93 | 37 ± 28.64 | 0.753 |
| Role limitations due to physical health | 57.74(0–100) | 30 ± 41.08 | 0.764 |
| Role limitations due to emotional problems | 75.57 ± 21.42 | 66.68 ± 40.82 | 0.744 |
| Energy/fatigue | 28 ± 21.38 | 43 ± 21.09 | 0.370 |
| Emotional well-being | 31.83 ± 5.25 | 40(36–68) | 0.099 |
| Social functioning | 27.5 ± 22.5 | 20 ± 14.25 | 0.577 |
| Pain | 46.67 ± 26.96 | 42.5 ± 21.14 | 0.814 |
| General health | 26.67 ± 20.82 | 24 ± 14.75 | 0.837 |
| Health change | 50(50–75) | 25 ± 17.68 | 0.042* |
| DASH (mean change) | 42.5 ± 12.92 | 28.04 ± 14.72 | 0.211 |
| Motoric (mean change) | 0 | 0 | |
*Mann–Whitney U test
The anatomical pathology samples taken through biopsy from patients during preoperative and postoperative following the intervention and biological agent administration. Variables assessed include inflammation, regeneration, and fibrosis, and each variable is graded from 0 to 3 for inflammation and regeneration, and grade 0–4 for fibrosis. In general, there was an increased in inflammation and regeneration with decreased fibrosis, and the changes were made to a different degrees. Similar trends were showed when the analysis was divided into each treatment group; however, the preoperative and postoperative changes in overall, UC-MSC, and secretome group were nonsignificant statistically.
Table 4 showed result of histologic analysis in overall cohort, UC-MSC, and secretome groups. From the analysis, there was no significant difference in the histologic outcomes (inflammation, regeneration, and fibrosis) in overall cohort between preoperative and postoperative cohort. There was also no significant difference in mean change of the histologic outcomes (inflammation, regeneration, and fibrosis) preoperative and postoperatively between UC-MSC and secretome groups. The histopathological examination is depicted in Fig. 3.
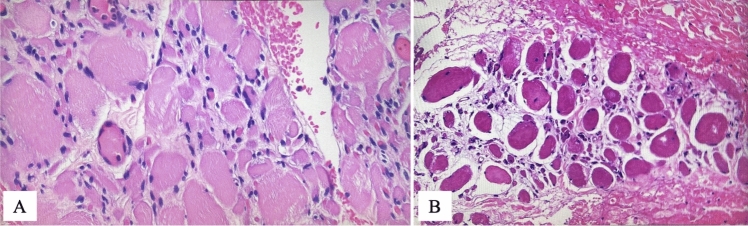
Fig. 3.
Histopathological findings: histological findings shown on the picture described, there were no alteration in term of inflammation, regeneration, and fibrosis between A preoperative and B postoperative conditions
Discussion
The use of stem cell therapy for brachial plexus injury (BPI) have several considerations. First, in all cases of BPI, there are three possible scenarios: nerves may be stretched but in continuity, ruptured, or avulsed. In stretched nerves, or neuropraxia, nerve can spontaneously recover. Nerves ruptured distal to the root entry zone are repairable because the distal stump has proliferating Schwann cells within the basal lamina that supports axonal regeneration. Nerve grafting of ruptured cervical spinal nerves is an accepted standard of treatment with useful clinical results [12].Root avulsions cause rapid cell death and are not repairable. Because the distal ruptured nerve stump sustains axon regeneration for a time, early application of appropriate stem cells at the site of nerve ruptures, and not root avulsions, may be the key to improve clinical outcome. The principles governing the timing of nerve reconstruction should apply to stem cell injections [13]. Second, autologous nerve grafts are currently used for repairing ruptured cervical spinal nerves to distal targets with good results. These nerve grafts serve as conduits containing Schwann cells that support axonal regeneration. The ability to use cells without harvesting grafts would be a benefit. It is here, where stem cell therapy has a role [12]. The underlying principle is that transplanted stem cells differentiate into needed cell types and promote nerve regeneration by functioning as support cells with their powerful paracrine effects, releasing growth factors promoting nerve regeneration [14]. The route of delivery is important. Intra-muscular bone marrow-derived mononuclear cell injections can increase myofiber diameters and motor unit amplitudes in chronic partially denervated muscle, but is unlikely to restore voluntary motor control in a complete BPI [15, 16]. Third, despite encouraging results in animal models, there is a lack of appropriately designed human studies on the efficacy and long-term safety of stem cell injections for brachial plexus injuries. The type, differentiation state, and ideal number of stem cells for optimal therapeutic effect in BPI need to be determined. It remains to be seen if data determined in animal and preclinical studies will translate well in humans. One can hope that stem cells may hasten the recovery of incomplete stretch injuries [17].
The NMJ is the peripheral synapse formed by the (a) nerve terminal of a motor neuron, (b) motor endplate of a muscle fiber, and (c) ensheathing terminal Schwann cells. In health, NMJs are dynamic structures with constant remodeling, and continuous innervation/denervation. A permanent morphological change in the NMJ may precede an irreversible denervation process, thought to be the earliest sign of degeneration in multiple human neuromuscular disorders, as well as part of the natural aging process [18].
UC-MSC implantation will become an efficacious treatment for brachial plexus injury. UC-MSC treatment which had been shown by numerous animal studies can save some level of neurological function following the injury. A lot of positive results following stem cells implantation seem to be related to salvage of the existing tissue rather than the repair or replacement of damaged tissue. The implanted cells release growth factors, cytokines, and hormones to provide most of the benefits. These proteins have paracrine effect following the transplantation of the stem cells/progenitor cells. These include neuroprotection, trophic support, guidance for axonal outgrowth, and glial scar control. Stem cell therapy has the ability to potentially repair muscle strength. The application of stem cells has been done in muscle-focused clinical studies of the heart and leg. Injection of stem cells in a partially denervated muscle was secure, with no adverse effects regarding vital signs, bone marrow aspiration sites, injection sites, or surgical wounds. Utilization of stem cells in brachial plexus injuries holds promise in enhancing the healing process, regeneration of the nerve, and the denervated muscles [19].
Previous report showed that after injection of stem cells, an increase in amplitude and number of phases of motor unit potentials were observed in the EMG findings, which suggestive of muscle reinnervation in the muscles. Such report concluded that there was augmented clinical benefits of the combination of cellular therapy and rehabilitation in patient suffered from brachial plexus injury [20]. Previous systematic review of animal studies also showed that stem cells implantation to brachial plexus injury showed its ability to regenerate nerve cells as evidenced by clinical, electrophysiological, and histopathological results [21].
Rodriquez et al. [22] performed a study to see the effect of bone marrow mesenchymal stem cells (BM-MSCs) associated with acellular nerve allograft in a rat model phrenic nerve transfer to musculocutaneous nerve in C5-C6 avulsion. They found that addition of BM-MSCs failed to provide a sufficient improvement in the parameters of nerve regeneration. They concluded that possible clinical application of acellular nerve allograft enriched with BM-MSCs should be optimized by the creation of a microenvironment that facilitate cell differentiation [22]. This may explain our result, in which we did not find any improvement in regeneration of the nerve after stem cells or secretome implantation because we did not modify the microenvironment.
In the study of stem cell therapy for brachial plexus injury, literature review identified one case series, two case reports, and one cohort which showed a promising results. Lee et al. [17] in 2023 described three adult traumatic brachial plexus injury (BPI) patients who had stem cell therapies. They concluded that stem cell injection did not make the patients to be stronger faster. They also did not certain whether any of the cases will have long-term complications from the injection. No functional improvement was noted at long-term follow-up despite claims reported by the commercial entities. Thus, they concluded that stem cell therapy for brachial plexus injury is at present unethical and unsafe [17]. Two case reports described the use of stem cell therapy in BPI. Sharma et al. [16] administered autologous BMMNCs intrathecally and intramuscularly in a patient with BPI, followed by multidisciplinary rehabilitation. At the follow-up assessment of 3 and 7 months after first cell transplantation, improvements were recorded in muscle strength and movements. Electromyography (EMG) performed after the intervention showed a response in biceps and deltoid muscles suggesting the process of reinnervation at the site of injury. The patient underwent second cell transplantation 8 months after the first transplantation. Muscle wasting had completely stopped with an increase in the muscle girth. No adverse effects were noted. Improvements were maintained for 4 years. They concluded that comprehensive randomized study for this type of injury is needed to establish the therapeutic benefits of cellular therapy [16]. Thakkar et al. [23] also reported a case of a patient with BPI who had complete sensory-motor loss for 16 years with right pseudo-meningocele at C5-D1 levels and extra-spinal extension up to C7-D1, with avulsion on magnetic resonance imaging and irreversible damage. On day 14, 2.8 ml stem cell inoculum was infused under local anesthesia in right brachial plexus sheath by brachial block technique under ultrasonography guidance with a 1.5-inch-long 23 gauge needle. They found no untoward effects, and the patient had sustained recovery with reinnervation over a follow-up of 4 years documented on electromyography-nerve conduction velocity study [23]. Hogendoorn et al. [15] presented a case series of nine patients with insufficient elbow flexion (i.e., partial denervation). Patients received intramuscular escalating doses of autologous bone marrow-derived mononuclear cells, combined with tendon transfers. They found that mononuclear cell injection in partly denervated muscle of brachial plexus patients is safe, and they concluded that the results suggest enhanced muscle reinnervation and regeneration [15].
In patients with BPI, the objective findings of the patients like muscle strength and sensory performance were generally considered instead of subjective symptoms like pain or feeling of well-being. However, patient may have a normal motor and sensory function with intractable pain, and in other side, a patient’s severe pain may be relieved but his/her motor status may not improve. Thus, when evaluating the outcome of surgery for brachial plexus injury, we must also take into account the subjective changes in the patients’ feelings such as emotional well-being, social functioning, pain, and general health [24]. Patients with BPIs seek surgical treatment to improve the functional status, pain, and quality of life (QoL). Motor and sensory outcomes are assessed by medical practitioners, while QoL and functional outcomes are best evaluated by the patients themselves. Only few studies evaluating the QoL outcomes in patients with BPI. As expected, patients with BPIs scored significantly worse on almost all QoL parameters compared with the general population. Our study found that after nerve transfer and implantation with either UC-MSC or secretome, significant postoperative improvements were observed in physical functioning, role limitations, energy/fatigue, emotional well-being, social functioning, pain, general health, and DASH scores, particularly in the overall cohort and the secretome group. When we compared the mean difference of clinical outcome from preoperative to postoperative between UC-MSC and secretome groups, the UC-MSC group showed better improvement of health change in SF-36 subgroup compared to secretome group. Despite this improvement, the motoric changes remained unchanged pre- and postoperatively across all groups. The result of this study may be in line with the study by Kretschmer et al. [25]. They found that 87% of the patients studied were satisfied with the result and 83% would undergo the procedure again; however, despite high satisfaction rate, patients remained considerably disable. This can be partially explained by previous study which found that the capability to work is regarded as one of the most important preconditions for good quality of life. A person’s occupation might be a more important indicator for satisfaction with one’s life situation than the degree of physical impairment, and occupational retraining is very important for these patients. Seventy percent of those who received occupational retraining returned to work permanently, and patient’s motivation is also important for re-employment. This is the reason why despite unchanged motoric outcome, patients can have improvement in physical functioning, role limitations, energy/fatigue, emotional well-being, social functioning, pain, general health, and DASH scores because with occupational training and motivation postoperatively [25].
From the analysis, there was no significant difference in the histologic outcomes (inflammation, regeneration, and fibrosis) in overall cohort between preoperative and postoperative cohort. There was also no significant difference in mean change of the histologic outcomes (inflammation, regeneration, and fibrosis) preoperative and postoperatively between UC-MSC and secretome groups. This reflected that implantation of either UC-MSC or secretome along with nerve transfer may provide clinical improvement, while to achieve histologic improvement, further conditioning should be performed.
Conclusion
Implantation of either UC-MSC or secretome along with nerve transfer may provide clinical improvement, while to achieve histologic improvement, further conditioning should be performed.
Funding
This study was supported by a research grant from Badan Riset dan Inovasi Nasional (BRIN), a national innovation research agency from Indonesia. The grant covered expenses related to the design, implementation, and analysis of the study.
Declarations
Conflict of interest
The authors stated that they have no conflicts of interest to declare.
Ethical approval
Prior to commencement, ethical clearance for this study was obtained from the Institutional Review Board (IRB) of Faculty of Medicine, University of Indonesia–Cipto Mangunkusumo National Hospital. The study protocol adhered to the ethical principles outlined in the Declaration of Helsinki.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.U.S. Preventive Services Task Force. Screening for gestational diabetes mellitus: {U}.{S}. {Preventive} {Services} {Task} {Force} recommendation statement. Ann Intern Med. 2008 May;148(10):759–65. [DOI] [PubMed]
- 2.Oliver JD, Beal C, Graham EM, Santosa KB, Hu MS (2020) Functioning free muscle transfer for brachial plexus injury: a systematic review and pooled analysis comparing functional outcomes of intercostal nerve and spinal accessory nerve grafts. J Reconstr Microsurg 36(8):567–571 [DOI] [PubMed] [Google Scholar]
- 3.Lin JAJ, Lu JCY, Chang TNJ, Sakarya AH, Chuang DCC (2023) Long-term outcome of 118 acute total brachial plexus injury patients using free vascularized ulnar nerve graft to innervate the median nerve. J Reconstr Microsurg 39(4):279–287 [DOI] [PubMed] [Google Scholar]
- 4.Mencl L, Waldauf P, Haninec P (2015) Results of nerve reconstructions in treatment of obstetrical brachial plexus injuries. Acta Neurochir (Wien) 157(4):673–680 [DOI] [PubMed] [Google Scholar]
- 5.Kang GHY, Yong FC (2022) Shoulder abduction reconstruction for C5–7 avulsion brachial plexus injury by dual nerve transfers: spinal accessory to suprascapular nerve and partial median or ulnar to axillary nerve. J Plast Surg Hand Surg 56(2):87–92 [DOI] [PubMed] [Google Scholar]
- 6.Hogendoorn S, Duijnisveld BJ, van Duinen SG, Stoel BC, van Dijk JG, Fibbe WE et al (2014) Local injection of autologous bone marrow cells to regenerate muscle in patients with traumatic brachial plexus injury: a pilot study. Bone Joint Res 3(2):38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semita IN, Utomo DN, Suroto H, Sudiana IK, Gandi P, Lee EY et al (2023) Human amniotic epithelial cell transplantation for the repair of injured brachial plexus nerve: evaluation of nerve viscoelastic properties. Neural Regen Res 36(1):2011–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurniawan A, Ivansyah MD, Dilogo IH, Hutami WD (2023) Umbilical cord mesenchymal stem cells combined with secretome for treating congenital pseudarthrosis of the Tibia: a case series. J Orthop Surg Traumatol 33:2881–2888 [DOI] [PubMed] [Google Scholar]
- 9.Dilogo IH, Fiolin J, Feby CA, Pawitan JA, Evah L (2022) The effect of secretome, xenogenic bone marrow-derived mesenchymal stem cells, bone morphogenetic protein-2, hydroxyapatite granule and mechanical fixation in critical-size defects of rat models. Arch Bone Jt Surg 17(1):17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wedderburn LR, Varsani H, Li CKC, Newton KR, Amato AA, Banwell B et al (2007) International consensus on a proposed score system for muscle biopsy evaluation in patients with juvenile dermatomyositis : a tool for potential use in clinical trials. Arthritis Rheum Arthritis Care Res 57(7):1192–201 [DOI] [PubMed] [Google Scholar]
- 11.Kölbel H, Preuße C, Brand L, Von Moers A, Della A, Markus M et al (2021) Inflammation, fibrosis and skeletal muscle regeneration in LGMDR9 are orchestrated by macrophages. Neuropathol Appl Neurobiol. 47:856–66 [DOI] [PubMed] [Google Scholar]
- 12.Bertelli JA, Ghizoni MF (2008) Results of grafting the anterior and posterior divisions of the upper trunk in complete palsies of the brachial plexus. J Hand Surg Am 33(9):1529–1540 [DOI] [PubMed] [Google Scholar]
- 13.Carlstedt T (2009) Nerve root replantation. Neurosurg Clin N Am 20(1):39–50 [DOI] [PubMed] [Google Scholar]
- 14.Saffari S, Saffari T, Ulrich D, Hovius S, Shin A (2021) The interaction of stem cells and vascularity in peripheral nerve regeneration. Neural Regen Res 16(18):1510–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogendoorn S, Duijnisveld B, Van DS, Stoel B, Van DJ, Fibbe W et al (2014) Local injection of autologous bone marrow cells to regenerate muscle in patients with traumatic brachial plexus injury: a pilot study. Bone Jt Res 3(2):38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma A, Sane H, Gokulchandran N, Badhe P, Pai S, Kulkarni P et al (2018) Cellular therapy for chronic traumatic brachial plexus injury. Adv Biomed Res 7(1):51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee EY, Spinner RJ, Mortazavi MM, Angius D, Adeeb N, Bishop AT et al (2023) Stem cell therapy for traumatic brachial plexus injury. Acta Neurochir (Wien). 165:2011–2014. 10.1007/s00701-023-05675-7 [DOI] [PubMed] [Google Scholar]
- 18.Maza AM, Jarvis S, Lee WC (2021) NMJ-Analyser identifies subtle early changes in mouse models of neuromuscular disease. Sci Rep 11(1):12251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumarwoto T, Suroto H, Mahyudin F, Utomo D, Romaniyanto F, Prijosedjati A et al (2022) Prospect of stem cells as promising therapy for brachial plexus injury: a systematic review. Stem Cells Cloning 22(15):29–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma A, Sane H, Gokulchandran N, Pai S, Kulkarni P, Jadav J et al (2018) Cellular therapy for chronic traumatic brachial plexus injury. Adv Biomed Res 7(1):51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Widodo W, Widyahening I, Pratama I, Kuncoro M (2024) Prospect of mesenchymal stem cells in enhancing nerve regeneration in brachial plexus injury in animals: a systematic review. Arch Bone Jt Surg 12(3):149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodríguez AG, Porto SAG, Melero NC, Arufe MC (2021) Acellular nerve graft enriched with mesenchymal stem cells in the transfer of the phrenic nerve to the musculocutaneous nerve in a C5–C6 brachial plexus avulsion in a rat model. Microsurgery 42(1):57–65 [DOI] [PubMed] [Google Scholar]
- 23.Thakkar U, Vanikar A, HargovindL T (2014) Co-infusion of autologous adipose tissue derived neuronal differentiated mes- enchymal stem cells and bone marrow derived hematopoietic stem cells, a viable therapy for post-traumatic brachial plexus injury: a case report. Biomed J. 37(4):237–240 [DOI] [PubMed] [Google Scholar]
- 24.Aras Y, Aydoseli A, Sabanci PA, Akcakaya MO, Alkir G, Imer M (2013) Functional outcomes after treatment of traumatic brachial plexus injuries: clinical study. Turk J Trauma Emerg Surg 19(6):521–528 [DOI] [PubMed] [Google Scholar]
- 25.Kretschmer T, Ihle S, Antoniadis G (2009) Patient satisfaction and disability after brachial plexus surgery. Neurosurgery 65(4):189–196 [DOI] [PubMed] [Google Scholar]