Abstract
Purpose
To investigate the bioavailability of vitamin B12 from nori and to evaluate the required dosage for improving vitamin B12 nutritional status in vegetarians not using supplements.
Methods
The study design is an open-label, parallel, dose-response randomized controlled trial. Thirty vegetarians were assigned to control (no nori), low-dose (5 g nori, aiming to provide 2.4 µg vitamin B12 per day), or high-dose (8 g nori, aiming to provide 4 µg vitamin B12 per day) groups. The primary outcome was changes in vitamin B12 status as measured by serum vitamin B12, holotranscobalamin (holoTC), homocysteine (Hcy), and methylmalonic acid (MMA), and a combined score of these four markers (4cB12 score) during the four-week intervention. Dietary vitamin B12 intakes were assessed at baseline and end of the trial with a 17-item food frequency questionnaire designed for vitamin B12 assessment. General linear model was used to compare least square means of changes in each biomarker of vitamin B12 status, among the three groups, while adjusting for respective baseline biomarker.
Results
After adjusting for baseline status, nori consumption led to significant improvement in serum vitamin B12 (among-group P-value = 0.0029), holoTC (P = 0.0127), Hcy (P = 0.0225), and 4cB12 (P = 0.0094). Changes in MMA did not differ significantly across groups, but showed within-group pre-post improvement in the low-dose group (median [p25, p75] = -339 [-461, -198] nmol/L). Vitamin B12 status appeared to plateau at low dose (5 g of nori), which compared with control group, improved serum vitamin B12 (lease square mean [95% CI] = + 59 [25, 93] pmol/L, P = 0.0014); holoTC (+ 28.2 [10.1, 46.3] pmol/L, P = 0.0035); Hcy (-3.7 [-6.8, -0.6] µmol/L, p = 0.0226); and 4cB12 score (+ 0.67 [0.24, 1.09], p = 0.0036). High-dose resulted in similar improvements. There was no significant difference between low-dose and high-dose groups in all biomarkers of vitamin B12.
Conclusions
Consuming 5 g of nori per day for 4 weeks significantly improved vitamin B12 status in vegetarians. A higher dose (8 g) may not confer additional benefits.
Clinical trial registration
ClinicalTrials.gov Identifier: NCT05614960. Date of registration: November 14th 2022.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00394-024-03505-9.
Keywords: Cobalamin, Purple laver, Nori, Vegetarian diet, Bioavailability
Introduction
Plant-based diets may be the most effective strategy and a necessary step in keeping green-house gas emission and other environmental burdens within the planetary boundary [1]. In the EPIC-Oxford cohort, dietary green-house gas emission of vegans was only 25% of high meat-eaters [2]. However, global adaptation of vegetarian or vegan diets could potentially worsen vitamin B12 deficiency to an unprecedented level [3, 4]. In a Taiwanese cohort, 26% of vegetarians versus 1% of omnivores were found to be vitamin B12 deficient (plasma vitamin B12 < 200pg/mL) [5]. Before launching plant-based diets at a global scale to combat climate change, local innovative food-based solutions for vitamin B12 are needed.
Edible algae and sea vegetables – common foods in East Asian cultures – have long been a controversial source of vitamin B12 for vegetarians as some algae may contain inactive analogues that compete for absorption and impair vitamin B12 status [6]. Amongst commonly consumed sea vegetables, purple laver (Neopyropia sp, previously Porphyra sp.) used to make nori, had been found to contain true vitamin B12 rather than analogues, and at a level that could easily achieve the recommended dietary allowance (RDA) of 2.4 µg, that it has been suggested as a source of vitamin B12 for vegetarians [7]. A previous study also showed that feeding nori to vitamin B12-deficient Wistar rats rendered urinary methylmalonic acid (MMA) – a functional marker that decreases when deficiency is dissolved – undetectable [8].
Western nutrition experts tend to advice against the use of sea vegetables for vitamin B12 [4, 9]. The vegetarian position paper of the Academy of Nutrition and Dietetics stated: “Fermented foods (such as tempeh), nori, spirulina, chlorella algae, and unfortified nutritional yeast cannot be relied upon as adequate or practical sources of B-12” [9]. In addition, a study suggested that while raw nori contains true B12 and dried nori may contain analogues [10]. In Taiwan, the most commonly consumed form is roasted nori. Whether vitamin B12 from roasted nori is bioavailable has never been tested through a well-designed randomized controlled trial in human. As each biomarker of vitamin B12 has its own limitation, inclusion of a panel of biomarkers – serum or plasma vitamin B12, holotranscobalamin (holoTC), homocysteine (Hcy) and MMA [11] – is needed to rigorously evaluate whether nori could be recommended as a food source of vitamin B12.
This study aimed to answer two questions: (1) could nori improve vitamin B12 status and therefore be recommended as a food source of vitamin B12 for vegetarians? (2) what is the optimal dosage if nori were to be used as a source of vitamin B12 for vegetarians? This study planned to test two dosages: 2.4 µg/d (RDA of vitamin B12 in USA and Taiwan) [12, 13], and 4 µg/d (the adequate intake [AI] recommended by European Food Safety Authority [EFSA]) [14].
Methods
Study design and participants
This dose-response trial utilized simple randomization to assign eligible participants at a ratio of 1:1:1 to the following groups: (1) control group; (2) low-dose group; (3) high-dose group. A random sequence of numbers (1 or 2 or 3, each corresponding to one group) was generated by the first and last authors, using Microsoft Excel; participants were enrolled following this random sequence, based on the time they joined the study. There was no blinding as participants had to consume provided nori (impossible to mask). The study advertised for participant recruitment through social media, including Facebook post of Taiwan Vegetarian Nutrition Society and Campus-wide email system and posters at Fu Jen Catholic University. Thirty vegetarians were recruited in November 2022. The trial was conducted from November to December 2022. Participants were asked to complete a demographic questionnaire (including age, sex, education, exercise habits, occupation, medical history, use of alcohol, cigarettes, and betel nuts) at enrollment.
The inclusion criteria were: age 20 to 60 years old, being vegetarian (vegan, ovo-vegetarian, lacto-vegetarian, lacto-ovo vegetarian) for at least 1 year, did not use supplements containing vitamin B12 or folate or fortified nutritional yeast in the past year. Participants were excluded if they reported having anemia, gastrointestinal diseases and surgeries, taking antacids or metformin in the past week or were alcoholic and not willing to abstain from alcoholic beverages. The study was conducted at Fu Jen Catholic University and the protocol was approved by the Institutional Review Board (approval number: C110211) at this university. All participants signed informed consent before joining the study. The trial was pre-registered at ClinicalTrials.gov (NCT05614960).
Intervention
The intervention lasted for four weeks. Control group received no nori and were instructed not to start the habit of consuming large amount of nori or any fortified foods and supplements. The intervention groups were provided with nori (for four weeks) on the day of first blood draw. Each package contained 26 sheets of nori. Low-dose group participants were provided with 5 packages and instructed to consume 4 sheets per day (5 g, initially estimated to contain 2.4 µg vitamin B12, corresponding to RDA in Taiwan and United States). High-dose group participants were provided with 8 packages and instructed to consume and 7 sheets per day (8 g, initially estimated to contain 4 µg vitamin B12, corresponding to AI in EFSA in European countries). Participants were instructed not to share this nori with family and friends, to maintain their usual diet, lifestyle and physical activities, and to continue avoiding any supplemental form of vitamin B12 (including multi-vitamins minerals) and nutritional yeast during the study period. A reminder of these study rules was printed on a post card (for participants) and on each package of nori provided. Participants who previously consume eggs, dairy, and fortified plant-milk were asked not to change the habits. The LINE smart phone application (a social media similar to What’s App, and widely used in Taiwan) had been used to regularly remind the participants to consume their prescribed daily nori.
Assessment of vitamin B12 content in nori
Vitamin B12 content of the nori samples were analyzed at the United Graduate School of Agricultural Sciences, Tottori University (Tottori, Japan). Nori samples were first extracted by KCN-boiling method, and vitamin B12 compounds were eluted and purified from extracts using Sep-Pak C18 cartridge and B12 immunoaffinity column. The purified compounds were analyzed using reversed-phase HPLC, as previously described [15].
During the pre-planning stage, four brands of commercially available unflavored roasted nori, and one fresh nori harvested from Penghu island near Taiwan were purchased and tested for vitamin B12 content, and all of them contained true vitamin B12 (rather than analogues), as detailed in Table S1. We chose one commercial brand with opaque packaging and containing the highest vitamin B12 (48.4 µg/100 g) for the intervention, as transparent packaging may expose nori to light and contribute to vitamin B12 photo-degradation.
However, when we sampled the nori from the batch used for the actual trial, it contained a lower amount of vitamin B12 (38.6 µg/100 g). This value would actually change the estimated vitamin B12 content to 1.9 and 3.1 µg for 5 g and 8 g of nori, respectively. These values were used for actual computation when assessing vitamin B12 intakes from nori. We also sampled the same brand of nori at different time throughout the year (Table S2).
Assessment of vitamin B12 nutritional status
The primary outcome of this trial was changes in vitamin B12 nutritional status (serum vitamin B12, holoTC, MMA, Hcy, and a combined score of these four markers) over the four-week intervention. Overnight fasting venous blood were collected (refrigerated at 4oC immediately) and sent to Chung-Yi Clinical Laboratory (New Taipei City, Taiwan) to assess serum vitamin B12, folate, and Hcy within the same day. Serum vitamin B12 and folate concentrations were analyzed using electrochemiluminescence immunoassay (Roche cobas e601). Hcy was analyzed using Chemiluminescent microparticle immuno assay (Abbott ARCHITECT 1L71/ABRL004/R4). The remaining blood samples (for MMA and holoTC) were centrifuged at 3000 rpm for 15 min at 4℃ shortly after collection, and stored at a -80℃ freezer for analysis of serum MMA and holoTC at the end of the trial. Serum MMA was analyzed by the Department of Chemistry, Fu Jen Catholic University (New Taipei City, Taiwan) using liquid chromatography with tandem mass spectrometry (LC–MS-MS) (Sigma-Aldrich M54058). Serum holoTC was analyzed using an ELISA kit (IBL-International) by Yi-Her Laboratory (Yilan, Taiwan). The Four combined index of vitamin B12 score (4cB12) score was calculated according to published Eqs. [16, 17] using serum vitamin B12 concentration, holoTC, Hcy, MMA, and age, as shown below:
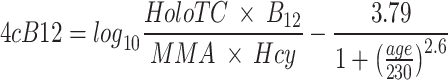 |
As improvement in vitamin B12 nutritional status would result in increases in serum vitamin B12 and holoTC, and decreases in functional biomarkers including both MMA and Hcy (raise during deficiency), the equation of 4cB12 score is a combined evaluation of all four biomarkers, and its increase indicates improvement in vitamin B12 nutritional status. A 4cB12 score < -1.5 indicates possible vitamin B12 deficiency [18]. The diagnosis cut points of vitamin B12 deficiency used in this study were serum vitamin B12 concentration < 148 pmol/L [19–21], holoTC < 35 pmol/L [22, 23], Hcy > 12 µmol/L [24–26], MMA > 271 nmol/L [12, 13], 4cB12 score < -1.5 [18].
Assessment of adherence and dietary vitamin B12 intakes
A quantitative food frequency questionnaire (FFQ) – that inquires both frequency and portion size – designed specifically to assess vitamin B12 intake – had been administered (through face-to-face interview) twice, once at baseline, and once at the end of the 4-week intervention. The FFQ includes use of supplements and nutritional yeast (in the past one year); and in the past one month, consumption of 15 main sources of vitamin B12 for vegetarians and vegans in Taiwan: milk, liquid yogurt, yogurt, cheese, eggs, nori, mushroom, kimchi, fermented tofu, and the six available brands of plant-milk that are fortified with vitamin B12. If participants reported use of any supplement that could potentially contain vitamin B12 (such as multi-vitamin or B-complex supplements), they were asked to provide photos of the brand and nutrition label of the supplement to ensure that it does not contain vitamin B12. Those who consume supplemental vitamin B12 in any form were excluded from the study.
The adherence score was calculated by total intake of nori from post-intervention FFQ, divided by the study-prescribed amount of nori (according to study group assignment) x 100%. Complete adherence (consuming all nori assigned by the study) would result in a score of 100% (maximum attainable), while consuming half of the nori would result in a score of 50%.
Statistical analysis
Statistical analysis was performed using SAS version 9.4 Software (SAS Institute, Cary, NC, USA). Intention-to-treat (ITT) approach was used to analyze data. Continuous variables of baseline characteristics were presented as mean ± SD or median (p25, p75), and categorical variables were expressed as frequencies and percentages. Comparison of baseline characteristics among three groups were performed using the ANOVA or Kruskal-Wallis test (continuous variables) and Fisher’s exact test (categorical variables). The within- and among-group changes in medians of dietary vitamin B12 intakes and biochemical parameters were compared using the Wilcoxon-signed rank test and Kruskal-Wallis test, respectively. P-values below 0.05 were considered statistically significant. Post-hoc comparisons between groups were conducted using Dwas, Steel, Critchlow-Fligner procedure when Kruskal-Wallis tests were significant. General linear model was used to compare least square means of changes in each biomarker of vitamin B12, while adjusting for respective baseline value (for example, comparing changes of holoTC in three groups as outcome, while adjusting for baseline holoTC). Log transformation was applied to improve normality when the residuals of linear regression showed deviation from normality as per graphical inspection.
G*Power version 3.1.9.4 was used to perform sample size estimation. For fixed-effect one-way ANOVA, alpha error = 0.05, power = 0.80, a sample size of 24 participants were needed to detect an effect size of 0.74 – estimated based on our preliminary data of another study (in which vegetarian participants adopting a vegan diet were instructed to consume 5 g of nori per day). We therefore planned for 30 participants (10 in each group), allowing potential drop out or non-adherence. We also conducted post-hoc power analysis for each of the vitamin B12 biomarkers using the effect size derived from the current study.
Graphical plots (violin plots and bar charts) were made using Matplotlib (version 3.8.3) in Python (version 3.12.2) [27].
Results
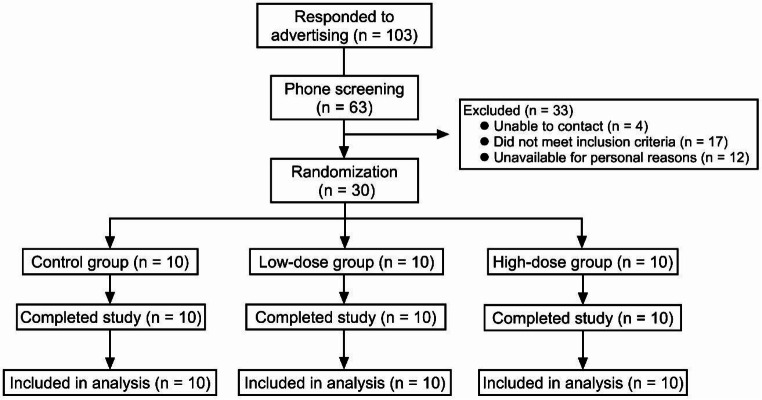
Figure 1 shows the flow chart of the study. All participants (ten in each group) completed the study. Adherence was good for all participants (consuming more than 85% of study-prescribed nori), except for one individual in the high-dose group who consumed only 30% of the prescribed nori (due to tiredness of the taste). No one reported any adverse events throughout the study. All participants were included in the analyses.
Fig. 1.
Flow chart of study participants
Participant characteristics
Table 1 presents the baseline demographic and nutritional characteristics of participants. 70% of the participants were women, and the majority had normal body weight (83% participants had BMI < 24 kg/m2, mean ± SD: 20.9 ± 3.2 kg/m2). Age ranged from 20 to 50 (mean ± SD: 31.4 ± 9.2) years old. The average duration of a vegetarian diet was 15.0 ± 9.3 years. Most of the participants (80%) were lacto-ovo vegetarians. The median 4cB12 scores in the three groups were at the level of low vitamin B12 status. Among the chosen cut points of deficiency, MMA > 271 nmol/L showed the highest proportion of participants with vitamin B12 deficiency. All baseline variables among three groups showed no significant differences.
Table 1.
Baseline characteristics of study participants
| Characteristics | Control group (n = 10) | Low-dose group (n = 10) | High-dose group (n = 10) | P-value |
|---|---|---|---|---|
| Women, n (%) | 8 (80.0) | 7 (70.0) | 6 (60.0) | 0.88 |
| Age (years) | 33.4 ± 10.0 | 31.3 ± 8.3 | 29.7 ± 9.8 | 0.68 |
| Weight (kg) | 56.5 ± 11.3 | 54.1 ± 7.3 | 62.1 ± 17.6 | 0.37 |
| Body mass index (kg/m2) | 21.0 ± 2.6 | 19.5 ± 2.6 | 22.0 ± 4.0 | 0.21 |
| Active physical activity*, n (%) | 1 (10.0) | 3 (30.0) | 5 (50.0) | 0.20 |
| Duration of vegetarian diets (years) | 19.4 ± 11.9 | 11.5 ± 7.0 | 14.0 ± 7.1 | 0.15 |
| Vegan, n (%) | 2 (20.0) | 3 (30.0) | 1 (10.0) | 0.85 |
| Serum vitamin B12 (pmol/L) | 242.1 [206.6, 282.7] | 174.5 [155.7, 203.7] | 183.4 [163.8, 233.9] | 0.17 |
| HoloTC (pmol/L) | 35.9 [21.7, 48.7] | 37.1 [11.2, 44.2] | 29.0 [14.1, 53.3] | 0.63 |
| Hcy (µmol/L) | 13.5 [12.2, 17.2] | 11.4 [10.2, 17.3] | 14.9 [11.2, 20.6] | 0.80 |
| MMA (nmol/L) | 671.8 [632.2, 789.6] | 864.6 [548.2, 934.3] | 398.4 [221.6, 1165.8] | 0.44 |
| 4cB12 score | -0.8 [-1.2, -0.7] | -0.9 [-2.0, -0.6] | -0.9 [-1.2, -0.5] | 0.80 |
| Serum folate (nmol/L) | 15.3 [13.8, 21.5] | 23.0 [19.9, 28.3] | 23.7 [18.1, 27.2] | 0.06 |
| Vitamin B12 deficiency, n (%) | ||||
| Serum vitamin B12 < 148 pmol/L | 1 (10.0) | 2 (20.0) | 1 (10.0) | 1.00 |
| HoloTC < 35 pmol/L | 5 (50.0) | 5 (50.0) | 7 (70.0) | 0.72 |
| Hcy > 12 µmol/L | 8 (80.0) | 5 (50.0) | 7 (70.0) | 0.50 |
| MMA > 271 nmol/L | 10 (100.0) | 9 (90.0) | 7 (70.0) | 0.29 |
| 4cB12 score < -1.5 | 1 (10.0) | 3 (30.0) | 2 (20.0) | 0.85 |
Continuous variables were compared using ANOVA for mean ± SD or Kruskal Wallis test for median [p25, p75]. Categorical variables were compared using Fisher’s exact test and expressed as frequency (percentage). *Defined as ≧ 3 physical activities per week. Abbreviations HoloTC holotranscobalamin, Hcy, homocysteine; MMA methylmalonic acid; 4cB12 score four combined index of vitamin B12 score
Dietary vitamin B12 intake
Table 2 summarizes dietary intakes of vitamin B12 as assessed by FFQ. Dietary vitamin B12 intake at baseline were similar in all three groups, and all had median intakes substantially lower than the RDA (2.4 µg/day). After 4 weeks of intervention, significant differences among groups were found mainly attributable to nori consumption. Dietary vitamin B12 intake increased from 0.3 µg/day to 2.0 µg/day (1.9 µg from nori) for low-dose and from 0.5 µg/day to 3.5 µg/day (3.1 µg from nori) for high-dose groups. Vitamin B12 from non-nori sources were almost the same before and during the intervention in all groups.
Table 2.
Dietary intakes of vitamin B12 in control and intervention groups
| Control group (n = 10) | Low-dose group (n = 10) | High-dose group (n = 10) | Among-group P-value# | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | P25 | P75 | Median | P25 | P75 | Median | P25 | P75 | |||
| Total dietary intakes of vitamin B12 including nori (µg/day)† | Pre | 0.8 | 0.7 | 2.4 | 0.3 | 0.2 | 1.0 | 0.5 | 0.4 | 0.8 | 0.21 |
| Post | 0.9 | 0.3 | 1.6 | 2.0 ** | 1.9 | 2.8 | 3.5 ** | 3.3 | 4.1 | 0.0003 | |
| Dietary intakes of vitamin B12 from foods other than nori (µg/day) | Pre | 0.8 | 0.7 | 2.4 | 0.2 | 0.2 | 1.0 | 0.5 | 0.3 | 0.8 | 0.16 |
| Post | 0.9 | 0.3 | 1.6 | 0.2 | 0.1 | 1.0 | 0.4 | 0.3 | 0.9 | 0.10 | |
Data are presented as median, p25, p75. *P-value < 0.05; **P-value < 0.01 for within-group changes between post-intervention and pre-intervention calculated by Wilcoxon signed-rank test. # Among-group P-value comparison using Kruskal-Wallis test. † Total dietary intake of vitamin B12 at post-intervention included intakes from both non-nori sources and study-prescribed nori provided for intervention groups (nori vitamin B12 calculated using 38.6 µg/100 g assessed by our study)
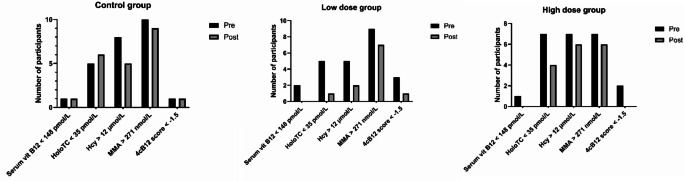
Changes in the number of participants with vitamin B12 deficiency
Figure 2 presents the changes in the number of participants with vitamin B12 deficiency over the study. The proportion with vitamin B12 deficiency was reduced after interventions in both low-dose and high-dose groups in all biomarker-diagnostic criteria. In contrast, the changes in number of participants with vitamin B12 deficiency were inconsistent across different biomarkers in the control group.
Fig. 2.
Number of vitamin B12 deficient individuals, pre- and post-intervention in each group, by different biomarkers. Abbreviations: HoloTC holotranscobalamin, Hcy homocysteine, MMA methylmalonic acid, 4cB12 score four combined index of vitamin B12 score
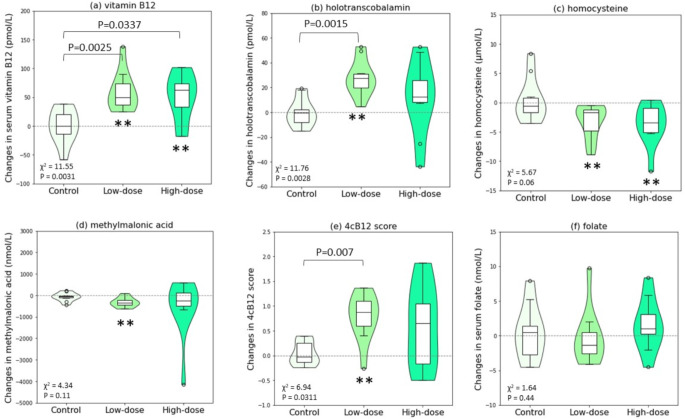
Changes in biochemical parameters throughout the study
The violin plots in Fig. 3(a) to (e) show the distribution of crude changes in vitamin B12 nutritional biomarkers throughout the study. Most of the biomarkers were approximately normal with only Hcy in low-dose group and MMA in high-dose group violating normality by Shapiro-Wilk test (P < 0.05). Significant difference across groups (as assessed by Kruskal Wallis test) was observed for serum vitamin B12, holoTC, and 4cB12 score. In control group, none of the biomarkers showed significant changes. Improvement in vitamin B12 nutritional status was evident in the low-dose group, as significant pre-post increases in serum vitamin B12 (median [p25, p75]: +49.8 [36.9, 77.5] pmol/L), holoTC (+ 27.3 [19.8, 31.1] pmol/L) were accompanied by significant decreases in Hcy (-1.7 [-5.3, -1.2] µmol/L) and MMA (-339 [-461, -198] nmol/L), and together resulted in a significant increase in 4cB12 score (+ 0.9 [0.6, 1.1]). All participants showed improvement in all four vitamin B12 biomarkers except one participant who experienced a slight increase in MMA; however, this participant had a very low MMA value to begin with, and that both pre- and post- MMA concentration (from 49 to 142 nmol/L) were well below the cut point for deficiency (> 271 nmol/L). The detailed changes of biomarkers for each individual could be found in Fig. S1. The high-dose group generally showed similar trends of improvement, though statistically significant pre-post improvement was observed only in serum vitamin B12 (+ 62 [31, 76] pmol/L) and Hcy (-3.5 [-5.3, -0.7]). For all the vitamin B12 nutritional biomarkers, there were no significant differences between low-dose and high-dose groups. Both within-group and among-group changes in serum folate were insignificant, as shown in Fig. 3(f).
Fig. 3.
Violin plots of distribution in changes in serum (a) vitamin B12, (b) holotranscobalamin, (c) homocysteine, (d) methylmalonic acid, (e) 4cB12 score, and (f) folate. *P-value < 0.05; **P-value < 0.01 for within-group changes comparing pre- and post-intervention, calculated by Wilcoxon signed-rank test. Across-group comparison were performed using Kruskal-Wallis test (χ2 and P-value shown in lower left corner); when this test is significant, between-group differences were calculated using Dwass, Steel, Critchlow-Fligner procedure. Abbreviations: 4cB12 score, Four combined index of vitamin B12 score
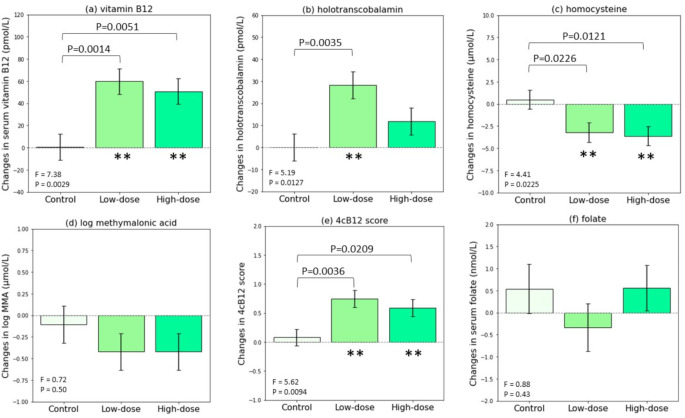
Figure 4(a) to (e) illustrate the least square means (standard errors) of changes in vitamin B12 biomarkers estimated through general linear model while adjusting for respective baseline status. Log transformation was applied to MMA to improve normality of the residuals. Significant differences across the groups were observed for serum vitamin B12, holoTC, Hcy, 4cB12 score but not log (MMA). Compared with control group, low-dose (5 g nori) group showed significant improvement in serum vitamin B12 (lease square mean [95% CI] = + 59 [25, 93] pmol/L, P = 0.0014); holoTC (+ 28.2 [10.1, 46.3] pmol/L, P = 0.0035); Hcy (-3.7 [-6.8, -0.6] µmol/L, P = 0.0226); and 4cB12 score (+ 0.67 [0.24, 1.09], P = 0.0036). High-dose group showed similar effects with statistical significance in serum vitamin B12 (+ 50 [16, 84] pmol/L, P = 0.0051), Hcy (-4.11 [-7.24, -0.98] µmol/L, P = 0.0121), and 4cB12 (+ 0.51 [0.08, 0.94], P = 0.0209) compared with control. We conducted post-hoc power analyses using the effect size and sample size of this current study, with alpha = 0.05, the power (in bracket) for each of the vitamin B12 biomarkers: serum vitamin B12 (0.93), holoTC (0.78), Hcy (0.71), log-transformed MMA (0.16), and 4cB12 (0.82). Both within-group and among-group changes in serum folate were insignificant, as shown in Fig. 4(f).
Fig. 4.
General linear model estimated least square means of changes in (a) serum vitamin B12, (b) holotranscobalamin, (c) homocysteine, (d) methylmalonic acid, (e) 4cB12 score, and (f) folate, while adjusting for respective baseline levels. *P-value < 0.05; **P-value < 0.01 for within-group changes comparing pre- and post-intervention, by t test provided by the general linear model. Abbreviations: 4cB12 score, Four combined index of vitamin B12 score
Discussion
This study suggests that at 5 g per day, nori may improve vitamin B12 nutritional status for vegetarians, as evidenced by improvement in serum vitamin B12, holoTC, Hcy, and 4cB12 score. A higher dose (8 g/d) did not confer additional benefits though no apparent harm either.
Bioavailability of vitamin B12 from nori
Our result provides clinical evidence to support previous chemical analyses that nori contains true vitamin B12 rather than inactive analogues [28]. If nori contains substantial amount of harmful analogues as previously suggested [6], functional markers such as Hcy and MMA would have worsen. The lowering of Hcy was unlikely influenced by folate nutritional status as serum folate did not change.
Dagnelie’s study over 30 years ago showed that five vegan children consuming algae experienced an increase of mean corpuscular value, indicating worsening of macrocytic anemia [6]. Participants in this study did not consume just nori but also spirulina and wakame, which had been reported to contain pseudo-vitamin B12 [29] and could negatively impact vitamin B12 status. The study used a FFQ to assess dietary intake, and potential error for misreporting could occur if the participants could not distinguish between different types of sea vegetables.
Yamada’s study fed raw and dried nori to college students and found that 40 g of dried nori increased urinary MMA and suggested that the drying process may have converted vitamin B12 to harmful analogues [10]. We used roasted nori (also quite dry) in our study but are unsure of how the detailed processing of nori in Taiwan and those Yamada used differ. We found that 8 g of unflavored nori was already beyond what some people could comfortably consume in a day that it is impractical to suggest vegetarians to consume the amount tested in Yamada’s study (40 g), though we could not rule out that at such an extreme intake (and with different food processing techniques), substantial analogues could be a problem.
Improvement in vitamin B12 relevant biomarkers in our study is comparable to (and perhaps better than) other vegetarian/vegan studies supplementing milk [30] whey powder [31] or vitamin B12-fortified toothpaste [32] in vegetarians and vegans. The responses in vitamin B12 biomarkers in our study were also comparable to those found in a meta-analysis of trials using oral vitamin B12 supplements (dosage ranging from 10 to 1500 µg/d), for MMA (-280 nmol/L) and Hcy (-3.3 µmol/L), though less potent for serum vitamin B12 (185 pmol/L) [33].
Overall, nori consumption improved vitamin B12 biomarkers – including both total and active vitamin B12 concentrations and functional markers – at levels comparable to other reliable sources, such as dairy, fortified toothpaste, and vitamin B12 supplements.
Possible reasons for lack of dose-response effect
The effect on vitamin B12 status appear to plateau at low-dose (5 g of nori per day), and there appears to be a lack of dose-response effect. Two reasons may potentially explain this observation.
First, vitamin B12 absorption is dependent on binding to the intrinsic factor (IF). The IF-B12 complex is absorb by cubilin-amnionless receptor via the endocytosis route in the ileum and this route is saturated at 2 µg of vitamin B12 per meal [11, 34]. According to our FFQ assessment, 6 of 10 participants (60%) in the low-dose group and 7 of 9 adherent participants (77%) in the high-dose group consumed all daily nori in one incidence. The dose of 5 g of nori (estimated to contain 1.9 µg vitamin B12/day) in one meal likely had reached the saturation point of vitamin B12, while a higher dose would have past the saturation point that the surplus may not be well absorbed. Our finding is echoed by another trial that found similar effects in low dose (350 µg/week) and high dose (2000 µg/week) vitamin B12 supplements in restoring vitamin B12 status in vegetarians and vegans with marginal deficiencies [35].
Second, there may potentially be variation in each package of nori, as these are natural food products rather than supplements or medication that could be manufactured with a high dosage precision. We surveyed the vitamin B12 content of the same brand of nori purchased on different dates, and found the vitamin B12 content ranged from 23.1 to 52.8 µg/100 g dry weight (Table S1 and S2).
Is nori a reliable food source of vitamin B12?
Most plant foods – tempeh or other fermented products, organic vegetables grown with manure fertilizers – are unreliable sources of vitamin B12, as their vitamin B12 contents depend on haphazard contamination or adventitious presences of vitamin B12-producing microbes. On the contrary, Takenaka et al.’s experiment showed that purple laver cultured aseptically (treated with antibiotics) in medium devoid of vitamin B12 contained 50 ± 2 µg of vitamin B12 per 100 g dried weight, suggesting that vitamin B12 in purple laver is not by contamination and that purple laver may be able to biosynthesize cobalamin from within [36].
One possible objection to recommending nori as a food source of vitamin B12 is the variability of vitamin B12 contents. We sampled and tested vitamin B12 contents of all major brands we could find and at six different times throughout the year and all of them appeared to contain substantial amount of vitamin B12. Even the sample with the lowest amount (23.1 µg/100 g), if consumed at 5 g/d, could provide about 1.2 µg (50% RDA). Other published data also consistently showed similar levels, ranging from 28.9 (seasoned and toasted) to 133.8 µg/100 g (dried purple laver) [15, 28, 36]. Although more sampling and testing are always warranted, the consistency of the results suggests that nori is likely a reliable source of vitamin B12. Our study found that 5 g of nori per day improved vitamin B12 intakes (from a meagre 0.3 µg to 2.0 µg [83% RDA]) in vegetarians, accompanied by improvement in vitamin B12 biomarkers.
However, consuming 5 g of nori a day did not completely eliminate vitamin B12 deficiency (depending on deficiency diagnosis criteria) in four weeks in our study, as this amount (providing 1.9 µg/day) may be insufficient. Studies using both factorial approaches and intakes associated with optimal biomarker levels had suggested that 4–20 µg per day may be needed to compensate for daily lost and to optimize vitamin B12 nutritional status [37, 38]. A trial also showed that supplementing 5.6 µg vitamin B12 per day (2.8 µg, twice daily) either through supplements or whey powder, for 8 weeks, improved but did not normalize vitamin B12 status in Indian lacto-ovo-vegetarians [31]. On the other hand, vegetarians and vegans in Adventist Health Study-2 showed excellent vitamin B12 status (even better than nonvegetarians of the same cohort), attributable to vitamin B12 from supplements and fortified foods [39]. Inclusion of vitamin B12 fortified foods could greatly increase food varieties (lessen the likelihood of consumption fatigue of nori), providing different options for different meals, and the increase in consumption frequency may enhance total vitamin B12 absorption.
One word of caution is that our study supports only the bioavailability of vitamin B12 in nori. Other algae, such as spirulina and wakame, have been found to contain analogues [29, 40] and should not be confused with nori. As the amount of vitamin B12 and analogues could vary substantially amongst different sea vegetables, each sea vegetable needs to be tested and recommended individually, and not lumped together as one food group. Besides nori, other promising sea vegetables, such as Wolffia globosa duckweed [41] and Taiwanese laver (hong-mao tai) [42] have been shown to increase vitamin B12 biomarker in human and warrant more rigorous testing.
Strengths and limitations
This study has several strengths. First, the randomized controlled dose-response design enabled us to investigate the effect of nori on vitamin B12 nutritional status with less bias than previous observational studies, and allowed us to identify an optimal dosage for recommendation and future research. Second, the biomarkers analyzed in our study were comprehensive and reliable for assessing vitamin B12 status. Third, the selection long-term vegetarians (not taking any vitamin B12 supplements) as participants enabled us to test effect of nori in correcting low vitamin B12 status due to inadequate intakes rather than malabsorption; most participants were highly motivated, collaborative, interested in the research question, and had good adherence. Moreover, at the time of this study, there were only very few foods fortified with vitamin B12 (only a few imported plant-based milks and none of the meat analogues) in Taiwan, that the chance of confounding by other fortified foods were low. In addition, we analyzed the vitamin B12 content in nori of major brands commercially available, and at different seasons throughout the year to evaluate the variation of vitamin B12 content in nori to support our understanding on the reliability and the generalizability of nori as a source of vitamin B12.
There are some limitations in the current study. First, the small sample was inadequately powered to detect the among-group differences in MMA. Second, blinding was difficult, as this was a food-based trial that it was impossible to create placebo identical in looks and tastes. Third, we tested only the duration of 4 weeks and thus unable to conclude on long-term effects. Fourth, vitamin B12 related biomarkers may be affected by genetics, gut microbiome, and other absorptive problems beyond our team’s capacity to assess, though we think these confounders would likely bias the findings toward the null. Fifth, the FFQ used in this study has not undergone a formal validation; however it included nearly all available food sources of vitamin B12 for vegetarians, and its correlations with biomarkers in an initial assessment in the present study (r = 0.30 for holoTC, r = − 0.46 for Hcy) were comparable to previous validation of FFQs (r = 0.33 for holoTC) [43] and (r = − 0.44 for Hcy) [44]. Sixth, vegetarian diets may contain analogues from other foods that hampers vitamin B12 nutritional status. Previous studies have reported analogues in wakame, spirulina, lion’s mane mushroom, and even sea animals such as abalone and escargot [29, 40, 45], but database of analogues is lacking and not well studied. Lastly, participants in this study were young and our results may not be applicable to older individuals.
Conclusion
This study suggests that that nori – in contrast to other algae – may improve vitamin B12 nutritional status of vegetarians at 5 g per day in 4 weeks, and may potentially be a novel option to support vitamin B12 needs for those choosing plant-based diets. Future studies using this dosage on larger sample sizes are warranted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- 4cB12
Four combined index of vitamin B12
- AI
Adequate intake
- FFQ
Food frequency questionnaire
- HoloTC
Holotranscobalamin
- Hcy
Homocysteine
- MMA
Methylmalonic acid
- RDA
Recommended dietary allowance
Author contributions
QNH and THC designed the research, conducted the research, analyzed data and wrote the manuscript. FW and KK: analyzed vitamin B12 content in nori. HLL and REH: analyzed serum MMA. THC had the primary responsibility for the final content. All authors read and approved the final manuscript.
Funding
This work was supported by National Science and Technology Council [grant number 111-2320-B-030 -011 -MY3 and MOST 109-2320-B-030 -013 -MY2]. The funder played no role in the conduct of the study, the analysis of the data, or the preparation of this manuscript
Data availability
As this study contains only a small number of participants, some of the personal info that may assist in deduction and reveal the identity of participants have been deleted in order to protect the privacy of these individuals.
Declarations
Competing interests
No potential conflict of interests was reported by all authors.
References
- 1.Springmann M, Clark M, Mason-D’Croz D, Wiebe K, Bodirsky BL, Lassaletta L, de Vries W, Vermeulen SJ, Herrero M, Carlson KM, Jonell M, Troell M, DeClerck F, Gordon LJ, Zurayk R, Scarborough P, Rayner M, Loken B, Fanzo J, Godfray HCJ, Tilman D, Rockström J, Willett W (2018) Options for keeping the food system within environmental limits. Nature 562(7728):519–525. 10.1038/s41586-018-0594-0 [DOI] [PubMed] [Google Scholar]
- 2.Scarborough P, Clark M, Cobiac L, Papier K, Knuppel A, Lynch J, Harrington R, Key T, Springmann M (2023) Vegans, vegetarians, fish-eaters and meat-eaters in the UK show discrepant environmental impacts. Nat Food 4(7):565–574. 10.1038/s43016-023-00795-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elmadfa I, Singer I (2009) Vitamin B-12 and homocysteine status among vegetarians: a global perspective. Am J Clin Nutr 89(5):1693S–1698S. 10.3945/ajcn.2009.26736Y [DOI] [PubMed] [Google Scholar]
- 4.Niklewicz A, Smith AD, Smith A, Holzer A, Klein A, McCaddon A, Molloy AM, Wolffenbuttel BHR, Nexo E, McNulty H, Refsum H, Gueant JL, Dib MJ, Ward M, Murphy M, Green R, Ahmadi KR, Hannibal L, Warren MJ, Owen PJ (2023) The importance of vitamin B(12) for individuals choosing plant-based diets. Eur J Nutr 62(3):1551–1559. 10.1007/s00394-022-03025-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu THT, Chang HR, Wang LY, Chang CC, Lin MN, Lin CL (2020) Vegetarian diet and incidence of total, ischemic, and hemorrhagic stroke in 2 cohorts in Taiwan. Neurology 94(11):e1112–e1121. 10.1212/wnl.0000000000009093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagnelie PC, van Staveren WA, van den Berg H (1991) Vitamin B-12 from algae appears not to be bioavailable. Am J Clin Nutr 53(3):695–697. 10.1093/ajcn/53.3.695 [DOI] [PubMed] [Google Scholar]
- 7.Watanabe F, Yabuta Y, Bito T, Teng F (2014) Vitamin B₁₂-containing plant food sources for vegetarians. Nutrients 6(5):1861–1873. 10.3390/nu6051861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takenaka S, Sugiyama S, Ebara S, Miyamoto E, Abe K, Tamura Y, Watanabe F, Tsuyama S, Nakano Y (2001) Feeding dried purple laver (nori) to vitamin B12-deficient rats significantly improves vitamin B12 status. Br J Nutr 85(6):699–703. 10.1079/bjn2001352 [DOI] [PubMed] [Google Scholar]
- 9.Melina V, Craig W, Levin S (2016) Position of the Academy of Nutrition and Dietetics: vegetarian diets. J Acad Nutr Diet 116(12):1970–1980. 10.1016/j.jand.2016.09.025 [DOI] [PubMed] [Google Scholar]
- 10.Yamada K, Yamada Y, Fukuda M, Yamada S (1999) Bioavailability of dried asakusanori (porphyra tenera) as a source of cobalamin (vitamin B12). Int J Vitam Nutr Res 69(6):412–418. 10.1024/0300-9831.69.6.412 [DOI] [PubMed] [Google Scholar]
- 11.Green R (2017) Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood 129(19):2603–2611. 10.1182/blood-2016-10-569186 [DOI] [PubMed] [Google Scholar]
- 12.Institute of Medicine (2006) Dietary reference intakes: the essential guide to nutrient requirements. National Academies, Washington, DC. 10.17226/11537 [Google Scholar]
- 13.Chen K, Weng M, Huang R, Lin B, Shiau S (2023) Dietary reference intakes: vitamin B12. edn. Ministry of Health Taiwan, Taipei, p 8 [Google Scholar]
- 14.EFSA Panel on Dietetic Products N, Allergies (2015) Scientific opinion on Dietary reference values for cobalamin (vitamin B12). EFSA J 13(7):4150. 10.2903/j.efsa.2015.4150 [Google Scholar]
- 15.Koseki K, Yoshimura R, Ido K, Katsuura K, Bito T, Watanabe F (2023) Determination of vitamin B(12) and Folate compounds in commercially available Edible Seaweed products. Front Biosci (Elite Ed) 15(2):10. 10.31083/j.fbe1502010 [DOI] [PubMed] [Google Scholar]
- 16.Jarquin Campos A, Risch L, Nydegger U, Wiesner J, Vazquez Van Dyck M, Renz H, Stanga Z, Risch M (2020) Diagnostic accuracy of Holotranscobalamin, vitamin B12, Methylmalonic Acid, and Homocysteine in Detecting B12 Deficiency in a large, mixed patient Population. Dis Markers 2020:7468506. 10.1155/2020/7468506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fedosov SN (2013) Biochemical markers of vitamin B12 deficiency combined in one diagnostic parameter: the age-dependence and association with cognitive function and blood hemoglobin. Clin Chim Acta 422:47–53. 10.1016/j.cca.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 18.Fedosov SN, Brito A, Miller JW, Green R, Allen LH (2015) Combined indicator of vitamin B12 status: modification for missing biomarkers and folate status and recommendations for revised cut-points. Clin Chem Lab Med 53(8):1215–1225. 10.1515/cclm-2014-0818 [DOI] [PubMed] [Google Scholar]
- 19.Hunt A, Harrington D, Robinson S (2014) Vitamin B12 deficiency. BMJ 349:g5226. 10.1136/bmj.g5226 [DOI] [PubMed] [Google Scholar]
- 20.Lee Y-P, Loh C-H, Hwang M-J, Lin C-P (2021) Vitamin B12 deficiency and anemia in 140 Taiwanese female lacto-vegetarians. J Formos Med Assoc 120(11):2003–2009. 10.1016/j.jfma.2021.04.007 [DOI] [PubMed] [Google Scholar]
- 21.Green R, Miller JW (2005) Vitamin B12 deficiency is the dominant nutritional cause of hyperhomocysteinemia in a folic acid-fortified population. Clin Chem Lab Med 43(10):1048–1051. 10.1515/cclm.2005.183 [DOI] [PubMed] [Google Scholar]
- 22.Rizzo G, Laganà AS, Rapisarda AMC, La Ferrera GMG, Buscema M, Rossetti P, Nigro A, Muscia V, Valenti G, Sapia F, Sarpietro G, Zigarelli M, Vitale SG (2016) Vitamin B12 among vegetarians: Status, Assessment and Supplementation. Nutrients 8(12):767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golding PH (2016) Holotranscobalamin (HoloTC, Active-B12) and Herbert’s model for the development of vitamin B12 deficiency: a review and alternative hypothesis. Springerplus 5(1):668. 10.1186/s40064-016-2252-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green R (2005) COBALAMINS. In: CABALLERO B, ALLEN L, PRENTICE A (eds) Encyclopedia of Human Nutrition, vol 4. Second edn. Academic Press, pp 401–406
- 25.Green R (2011) Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. Am J Clin Nutr 94(2):666S–672S. 10.3945/ajcn.110.009613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrmann W, Obeid R, Schorr H, Geisel J (2005) The usefulness of holotranscobalamin in predicting vitamin B12 status in different clinical settings. Curr Drug Metab 6(1):47–53. 10.2174/1389200052997384 [DOI] [PubMed] [Google Scholar]
- 27.Hunter JD (2007) Matplotlib: a 2D Graphics Environment. Comput Sci Eng 9(3):90–95. 10.1109/MCSE.2007.55 [Google Scholar]
- 28.Miyamoto E, Yabuta Y, Kwak CS, Enomoto T, Watanabe F (2009) Characterization of vitamin B12 compounds from Korean purple laver (Porphyra sp.) products. J Agric Food Chem 57(7):2793–2796. 10.1021/jf803755s [DOI] [PubMed] [Google Scholar]
- 29.Watanabe F, Katsura H, Takenaka S, Fujita T, Abe K, Tamura Y, Nakatsuka T, Nakano Y (1999) Pseudovitamin B(12) is the predominant cobamide of an algal health food, spirulina tablets. J Agric Food Chem 47(11):4736–4741. 10.1021/jf990541b [DOI] [PubMed] [Google Scholar]
- 30.Mahalle N, Bhide V, Greibe E, Heegaard CW, Nexo E, Fedosov SN, Naik S (2019) Comparative bioavailability of synthetic B12 and dietary vitamin B12 Present in cow and Buffalo milk: a prospective study in Lactovegetarian indians. Nutrients 11(2). 10.3390/nu11020304 [DOI] [PMC free article] [PubMed]
- 31.Naik S, Mahalle N, Greibe E, Ostenfeld MS, Heegaard CW, Nexo E, Fedosov SN (2019) Cyano-B12 or whey powder with endogenous Hydroxo-B12 for supplementation in B12 deficient lactovegetarians. Nutrients 11(10). 10.3390/nu11102382 [DOI] [PMC free article] [PubMed]
- 32.Siebert AK, Obeid R, Weder S, Awwad HM, Sputtek A, Geisel J, Keller M (2017) Vitamin B-12-fortified toothpaste improves vitamin status in vegans: a 12-wk randomized placebo-controlled study. Am J Clin Nutr 105(3):618–625. 10.3945/ajcn.116.141978 [DOI] [PubMed] [Google Scholar]
- 33.Hoey L, Strain JJ, McNulty H (2009) Studies of biomarker responses to intervention with vitamin B-12: a systematic review of randomized controlled trials. Am J Clin Nutr 89(6):1981s–1996s. 10.3945/ajcn.2009.27230C [DOI] [PubMed] [Google Scholar]
- 34.Paul C, Brady DM (2017) Comparative bioavailability and utilization of Particular forms of B(12) supplements with potential to Mitigate B(12)-related genetic polymorphisms. Integr Med (Encinitas) 16(1):42–49 [PMC free article] [PubMed] [Google Scholar]
- 35.Del Bo C, Riso P, Gardana C, Brusamolino A, Battezzati A, Ciappellano S (2019) Effect of two different sublingual dosages of vitamin B(12) on cobalamin nutritional status in vegans and vegetarians with a marginal deficiency: a randomized controlled trial. Clin Nutr 38(2):575–583. 10.1016/j.clnu.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 36.Takenaka S, Takubo K, Watanabe F, Tanno T, Tsuyama S, Nanano Y, Tamura Y (2003) Occurrence of coenzyme forms of vitamin B12 in a cultured purple laver (Porphyla Yezoensis). Biosci Biotechnol Biochem 67(11):2480–2482. 10.1271/bbb.67.2480 [DOI] [PubMed] [Google Scholar]
- 37.Bor MV, von Castel-Roberts KM, Kauwell GP, Stabler SP, Allen RH, Maneval DR, Bailey LB, Nexo E (2010) Daily intake of 4 to 7 µg dietary vitamin B-12 is associated with steady concentrations of vitamin B-12–related biomarkers in a healthy young population. Am J Clin Nutr 91(3):571–577. 10.3945/ajcn.2009.28082 [DOI] [PubMed] [Google Scholar]
- 38.‘t Doets EL (2013) Veld PH, Szczecińska A, Dhonukshe-Rutten RA, Cavelaars AE, van ‘t Veer P, Brzozowska A, de Groot LC Systematic review on daily vitamin B12 losses and bioavailability for deriving recommendations on vitamin B12 intake with the factorial approach. Ann Nutr Metab 62 (4):311–322. 10.1159/000346968 [DOI] [PubMed]
- 39.Damayanti D, Jaceldo-Siegl K, Beeson WL, Fraser G, Oda K, Haddad EH (2018) Foods and Supplements Associated with vitamin B(12) biomarkers among vegetarian and non-vegetarian participants of the Adventist Health Study-2 (AHS-2) calibration study. Nutrients 10(6). 10.3390/nu10060722 [DOI] [PMC free article] [PubMed]
- 40.Yamada S, Shibata Y, Takayama M, Narita Y, Sugawara K, Fukuda M (1996) Content and characteristics of vitamin B12 in some seaweeds. J Nutr Sci Vitaminol (Tokyo) 42(6):497–505. 10.3177/jnsv.42.497 [DOI] [PubMed] [Google Scholar]
- 41.Kaplan A, Zelicha H, Tsaban G, Yaskolka Meir A, Rinott E, Kovsan J, Novack L, Thiery J, Ceglarek U, Burkhardt R, Willenberg A, Tirosh A, Cabantchik I, Stampfer MJ, Shai I (2019) Protein bioavailability of Wolffia globosa duckweed, a novel aquatic plant - A randomized controlled trial. Clin Nutr 38(6):2576–2582. 10.1016/j.clnu.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 42.Chiu THT, Kao Y-C, Wang L-Y, Chang H-R, Lin C-L (2022) A Dietitian-Led Vegan Program May improve GlycA, and other Novel and traditional cardiometabolic risk factors in patients with dyslipidemia: a pilot study. Front Nutr 9. 10.3389/fnut.2022.807810 [DOI] [PMC free article] [PubMed]
- 43.Fraser GE, Jaceldo-Siegl K, Henning SM, Fan J, Knutsen SF, Haddad EH, Sabaté J, Beeson WL, Bennett H (2016) Biomarkers of Dietary Intake are correlated with corresponding measures from repeated Dietary recalls and food-frequency questionnaires in the Adventist Health Study-2. J Nutr 146(3):586–594. 10.3945/jn.115.225508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mogul DB, Brereton N, Carson KA, Pittarelli M, Daniel H, Torbenson M, Schwarz KB (2018) Development of a Dietary Methyl Donor Food frequency questionnaire to assess folate and vitamin B(12) status in children with chronic Hepatitis B Virus infection. J Pediatr 203:41–46e42. 10.1016/j.jpeds.2018.07.088 [DOI] [PubMed] [Google Scholar]
- 45.Watanabe F, Bito T (2018) Vitamin B(12) sources and microbial interaction. Exp Biol Med (Maywood) 243(2):148–158. 10.1177/1535370217746612 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
As this study contains only a small number of participants, some of the personal info that may assist in deduction and reveal the identity of participants have been deleted in order to protect the privacy of these individuals.