Abstract
Background
To evaluate the efficacy and safety of anlotinib combined with chemotherapy in the treatment of advanced soft tissue sarcoma (STS).
Methods
A total of 14 patients with advanced STS consisting of 4 liposarcoma, 3 undifferentiated polymorphous sarcoma, 2 synovial sarcoma, 1 Extraosseous ewing sarcoma, 1 spindle cell sarcoma, 1 Sarcomatoid malignant mesothelioma, 1 hemangiosarcoma, and 1 fibrosarcoma, were treated with anlotinib plus chemotherapy. The anlotinib was combined with chemotherapy as the first-line treatment in 13 patients, and as second-line treatment in 1 patient. Chemotherapy regimens were based on anthracyclines and ifosfamide, and other drugs included paclitaxel, and so on. Efficacy and safety were evaluated every 2 treatment cycles.
Results
According to the stage of AJCC, 9 patients were stage III and 5 patients were stage IV. The average cycle of treatment is 3.86. Among the 14 patients, 2 cases had received surgical treatment after neoadjuvant chemothrapy, 5 cases had partial response (PR), 7 cases had stable disease (SD), and 2 cases had progressive disease (PD). The overall response rate (ORR) was 35.7% (5/14). Patients who had not underwent surgical treatment were with a disease control rate (DCR) of 83.3% (10/12). The median progression free survival (PFS) was 8.25 months. The common treatment-related adverse effects included bone marrow suppression, nausea, vomiting and hypertension. Three patients had severe adverse effect, which was febrile neutropenia. No treatment-related death was found.
Conclusions
Anlotinib combined with chemotherapy in the treatment of STS is effective and tolerable, which is a promising strategy. It is worthy of further clinical research.
Keywords: Anlotinib, Chemotherapy, Advanced soft tissue sarcoma
Introduction
Soft tissue sarcoma (STS) is a malignant tumor originated from mesenchymal tissues. Although the incidence of STS is rare, accounting for less than 1% of adult malignant tumors and 15% of pediatric malignant tumors. The subtypes of STS are extremely abundant. According to the standard of WHO, STS is divided into more than 50 types [1–3]. Due to the heterogeneity of STS, the treatment is difficult, and the prognosis is poor. The main treatments of STS are surgery, radiotherapy and chemotherapy. Surgery is the standard regimen of resectable patients, while patients with improper surgery are at a high risk of recurrence and metastasis. For patients with advanced and metastatic STS, systematic chemotherapy is the primary treatment and it could effectively prolong the overall survival [4]. Standard first line chemotherapy includes doxorubicin with or without ifosfamide, the objective response rate is 25–30% and the median overall survival is approximately 12–17 months [5].
Neovascularization is an important process to promote tumor growth, proliferation and metastasis [6]. VEGF, PDGF and FGF is the main pathways to promote angiogenesis. Drugs like pazopanib, regorafenib and anlotinib, are relating to the pathways mentioned above, and also exhibiting excellent anti-tumor effects [7–9]. Anlotinib is a new, orally administered tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptors (PDGFR), and c-kit [10]. It has been approved for the treatment of STS in China. Its application has attracted more and more attention due to the significant efficacy and safety [11].
Because of the limited effects of doxorubicin-based chemotherapy regimen in the treatment of STS [12], we attempt to investigate the anlotinib combined with chemotherapy to improve outcomes. In this article, we retrospectively analyzed 14 cases to explore the effectiveness and safety of the combined regimen in first and second line treatment of STS and provide new treatment options for advanced STS.
Patients and methods
Patient characteristics
Fourteen patients with advanced STS were enrolled from Fudan University Shanghai Cancer Center Minhang Branch between July 2019 and March 2020. All patients had available imaging and pathological examination data. All patients received written informed consents before the combination therapy.
Procedures
Advanced STS patients received chemotherapy combined anlotinib. Chemotherapy regimens were based on anthracyclines and ifosfamide, and other drugs included paclitaxel, and so on. Anlotinib were taken orally 12 mg once a day for 14 days and 7 days off, 21 days for a treatment cycle. Efficacy and safety were evaluated every 2 treatment cycles until PD or unacceptable toxicity.
Safety and outcome assessment
Efficacy assessment were conducted by RECIST 1.1. Disease control rate (DCR) is defined as the total percentage of patients with stable disease (SD), partial response (PR), and complete response (CR). Objective response rate is defined as the sum of patients with PR and CR. From the initiation of combination therapy to any cause of tumor progression or death is defined as progression free survival (PFS). In addition, treatment-related adverse events were evaluated in accordance with Common Terminology Criteria for Adverse Events (CTCAE) (vision 4.3).
Statistic and analysis
SPSS software (version 14.0) was used to analyze the data, describe variables related to treatment-related toxicity and patient characteristics. Median progression free survival was calculated by Kaplan–Meier method.
Ethics
The study was approved by the Ethics Committee of the Shanghai Cancer Hospital Affiliated to Fudan University. All procedures performed in studies involving human participants are in compliance with the ethical standards of the institution and/or the National Research Council and the Declaration of Helsinki. Since this is a retrospective study, no informed consent is required. Patient data used in the study is confidential.
Results
Patient characteristics
Table 1 summarized the baseline characteristics of advanced STS patients, including sex, age, pathological type, AJCC stage, metastatic site, and history of treatments. 9 patients were male and 5 patients were female. Pathological types included LPS (n = 4), UPS (n = 3), SS (n = 2), EES (n = 1), SCS (n = 1), SMM (n = 1), HS (n = 1) and FS (n = 1). Nine patients were with stage III, and five were with stage IV. Four patients suffered from pulmonary metastasis, and one patient suffered from peritoneum metastasis. Twelve patients had previously received surgery and four patients received chemotherapy.
Table 1.
Patient demographics and clinical characteristics
| Case | Sex | Age | Histology | AJCC staging | Metastatic site | Previous treatment | Therapeutic regimen | Response |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 48 | LPS | IIIB | – | SUR + CHE | AIM + Anlotinib | PD |
| 2 | M | 72 | LPS | IIIA | – | SUR + CHE | AIM + Anlotinib | SD |
| 3 | F | 33 | LPS | IV | Lung | SUR | AIM + Anlotinib | SD |
| 4 | F | 43 | LPS | IIIA | – | SUR | AIM + Anlotinib | PR |
| 5 | M | 38 | UPS | IV | Lung | SUR | AIM + Anlotinib | SD |
| 6 | M | 81 | UPS | IIIA | – | – | A + Anlotinib | PR |
| 7 | M | 69 | UPS | IV | Lung | SUR | AIM + Anlotinib | PD |
| 8 | M | 40 | SS | IIIB | – | SUR | AIM + Endostar + Anlotinib | PR |
| 9 | F | 39 | SS | IIIA | – | – | AIM + Anlotinib | SD |
| 10 | F | 52 | EES | IV | Peritoneum | SUR + CHE | A + Anlotinib | SD |
| 11 | M | 65 | SCS | IIIB | – | SUR | AIM + Anlotinib | PR |
| 12 | F | 72 | SMM | IIIB | – | SUR | AIM + Anlotinib | PR |
| 13 | M | 47 | HS | IV | Lung | SUR + CHE | Paclitaxel + Anlotinib | SD |
| 14 | M | 41 | FS | IIIB | – | SUR | AIM + Anlotinib | SD |
AIM anthracyclines + ifosfamide + mesna, SUR surgery, CHE chemotherapy, PD progressive disease, SD stable disease, PR partial response
Efficacy
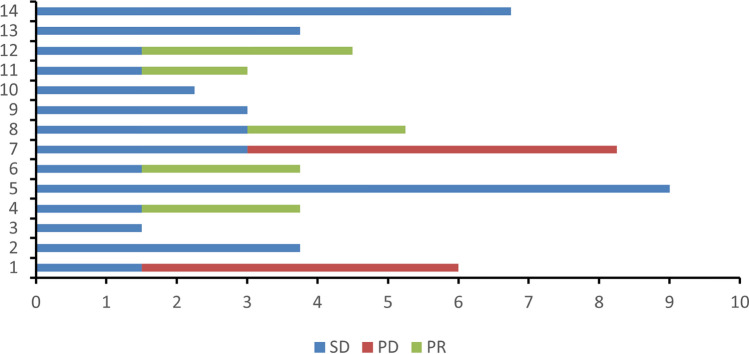
The average cycles of treatment were 3.86. Among the 14 patients, 5 had partial response (PR), 7 had stable disease (SD), and 2 had progressive disease (PD) (Fig. 1). The overall response rate (ORR) was 35.7% (5/14). 2 cases of stage III patients received surgical treatment after neoadjuvant chemotherapy. Patients who had not underwent surgical treatment were with a disease control rate (DCR) of 83.3% (10/12). The median progression free survival (PFS) was 8.25 months (Fig. 2; 95% CI 4.88–11.62). All 14 patients were alive, and no deaths had occurred.
Fig. 1.
Time from treatment of patients (months)
Fig. 2.
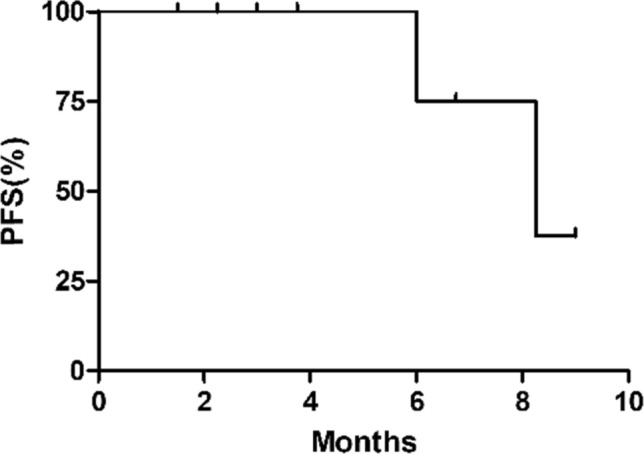
The progression free survival of patients who had not underwent surgical treatment
Adverse events
Common treatment-related adverse events were shown in Table 2. The Grade 3–4 adverse event is neutropenia (n = 5, 35.7%). Most of treatment-related adverse events are grade 1–2, including neutropenia, anemia, thrombocytopenia, hypertension, nausea, vomiting and ALT elevation. No patient discontinued treatment with combined regimens due to adverse events. There were no treatment-related death, as well as new treatment-related adverse events. The combined chemotherapy were well tolerated.
Table 2.
Toxicity profile (N = 14, n (%))
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Neutropenia | 7 (50.0) | 2 (14.3) | 2 (14.3) | 3 (21.4) |
| Anemia | 5 (35.7) | 4 (28.6) | 0 | 0 |
| Thrombocytopenia | 3 (21.4) | 2 (14.3) | 0 | 0 |
| Hypertension | 1 (7.1) | 1 (7.1) | 0 | 0 |
| Nausea/vomiting | 6 (42.9) | 2 (14.3) | 0 | 0 |
| ALT elevation | 2 (14.3) | 1 (7.1) | 0 | 0 |
Neoadjuvant chemotherapy
It was noted that 2 of 14 patients achieved PR after combined treatment, and sequentially received surgical resection with R0. Details were shown in Table 3.
Table 3.
Individual patient data of neoadjuvant chemotherapy
| Name | Case 1 | Case 2 |
|---|---|---|
| Sex | Male | Female |
| Age | 40 | 72 |
| Histologic category | Synovial sarcoma | Sarcomatoid malignant mesothelioma |
| Surgery history | Yes | No |
| Chemotherapy history | No | No |
| Maximum diameter of tumor | 5.8 cm | 12.4 cm |
| Chemotherapy | AIM + Endostar + Anlotinib | AIM + Anlotinib |
| First time | September 17th, 2019 | September 17th, 2019 |
| Toxicity profile | – | Nausea/Vomiting (I) |
| Second time | October 9th, 2019 | October 9th, 2019 |
| Toxicity profile | – | Nausea/Vomiting (I) |
| Maximum diameter of tumor | 3.5 cm | 7.5 cm |
| Response | PR | PR |
| Third time | October 31th,2019 | October 31th,2019 |
| Toxicity profile | – | Febrile neutropenia (IV) |
| Fourth time | 2019.11.22 | – |
| Toxicity profile | Neutropenia (I) | – |
| Maximum diameter of tumor | 2.8 cm | 8.1 cm |
| Response | PR | PR |
| Surgery | December 23th, 2019(R0) | December 16th, 2019(R0) |
One case was a synovial sarcoma, who had a history of surgery but no chemotherapy. The patient was treated with AIM + Endostar + Anlotinib. The tumor with a maximum diameter of 5.8 cm. After 2 cycles of chemotherapy, the tumor was significantly reduced with a maximum diameter of 3.5 cm, reaching PR. The patient had good chemotherapy effect, and no obvious adverse reactions. Chemotherapy was continued for 2 cycles. MRI examination was performed again. The maximum tumor diameter was 2.8 cm and achieved PR. In the 4 chemotherapy cycles, only the last chemotherapy showed grade 1 deficiency neutropenia. The patient received a R0 surgery resection sequentially.
Another case was a malignant mesothelioma, with a maximum diameter of 12.4 cm at the time of initial diagnosis, and with no history of surgery or chemotherapy. On september 17th, 2019, the AIM + Anlotinib was performed. After 2 cycles of chemotherapy, the tumor was significantly reduced with a maximum diameter of 7.5 cm, reaching PR. In this period, grade 1 nausea and vomiting had occurred. After the third chemotherapy, the patient suffered from grade 4 febrile neutropenia and the supportive treatment was given. Preoperative abdominal CT evaluation showed that the tumor was slightly larger than before, with a maximum diameter of 8.1 cm. The evaluation result was still PR. The patient received a R0 surgery resection eventually.
Discussion
Over the past decade, main treatment of advanced STSs is palliative chemotherapy. Anthracyclines are standard first-line treatment options. Judson et al. reported that doxorubicin combined with ifosfamide significantly improves ORR (26.5 vs 13.6%) and proglongs PFS (7.4 months vs 4.6 months, HR = 0.74, P = 0.003) compared to ifosfamide alone. However, adverse events are also increased apparently, and OS is similar (14.3 months vs 12.8 months, P = 0.076). When patients experience failure of first-line chemotherapy, there are few therapeutic drugs available. According to pathological types and individual conditions, we can choose Dacarbazine, gemcitabine, temozolomide, vincristine, cyclophosphamide and paclitaxel as second-line chemotherapy. But the prognosis of STS patients is still poor [13].
Previous studies have shown that anlotinib can be effective in different kinds of STSs, especially in Synovial sarcoma, leiomyosarcoma and alveolar soft tissue sarcoma [9]. A Phase II trial proved that 166 advanced STS patients received alotinib had an twelve-week PFS rates of 68%, a median PFS time of 5.6 months, and a OS time of 12 months, which was similar to paients received paczopanil and Regominib. The trial also suggested that the most common grade 1 and 2 adverse events were Triglyceride elevation (44%), Hand-foot skin reaction (43%) and Hypertension (42%).This study concluded that anlotinib showed better efficacy and tolerance in STSs patients experience failure of conventional chemotherapy [9].
Further research showed that anti-angiogenesis therapy combined with cytotoxic chemotherapy could effectively overcome chemotherapy resistance and be beneficial to tumor growth inhibition [13]. The efficacy of bevacizumab combined with gemcitabine and docetaxel in the treatment of STS was relatively good [14, 15]. Verschraegen CF reported a Phase I trial showed that bevacizumab combined with docetaxel and gemcitabine in 38 patients with advanced STS had an ORR of 31%, including 5 CR, 6 PR, and 18 SD [14]. Reported by the Spanish sarcoma study group, a prospective randomized phase II study in 35 advanced STS patients received sorafenib combined with ifosfamide, which proved to be a promising therapy, with 3 month and 6 month PFS rates of 66% and 37%, a median PFS time of 4.8 months, and a OS time of 16.2 months [16].
In our study, 5 achieved PR, 7 achieved SD, 2 achieved PD among 14 STSs patients. The ORR was 35.7% (5/14) and DCR was 83.3% (10/12).The median PFS achieved 8.25 months. Common treatment-related adverse effects included bone marrow suppression, nausea, vomiting and hypertension. Three patients had severe adverse effect, which was febrile neutropenia. ALTER-S006 is a multicentre, open-label, single-arm, phase 2 trial to evaluate the efficacy and safety of anlotinib as a maintenance treatment after chemotherapy in STS. The study enrolled 49 patients and showed that median PFS was 9.1 months, and the 1-year OS rate was 98.0%. The best ORR and DCR were 16 and 94%, respectively. Most of the treatment-related adverse events were grade 1–2. The results of our retrospective study are similar to this clinical study [17].
There is no consensus on the efficacy of neoadjuvant chemotherapy in the treatment of STS. The result of clinical studies at present is different. Gortzak et al.argued that there was no difference in efficacy between surgery alone and neoadjuvant chemotherapy combined with surgery in high-risk STS, The 5-year survival rates of the surgery group and the surgery combined with neoadjuvant chemotherapy group were 64 and 65% respectively (P = 0.22) [18]. Another retrospective study reported that only high-risk soft tissue sarcoma patients with tumor diameter greater than 10 cm could benefit from neoadjuvant chemotherapy [19]. Delaney et al. reported that compared with the surgery alone group, neoadjuvant chemotherapy of MAID (mesna, doxorubicin, ifosfamide, dacarbazine) combined with surgery could significantly improve the 5-year survival rate of high-risk patients with STS patients (58 vs 87%, P = 0.0003) [20]. In addition, neoadjuvant chemotherapy may induce significant short-term adverse events [21].
In the reasearch, 2 advanced STS patients who received surgical treatment after alotinib combined with chemotherapy achieved PR. As a result, we believe that the chemotherapy regimen is effective in the treatment of advanced STS patients. Although the results are delightful, there are still limitations in this study. For example, the total sample size is small. Our research was retrospective, thus going along with the inherent problems of retrospective research including selection bias, recall bias, confounding variables and incomplete data. Further studies are needed to verify the effectiveness and safety of combined regimens in STS.
Conclusion
Overall, the therapeutic effect of chemotherapy plus anlotinib in STS significantly improved ORR and PFS, compared with previous results and the adverse events are controllable, which provided a new way for the treatment of STS.
Abbreviations
- STS
Advanced soft tissue sarcoma
- PR
Partial response
- SD
Stable disease
- PD
Progressive disease
- ORR
Overall response rate
- DCR
Disease control rate
- PFS
Progression free survival
- VEGFR
Vascular endothelial growth factor receptor
- FGFR
Fibroblast growth factor receptor
- PDGFR
Platelet-derived growth factor receptors
- CTCAE
Common terminology criteria for adverse events
- MAID
Mesna, doxorubicin, ifosfamide, dacarbazine
- AIM
Anthracyclines, ifosfamide, mesna
- SUR
Surgery
- CHE
Chemotherapy
Author contributions
Ying Ni, Yabing Dong, Aijun Jiang: Conceptualization, Investigation, Methodology, Writing – original draft; Hui Liang: Conceptualization, Formal analysis, Investigation, Resources, Writing – review & editing; Wei Zhang: Conceptualization, Methodology, Project administration, Writing – review & editing; Qun Zhang: Investigation, Validation; Zhantong Wang: Investigation, Methodology, Validation.
Funding
This work was supported by Minhang District Health Commission Scientific research project [2019MW14].
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or its supplementary materials].
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying Ni, Yabing Dong and Aijun Jiang have contributed equally to this paper.
Contributor Information
Hui Liang, Email: 13564641266@163.com.
Wei Zhang, Email: zw-mitchell@hotmail.com.
References
- 1.Doyle LA. Sarcoma classification: an update based on the 2013 world health organization classification of tumors of soft tissue and bone. Cancer. 2014;120:1763–74. [DOI] [PubMed] [Google Scholar]
- 2.Sinha S, Peach AH. Diagnosis and management of soft tissue sarcoma. BMJ. 2010;341:c7170. [DOI] [PubMed] [Google Scholar]
- 3.Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353:701–11. [DOI] [PubMed] [Google Scholar]
- 4.Stoeckle E, Coindre JM, Kind M, Kantor G, Bui BN. Evaluating surgery quality in soft tissue sarcoma. Recent Results Cancer Res. 2009;179:229–42. [DOI] [PubMed] [Google Scholar]
- 5.Tap WD, Papai Z, Van Tine BA, Attia S, Ganjoo KN, Jones RL, et al. Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft-tissue sarcoma (TH CR-406/SARC021): an international, multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2017;18:1089–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–86. [DOI] [PubMed] [Google Scholar]
- 8.Mir O, Brodowicz T, Italiano A, Wallet J, Blay JY, Bertucci F, et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17:1732–42. [DOI] [PubMed] [Google Scholar]
- 9.Chi Y, Fang Z, Hong X, Yao Y, Sun P, Wang G, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft tissue sarcoma. Clin Cancer Res. 2018;24:5233–8. [DOI] [PubMed] [Google Scholar]
- 10.Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie C, Wan X, Quan H, Zheng M, Fu L, Li Y, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018;109:1207–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Judson I, Verweij J, Gelderblom H, Hartmann JT, Schöffski P, Blay JY, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415–23. [DOI] [PubMed] [Google Scholar]
- 13.Vo KT, Matthay KK, DuBois SG. Targeted antiangiogenic agents in combination with cytotoxic chemotherapy in preclinical and clinical studies in sarcoma. Clin Sarcoma Res. 2016;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verschraegen CF, Arias-Pulido H, Lee SJ, Movva S, Cerilli LA, Eberhardt S, et al. Phase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in datients with advanced or recurrent soft tissue sarcoma: the Axtell regimen. Ann Oncol. 2012;23:785–90. [DOI] [PubMed] [Google Scholar]
- 15.Kuo C, Kent PM, Logan AD, Tamulonis KB, Dalton KL, Batus M, et al. Docetaxel, bevacizumab, and gemcitabine for very high risk sarcomas in adolescents and young adults: a single-center experience. Pediatr Blood Cancer. 2017. 10.1002/pbc.26265. [DOI] [PubMed] [Google Scholar]
- 16.García Del Muro X, Maurel J, Martínez Trufero J, Lavernia J, López Pousa A, de Las PR, et al. Phase II trial of ifosfamide in combination with the VEGFR inhibitor sorafenib in advanced soft tissue sarcoma: a Spanish group for research on sarcomas (GEIS) study. Invest New Drugs. 2018;36:468–75. [DOI] [PubMed] [Google Scholar]
- 17.Bushu X, Pan Q, Pan H, Li H, Li X, Chen J, Pang D, et al. Anlotinib as a maintenance treatment for advanced soft tissue sarcoma after first-line chemotherapy (ALTER-S006): a multicentre, open-label, single-arm, phase 2 trial. eClinicalMedicine. 2023;64:102240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gortzak E, Azzarelli A, Buesa J, Bramwell VH, van Coevorden F, van Geel AN, et al. A randomised phase II study on neoadjuvant chemotherapy for ‘High-Risk’ adult soft-tissue sarcoma. Eur J Cancer. 2001;37:1096–103. [DOI] [PubMed] [Google Scholar]
- 19.Grobmyer SR, Maki RG, Demetri GD, Mazumdar M, Riedel E, Brennan MF, et al. Neoadjuvant chemotherapy for primary high-grade extremity soft tissue sarcoma. Ann Oncol. 2004;15:1667–72. [DOI] [PubMed] [Google Scholar]
- 20.DeLaney TF, Spiro IJ, Suit HD, Gebhardt MC, Hornicek FJ, Mankin HJ, et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2003;56:1117–27. [DOI] [PubMed] [Google Scholar]
- 21.Mullen JT, Kobayashi W, Wang JJ, Harmon DC, Choy E, Hornicek FJ, et al. Long-term follow-up of patients treated with neoadjuvant chemotherapy and radiotherapy for large, extremity soft tissue sarcomas. Cancer. 2012;118:3758–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or its supplementary materials].