Abstract
Granulomatous amebic encephalitis (GAE), an infection of immunocompromised hosts, is almost uniformly fatal. A case of GAE in a patient who failed to mount a serologic response to Acanthamoeba polyphaga is presented. Although Acanthamoeba polyphaga that is sensitive to multiple antimicrobials grew from brain tissue, an inability to make a premortem diagnosis precluded therapy.
CASE REPORT
A 70-year-old female presented for medical attention with lethargy and seizures. Symptoms began 36 days earlier when she was hospitalized with syncope. An evaluation at that time included magnetic resonance imaging (MRI), revealing a focal area of edema in the right temporal lobe consistent with an acute infarction, and she was discharged to a rehabilitation facility with a diagnosis of cerebrovascular accident. She was readmitted with worsening ataxia, lethargy, and generalized seizures. Her past medical history included chronic neutropenia secondary to myelodysplastic syndrome, hypogammaglobulinemia treated with monthly intravenous gamma globulin, asplenia, steroid-dependent discoid lupus (prednisone, 20 mg twice a day), and diabetes mellitus.
On exam, the patient was afebrile and hemodynamically stable. An ocular exam did not reveal papilledema. Her neck was supple. She was oriented only to person. A neurological examination was otherwise nonfocal. Laboratory studies included a platelet count of 355 × 109/liter, a hematocrit of 32.8%, and a white blood cell count of 2.8 × 109/liter, which was unchanged from her baseline leukopenia. A lumbar puncture (Table 1) was significant for lymphocytic pleocytosis and hypoglycorrhacia. A repeat MRI revealed intense contrast enhancement of the right posterior temporal lobe, with two new contrast-enhancing areas in the medial temporal lobe. A stereotactic biopsy of the right temporal lobe performed on day 42 was nondiagnostic, with results showing a reactive gliosis with lymphocytic infiltrate in the leptomeninges, but no amebae or granulomas, and negative bacterial, fungal, and mycobacterial cultures.
TABLE 1.
Cerebrospinal fluid parametersa
| Day after initial presentation | No. of WBC/mm3 | No. of RBC/mm3 | Differential (%)
|
Glucose (mg/dl) | Protein (mg/dl) | ||
|---|---|---|---|---|---|---|---|
| N | L | M | |||||
| 38 | 25 | 1 | 10 | 90 | 19 | 86 | |
| 48 | 27 | 0 | 19 | 79 | 2 | 14 | 88 |
| 58 | 48 | 115 | 89 | 9 | 124 | ||
| 65 | 26 | 0 | 89 | 7 | 166 | ||
WBC, white blood cells; RBC, red blood cells; N, neutrophils; L, lymphocytes; M, monocytes.
The patient's hospital course was significant for progressive obtundation, requiring intubation for airway protection. The results of repeat lumbar punctures are provided in Table 1. Serial MRI revealed persistent abnormal signal intensity in the temporal lobes, with new areas of uptake in the right basal ganglia, pons, and left occiput. Extensive diagnostic evaluation was unrevealing except for a stable elevation in titers of antibodies to Mycoplasma pneumoniae (1.425 in the acute phase and 1.645 in the convalescent phase) and Ehrlichia chaffeensis (1:128 in the acute phase and 1:128 in the convalescent phase) and evidence of prior Epstein-Barr virus infection (viral capsid antigen immunoglobulin G titer of >10, viral capsid antigen immunoglobulin M titer of <10, and EBNA titer of >10). Despite empirical treatment with acyclovir, decadron, and plasmaphoresis, the patient died 100 days after her initial presentation.
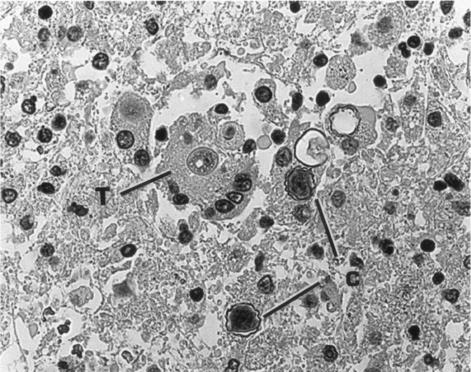
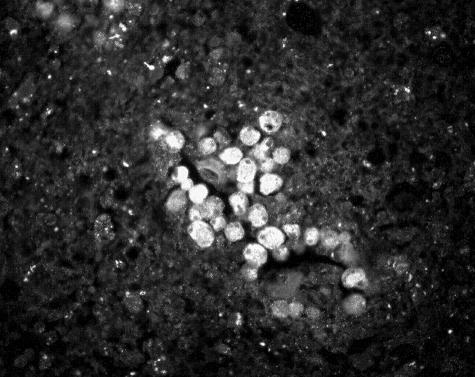
Hematoxylin and eosin-stained sections of the patient's brain obtained at autopsy revealed both trophic and encysted amebae, as described in a previous report (34). Cysts were readily identifiable by their characteristic thick double-walled structure. Indirect immunofluorescence and immunoperoxidase staining (6) revealed large numbers of amebae in perivascular regions of the brain tissue (Fig. 1 and 2).
FIG. 1.
Immunoperoxidase-stained section of brain tissue showing both trophic (T) and cystic (C) stages of Acanthamoeba. The cysts are readily recognizable by their thick, wavy walls. Magnification, ×300.
FIG. 2.
Indirect immunofluorescence staining of a cluster of trophic amebae seen in the perivascular area of a section through brain tissue. Magnification, ×225.
Indirect immunofluorescence staining was performed on premortem serum samples using Acanthamoeba castellanii, Acanthamoeba polyphaga, and Acanthamoeba culbertsoni as antigens. Trophic amebae from bacterium-free cultures were fixed in 1% formalin and dried on multiwell slides. Serum samples from the patient were applied to the amebae in dilutions of 1:2 to 1:4,096 as previously described (35). The wells were then treated with goat antihuman fluorescein isothiocyanate serum conjugate, followed by washing, mounting, and observation with a fluorescence microscope. Serum obtained 48 days after the patient's initial presentation yielded antibody titers of 1:16 to A. castellanii, 1:8 to A. polyphaga, and 1:16 to A. culbertsoni. A subsequent sample, obtained 64 days after the patient's presentation, yielded an antibody titer of 1:32 to A. castellanii. Serum from an asymptomatic control run in parallel with the paired specimens demonstrated a titer of antibody to A. castellanii of 1:32.
Amebae were isolated from brain tissue obtained at autopsy. A sample of brain tissue was macerated in sterile phosphate-buffered saline, and the suspension was applied to the surface of a nonnutrient agar petri plate that had been streaked with a suspension of Escherichia coli. After 2 days of incubation at 30°C, inverted microscopy revealed the presence of amebae feeding on the bacteria and moving over the agar surface, ultimately undergoing encystment to produce thick-walled cysts. Bacterium-free (axenic) cultures of trophic amebae were subsequently established by transfer to proteose-peptone-yeast extract-glucose medium supplemented with fetal calf serum and a vitamin supplement at 30°C (22). Penicillin-streptomycin was added to kill any bacteria that were carried forward into the axenic medium. The isolate was identified as Acanthamoeba polyphaga based on the cyst morphology. When tested for growth at different temperatures (30°C, 33°C, and 37°C), the amebae grew best at 30°C. Similar attempts to isolate the ameba from cerebrospinal fluid obtained on day 65 after the patient's presentation, both in the presence of bacteria and into axenic proteose-peptone-yeast extract-glucose medium, were unsuccessful.
Cultured amebae were tested for sensitivity to the following antimicrobial agents at 1, 5, and 10 μg/ml: amphotericin B, azithromycin, clarithromycin, fluconazole, flucytosine, pentamidine isethionate, and sulfadiazine. Sensitivity was determined by the growth or the absence of growth of amebae on monkey kidney cells (23). Ameba growth was strongly inhibited by fluconazole (all concentrations), azithromycin (all concentrations), and pentamidine (all concentrations) and less so by amphotericin B (5 and 10 μg/ml only). No inhibition was seen with clarithromycin, flucytosine, and sulfadiazine.
Acanthamoeba spp. are ubiquitous in the environment and are highly tolerant of a wide range of growth conditions from sea to tap waters, tropical to arctic soils, aquatic waste dump sites, and cooling towers of air conditioning systems. In the home, they can be recovered from humidifiers, aquariums, biofilms in sink drains and water faucets, and soil in potted plants. Amebae have been isolated from the nasal mucosae of various groups of healthy individuals, including military recruits, students, and children, suggesting that while colonization and subclincal infections are relatively common, invasive disease is, fortunately, a rarity (3, 12, 14).
Granulomatous amebic encephalitis (GAE) presents as a subacute but progressive meningoencephalitis that is almost universally fatal (15). While there are reports of infections in immunocompetent individuals (27, 28), the majority of cases of GAE have occurred in immunocompromised hosts. Case reports of GAE in patients with human immunodeficiency virus/AIDS (18, 26, 33) and patients who have undergone organ transplantation (2, 16, 29, 30) likely reflect an increased incidence of GAE due to a larger population of susceptible individuals. The preponderance of GAE among patients with impaired T-cell immunity, coupled with experimental data showing T-lymphocyte proliferation among healthy volunteers exposed to Acanthamoeba antigens (32), implicates deficits in cell-mediated immunity as an important risk factor for GAE. The patient in this report had impaired T-cell immunity based on chronic steroid use for systemic lupus erythematosis, which has been reported in previous fatal cases of GAE (9, 10, 20).
Diagnosis of amebic meningoencephalitis is typically made by recognition of trophozoites and cysts on examination of brain tissue. In this case, a stereotactic biopsy performed premortem was nondiagnostic. Granuloma formation, the pathological hallmark of GAE, may be absent or diminished in immunocompromised individuals (13, 30). False-negative biopsy results have previously been reported due to sampling error (19), failure to recognize amebae on the initial review (8, 30), or misidentification of the organisms as reactive histiocytes (25) or yeasts (31). Because the pathological diagnosis of acanthamoebiasis may be elusive, particularly if limited specimens are obtained, alternative diagnostic methods are needed.
Low-level antibody titers to Acanthamoeba spp. are found in 50 to 100% of asymptomatic individuals, suggesting that occult infection is common (4, 5, 7). Significant elevations in titers have been reported among patients with acanthamoebic keratitis (1) and Acanthamoeba meningoencephalitis (9), raising the possibility that serology may provide a noninvasive method for early diagnosis of GAE in a clinically compatible case. Positive antibody titers in Acanthamoeba GAE are typically 1:128 and higher (G. S. Visvesvara, personal communication). In this study, testing for serum antibodies using three different species of Acanthamoeba gave consistently low titers (A. castellanii, 1:16; A. polyphaga, 1:8; and A. culbertsoni, 1:16) comparable to the levels of titers detected in the asymptomatic control. There was no significant increase in titers (acute phase, 1:16; convalescent phase, 1:32) despite sufficient time between the serial samples for a rise in antibodies to develop. We postulate that the failure to develop a serologic response may have been due to the underlying diagnosis of hypogammaglobulinemia and that treatment with high-dose corticosteroids may have blunted the humoral immune response, as has been previously reported (14). Of note, however, is that detection of stably elevated titers of antibodies to Mycoplasma pneumoniae and Ehrlichia chaffeensis suggests that this patient was able to mount an immunologic response to other infectious agents in the past.
Culture of Acanthamoeba has a limited role in diagnosis but is useful for speciation and determination of antimicrobial susceptibility. The ameba was successfully isolated from macerated brain tissue obtained at biopsy and identified as A. polyphaga based on cyst morphology. Optimal growth of the organism in vitro was at 30°C, consistent with other clinical isolates of Acanthamoeba which grow best below mammalian body temperature (23). As was the case in this report, isolation of amebae from cerebrospinal fluid is uncommon (11, 28).
PCR of corneal scrapings has been reported as both sensitive and specific for the diagnosis of Acanthamoeba keratitis (17); however, the role of molecular testing on either cerebrospinal fluid or brain tissue for a diagnosis of amoebic infection has not been defined. In the current report, rRNA gene sequencing was used to identify the isolate as a member of the T4 group of Acanthamoeba spp. (Gregory C. Booton, personal communication). Of note, amebas of the T4 group of Acanthamoeba spp. are detected in the majority of systemic and ocular infections (24), suggesting either (i) that T4 amebae may be more virulent than members of other groups of acanthamoebae found in the environment or (ii) that they are more commonly encountered in the environment and, therefore, are more likely to infect immunocompromised hosts.
Could earlier diagnosis leading to the initiation of treatment have altered the fatal outcome? Antimicrobial treatment of GAE is largely empirical, and as yet, there are no standardized treatment recommendations. The rare reports of long-term survivors among patients with GAE (26-28) and disseminated acanthamoebiasis (16, 25, 29) who are treated with a combination of antibiotic regimens support aggressive therapy. Often, however, the same antibiotic regimens have been used unsuccessfully in other patients, suggesting that early diagnosis, virulence of the agent, infective dose, and host immune factors all play a role in determining the outcome of GAE. Among the drugs that have been used with success in treating GAE cases are pentamidine isethionate, imidazoles, triazoles, flucytosine, amphotericin B, sulfa-containing antibiotics, and macrolides (21). The isolate grown from our patient was resistant to a number of these agents, making determination of susceptibility critical for optimizing the antibiotic regimen.
The current report illustrates the difficulty in making a diagnosis of GAE premortem. While GAE should be included in the differential diagnosis of any immunocompromised patient presenting with a subacute and progressive central nervous system syndrome, in this case, serologic testing for Acanthamoeba spp. performed on premortem specimens and stereotactic brain biopsy were nondiagnostic, precluding initiation of empirical antibiotic therapy. The antimicrobial resistance pattern of the isolate ultimately cultured from the patient's brain underscores the need for both early diagnosis and standardized methods for testing antimicrobial susceptibility if progress is to be made in decreasing the case fatality rate of GAE.
Acknowledgments
We thank Vedran Uschuplich and Darinka Mileusnic (Department of Pathology, University of Tennessee Medical Center, Knoxville, TN), who initially identified amebae in the brain tissue; Delia Woods and Diane Levine (Department of Preventive Medicine, Vanderbilt University, Nashville, TN) for collecting the clinical data; Gregory C. Booton (Department of Evolution, Ecology, and Organismal Biology, Ohio State University, Columbus, Ohio) for identification of the Acanthamoeba isolate; the Infectious Diseases Pathology Group (Centers for Disease Control and Prevention, Atlanta, Georgia) for immunohistochemical confirmation of Acanthamoeba in brain tissue; and Carol Glaser (Viral and Rickettsial Diseases Laboratory, California Department of Health, Richmond, CA) for review of the manuscript.
This work was supported in part by the Emerging Infections Program cooperative agreement (U50/CCU416123-04) with the Centers for Disease Control and Prevention.
REFERENCES
- 1.Alizadeh, H., S. Apte, M. S. El-Agha, L. Li, M. Hurt, K. Howard, H. D. Cavanagh, J. P. McCulley, and J. Y. Niederkorn. 2001. Tear IgA and serum IgG antibodies against Acanthamoeba in patients with Acanthamoeba keratitis. Cornea 20:622-627. [DOI] [PubMed] [Google Scholar]
- 2.Anderlini, P., D. Przepiorka, and M. Luna. 1994. Acanthamoeba meningoencephalitis after bone marrow transplantation. Bone Marrow Transplant. 14:459-461. [PubMed] [Google Scholar]
- 3.Badenoch, P., T. Grimmond, J. Cadwgan, S. Deayton, M. Essery, and B. Hill. 1988. Nasal carriage of free-living amoebae. Microb. Ecol. Health Dis. 1:209-211. [Google Scholar]
- 4.Cerva, L. 1989. Acanthamoeba culbertsoni and Naegleria fowleri: occurrence of antibodies in man. J. Hyg. Epidemiol. Microbiol. Immunol. 33:99-103. [PubMed] [Google Scholar]
- 5.Chappell, C. L., J. A. Wright, M. Coletta, and A. L. Newsome. 2001. Standardized methods of measuring Acanthamoeba antibodies in sera from healthy human subjects. Clin. Diagn. Lab. Immunol. 8:724-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Culbertson, C. G., and K. Harper. 1977. Immunoperoxidase staining of E. histolytica and soil amebas in formalin-fixed tissue. Am. J. Clin. Pathol. 68:529-530. [DOI] [PubMed] [Google Scholar]
- 7.Cursons, R. T. M., T. J. Brown, E. A. Keys, K. M. Moriarty, and D. Till. 1980. Immunity to pathogenic free-living amoebae: role of humoral antibody. Infect. Immun. 29:401-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deetz, T., M. Sawyer, G. Billman, F. Schuster, and G. Visvesvara. 2003. Successful treatment of Balmuthia amoebic encephalitis: presentation of 2 cases. Clin. Infect. Dis. 37:1304-1312. [DOI] [PubMed] [Google Scholar]
- 9.Grunnet, M., G. Cannon, and J. Kushner. 1981. Fulminant amebic meningoencephalitis due to Acanthamoeba. Neurology 31:174-177. [DOI] [PubMed] [Google Scholar]
- 10.Koide, J., E. Okusawa, T. Ito, S. Mori, T. Takeuchi, S. Itoyama, and T. Abe. 1998. Granulomatous amoebic encephalitis caused by Acanthamoeba in patient with systemic lupus erythematosis. Clin. Rheumatol. 17:329-332. [DOI] [PubMed] [Google Scholar]
- 11.Lalitha, M. K., V. Anandi, A. Srivastava, K. Thomas, A. M. Cherian, and S. M. Chandi. 1985. Isolation of Acanthamoeba culbertsoni from a patient with meningitis. J. Clin. Microbiol. 21:666-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawande, R., S. Abraham, I. John, and L. Egler. 1979. Recovery of soil amebas from the nasal passages of children during the dusty harmattan period in Zaria. Am. J. Pathol. 71:201-203. [DOI] [PubMed] [Google Scholar]
- 13.Marciano-Cabral, F., and G. Cabral. 2003. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 16:273-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez, A. 1982. Acanthamoebiasis and immunosuppression. Case report. J. Neuropathol. Exp. Neurol. 41:548-557. [DOI] [PubMed] [Google Scholar]
- 15.Martinez, A. 1985. Free-living amebas: natural history, prevention, diagnosis, pathology, and treatment of disease. CRC Press, Boca Raton, Fla.
- 16.Oliva, S., M. Jantz, D. Tiernan, D. Cook, and M. Judson. 1999. Successful treatment of widely disseminated acanthamoebiasis. South. Med. J. 92:55-57. [DOI] [PubMed] [Google Scholar]
- 17.Pasricha, G., S. Sharma, P. Garg, and R. K. Aggarwal. 2003. Use of 18S rRNA gene-based PCR assay for diagnosis of Acanthamoeba keratitis in non-contact lens wearers in India. J. Clin. Microbiol. 41:3206-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivera, M., and T. Padhya. 2002. Acanthamoeba: a rare primary case of rhinosinusitis. Laryngoscope 112:1201-1203. [DOI] [PubMed] [Google Scholar]
- 19.Rowen, J., C. Doerr, H. Vogel, and C. Baker. 1995. Balamuthia mandrillaris: a newly recognized agent for amebic meningoencephalitis. Pediatr. Infect. Dis. J. 14:705-710. [PubMed] [Google Scholar]
- 20.Sangruchi, T., A. J. Martinez, and G. Visvesvara. 1994. Spontaneous granulomatous amebic encephalitis: report of four cases from Thailand. Southeast Asian J. Trop. Med. Public Health 25:309-313. [PubMed] [Google Scholar]
- 21.Schuster, F., and G. Visvesvara. 2003. Amebic encephalitides and amebic keratitis caused by pathogenic and opportunistic free-living amebas. Curr. Treat. Options Infect. Dis. 5:273-282. [Google Scholar]
- 22.Schuster, F. L. 2002. Cultivation of pathogenic and opportunistic free-living amebas. Clin. Microbiol. Rev. 15:342-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuster, F. L., and G. S. Visvesvara. 1998. Efficacy of novel antimicrobials against clinical isolates of opportunistic amebas. J. Eukaryot. Microbiol. 45:612-618. [DOI] [PubMed] [Google Scholar]
- 24.Schuster, F. L., and G. S. Visvesvara. 2004. Free-living amoebae as opportunistic pathogens of humans and animals. Int. J. Parasitol. 34:1001-1027. [DOI] [PubMed] [Google Scholar]
- 25.Schwarzwald, H., P. Shah, J. Hicks, M. Levy, M. L. Wagner, and M. W. Kline. 2003. Disseminated Acanthamoeba infection in a human immunodeficiency virus-infected infant. Pediatr. Infect. Dis. J. 22:197-199. [PubMed] [Google Scholar]
- 26.Seijo Martinez, M., G. Gonzalez-Mediero, P. Santiago, A. Rodriguez de Lope, J. Diz, C. Conde, and G. S. Visvesvara. 2000. Granulomatous amebic encephalitis in a patient with AIDS: isolation of Acanthamoeba sp. group II from brain tissue and successful treatment with sulfadiazine and fluconazole. J. Clin. Microbiol. 38:3892-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma, P., P. Gupta, M. Murali, and V. Ramachandran. 1993. Primary amebic meningoencephalitis caused by Acanthamoeba: successfully treated with cotrimaxazole. Indian Pediatr. 30:1219-1222. [PubMed] [Google Scholar]
- 28.Singhal, T., A. Bajpai, and V. Kalra. 2001. Successful treatment of Acanthamoeba meningitis with combination oral antimicrobials. Pediatr. Infect. Dis. J. 20:623-627. [DOI] [PubMed] [Google Scholar]
- 29.Slater, C., J. Sickel, G. Visvesvara, R. Pabico, and A. Gaspari. 1994. Successful treatment of disseminated Acanthamoeba infection in an immunocompromised patient. N. Engl. J. Med. 331:85-87. [DOI] [PubMed] [Google Scholar]
- 30.Steinberg, J. P., R. L. Galindo, E. S. Kraus, and K. G. Ghanem. 2002. Disseminated acanthamoebiasis in a renal transplant recipient with osteomyelitis and cutaneous lesions: case report and literature review. Clin. Infect. Dis. 35:e43-e49. [DOI] [PubMed] [Google Scholar]
- 31.Tan, B., M. Weldon-Linne, D. Rhone, C. Penning, and G. Visvesvara. 1993. Acanthamoeba infection presenting as skin lesions in patients with the acquired immunodeficiency syndrome. Arch. Pathol. Lab. Med. 117:1043-1046. [PubMed] [Google Scholar]
- 32.Tanaka, Y., S. Suguri, M. Harada, T. Hayabara, K. Suzumori, and N. Ohta. 1994. Acanthamoeba-specific human T-cell clones isolated from healthy individuals. Parasitol. Res. 80:549-553. [DOI] [PubMed] [Google Scholar]
- 33.Torno, M. S., Jr., R. Babapour, A. Gurevitch, and M. D. Witt. 2000. Cutaenous acanthamoebiasis in AIDS. J. Am. Acad. Dermatol. 42:351-354. [DOI] [PubMed] [Google Scholar]
- 34.Uschuplich, V., D. Mileusnic, and M. Johnson. 2004. Progressive fatal encephalopathy in an immunosuppressed patient with a history of discoid lupus erythematosus. Arch. Pathol. Lab. Med. 128:e109-e111. [DOI] [PubMed] [Google Scholar]
- 35.Visvesvara, G., F. Schuster, and A. Martinez. 1993. Balamuthia mandrillaris, N.G., N. Sp., agent of amebic meningoencephalitis in humans and other animals. J. Eukaryot. Microbiol. 40:504-514. [DOI] [PubMed] [Google Scholar]