Abstract
A growing body of evidence indicates there is an increasing incidence of cognitive dysfunction in patients after coronavirus disease 2019 (COVID-19) infection. However, still lack diagnostic tools, which allow us to predict prognosis in such cases and improve the stratification of the disease. This study aims to evaluate the usefulness of the biomarkers that could allow to predict the severity and progression of COVID-19 in patients with post-COVID syndrome and cognitive problems. Data regarding clinical history, pre-existing conditions, chest CT scan, and therapy (remdesivir, steroids) were acquired. A total of 44 patients with hospitalized COVID-19, and healthy controls were enrolled in the investigation, and serum blood was obtained. After 6 months of observations, patients with COVID-19 were divided into two groups: first - without post-COVID syndrome and memory complaints, and second - with post-COVID and cognitive problems. Measurements of YKL-40 and MR-pro-ADM were taken in the serum with enzyme immunoassay kits at the time of admission (visit 1) and 6 months after discharge from the hospital (visit 2). Significantly higher concentrations of YKL-40 were found in patients with COVID-19 as compared to healthy individuals (p = 0.016). Moreover, YKL-40 ratio allowed to differentiate patients with and without post-COVID syndrome (median: 0.94 vs. 1.55, p = 0.004). Additionally, COVID-19 patients with dyspnea presented significantly elevated levels of MR-pro-ADM as compared to the group of COVID-19 survivors without dyspnea (p = 0.015). In the group of patients without post-COVID syndrome, the concentrations of YKL-40 and MR-pro-ADM decreased after treatment as compared to levels before therapy (77 vs. 36 ng/ml and 607 vs. 456 pmol/L). However, in patients with post-COVID syndrome and cognitive problems, the levels of both markers did not alter 6 months after hospital discharge in comparison to basal levels. Furthermore, after dexamethasone treatment the YKL-40 concentrations declined significantly (p = 0.003) in patients with COVID-19. This study demonstrated the predictive usefulness of YKL-40 as an indicator of successful treatment in patients with COVID-19 infection allowing risk stratification of hospitalized patients. It seems that indicators of neuroinflammation might have the potential to track development of cognitive complaints, however, it requires further investigations.
Keywords: Cognitive dysfunction, COVID-19, MR-pro-ADM, YKL-40
Subject terms: Neuroscience, Biomarkers
Introduction
Increasing literature data reported cognitive dysfunctions in patients who contracted coronavirus disease 2019 (COVID-19) infection, that are also called brain fog1,2. It is suggested that the prevalence of post–COVID–19 cognitive impairment is higher in patients who stay in the hospital and it was estimated at about 20% 3. However, findings from the first studies reported that significant cognitive impairment was observed even in asymptomatic and mild cases of COVID-19 4. The predominant symptoms among hospitalized patients were impairments in executive functioning, processing speed, category fluency, memory encoding, and recall3. It has been revealed that neurological complications are present in above 36% of patients with COVID-19 5. The decline of cognitive functions after COVID could be a serious not only medical but also social problem, because it may influence the functional, psychological as well as occupational outcomes of infected patients. The mechanisms underlying potential cognitive impairment after COVID-19 infection remain poorly understood4. Therefore, future studies are needed to identify the pathological mechanisms leading to cognitive dysfunction as well as diagnostic tools allowing for the prediction of disease severity or facilitating decision-making about possible treatment. Important aspects that could contribute to cognitive impairment in patients with COVID-19 are systematic inflammation infecting the central nervous system (CNS), a storm of intracranial cytokines mediated by blood-brain barrier permeabilization, and vascular involvement6. Studies confirmed that neuropsychiatric complications in COVID-19 are related to overactivation of immune response and neuroinflammation. Additionally, the assessment of neurological changes via MRI three months after COVID-19 infection showed significant structural changes that were linked with prolonged neurological symptoms such as cognitive deficits and anosmia5. The emerging evidence from experimental studies indicated that there is multiple pathways by which respiratory infection can influence the physiology of the central nervous system (CNS). One of the most common last years respiratory infections that was related to CNS damage and induced neurotoxicity, have been COVID-197. A growing body of evidence hypothesized that the lung-brain axis, which is a link pulmonary microbes to neurodegenerative disorders and behavioral changes, could be a pivotal causative factor in neurodegenerative disorders8. It has been observed that inflammation in the lung is regulated by acquired microbiota, which affects the local nerve and immune microenvironment through various mechanisms9. In COVID-19 the main pathomechanism is related to the receptor angiotensin converting enzyme-2 (ACE2), which is expressed in both lung and brain tissues. The viral mechanism in the lung-brain-axis is postulated to be by the olfactory epithelium or via a trans-synaptic channel (trigeminal nerves) upon infecting the peripheral nerve terminals. The direct result of the injury in the CNS might be by neuro-inflammation and oxidative stress. Other neuronal-related pathways in which a virus may enter the CNS is via the vasculature, the cerebrospinal fluid, and the lymphatic system10. Moreover, other crucial mechanisms, indicated that the lung microbiome can profoundly affect microglial function, and modulate the expression of inflammatory mediators and activation markers9.
Recent findings suggest that Mid regional pro-adrenomedullin (MR-pro-ADM) could be a predictive factor of disease progression and mortality in COVID-19 patients11,12, however, is a lack of data on this protein in the context of cognitive decline. MR-pro-ADM is involved in endothelial barrier regulation and stabilization of microcirculation, vascular permeability, and inflammation thus it could be a promising biomarker of organ dysfunction13. Studies on patients with sepsis revealed that increased concentration of MR-pro-ADM may indicate severe illness and potential for further progression, whereas, lower levels were interpreted as a lack of endothelial damage and better prognosis for patient14. Moreover, Hupf et al. reported that significantly higher RNA expression of MR-pro-ADM was found in COVID-19 patients who died as compared to individuals who survived15. It was demonstrated that increased levels of MR-pro-ADM in patients with COVID could be a valuable marker of treatment efficiency, predicting potential complications during therapy, unfavorable disease progression, as well as avoiding admission to hospital when this is not necessary16. The prognostic value of this biomarker was demonstrated by Spain researchers, who revealed that low MR-pro-ADM levels could indicate lower mortality risk and the necessity of less intensive therapy17.
Another mechanism potentially involved in dysfunction in the CNS in COVID-19 patients was increased astrogliosis and microglial density, as well as reactivity18. Chitinase-3-like protein (YKL-40) is a glycoprotein primarily expressed in astrocytes and is considered a common marker of neuroinflammation and neurodegeneration19,20. Increased expression of YKL-40 by reactive astrocytes was found in several neurodegenerative diseases, as well as in acute respiratory distress syndrome (ARDS) or COVID-19 infection18,21. YKL-40 is known to be involved in extracellular matrix (ECM) remodeling in various disorders, including acute and chronic inflammatory lung disorders22. Interestingly, YKL-40 has been found to stimulate the expression of angiotensin-converting enzyme 2 and host proteases implicated in SARS-CoV-2 infectivity23. Additionally, inhibition of this protein augmented SARS-CoV-2 infection in epithelial cells, including infection with omicron variants24. YKL-40 could be an indicator of pulmonary fibrosis, as remaining elevated levels of YKL-40 a few months after infection correlated with reduced lung function25. Moreover, evidence from one of the first studies indicate that YKL-40 might be a marker of disease severity and mortality in patients with COVID-19 18. However, a study De Lorenzo et al. suggests that YKL-40 is not a useful marker in the prediction of post-COVID-19 sequelae26.
Considering that both mentioned proteins reflect primary pathological mechanisms of COVID infection the aim of the present study was to investigate the longitudinal performance of YKL-40 and MR-pro-ADM in clinical evaluation of patients contracted COVID-19 infection, particularly with developing neurological manifestation, such as memory problems. We hypothesized that due to involvement of YKL-40 in neuroinflammation, and MR-pro-ADM in endothelial dysfunction, crucial pathomechanisms of neurodegeneration, both these proteins could be applied as potential biomarkers in the assessment of developing cognitive dysfunction in patients after COVID-19 infection.
Results
Characteristics of the study population
The demographic and clinical characteristic group of patients with COVID-19 before treatment is demonstrated in Table 1. The whole study group included 36 patients with COVID-19 and 8 volunteers donating blood from a local Blood Donation Centre. All patients with COVID-19 enrolled were tested positive for SARS-CoV-2 and had serum antibody positivity, and features of respiratory infection, evaluated with radiologic chest CT, and other clinical signs. Moreover, study participants had no history of dementia. Regarding the protocol of our study patients with COVID-19 were examined twice: firstly, after administration in the hospital (during diagnostics process) and secondly, six months after therapy on follow-up visit.
Table 1.
Baseline characteristics of the patients with COVID-19, laboratory and clinical findings at hospital admission.
| Sociodemographic and clinical data | Whole study group of patients with COVID-19 | Group 1 patients without post-COVID syndrome | Group 2 patients with post-COVID syndrome | p value (M-W test) gr.1 vs. gr.2 |
|---|---|---|---|---|
| Number of participants | 36 | 16 | 20 | |
| Age, years (median, range) | 56 (36–67) | 47 (36–65) | 57 (34–65) | 0.18 |
| Length of hospitalization, days (median, range) | 10 (0–25) | 9 (6–25) | 11 (0–25) | 0.98 |
| Symptoms, N (%) | ||||
| Dyspnea | 17 (61) | 9 (69) | 8 (53) | 0.49 |
| Fever | 22 (79) | 11 (85) | 11 (73) | 0.62 |
| Cough | 20 (71) | 9 (69) | 11 (73) | 0.86 |
| Sore throat | 3 (11) | 2 (15) | 1 (7) | 0.72 |
| Weakness | 21 (75) | 10 (77) | 11 (73) | 0.89 |
| Olfactory disorders | 4 (14) | 2 (15) | 2 (13) | 0.93 |
| Diarrhea | 3 (11) | 1 (8) | 2 (13) | 0.82 |
| Headache | 5 (18) | 2 (15) | 3 (20) | 0.85 |
| Comorbidities, N (%) | ||||
| Hypertension | 10 (28) | 3 (19) | 7 (35) | 0.56 |
| Cardiological diseases | 1 (3) | 0 (0) | 1 (5) | 0.86 |
| Vascular diseases | 2 (6) | 1 (6) | 1 (5) | 0.96 |
| Diabetes | 1 (3) | 0 (0) | 1 (5) | 0.86 |
| Obesity | 3 (8) | 2 (13) | 1 (5) | 0.76 |
| Endocrine diseases | 4 (11) | 2 (13) | 2 (10) | 0.93 |
| Rheumatological diseases | 4 (11) | 1 (6) | 3 (15) | 0.75 |
| Gastrointestinal diseases | 2 (6) | 1 (6) | 1 (5) | 0.96 |
| Renal diseases | 3 (8) | 1 (6) | 2 (10) | 0.90 |
| Laboratory examinations | ||||
| Leukocytes, µL− 1 (median, range) | 5.58 (3.02–17.31) | 4.75 (3.02–17.31) | 5.67 (3.62–8.84) | 0.75 |
| Platelets, x 1000 µL− 1 (median, range) | 198.50 (105–444) | 220 (105–444) | 192 (125–267) | 0.41 |
| C-reactive protein, mg/dl (median, range) | 12.83 (0.9-134.2) | 16.3 (0.9-134.2) | 10.145 (1.4-133.23) | 0.75 |
| Procalcitonin µg/L, (median, range) | 0.05 (0.02–0.5) | 0.05 (0.03–0.5) | 0.05 (0.02–0.2) | 0.78 |
| Ferritin µg/L, (median, range) | 257.3 (3.7–2838) | 208.7 (3,7-1242) | 642.15 (10,9-2838) | 0.15 |
| IL-6 pg/ml, (median, range) | 6,5 (1,5-196,8) | 5 (1,5-113,6) | 12,7 (1,5-196.8) | 0.49 |
| Antibodies anti-SARS-CoV-2 Trimer I, (median, range) | 360 (0-2080) | 514.5 (4.81–2080) | 170 (0-2080) | 0.60 |
| Antibodies anti-SARS-CoV-2 Trimer II, (median, range) | 1390 (0.87–2080) | 896 (188–2080) | 1410 (0.87–2080) | 0.43 |
| Imaging, N (%) | ||||
| CT typical changes (ground glass opacities and crazy-paving pattern) | 22 (61) | 9 (56) | 13 (65) | 0.69 |
| Treatment, N (%) | ||||
| Actemra/RoActemra (tocilizumab) | 2 (6) | 1 (10) | 1 (7) | 0.89 |
| Neoparin (endoxaparinum natricum) | 20 (56) | 8 (80) | 12 (80) | 1.0 |
| Remdesivir | 4 (11) | 1 (10) | 3 (20) | 0.68 |
| Dexamethasone (glucocorticosteroid) | 14 (39) | 8 (80) | 6 (40) | 0.10 |
| Budesonide (glucocorticosteroid) | 2 (6) | - | 2 (13) | - |
Abbreviations: COVID-19 - Coronavirus disease 19, N-number of patients, IL-6 – interleukin 6, CT- computed tomography scan.
Patients with COVID were divided into two subgroups:
group I - patients without post-COVID-19 syndrome (n = 16).
group II - patients with post-COVID-19 syndrome (n = 20) and memory complaints.
Patients from the first group were younger (median of age − 47 years) than patients from the second group (median of age − 57 years), with the women predominance in both groups. Patients with post-COVID syndrome had slightly longer hospitalization than patients without post-COVID manifestation (median 11 days vs. 9 days). The study group was homogenous regarding the clinical picture and presented symptoms such as dyspnea, fever, cough, sore throat, weakness, olfactory disorders, diarrhea, and headache. The administrated treatment included: Actemra/RoActemra (tocilizumab), Neoparin (endoxaparinum natricum), Remdesivir, Dexamethasone (glucocorticosteroid), Budesonide (glucocorticosteroid). The patients were treated with dexamethasone during hospital stay to release of the symptoms and the treatment lasted no longer than 7 days. Patients without post-COVID syndrome presented higher concentrations of C-reactive protein, whereas in patients with post-COVID increased numbers of leukocytes, and elevated levels of ferritin and interleukin 6 (IL-6) were found (Table 1). Higher baseline concentrations of anti-SARS-CoV-2 antibodies were observed in patients without post-COVID syndrome as compared to patients not demonstrating such manifestation (group II) (Table 1).
Quantitative assessment of tested biomarkers
Significantly higher concentrations of YKL-40 were observed in the whole study group of patients with COVID-19 (median 44 ng/ml) in comparison to healthy subjects (median 29 ng/ml, p = 0.016), whereas the levels of MR-pro-ADM were not different between both study groups (median 628 pmol/l vs. 725 pmol/l, respectively, p = 0.222). Only the concentration of YKL-40 differentiates significantly between the three study groups (p = 0.012 in the Kruskal-Wallis test (K-W test)), whereas the levels of MR-pro-ADM were not statistically different in comparison three study groups (p = 0.311 in K-W test). Our findings revealed significantly higher basal levels of YKL-40 (before treatment) in the group of patients without post-COVID syndrome as compared to controls (medians: 77 vs. 28,6 ng/ml, p = 0.008 in post-hoc test), however, the difference in concentrations of YKL-40 between group of patients with post-COVID and controls (median: 37 ng/ml, p = 0.303 in post-hoc test), as well as between patients with post-COVID and without post-COVID syndrome (p = 0.187 in post-hoc test) were not significant. The comparative analysis of basal levels of MR-pro-ADM (visit 1 – at hospital admission) did not reveal significant differences between any of the tested groups (controls vs. patients with COVID median: 656 pmol/l, p = 0.786 in the post-hoc test; controls vs. patients without COVID median: 607 pmol/l, p = 0.200 in post-hoc test; with post-COVID and without post-COVID syndrome p = 0.725).
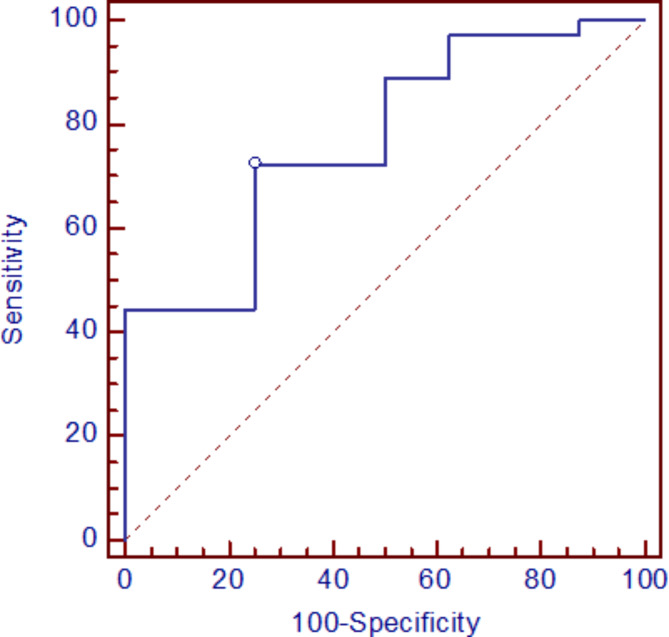
The Receiver Operating Characteristic (ROC) curve analysis indicates that YKL-40 protein concentration in the serum differentiates COVID-19 patients from the control group at the cut-off 35 ng/ml with specificity equal to 75% and sensitivity 72%, AUC = 0.771, p = 0.003 (Fig. 1).
Fig. 1.
The ROC curve for quantitative serum levels of YKL-40 in patients with COVID-19 in comparison to healthy controls.
Moreover, the correlations between tested biomarkers and white blood cells (WBC), platelets (PLT), and concentrations of C-reactive protein (CRP), procalcitonin (PCT), ferritin, as well as IL-6 were examined. The basal levels of YKL-40 in the group of patients with post-COVID syndrome correlated with pathological changes in the lung assessed by computed tomography (r = 0.558, p < 0.05). In comparison, the concentrations of MR-pro-ADM before treatment were associated with procalcitonin levels in post-COVID syndrome (r = 0.611, p < 0.05).
Relationships between symptoms and tested biomarkers
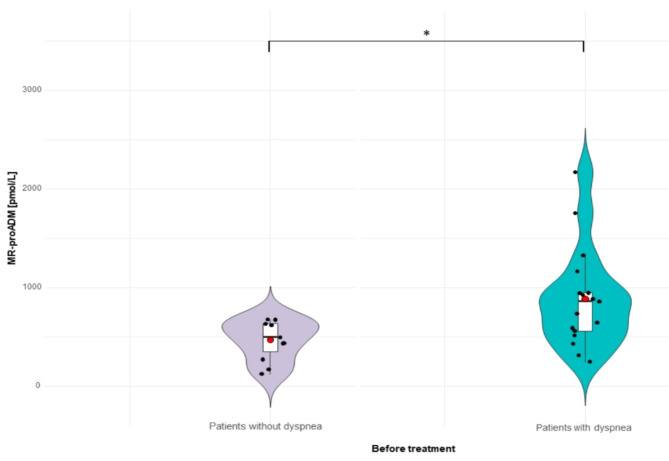
To assess whether there is any relationship between levels of tested biomarkers and clinical symptoms, patients with COVID-19 were divided into subgroups regarding the clinical features. In patients before therapy the most common manifestation were dyspnea, fever, cough, weakness, headache and olfactory disorders, whereas on follow-up visit (after six months of observation) in the group of patients with post-COVID-19 syndrome, the most frequent signs were memory complains, headaches and dizziness, changes to smell and taste, difficulty thinking and concentrating, as well as difficulties in choosing words. We focus on the comparison of the differences in tested biomarkers levels in the groups of patients with selected symptoms, typical for the disease, such as dyspnea or olfactory disorders, as well as characteristics for neurological manifestations developed after COVID-19 infection, including memory complaints, problems with concentration, and headaches and dizziness. Among patients suffering from dyspnea (n = 17) significantly lower concentrations of MR-pro-ADM were revealed on the visit 1 in comparison to the group without such symptom (n = 11) (median; 863 pmol/L vs. 498 pmol/L, p = 0.015 (Fig. 2)). The levels of YKL-40 did not differentiate significantly between groups that manifested such symptom and subjects without dyspnea (median: 47 vs. 37 ng/ml, p = 0.88).
Fig. 2.
The baseline concentrations of MR-pro-ADM in patients with dyspnea and subjects without dyspnea measured at hospital admission (visit 1 - before treatment). *p value between levels of MR-pro-ADM before therapy in patients with and without dyspnea p = 0.015.
ROC curve analysis indicates that serum MR-pro-ADM protein concentrations differentiate COVID-19 patients with dyspnea from COVID-19 patients without dyspnea at the cut-off equal 678 pmol/L, with specificity 100% and sensitivity 59%, whereas AUC was 0.775, p = 0.002 (Fig. 3).
Fig. 3.
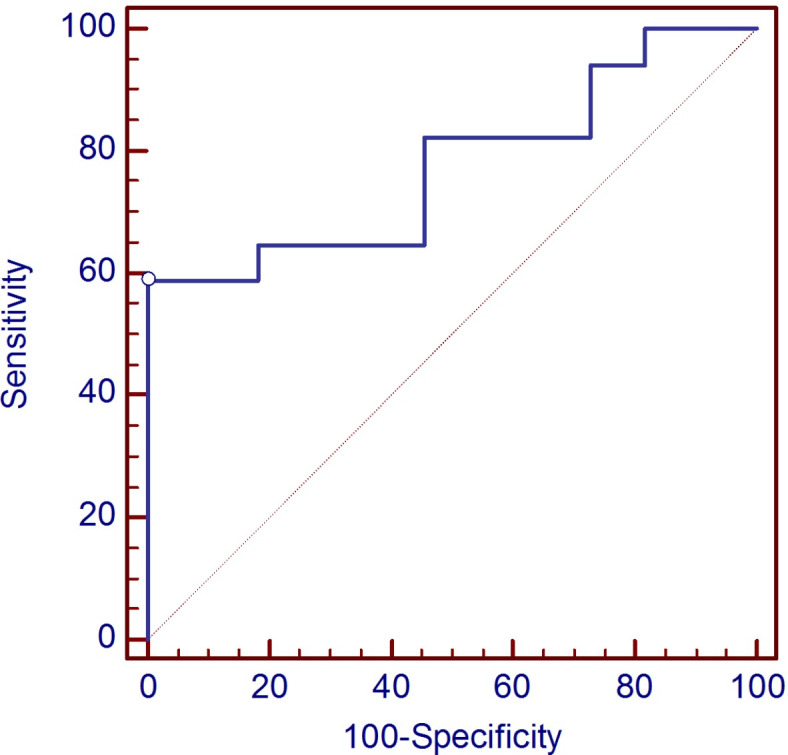
The receiver operating characteristic (ROC) curve for MR-pro-ADM levels in differentiation patients with dyspnea and patients without dyspnea.
Similar findings for both markers (MR-pro-ADM and YKL-40) were noticed in comparison to patients with olfactory disorders and the group of patients who did not present such symptoms. None of tested biomarkers revealed significant difference in concentration between patients manifested olfactory disorder and subjects without such symptoms (medians for MR-pro-ADM: 592 vs. 643 pmol/L, p = 0.69 and for YKL-40: 67 vs. 42 ng/ml, p = 0.29).
The concentrations of YKL-40 in patients with selected neurological symptoms were lower as compared to subjects without such clinical manifestation, however the difference was not statistically significant (patients with memory complains, median 36,68 ng/ml vs. 70,89 ng/ml, p = 0.178, group with headaches and dizziness, median 37,08 ng/ml vs. 60,76 ng/ml, p = 0.320, as well as patients with concentration problems, median 36,07 ng/ml vs. 44,69 ng/ml, p = 0.625). In agreement with the YKL-40 findings, concentrations of MR-pro-ADM were not significantly different between groups of patients with cognitive dysfunction (post-COVID syndrome) and individuals without neurological complication (patients with memory complains, median 655,90 vs. 614,47 pmol/L, p = 0.54, group with headaches and dizziness, median 636,25 vs. 592,29 pmol/L, p = 0.957, as well as patients with concentration problems, median 498,30 vs. 637,02 pmol/L, p = 0.657).
Assessment of concentration changes of tested biomarkers after six months of observation (follow-up visit)
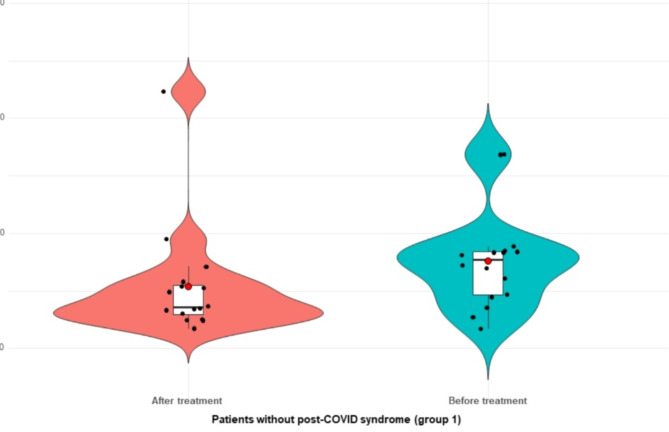
In all patients with COVID-19, the concentrations of YKL-40 and MR-pro-ADM decreased after six month of observation (visit 2) (median 39 ng/ml and 499 pmol/l, respectively), although the differences were not statistically significant (for YKL-40 p = 0.226 and MR-pro-ADM p = 0.119). Similarly, in patients without post-COVID syndrome concentrations of both biomarkers had fallen six months after hospitalization (visit 2, median of levels: 36 ng/ml vs. 456 pmol/l, respectively), however, significant differences were noticed only for YKL-40 (p = 0.008, for MR-pro-ADM p value = 0.326) (Fig. 4). In the group of subjects with post-COVID syndrome, no significant differences in levels of both proteins between visit 1 and visit 2 were found (medians for MR-pro-ADM 656 vs. 549 pmol/l, p = 0.171 and YKL-40 37 vs. 40 ng/ml, p = 0.295). In both groups of patients, the concentrations of CRP protein significantly decreased six months of observation (p = 0.012), additionally, in patients with post-COVID syndrome (group 2) reduced levels of IL-6 and ferritin on visit 2 were noticed, however, the difference were not statistically significant (p = 0.24, p = 0.44) (Table 2).
Fig. 4.
The concentrations of YKL-40 (ng/mL) in patients without post-COVID syndrome (group 1) at admission to hospital (visit 1) and after 6-months of observation (visit 2) *p value < 0.05.
Table 2.
Characteristics of laboratory variables in patients without post-COVID (group 1) and patients with post-COVID syndrome (group 2) during 6 months follow-up (visit 2).
| Variables on follow-up visit (6 months after treatment) | Group 1 patients without post-COVID | Group 2 patients with post-COVID | p-value group 1 vs. group 2 (visit 2) |
|---|---|---|---|
| Leukocytes, µL-1 (median, range) | 7.81 (3.35–15.81) | 6.05 (4.04–10.73) | 0.077 |
| p value (visit 1 vs. visit 2) | 0.020 | 0.300 | |
| Platelets, x 1000 µL-1 (median, range) | 367 (142–489) | 223 (126–412) | 0.004 |
| p value (visit 1 vs. visit 2) | 0.004 | 0.124 | |
| C-reactive protein, mg/dl (median, range) | 1.69 (1.00-93.7) | 2.05 (0.86–29.2) | 0.649 |
| p value (visit 1 vs. visit 2) | 0.009 | 0.012 | |
| Procalcitonin, (median, range) | 0.23 (0.02–0.25) | 0.05 (0.04–0.05) | 0.628 |
| p value (visit 1 vs. visit 2) | 0.003 | 0.654 | |
| Ferritin µg/L, (median, range) | 367.5 (12-1031) | 292.75 (9.6–2556) | 0.862 |
| p value (visit 1 vs. visit 2) | 0.161 | 0.441 | |
| IL-6 pg/ml, (median, range) | 7.95 (1.5–15.8) | 2.25 (1.5-634.5) | 0.570 |
| p value (visit 1 vs. visit 2) | 0.108 | 0.236 |
Abbreviations: COVID-19 - Coronavirus disease 19, IL-6 – interleukin 6.
The ratios for YKL-40 and MR-pro-ADM levels measured after first and second visit in the group I (median for YKL-40 1:2 = 1,56 and MR-pro-ADM 1:2 = 1,18) and group II (median for YKL-40 1:2 = 0,95 and MR-pro-ADM 1:2 = 1,15) were calculated. In the group of patients without post-COVID syndrome the YKL-40 ratio was significantly higher as compared to post-COVID syndrome (p = 0,004). Similar difference was found for MR-pro-ADM ratio (p = 0.789).
ROC curve analysis indicates that YKL-40 protein concentration ratio -YKL-40 1/2 differentiates the group of patients with post-COVID syndrome from patients without post-COVID syndrome at the cut-off equal 0.998, and with specificity 87.5% as well as sensitivity 65%, AUC = 0.775 (p = 0.0009) (Fig. 5).
Fig. 5.
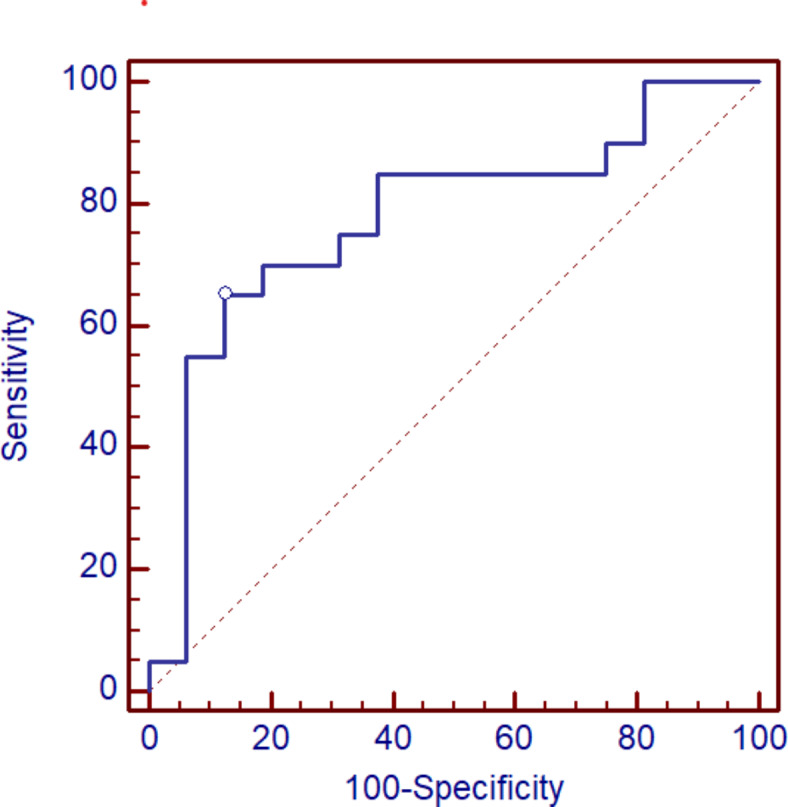
ROC curve for YKL-40 1/2 ratio in differentiation between patients without post-COVID syndrome (group 1) and with post-COVID syndrome (group 2).
Relationships between type of medication and levels of tested biomarkers
In the group of patients with COVID, the analysis of both markers was also performed depend the type of administrated treatment. The higher basal levels of YKL-40 and ratio of YKL-40 1:2 were observed in the patients treated with dexamethasone (group B) in comparison to the group without such treatment (group A) (Table 3), although, a significant difference was observed only for YKL-40 1:2 ratio (p = 0.267 vs. p = 0.013). Moreover, in dexamethasone-treated patients concentrations of YKL-40 decreased significantly on visit 2 (p = 0.05) (Table 3). The levels of MR-pro-ADM were similar in both groups before treatment (p = 0.689), additionally, the concentration of the protein decreased in both groups (gr. A vs. B) after therapy (Table 3), although the differences were not statistically significant (Table 3). ROC curve analysis indicates that serum YKL-40 1/2 ratio differentiates COVID-19 patients treated with dexamethasone from patients without such treatment at the cut-off at 0.97, specificity was equal to 73% and sensitivity 86%, AUC equal 0.792, p = 0.003 (Fig. 6).
Table 3.
The concentrations of tested biomarkers in patients with COVID-19 treated with dexamethasone and without such treatment.
| Variables | Group A without dexamethasone (n = 22) | p value gr.A v.1 vs. v.2 | Group B with dexamethasone (n = 14) | p value gr.B v.1 vs. v.2 | ||
|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Visit 1 | Visit 2 | |||
| YKL-40, ng/ml (median, range) | 36.63 (8.31-185.86) | 37.11 (8.98-279.29) | 0.566 | 59.56 (17.25-177.45) | 33.66 (15.52-233.43) | 0.05 |
| YKL-40 1:2 ratio, (median, range) | 0.77 (0.13–2.30) | 1.41 (0.32–5.50) | ||||
| MR-pro-ADM, pmol/l (median, range) | 635.48 (127.11-1757.72) | 475.15 (338.22-1485.09) | 0.130 | 620.52 (174.27-2173.03) | 536.33 (194.91-2393.74) | 0.509 |
| MR-pro-ADM 1:2 ratio, (median, range) | 1.16 (0.38–1.75) | 1.10 (0.38–3.96) | ||||
Abbreviations: MR-pro-ADM - Mid regional pro-adrenomedullin, YKL-40 - Chitinase-3-like protein 1.
Fig. 6.
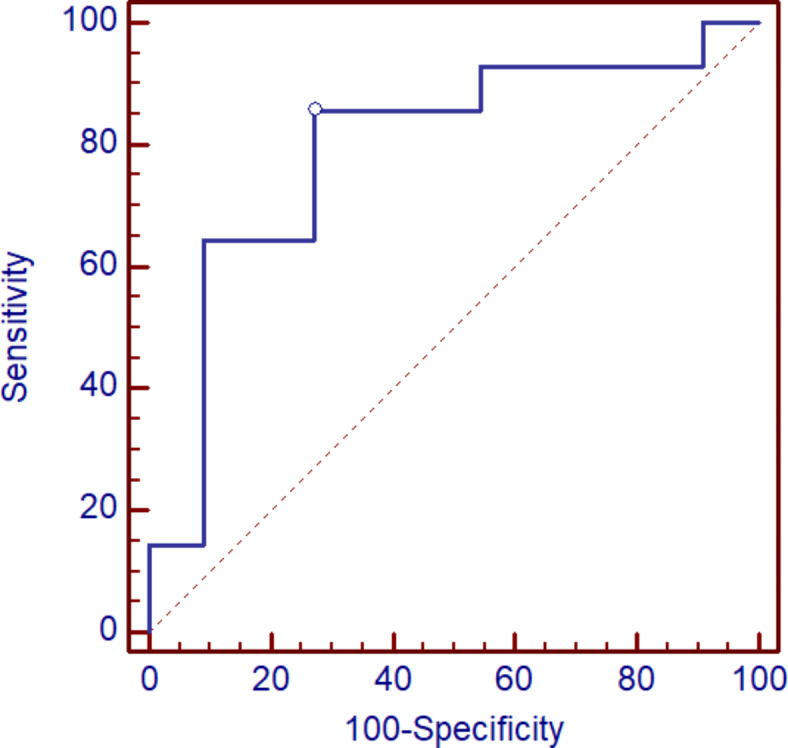
The ROC curve for serum YKL-40 1/2 ratio in differentiation patients with COVID-19 treated with dexamethasone and group of patients without such medication.
A similar analysis was performed for remdesivir, but not significant differences in the concentrations of YKL-40 and MR-pro-ADM between groups of patients treated with remdesivir and patients without such medication were found (median for YKL-40–30 ng/ml vs. 45 ng/ml, p = 0.295, and for MR-pro-ADM 587 pmol/L vs. 635 pmol/L, p = 0.694 respectively).
Discussion
In the present study, we investigated proteins engaged in vascular permeability, inflammation, and lung fibrosis, as well as pathological processes in the central nervous system (CNS) such as MR-pro-ADM and YKL-40 in patients with COVID-19, who developed in time post-COVID syndrome, neurological abnormalities and complained on so called “post-COVID-19 brain fog” and in the group of patients without such symptoms, at hospital admission and six months after hospital discharge to establish whether YKL-40 or MR-pro-ADM could be helpful indicators of neurological complications after COVID-19 infection. The findings of our study revealed higher concentrations of YKL-40 in patients without post-COVID syndrome as compared to the group with post-COVID and neurological manifestation, however, the difference was not significant. Moreover, only YKL-40 1/2 ratio allows to differentiate patients with post-COVID syndrome and cognitive dysfunction. Additionally, the concentration of YKL-40 decreased statistically in patients without post-COVID syndrome in 6-month follow-up. Interestingly, significant differences in the levels of this protein and YKL-40 1/2 ratio were found between patients treated with dexamethasone and group without such medication. Our results indicate that MR-pro-ADM allows to differentiate patients with dyspnea and subjects without such manifestation, as significantly higher levels of MR-pro-ADM were found in patients with dyspnea. The advantage of our longitudinal study is the possibility of tracking changes in the concentrations selected biomarkers in time to evaluate the potential diagnostic and prognostic utility of these proteins in the post-COVID stage with neurological manifestation. It should, however, be stressed that tracking the dynamics of markers is more important for predicting cognitive outcomes than isolated assessment. Since the number of reported neurological complications in COVID-19 survivals is rapidly increasing the investigation of potential mechanisms is necessary, thus such studies seem to be crucial. The mechanisms underlying potential neurological dysfunctions after COVID-19 infection are still unknown and may include the direct impact of cellular damage caused by viral infection, secondary inflammatory responses, reduced angiotensin-converting enzyme 2 (ACE2) activity that modulate neuroprotective and neuroimmunomodulatory functions, oxidative stress, and hypoxia, sepsis, and/or multi-organ damage related to severe COVID-194,27. The main mechanism leading to cognitive impairment in COVID-19 patients is considered to be systemic inflammation infecting the central nervous system (CNS) and a storm of intracranial cytokines mediating blood-brain barrier permeability4,28. Glial cells including microglia and astrocytes, main players in neuroinflammation, might be a possible target of COVID-19 viruses as higher expression of angiotensin-converting enzyme 2 (ACE2)29. Glial cells are responsible for fighting viruses through the recruitment of immune cells and the activation release of antiviral cytokines. Persisting viral infection may result in the overactivation of microglial cells, which may be responsible for neuronal injury or T cell/astrocytes-induced neurotoxicity28. Moreover, an alternative hypothesis postulates that vascular involvement could also affect the onset of neurocognitive manifestation4. Additionally, SARS-COV-2 colocalizes with the phosphorylated tau protein, causing neuronal death and disturbing the distribution of tau, such chronic pathomechanism may potentially result in neurodegeneration30. Growing evidence indicates that COVID-19 infection may be responsible for many psychiatric problems, and neurological consequences including anxiety, depression, stroke, ataxia, cognitive impairment, as well as dysautonomia, and the sympathetic storm caused by cholinergic dysfunction31–36. Studies showed that syndrome coronavirus type 2 (SARS-CoV-2) spike protein may act as a neurotoxin on nicotinic acetylcholine receptors (nAChR), after binding to receptors cause hyperinflammation and cell injury34. Bearing in mind above mentioned facts, we hypothesize that the elevation of neuroinflammation and vascular dysfunction markers can be linked to the incidence of neurological complications, including memory problems assessed in patients with COVID-19. Thus we evaluated potential clinical utility of YKL-40 and MR-pro-ADM in patients with memory problems developed after COVID-19 infection.
In agreement with other investigations37,38 we noticed increased serum concentrations of YKL-40 in patients with COVID-19 in comparison to healthy controls. Additionally, the significant difference was noticed in concentration of YKL-40 in the group without post-COVID as compared to controls. Moreover, we demonstrated that YKL-40 presents a well diagnostic performance in the discrimination of COVID-19 patients from controls. It is postulated that YKL-40 elevates in the early phase of COVID-19 infection as a part of the beginning inflammatory response by the host organism recognizing the SARS-COV-2 virus38. The enhanced expression of YKL-40 in bronchiolar epithelial cells and alveolar macrophages located close to fibrotic lesions in patients with idiopathic pulmonary fibrosis (IPF) indicates the pivotal role of this protein in pulmonary tissue remodeling during damage and repair processes in COVID-1937. Findings Kamle et al.23 demonstrated that expression of YKL-40 was related to potentiated SARS-COV2 infection in epithelial cells. Thus, one can speculate that YKL-40 is potentially involved in COVID-19 by promoting persisting pathological mechanisms in the lungs. The diagnostic approach of YKL-40 as a prognostic marker of COVID-19 was widely discussed in the literature25,37,38. Kimura et al. showed that YKL-40 presents a better ability to discriminate favorable and adverse prognoses in patients with COVID-19 than other markers. Elevated serum levels of YKL-40 allowed to identification of COVID-19 patients with severe pneumonia at high risk of mortality37.
Considering YKL-40 is involved in neuroinflammation and development of impairment of cognitive functions we assessed the clinical usefulness of this protein in patients with COVID-19 with memory problems. Unexpectedly, we found higher concentrations of YKL-40 in patients without post-COVID syndrome as compared to the group of patients with post-COVID syndrome and memory problems. Moreover, we did not revealed the difference of tested biomarkers in patients with different neurological symptoms probably due to small subgroups of patients with specific symptoms. Elevated levels of YKL-40 in patients without post-COVID syndrome could be related to more potentiated inflammatory process in this group, as higher concentrations of C-reactive protein in this group of patients were noticed. YKL-40 in patients without post-COVID syndrome may be a critical factor in the development of the COVID infection. Such results are in line with other studies39. On the other hand, the youngest age of these patients should also be bear in mind when interpreting the results, since age is a risk factor for neurological manifestation, including cognitive decline in the general population, as well in post-COVID. Patients with post-COVID syndrome were older than the first group. People in more advanced age have less cognitive reserve, and they are more burdened with comorbidities that could contribute to the development of neurological complications39. However, the significance of this protein as a predictive factor of neurocognitive complications in survivors with post-COVID syndrome requires further studies. Essential to answer the question raised seems to be investigations of new biomarkers in blood and cerebrospinal fluid (CSF) of COVID-19 survivals with neurological complications, and a comparison of the dynamic changes of concentrations biomarkers with the severity of the disease, existing symptoms, comorbidities, and cognitive status assessed by psychological tests. Furthermore, our study revealed that the YKL-40 ratio presents a better diagnostic accuracy in the discrimination of a group of patients with post-COVID syndrome and neurological dysfunction from patients, who did not manifest symptoms of post-COVID syndrome than the individual measurement of biomarkers. This finding highlights the significance of longer observation for the identification of post-COVID complications, and assessment of the dynamic changes of biomarker levels, to establish possible prognosis of the patients.
Interestingly, our findings revealed elevated baseline levels of CRP and ferritin in patients with COVID-19 infection at hospital admission, although, CRP concentrations were higher in patients without post-COVID, whereas ferritin levels in the group with post-COVID syndrome. We speculated that increased ferritin levels in post-COVID syndrome could be a result of smoldering inflammation and extensive immunological response, however, it might be also related to neurological pathomechanisms, that forecast the decline of cognitive functions. It seems that at the onset of COVID ferritin could have dual functions and may enhance inflammatory pathways by the activation of NLRP3 inflammasome and consequent synthesis of pro-inflammatory molecules, as well as could act as a protective factor that prevents cell death induced by oxidative stress and neurotoxic proteins40–42. On the other hand, some experimental evidence confirms that ferritin and iron could be risk factors for neurodegenerative diseases. The main function of ferritin is to store cellular iron in redox inactive form, protecting the cell from potential iron-dependent radical damage and allowing iron release according to cellular demand43. About 70% of the iron in the brain is associated with myelin that contains ferritin44,45. Studies on animal models with dysregulated iron metabolism due to genetic deficiencies in iron regulatory proteins have shown that increased ferritin levels could be associated with myelin breakdown as a consequence of damage to oligodendrocytes and white matter46. Moreover, it has been established that the genetically induced mutation in ferritin causes its loss of function, leads to decreased ability of ferritin to bind iron within the iron core of ferritin. Elevated iron levels promote oxidative damage in the brain and protein oligomerization, which are common pathomechanisms of cognitive impairment and development of the neurodegenerative diseases43. Importantly, in interpreting the ferritin results, age also should be considered. During normal aging, about 45% of myelinated fiber length is damaged, particularly from regions such as the frontal lobe that contain the highest percentage of thinner fibers that lose their myelin sheaths with age, especially in individuals with ApoE4 genotype, which could influence higher ferritin levels47. In our investigation we did not confirm significantly different levels of MR-pro-ADM in patients with COVID-19 as compared to controls. However, significantly higher protein concentrations were found in patients with dyspnea than in the group without such manifestation, which is in agreement with other studies. Lack of difference in the levels of the biomarker in the whole group of COVID-19 could be related to a lower number of examined subjects, particularly in the control group. On the other hand, considering that MR-pro-ADM is involved in inflammatory response and allows to reflect the progression from sepsis to septic shock we postulated that the difference between our findings and other investigations could arise from less intensive inflammatory processes in our patients and less severity of COVID-19 in our population in comparison to other cohorts. It seems that COVID-19 patients in our study were affected by a moderate severity course of the disease as compared to populations from other investigations11,13,17,48. Literature data reported that MR-pro-ADM, a biomarker of endothelial dysfunction could be a useful indicator of COVID-19 severity. Some studies revealed that higher concentrations of MR-pro-ADM may be useful tool for monitoring disease progression and stratification of the risk of critical illness or death46,47. Increased concentration of MR-pro-ADM was found in 74% of patients with COVID-19 with critical illness as compared to those without49. In agreement with that observation are findings from the study by Benedetti et al., the authors revealed that mean concentrations of MR-pro-ADM in patients who died were significantly higher (3,5 nmol/l) than in those who survived (0,80 nmol/l)48, Study Guadiana-Romualdo et al. showed that MR-pro-ADM could be a valuable biomarker for the prediction of mid- or short-term mortality in confirmed COVID-19. Higher mortality was noticed in patients with MR-pro-ADM above 0,80 nmol/l in comparison to those with protein levels below/equal to 0,80 nmol/l. It is suggested that lower levels of MR-pro-ADM allow identified patients with lower mortality risk who could be treated less intensively. In our study levels of MR-pro-ADM in patients with post-COVID syndrome as well as patients without post-COVID-19 were much lower than in other investigations11,13,50,51, such results may reflect less severity of the disease and could be related to a better prognosis.
We evaluated the long-term dynamics of YKL-40 and MR-pro-ADM in patients with COVID-19 at admission and six months after hospitalization. We found not significant decreased levels both markers after treatment in COVID-19 patients. However, the significantly lower levels was noticed only for YKL40 in the group of patients without post-COVID infection. Additionally, only YKL 40 ratio presented promising diagnostic performance in differentiation individuals with post-COVID and without this syndrome, as significantly higher YKL 40 ratio in patients without post-COVID was found. An important aspect that should be considered in the interpretation of the alterations of the potential biomarkers in COVID-19 patients with neurological manifestations is applied anti-COVID-19 treatment, that could affect COVID-19 related neurodysfunction. Since it remains unknown how SARS-CoV-2-related therapies affect COVID-19-associated neuroinflammation and neurodegeneration, as well as the dynamics of protein reflecting such pathological changes, studies that provide a deeper understanding of such relationships are necessary. The tracking of changes in the concentrations of the biomarkers in time could be more valuable for predicting some neurological complications than isolated outcomes. More diminished levels of YKL-40 in patients without COVID-19 syndrome after treatment could be related to an overall favorable response to therapy. This finding indicates the clinical value of YKL-40 protein as a predictive biomarker in the assessment of the patient’s prognosis after therapy. Additionally, it should be noticed that concentrations of this protein in patients with post-COVID syndrome and neurocognitive symptoms did not changed after therapy, which might be associated with weaker immunological response and less effectiveness in therapy in this group of subjects. On the other hand, sustained constantly higher levels of YKL-40 in patients with post-COVID syndrome after 6 months may suggest an ongoing pathological process, which in time could lead to cognitive decline. It might be possible that some indirect pathways exist that drive YKL-40-mediated brain dysfunction. In agreement with these findings is the study Laudanski et al., which demonstrated that elevated concentrations of YKL-40, NCAM-1, and CCL23 may reflect the beginning and accelerating neurodegenerative processes after COVID-19. The authors suggested that in post-COVID, patterns of biomarker changes could be essential for the prediction of the disease52. Interestingly, the neurodegeneration process initiated by the viral infection seems to continue despite the decrease in biomarkers indicating such pathology. Kanberg et al. revealed that the neurological symptoms persisted despite the decreasing concentrations of GFAP and NFL six months after COVID-19 53. Under that reasoning, longitudinal studies assessing a panel of neurodegenerative biomarkers changes for a longer time in a cohort of survivors of COVID-19 could be valuable and necessary.
We compared the concentrations of YKL-40 and MR-pro-ADM in patients treated with dexamethasone and remdesivir. Only in the group with dexamethasone, significant changes for the YKL-40 ratio were demonstrated as compared to non-dexamethasone group, furthermore, after treatment the levels of the protein declined. Additionally, the higher concentrations of inflammatory indicators (such as CRP and IL-6) and antibodies anti-SARS-CoV-2 at hospital admission in the dexamethasone-treated group before therapy were observed, as were the levels of YKL-40, may imply the higher degree of inflammation in this group of patients than in the group without dexamethasone. Given above mentioned facts we postulate that the significantly decreased concentration of YKL-40 after treatment with dexamethasone as compared to the findings in the non-dexamethasone group may be related to an effective response to anti-inflammatory treatment in this group of patients. One of the hypotheses explaining the findings is that dexamethasone could influence on restriction of neurological complications. In agreement with our findings, are also postulates of other researchers, who suggested that treatment diminishing the development of the infection (such as remdesivir), or regulating the immunological response (such as steroids) might have beneficial outcomes in COVID-19 patients, it may also regard to neurocognitive manifestations54–56.
We are sensible that this study entails some limitations. The first is a lack of variants analysis, however, the sample size was too small to perform stratification. Secondly, the patients with neurocognitive symptoms and memory problems from the group of post–COVID-19 were not examined by neuropsychological tests, but only on self-reporting symptoms of post-COVID-19 syndrome, such as brain fog, memory problems, etc., thus drawn conclusions should be interpreted with some caution. Additionally, before the COVID-19 infection, the cognitive status of the patients was not tested, however, during the interview, patients with symptoms of dementia or memory problems before the COVID-19 infection were excluded. Moreover, further validation studies on a larger independent group of patients are necessary. Third a longer follow-up may provide more information about the deterioration of neurocognitive functions and potential relationships with changes in some proteomic molecules in time. Future studies should examine the long-term post–COVID-19 cognitive deterioration and the relationships with biochemical tests, neuroimaging findings, as well as neuropsychological tests to assess potential mechanisms.
Conclusion
Despite the limitations, the present study indicates that the diagnostic performance of inflammatory markers, such as YKL-40 and MR-pro-ADM is more prominent in the assessment of the severity of diseases than in the evaluations of neurological complications. However, considering weaker diminished levels of YKL-40 gradually after therapy in patients with post-COVID syndrome with neurological manifestation, we can speculate that this protein may contribute to the development of neurocognitive abnormalities. The findings of the presented study revealed that cognitive problems might appear even after moderate COVID-19 infection in patients with post-COVID phase, and one of the potential risk factor to predict their development could be ferritin. Additionally, YKL-40 has potential as a predictive biomarker, the assessment of the protein after therapy allows forecasts of clinical efficacy in COVID-19 survivals.
Methods
Study participants
The study population involved 44 subjects, including 36 patients with COVID-19 and 8 healthy individuals, as the control group. The control group consists of healthy volunteers donating blood from a local Blood Donation Centre in Bialystok. Individuals from the control group were free of comorbidities, and not taking any medications. Control samples were taken from healthy patients from the pre-pandemic era, in whom inflammatory and neurodegenerative diseases, treatment with corticosteroids, immunosuppressive agents, or any biological therapy were excluded. Patients were diagnosed and treated at the Department of Infectious Diseases and Neuroinfectious of the Medical University of Bialystok, Poland. At the time of the diagnosis (visit1) data from the clinical history were recorded, CT was performed to confirm the changes in the lung, and routine laboratory tests were done. Next six months after discharge from the hospital the follow-up visit was scheduled (visit 2) with assessment of clinical symptoms and examination of the biochemical tests, including newly biomarkers, were performed. All patients with COVID-19 treated during the time of the study were analyzed and divided into two groups based on their clinical complications connected with cognitive dysfunction and memory problems: group I: patients without post-COVID-19 syndrome (n = 16; 12 women, 4 men, age 49 ± 9.1 years), and group II: patients with post-COVID-19 syndrome (n = 20: 15 women and 5 men, mean age 53 ± 9.7 years). The post-COVID syndrome group included patients with varoius symptoms ongoing for more than three months and fulfilled the criteria of the syndrome according to WHO: post-COVID-19 condition, commonly known as long COVID, can affect anyone exposed to SARS-CoV-2, regardless of age or severity of original symptoms. According to WHO post-COVID is defined as the continuation or development of new symptoms 3 months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least 2 months with no other explanation. While common symptoms of long COVID-19 can include fatigue, shortness of breath, and cognitive dysfunction. The following symptoms were also seen in the patients facial pain, muscle issues, neuralgia, fatigue, insomnia, changes to smell and taste, headache, dizziness, memory complaints, and problems with concentration. From the study group were excluded patients with memory problems, depression, and any neurological diseases, including neurodegenerative diseases that patients suffered from before the COVID-19 infection, as well as treated with corticosteroids, immunosuppressive agents, or any biological therapy to avoid the bias of the study. Patients not fulfilling the criteria of post-COVID were classified as a group without post-COVID syndrome. Patients and healthy individuals voluntarily agreed to participate in the study and gave their written informed consent. The Local Bioethics Committee of the Medical University of Bialystok approved the study.
Method selection of the chest computed tomography (CT) results in COVID-19 patients
Computed tomography imaging is considered as the most effective method for detecting lung abnormalities, particularly in the early stage of the COVID-19 disease. Thus all patients with COVID-19 were tested by CT to confirm the diagnosis. As a CT typical changes were described the ground glass opacities and crazy-paving. The findings were interpreted according to the radiological scale – the commonly used scale, the area of the lungs affected was divided into 25 points. The overall CT score was the sum of the points from each lobe and ranged from 0 to 25 points. Lungs were divided into five lobes, and each lobe was assessed individually (CT score from 0 to 5) depending on the percentage of the involved lobe: score 0–0% involvement; score 1 – less than 5% involvement; score 2–5–25% involvement; score 3–26–49% involvement; score 4–50–75% involvement; score 5 – greater than 75% involvement.
Blood sampling protocol and quantitative measurements
Peripheral venous blood was drawn into pyrogen-free blood tubes without any anticoagulant. Samples were allowed to clot at room temperature before centrifugation, and next aliquoted and stored at – 80 C until analysis. Routine laboratory tests (i.e. complete blood count with total leukocyte and platelets counts, C-reactive protein (CRP), ferritin, IL-6) were measured at the hospital laboratory. The concentration of YKL-40 (ng/ml) in the serum was determined using the Quidel test MicroVueTM YKL-40 enzyme immunoassay kit (San Diego, USA), whereas serum levels of MR-pro-ADM were assessed by FineTest kit (Wuhan, China). The quantitative determination of the tested proteins was performed in duplicate, following manufacturers’ procedures in the Department of Neurodegeneration Diagnostics. Serum concentrations of YKL-40 and MR-pro-ADM were measured twice: on admission (visit 1) and 6 months after treatment (visit 2).
Statistical methods
The statistical analysis was performed using StatSoft, Inc. (2015) STATISTICA (Data Analysis Software System), Version 13 and MedCalc Software Ltd Version 11.3. and R 4.2.3 statistical software, with the PMCMRplus package. The normality of variables was tested by Shapiro-Wilk test, as the concentrations of the tested proteins did not follow a normal distribution statistical analysis was performed using nonparametric tests, and all the data were reported as median and range. For examining biomarker levels between three groups (control, with post-COVID syndrome, and without post-COVID syndrome) the Kruskal-Wallis test was used. Moreover, significant differences between the levels of the tested proteins were analyzed using the post hoc Dwass–Steel–Critchlow–Fligner test to verify which groups were different. To compare serum biomarker levels between two groups Mann-Whitney U test and Wilcoxon-matched pair test were performed. Receiver-operating characteristic curve (ROC) was used to assess the diagnostic performance of tested biomarkers. Correlations between tested proteins and symptoms, imaging tests, and laboratory parameters were assessed by the Spearman rank test. A p-value of less than 0.05 was established as statistically significant.
Author contributions
A.KP. performed the experiments, contributed to the interpretation of results, and wrote the current manuscript. A.MM. designed the experiment, wrote preliminary documents on the part of the results presented here and got the funding. P.C and E.K. collected the biological material and contributed to the interpretation of results. JA. created a clinical database of patients and participated in preparing answers to reviewers. B.M., A.MM. supervised and corrected this manuscript.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Calabria, M. et al. Post-COVID-19 fatigue: The contribution of cognitive and neuropsychiatric symptoms. J. Neurol.269, 3990–3999 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ceban, F. et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun.101, 93–135 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker, J. H. et al. Assessment of cognitive function in patients after COVID-19 infection. JAMA Netw. Open4, e2130645 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crivelli, L. et al. Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis. Alzheimer’s Dementia18, 1047–1066 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batiha, G. E. S., Al-kuraishy, H. M., Al-Gareeb, A. I. & Welson, N. N. Pathophysiology of post-COVID syndromes: A new perspective. Virol. J.10.1186/s12985-022-01891-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soung, A. L. et al. COVID-19 induces CNS cytokine expression and loss of hippocampal neurogenesis. Brain145, 4193–4201 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Kuraishy, H. M. et al. Mechanistic insight and possible mechanism of seizure in Covid-19: The nuances and focal points. CNS Neurol. Disord. Drug Targets22, (2022). [DOI] [PubMed]
- 8.Bajinka, O., Simbilyabo, L., Tan, Y., Jabang, J. & Saleem, S. A. Lung-brain axis. Crit. Rev. Microbiol.48, 257–269 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Azzoni, R. & Marsland, B. J. The lung-brain axis: A new frontier in host-microbe interactions. Immunity.10.1016/j.immuni.2022.03.015 (2022). [DOI] [PubMed] [Google Scholar]
- 10.Nuzzo, D. & Picone, P. Potential neurological effects of severe COVID-19 infection. Neurosci. Res.. 10.1016/j.neures.2020.06.009 (2020). [DOI] [PMC free article] [PubMed]
- 11.Montrucchio, G. et al. Effectiveness of mid-regional pro-adrenomedullin (MR-proADM) as prognostic marker in COVID-19 critically ill patients: An observational prospective study. PLoS ONE16, e0246771 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montrucchio, G. et al. Effectiveness of mid-regional pro-adrenomedullin, compared to other biomarkers (including lymphocyte subpopulations and immunoglobulins), as a prognostic biomarker in COVID-19 critically ill patients: New evidence from a 15-month observational prospective. Front. Med.10, (2023). [DOI] [PMC free article] [PubMed]
- 13.Sozio, E. et al. MR-proADM as prognostic factor of outcome in COVID-19 patients. Sci. Rep.11, (2021). [DOI] [PMC free article] [PubMed]
- 14.Elke, G. et al. The use of mid-regional proadrenomedullin to identify disease severity and treatment response to sepsis—A secondary analysis of a large randomised controlled trial. Crit. Care22, 79 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hupf, J. et al. RNA-expression of adrenomedullin is increased in patients with severe COVID-19. Crit. Care24, 527 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fialek, B. et al. Systematic review with meta-analysis of mid-regional pro-adrenomedullin (MR-proadm) as a prognostic marker in Covid-19-hospitalized patients. Ann. Med.55, 379–387 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García de Guadiana-Romualdo, L. et al. Circulating MR-proADM levels, as an indicator of endothelial dysfunction, for early risk stratification of mid-term mortality in COVID-19 patients. Inte. J. Infect. Dis.111, 211–218 (2021). [DOI] [PMC free article] [PubMed]
- 18.Madden, N. et al. The link between SARS-CoV-2 related microglial reactivity and astrocyte pathology in the inferior olivary nucleus. Front. Neurosci.17, 1198219 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lananna, B. V. et al. Chi3l1/YKL-40 is controlled by the astrocyte circadian clock and regulates neuroinflammation and Alzheimer’s disease pathogenesis. Sci. Transl. Med.12, (2020). [DOI] [PMC free article] [PubMed]
- 20.Ferrari-Souza, J. P. et al. Astrocyte biomarker signatures of amyloid-β and tau pathologies in Alzheimer’s disease. Mol. Psychiatry27, 4781–4789 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonneh-Barkay, D., Wang, G., Starkey, A., Hamilton, R. L. & Wiley, C. A. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. J. Neuroinflamm.7, 34 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy, S. L. et al. Circulating markers of extracellular matrix remodelling in severe COVID-19 patients. J. Intern Med.294, 784–797 (2023). [DOI] [PubMed] [Google Scholar]
- 23.Kamle, S. et al. Chitinase 3-like-1 is a therapeutic target that mediates the effects of aging in COVID-19. JCI Insight6, (2021). [DOI] [PMC free article] [PubMed]
- 24.Kamle, S. et al. Host chitinase 3-like-1 is a universal therapeutic target for SARS-CoV-2 viral variants in COVID-19. Elife11, (2022). [DOI] [PMC free article] [PubMed]
- 25.Sánchez-Díez, S. et al. Biomarker profiles associated with COVID-19 severity and mortality. Curr. Issues Mol. Biol.45, 1998–2012 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Lorenzo, R. et al. Post-COVID trajectory of pentraxin 3 plasma levels over 6 months and their association with the risk of developing post-acute depression and anxiety. CNS Drugs38, 459–472 (2024). [DOI] [PubMed] [Google Scholar]
- 27.Bandala, C. et al. Putative mechanism of neurological damage in COVID-19 infection. Acta Neurobiol. Exp81, 69–79 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Batiha, G. E. S. et al. Targeting of neuroinflammation by glibenclamide in Covid-19: old weapon from arsenal. Inflammopharmacology. 10.1007/s10787-022-01087-8 (2023). [DOI] [PMC free article] [PubMed]
- 29.Al-kuraishy, H. M. et al. Pirfenidone and post-Covid-19 pulmonary fibrosis: Invoked again for realistic goals. Inflammopharmacology. 10.1007/s10787-022-01027-6 (2022). [DOI] [PMC free article] [PubMed]
- 30.Ramani, A., Pranty, A. I. & Gopalakrishnan, J. Neurotropic effects of SARS-CoV-2 modeled by the human brain organoids. Stem Cell Rep.. 10.1016/j.stemcr.2021.02.007 (2021). [DOI] [PMC free article] [PubMed]
- 31.Ransing, R. et al. Can COVID-19 related mental health issues be measured? Brain Behav. Immunity. 10.1016/j.bbi.2020.05.049 (2020). [DOI] [PMC free article] [PubMed]
- 32.Şahan, E., Ünal, S. M. & Kırpınar, İ. Can we predict who will be more anxious and depressed in the COVID-19 ward? J. Psychosom Res.140, (2021). [DOI] [PMC free article] [PubMed]
- 33.Al-kuraishy, H. M. et al. Covid-19-induced dysautonomia: A menace of sympathetic storm. ASN Neuro. 10.1177/17590914211057635 (2021). [DOI] [PMC free article] [PubMed]
- 34.Nadwa, E. H. et al. Cholinergic dysfunction in COVID-19: Frantic search and hoping for the best. Naunyn-Schmiedeberg’s Arch. Pharmacol. vol. 396. 10.1007/s00210-022-02346-9 (2023). [DOI] [PMC free article] [PubMed]
- 35.Boyraz, R. K., Şahan, E., Boylu, M. E. & Kırpınar, İ. Predictors of long-term anxiety and depression in discharged COVID-19 patients: A follow-up study. World J. Clin. Cases10, (2022). [DOI] [PMC free article] [PubMed]
- 36.Serrano-Castro, P. J. et al. The cognitive and psychiatric subacute impairment in severe Covid-19. Sci. Rep.12, (2022). [DOI] [PMC free article] [PubMed]
- 37.Kimura, Y. et al. Identification of serum prognostic biomarkers of severe COVID-19 using a quantitative proteomic approach. Sci. Rep.11, (2021). [DOI] [PMC free article] [PubMed]
- 38.De Lorenzo, R. et al. Chitinase-3-like protein-1 at hospital admission predicts COVID-19 outcome: A prospective cohort study. Sci. Rep.12, 7606 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Damiano, R. F. et al. Cognitive impairment in long-COVID and its association with persistent dysregulation in inflammatory markers. Front. Immunol.14, (2023). [DOI] [PMC free article] [PubMed]
- 40.Zhang, N., Yu, X., Xie, J. & Xu, H. New Insights into the Role of Ferritin in Iron Homeostasis and Neurodegenerative Diseases. Mol. Neurobiol.. 10.1007/s12035-020-02277-7 (2021). [DOI] [PubMed]
- 41.Ruscitti, P. & Giacomelli, R. Ferritin and Severe COVID-19, from clinical observations to pathogenic implications and therapeutic perspectives. Isr. Med. Assoc. (2020). [PubMed]
- 42.Yang, H. et al. Mitochondrial ferritin in neurodegenerative diseases. Neurosci. Res.. 10.1016/j.neures.2013.07.005 (2013). [DOI] [PubMed]
- 43.Friedman, A., Arosio, P., Finazzi, D., Koziorowski, D. & Galazka-Friedman, J. Ferritin as an important player in neurodegeneration. Parkins. Relat. Disord.. 10.1016/j.parkreldis.2011.03.016 (2011). [DOI] [PubMed]
- 44.Quintana, C. et al. Study of the localization of iron, ferritin, and hemosiderin in Alzheimer’s disease hippocampus by analytical microscopy at the subcellular level. J. Struct. Biol.153, (2006). [DOI] [PubMed]
- 45.de los Monteros, A. E. et al. Dietary iron and the integrity of the developing rat brain: A study with the artificially-reared rat pup. Cell Mol. Biol.46, (2000). [PubMed]
- 46.Bartzokis, G. et al. Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol. Aging28, (2007). [DOI] [PubMed]
- 47.Bartzokis, G. et al. Apolipoprotein E genotype and age-related myelin breakdown in healthy individuals: Implications for cognitive decline and dementia. Arch. Gen Psychiatry63, (2006). [DOI] [PubMed]
- 48.Sozio, E. et al. Identification of COVID-19 patients at risk of hospital admission and mortality: A European multicentre retrospective analysis of mid-regional pro-adrenomedullin. Respir. Res.23, (2022). [DOI] [PMC free article] [PubMed]
- 49.Benedetti, I. et al. High levels of mid-regional proadrenomedullin in ARDS COVID-19 patients: The experience of a single, italian center. Eur. Rev. Med. Pharmacol. Sci.25, (2021). [DOI] [PubMed]
- 50.Oblitas, C.-M. et al. Mid-regional pro-adrenomedullin, methemoglobin and carboxyhemoglobin as prognosis biomarkers in critically ill patients with COVID-19: An observational prospective study. Viruses13, 2445 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lippi, G. & Henry, B. M. Pooled analysis of mid-regional pro-adrenomedullin values in COVID-19 patients with critical illness. Intern. Emerg. Med.16, 1723–1725 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laudanski, K. et al. Dynamic changes in central and peripheral neuro-injury vs. Neuroprotective serum markers in covid-19 are modulated by different types of anti-viral treatments but do not affect the incidence of late and early strokes. Biomedicines9, (2021). [DOI] [PMC free article] [PubMed]
- 53.Kanberg, N. et al. Neurochemical signs of astrocytic and neuronal injury in acute COVID-19 normalizes during long-term follow-up. EBioMedicine70, 103512 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roback, J. D. & Guarner, J. Convalescent plasma to treat COVID-19. JAMA323, 1561 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Rezagholizadeh, A., Khiali, S., Sarbakhsh, P. & Entezari-Maleki, T. Remdesivir for treatment of COVID-19; an updated systematic review and meta-analysis. Eur. J. Pharmacol. vol. 897. 10.1016/j.ejphar.2021.173926 (2021). [DOI] [PMC free article] [PubMed]
- 56.Wu, C. et al. Corticosteroid therapy for coronavirus disease 2019-related acute respiratory distress syndrome: A cohort study with propensity score analysis. Crit. Care24, (2020). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.