Abstract
Candida dubliniensis is an uncommon cause of bloodstream infection. We describe the first reported case of endocarditis caused by C. dubliniensis and the use of a rapid and novel real-time PCR assay based on the internal transcribed spacer 2 variable region of the rRNA operon that was used to identify this organism.
CASE REPORT
A 30-year-old intravenous drug user infected with hepatitis C but human immunodeficiency virus antibody negative and who had last injected heroin 3 days prior to admission presented to the Emergency Department in a confused and agitated state. He was obviously jaundiced and dehydrated on admission and had a fever of 39.4°C. Examination revealed a petechial rash, a systolic and diastolic murmur (that was later noted not to have been present on a previous admission), and tender hepatomegaly. Laboratory studies revealed a white blood count of 15,000 cells/mm3 (87% neutrophils), abnormal liver function, and a disseminated intravascular coagulopathy. The initial clinical impression was of decompensated liver disease secondary to sepsis of unknown source—possibly meningitis or endocarditis.
Following a septic screen that included blood cultures, the patient was treated empirically with intravenous vancomycin (1 g, twice per day) and cefotaxime (2 g, six times per day). The clinical course was complicated by increasing confusion, abdominal pain, and deteriorating liver, respiratory, cardiovascular, and renal function that required supportive care in the Intensive Therapy Unit. Radiological investigations revealed three small intracerebral lesions (consistent with abscesses) and infarcted lesions of the spleen and both kidneys. A transesophageal echocardiogram revealed severe aortic incompetence with a possible vegetation on the anterior valve cusp leaflet.
Four sets of blood cultures were obtained in BACTEC Plus Aerobic/F and BACTEC Plus Anaerobic/F medium during the first 10 days of admission. A coagulase-negative staphylococcus was recovered from the admission blood culture, but a yeast was recovered from the aerobic bottle of three subsequent blood cultures taken 2, 7, and 9 days postadmission. Following identification of a yeast on day 3, liposomal amphotericin B (5 mg/kg of body weight once daily) was added to the patient's antimicrobial therapy. In view of continued deterioration and the lack of surgical options, caspofungin (50 mg on the first day and 35 mg daily thereafter) was added. Despite intensive supportive care and antifungal therapy the patient died 17 days postadmission. Consent for a post mortem examination was obtained and confirmed the ante mortem findings of multiple areas of septic emboli and infarction of both kidneys, spleen, and brain. Septic emboli composed of a mass of fungi were seen within a number of vessels. Fungal vegetations on the anterior leaflet of the aortic valve were also confirmed.
The three yeasts obtained from all three blood cultures were germ tube positive and produced abundant chylamydospores. All isolates were tested with the API ID32C yeast identification system (BioMérieux, Marcy-l-Étoile, France), and all yielded the profile 7142100015 after 48 h of incubation at 37°C, corresponding to an excellent identification for Candida dubliniensis by use of the API APILAB version 2 database (BioMérieux). The yeasts did not assimilate xylose or α-methyl-d-glucoside. Furthermore, these isolates did not grow at 42°C and were dark green in color on CHROMagar Candida medium following 48 h of growth at 37°C, characteristic features of many C. dubliniensis isolates (9, 11, 12). Determinations of MICs of fluconazole and amphotericin B were performed at the Mycology Reference Laboratory of the Health Protection Agency, Kingsdown, Bristol, UK, and MICs of both drugs were 0.25 mg/liter, a result which is considered to identify a sample as susceptible.
Many studies have shown that it is often difficult to discriminate between C. dubliniensis and the closely related species C. albicans. Therefore, we confirmed the identity of these isolates as C. dubliniensis by developing a rapid real-time multiplex PCR identification method. The method used a biprobe fluorescence resonance energy transfer (FRET) strategy on a Roche Lightcycler. Candida reference and type strains employed were C. albicans SC5314, Candida parapsilosis HEM20, Candida tropicalis NCPF3111, Candida glabrata 11088A, Candida krusei CDC259-75 (a kind gift from Christine Morrison, Centers for Disease Control and Prevention, Atlanta, Ga.), Candida kefyr NCPF3234, and the C. dubliniensis type strain CD36. Candida strains were routinely grown on potato dextrose agar (Oxoid, Hampshire, UK) and CHROMagar Candida medium (CHROMagar, Paris, France) for 48 h at 37°C, and DNA was extracted by a rapid and simple boiling procedure (2). C. albicans- and C. dubliniensis-specific Lightcycler probes were designed following ClustalX alignment (13) of the rRNA locus from seven medically important Candida species (C. albicans, C. parapsilosis, C. tropicalis, C. glabrata, C. krusei, C. kefyr, and C. dubliniensis) and Aspergillus fumigatus. Universal oligonucleotide primers ITS3 (5′-GCATCGATGAAGAACGCAGC-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) bind to the conserved fungal 5.8S and 28S rRNA genes, respectively, and amplify the entire variable internal transcribed spacer 2 (ITS2) region, generating fragments of between 350 to 450 bp dependent on the particular Candida species employed (14).
The probes employed were as follows: for C. albicans, CA-FLU (5′-AATGGCTTAGGTCTAACCAAAAACATT-FL labeled at the 3′ end with fluorescein [FL]) (TibMOLBIOL, Berlin, Germany) and LC640-CA (LC Red640-TTGCGGCGGTAACGTCCACCA-PH labeled at the 5′ end with Lightcycler Red 640 and synthesized with a blocking phosphate group [PH] at the 3′ end to prevent extension); for C. dubliniensis, DUB-FLU (5′-AATGGCTTAGGTGTAACCAAAAACATT-FL labeled at the 3′ end with fluorescein) and LC705-DUB (LC Red705-TAAGGCGGTCTCTGGCGTCGCCCATTT-PH labeled at the 5′ end with Lightcycler Red 705 and again synthesized with a blocking phosphate group at the 3′ end).
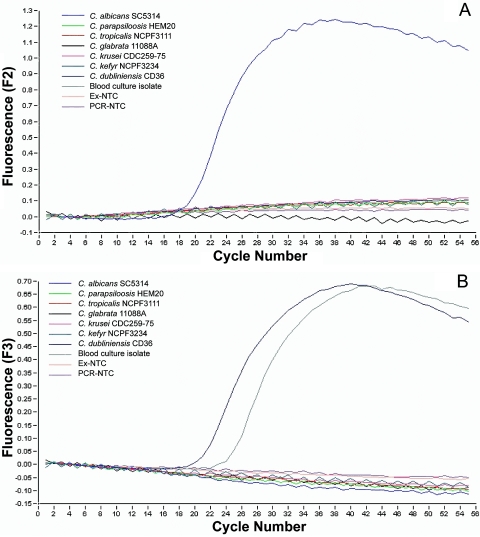
For amplicon detection a Lightcycler DNA master hybridization probe kit (Roche, East Sussex, UK) was employed as described by the manufacturer. The ready-to-use PCR mixture contained 1× Lightcycler hybridization reaction buffer, Taq DNA polymerase, deoxynucleoside triphosphate mix, 4 mM MgCl2, 1.0 μM concentrations of ITS3 and ITS4 (each), 0.2 μM concentrations of each of the probes, and 2 μl of the template in a final volume of 20 μl. Samples were loaded into glass capillary cuvettes which were filled by pulse centrifugation at 2,000 rpm in a microcentrifuge. The Lightcycler was programmed for 1 cycle of 95°C for 10 min, followed by 55 cycles of 95°C for 10 s, 55°C for 10 s, and 72°C for 20 s with monitoring of fluorescence gain during the annealing phase. Prospective molecular identification of the first Candida blood culture isolated from this patient was carried out employing the new real-time PCR technique described above. As shown in Fig. 1A, a single characteristic sigmoidal curve was obtained from the C. albicans reference strain SC5314 when F2 fluorescence emission from the CA-FLU and LC640-CA probes was plotted and no cross-reactivity was observed with the other Candida strains and no template control (NTC) for extraction and the PCR, demonstrating good test specificity. As shown in Fig. 1B, the C. dubliniensis ITS2-specific DUB-FLU and LC705-DUB biprobe system generated two sigmoidal curves from the CD36 C. dubliniensis strain and the blood culture isolate when fluorescence gain per cycle number was plotted as a function of the F3 channel, demonstrating that this blood culture isolate is the yeast C. dubliniensis. Very high species specificity was again achieved, as none of the other Candida strains, including the closely genotypically and phenotypically related C. albicans strain, reacted with the C. dubliniensis-specific probes.
FIG. 1.
A. Amplification profile generated by the C. albicans-specific FRET-based probes CA-FLU and LC640-CA in a real-time Lightcycler PCR assay, with F2 channel fluorescence plotted against cycle number showing a sigmoidal increase in F2 fluorescence emission with the C. albicans strain SC5314. Ex-NTC, extraction-no template control; PCR-NTC, PCR-no template control. B. Amplification profile generated by the C. dubliniensis-specific FRET-based probes CD-FLU and LC705-CD in a real-time Lightcycler PCR assay, with F3 channel fluorescence plotted against cycle number demonstrating an increase in F3 fluorescence intensity with the C. dubliniensis type strain CD36 and the original blood culture isolate from the patient.
Candida dubliniensis was first described in 1995 among oropharyngeal isolates from human immunodeficiency virus (HIV)-infected individuals (12). C. dubliniensis is most frequently found in the oral cavity of HIV-infected individuals and as an oral commensal organism in a small minority of the normal healthy population. Subsequent studies have confirmed that this species of Candida is prevalent throughout the world, is not confined to patients coinfected with HIV, and may cause invasive as well as mucocutaneous disease. However, bloodstream infection caused by C. dubliniensis is uncommon. In one prospective study from the UK, it accounted for only 3 of 136 (2.2%) yeast-positive blood cultures (11). It has been reported to cause fungemia among patients with a wide variation in underlying disease, including HIV-infected individuals, neutropenic patients receiving cancer chemotherapy, and bone marrow and solid organ transplant recipients. It is also a reported cause of catheter-related fungemia. At least three patients with C. dubliniensis and severe liver disease have been described. The patient described in the present report had liver disease caused by hepatitis C but was also an injecting drug user, which may have provided a portal of entry for Candida yeast into the bloodstream.
This report represents the first published case of endocarditis caused by C. dubliniensis. Mortality from Candida species endocarditis is high if no surgical intervention is undertaken, and although there is some evidence that C. dubliniensis is less virulent than C. albicans (11), this case demonstrates that a fatal outcome may occur if the focus of infection cannot be removed. In addition it is noteworthy that the autopsy findings demonstrated multiple septic emboli that were composed of a mass of fungal hyphae consistent with Candida species. Current Infectious Diseases Society of America guidelines for the treatment of fungal endocarditis recommend surgical valve replacement and treatment with amphotericin B with or without flucytosine (6).
MIC results of 0.25 mg/liter, a value which is considered to signify susceptibility in an isolate, for both fluconazole and amphotericin B in this report are in keeping with findings reported in previous publications. Fluconazole resistance has been reported for C. dubliniensis (5, 10), and the vast majority of isolates tested to date are susceptible to commonly used antifungal drugs, including azoles and amphotericin B (8). Although the MIC of caspofungin was not available for this isolate, previously described MIC data suggest that echinocandin antifungals (including caspofungin) are highly active against isolates of C. dubliniensis (7, 8).
Candida dubliniensis has proven to be difficult to distinguish from C. albicans by routine laboratory methods, mainly phenotypic tests such as carbohydrate substrate assimilation tests and growth under specific environmental conditions, and as a result confirmation by molecular methods has been recommended by a number of authors (1, 2, 4, 15). Previous studies have shown that identification of C. dubliniensis using substrate assimilation profile analysis can be problematic, mainly because the C. dubliniensis assimilation profiles included in the databases of commercially available yeast identification systems such as the API ID 32C system do not include all isolates of C. dubliniensis (9). However, Candida dubliniensis was suspected in this case because of the lack of assimilation of xylose or α-methyl-d-glucoside, which has been shown to be useful in discriminating between these two yeasts (3, 9). In addition, the inability of C. dubliniensis to grow at elevated temperature (42°C) and its growth as dark green colonies on CHROMagar Candida medium also suggested that the isolate in question was actually C. dubliniensis rather than C. albicans. However, while these phenotypic tests are certainly helpful, identification employing them is far from absolute. Therefore, definitive identification is usually determined using genotypic-based molecular techniques, especially PCR.
Multiplex PCR technology can be used to simultaneously detect multiple microorganisms as well as drug resistance genes and offers significant advantages with respect to the rapidity, specificity, and sensitivity of existing assays. Several conventional PCR tests for the identification of C. dubliniensis have been reported (1, 2, 4, 15). One of the most extensively used of these employs primers based on the C. dubliniensis ACT1-associated intron to distinguish C. dubliniensis isolates from the closely related C. albicans (2). A variety of other sequence targets have been identified as being sufficiently divergent between the two yeast species to allow the design of species-specific primers. These include the genes encoding mitochondrial cytochrome B (1), topoisomerase II (4) and the rRNA operon (15). The real-time multiplex PCR assay described here is more rapid than the routinely employed molecular and biochemical assays currently utilized in clinical microbiology laboratories (1, 2, 3, 4, 9, 15) and in conjunction with existing carbohydrate assimilation tests allows a more rapid and straightforward probe-based molecular confirmation assay of these phenotypic tests.
REFERENCES
- 1.Biswas, S. K., K. Yokoyama, L. Wang, K. Nishimura, and M. Miyaji. 2001. Identification of Candida dubliniensis based on the specific amplification of mitochondrial cytochrome b gene. Nippon Ishinkin Gakkai Zasshi 42:95-98. [DOI] [PubMed] [Google Scholar]
- 2.Donnelly, S. M., D. J. Sullivan, D. B. Shanley, and D. C. Coleman. 1999. Phylogenetic analysis and rapid identification of Candida dubliniensis based on analysis of ACT1 intron and exon sequences. Microbiology 145:1871-1882. [DOI] [PubMed] [Google Scholar]
- 3.Gales, A. C., M. A. Pfaller, A. K. Houston, S. Joly, D. J. Sullivan, D. C. Coleman, and D. R. Soll. 1999. Identification of Candida dubliniensis based on temperature and utilization of xylose and α-methyl-d-glucoside as determined with the API 20C Aux and Vitek YBC systems. J. Clin. Microbiol. 37:3804-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanbe, T., T. Horii, T. Arishima, M. Ozeki, and A. Kikuchi. 2002. PCR-based identification of pathogenic Candida species using primer mixes specific to Candida DNA topoisomerase II genes. Yeast 19:973-989. [DOI] [PubMed] [Google Scholar]
- 5.Moran, G. P., D. J. Sullivan, M. C. Henman, C. E. McCreary, B. J. Harrington, D. B. Shanley, and D. C. Coleman. 1997. Antifungal drug susceptibilities of oral Candida dubliniensis isolates from human immunodeficiency virus (HIV)-infected and non-HIV-infected subjects and generation of stable fluconazole-resistant derivatives in vitro. Antimicrob. Agents Chemother. 41:617-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pappas, P. G., J. H. Rex, J. D. Sobel, S. G. Filler, W. E. Dismukes, T. J. Walsh, and J. E. Edwards. 2004. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 38:161-189. [DOI] [PubMed] [Google Scholar]
- 7.Pfaller, M. A., D. J. Diekema, S. A. Messer, R. J. Hollis, and R. N. Jones. 2003. In vitro activities of caspofungin compared with those of fluconazole and itraconazole against 3,959 clinical isolates of Candida spp., including 157 fluconazole-resistant isolates. Antimicrob. Agents Chemother. 47:1068-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfaller, M. A., S. A. Messer, S. Gee, S. Joly, C. Pujol, D. J. Sullivan, D. C. Coleman, and D. R. Soll. 1999. In vitro susceptibilities of Candida dubliniensis isolates tested against the new triazole and echinocandin antifungal agents. J. Clin. Microbiol. 37:870-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pincus, D. H., D. C. Coleman, W. R. Pruitt, A. A. Padhye, I. F. Salkin, M. Geimer, A. Bassel, D. J. Sullivan, M. Clarke, and V. Hearn. 1999. Rapid identification of Candida dubliniensis with commercial yeast identification systems. J. Clin. Microbiol. 37:3533-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruhnke, M., A. Schmidt-Westhausen, and J. Morschhauser. 2000. Development of simultaneous resistance to fluconazole in Candida albicans and Candida dubliniensis in a patient with AIDS. J. Antimicrob. Chemother. 46:291-295. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan, D. J., G. P. Moran, E. Pinjon, A. Al-Mosaid, C. Stokes, C. Vaughan, and D. C. Coleman. 2004. Comparison of the epidemiology, drug resistance mechanisms, and virulence of Candida dubliniensis and Candida albicans. FEMS Yeast Res. 4:369-376. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan, D. J., T. J. Westerneng, K. A. Haynes, D. E. Bennett, and D. C. Coleman. 1995. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology 141:1507-1521. [DOI] [PubMed] [Google Scholar]
- 13.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTALX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White, T. J., T. D. Burns, S. B. Lee, and J. W. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols. Academic Press, San Diego, Calif.
- 15.Williams, D. W., W. A. Coulter, M. J. Wilson, A. J. Potts, and M. A. Lewis. 2001. Identification of Candida dubliniensis, based on ribosomal DNA sequence analysis. Br. J. Biomed. Sci. 58:11-16. [PubMed] [Google Scholar]