Abstract
Heart failure is a highly prevalent disease, which courses with frequent readmissions, mainly by Acute Heart Failure (AHF). Reduced renal function is associated with increased mortality in patients with HF. Therefore, an accurate and precise evaluation of renal function in patients with HF is crucial. The error of estimated GFR (eGFR) is wide and common, showing a ± 30% variability compared to measured GFR (mGFR). However, there is no evidence on the error of formulas in reflecting real renal function and particularly the consequences of this error in patients with AHF. This is a prospective study comparing the impact of mGFR versus eGFR in the onset of cardiovascular (CV) outcomes in patients with AHF. This was tested with cox survival analysis. Measured GFR was determined by the plasma clearance of iohexol-dbs and eGFR by Cockroft-Gould, MDRD, CKD-EPI creatinine, CKD-EPI cystatin-C and CKD-EPI creatinine + cystatin-C equations formulas. Also the agreement between mGFR and eGFR was analyzed. A total of 90 patients were included. Average age was 66 (± 12 years) and 52 (58%) were male. Of them 53 patients (59%) had a cardiovascular event during follow-up, 22 fatal (41%). The agreement between mGFR and eGFR indicated moderate precision and accuracy (concordance correlation coefficient of 0.77; CI = 0.73–0.82). In multiple cox survival analysis, mGFR was significantly associated with cardiovascular events together with NTproBNP, BMI, LVEF and previous coronary artery disease (p = 0.037; HR = 0.98, 95% CI = 0.95–0.99). Estimated GFR by formulas was not significant. In patients with AHF the error of formulas is large, frequent and random, also, mGFR and not eGFR predicted future CV events. The error of eGFR may have clinical consequences in specific subpopulations.
Keywords: Acute heart failure, Glomerular filtration rate, Renal function, Cardiovascular outcomes
Subject terms: Cardiology, Nephrology
Introduction
Heart failure (HF) is a frequent clinical syndrome affecting 1–2% of the adult population in Europe1. The diagnosis of HF portends a 20% risk for mortality at 1 year2 which increases up to 60%3,4 at 5 years. The disease is marked by frequent readmissions, mainly due to acute heart failure (AHF)5,6. These admissions are a major public health problem, causing patient disability and increasing economic costs7. Thus, understanding the pathogenesis and risk factors of HF may help improve clinical management and survival in this population.
Risk factors for AHF include age8, diabetes, atrial fibrillation, obesity, poorly controlled hypertension, chronic obstructive pulmonary disease, iron deficiency or anaemia9,10 and chronic kidney disease (CKD)11. The latter is a major factor for morbidity and mortality in patients with HF12,13. Reduced Glomerular Filtration Rate (GFR) could be as relevant as a low left ventricular ejection fraction in the evolution of HF 14. Moreover, this picture is complicated by the fact that around 50% of the HF patients have CKD15,16 with 29% of them having moderate to severe renal impairment12. Several meta-analyses observed that worsening renal function is associated with increased mortality in patients with HF12,13,17,18. All these aspects make an accurate and precise assessment of renal function a crucial aspect of the clinical evaluation of patients with HF.
In clinical practice, renal function is estimated by serum creatinine, cystatin-C or formulas that use these markers. More than 70 formulas have been published to estimate GFR using serum creatinine and/or cystatin-C. However, the reliability of these formulas has been widely questioned. The error of estimated GFR (eGFR) is wide and common, showing a ± 30% of variability compared to measured GFR (mGFR). This has been found in different populations, such as CKD19,20, type 2 diabetes mellitus 21,22, renal and no-renal transplantation 23–25, cancer patients 26,27 and chronic liver disease 28–30. Evidence on the variability of eGFR by formulas in patients with HF is scarce. Two studies showed that formulas either overestimated or underestimated renal function in patients with CHF 31,32. However, in patients with AHF there is no definitive evidence on the magnitude and frequency of the error of formulas and particularly the clinical consequences of this error, being these the main objectives of this study.
Methods
In this prospective study we analyzed the error of formulas that estimate GFR and the impact of this error in the evaluation of the association between GFR and long-term cardiovascular (CV) events in patients with AHF. We evaluated a group of patients with AHF who underwent the plasma clearance of iohexol, a gold-standard method to measure renal function. At the same time eGFR was assessed by a group of creatinine- and cystatin-C–based equations. During follow-up major CV events were recorded. The protocol follows the Helsinki declaration and was approved by the Ethic Committee of the Hospital Universitario de Canarias.
Patients
We evaluated a group of patients with AHF admitted in the Acute Cardiovascular Care Unit of the Cardiology Department of the Hospital Universitario de Canarias (Tenerife, Spain). Inclusion criteria were: age > 18 years; AHF requiring admission based on the European clinical guidelines33; clinical stability at least 48 h following admission, defined as an amelioration of the signs of congestion i.e. stable or lowering diuretic dose, oxygen requirement, no need of inotropes/vasopressor and a SBP ≥ 90 mmHg with no evidence of blood pressure fluctuation. Also no signs of clinical hypoperfusion were allowed, including lactate level when required. Exclusion criteria were: clinical instability at inclusion defined as the presence of cardiogenic shock; need for invasive mechanical ventilation, mechanical circulatory support or concomitant active infectious disease; allergy to iodine or a history of adverse reactions to contrast media; active malignancy; acute kidney injury needing dialysis; severe psychiatric disease; pregnancy and lactation; anorexia nervosa or any other cause of cachexia and hypercatabolic states i. e. severe hyperthyroidism. Informed consent was obtained from all subjects.
Procedures
Treatment of AHF
All patients after admission were treated with standard therapy according to current AHF guidelines33, which includes diuretics, oxygen and/or vasodilators. In special cases, inotropes, vasopressors or non-invasive mechanical ventilation were used.
Measured GFR—Plasma clearance of iohexol
At inclusion all patients underwent the plasma clearance of iohexol in conditions of clinical stability as defined in the inclusion criteria. The GFR was determined by plasma clearance of iohexol as previously described34. Briefly, 5 mL of the iohexol solution (Omnipaque 300, GE Healthcare) was injected intravenously during 2 min. Blood samples were then taken at 120, 180, 240, 300, 360, 420, and 480 min for patients with eGFR (aMDRD) of 40 mL/min or less; and at 120, 150, 180, 210, and 240 min for those with eGFR greater than 40 mL/min. Iohexol was measured in plasma or dried blood spots (DBS)18. Both methods (plasma or DBS) are interchangeable18. Iohexol was measured by HPLC–UV at the Laboratory of Renal Function of the ULL (http://lfr.ecihucan.es/). The clearance of iohexol was calculated according to a 1 compartment model (CL1) by the formula: CL1 = Dose/ AUC, where AUC is the area under the plasma concentration time curve from time equal zero to infinity. The plasma clearances were then corrected using the Bröchner-Mortensen equation35.
Estimated GFR by formulas
Creatinine (mg/dL) was measured by isotopic dilution mass spectrometry–traceable creatinine (enzymatic assay) using the cobas c711 module (Roche Diagnostics). cystatin-C levels were determined by immunonephelometry (BN II System, Siemens Healthcare), calibrated with ERM-DA471/IFCC.
Estimated GFR was evaluated by the following equations: Cockroft Gault36, MDRD37, CKD-EPI creatinine38, CKD-EPI cystatin-C39 and CKD-EPI creatinine + cystatin-C39. These formulas were selected since they are the most used equations available in 2023. The agreement between formulas and measured GFR was evaluated with the formulas adjusted and unadjusted for body surface area (BSA). When estimated GFR was already adjusted, we reversed the adjustment of the result, that is, MDRD and CKD-EPI by applying the following formula GFR unadjusted = GFR adjusted × BSA/1.7340.
Clinical variables and laboratory analysis
The following variables were collected: age, gender, weight (kg), height (cm), body mass index (BMI), hypertension, dyslipidemia, diabetes mellitus, previous cardiovascular disease, chronic kidney disease defined (eGFR < 60 mL/min) and smoker status. Laboratory variables were recorded simultaneously within the plasma clearance of iohexol and included serum creatinine, cystatin-C, hemoglobin, Quick-Time, Prothrombin activity, sodium, potassium, NT-proBNP, troponin and C- reactive protein. The highest NT-proBNP value before patient inclusion was recorded.
Specific heart failure-related variables
Etiology, duration and triggering causes of HF were recorded, as well as ejection fraction, diastolic function (E/a; E/é), presence of atrial fibrillation, left bundle branch block, pacemaker stimulation, etc. Also previous treatments were collected: aspirin, betablockers, ACEi/ARAII, furosemide dose, other diuretics (thiazide, chlorthalidone), aldactone, eplerenone and statins. Echocardiographic variables were recorded at the time of patient inclusion and include: telediastolic diameter of the LV, left ventricular ejection fraction (measured using the Simpson biplane method), left ventricle outflow tract VTI, presence and degree of mitral regurgitation and estimation of systolic pulmonary pressure. Congestion was not evaluated from echocardiography or lung ultrasound point of view, but we included data about diastolic function, NTproBNP—a marker of congestion and myocardial stress-.
Statistical analysis
Agreement analysis between estimated and measured GFR
The error of formulas was evaluated by specific statistics for continuous data, including the concordance correlation coefficient (CCC), total deviation index (TDI), coverage probability (CP)41. The CCC varies from 0 to 1 and combines meaningful components of accuracy and precision. A CCC of 0.90 reflects optimal concordance between measurements. The TDI captures a large proportion of data within a boundary for allowed differences between 2 measurements. Empirical TDI was calculated for a theoretical TDI of 10% and a CP of 90%. We defined a priori that acceptable bias between eGFR and mGFR should be at least 10%, and that 90% of the estimations should be included within these limits. This is based on previous reports and the reproducibility of measured GFR considering different methods42. Coverage probability varies from 0 to 1 and estimates whether a given TDI is less than a pre-specified fixed percentage.
Association between estimated or measured GFR and major CV outcomes
The evolution of the patients enrolled was assessed by reviewing the electronic clinical charts. The main outcome was the first onset of fatal and nonfatal major cardiovascular events occurring after the first episode of AHF. These included: (a) coronary artery disease: unstable angina, acute coronary syndrome, the need of coronary revascularization either percutaneously than surgical; (b) AHF: advanced heart failure requiring admission; (c) sudden cardiac death; (d) cerebrovascular disease: stroke or transient ischemic attack; (e) peripheral vascular disease: symptoms of intermittent claudication, amputation or need of re-vascularization; (f) heart transplant or need of long-term ventricular assist device implantation. The causes of non-cardiovascular death were recorded.
The association between eGFR or mGFR and the onset of CV events was evaluated with cox-survival analysis. In the simple analysis, all possible variables with a proven or possible association with CV events were included, such as: age, sex, duration of heart failure syndrome, LVEF, smoking, diabetes mellitus type 2, dyslipidemia, previous history of cardiovascular events, body mass index, serum creatinine, cystatin-C, eGFR, mGFR, treatments (diuretics, betablockers or ACE inhibitors), NT-proBNP and us-Trop T levels and renal function, estimated or measured.
For the multiple cox analysis models, all the significant variables tested in the simple analysis were included. Also, other confounders with proven association with cardiovascular events were included irrespective of the results of simple cox analysis, such as NTproBNP and LVEF. One covariate was included every 8 events.
Three different multiple cox survival models were created. i) the main model including mGFR; ii) the second model replacing mGFR by serum creatinine or by cystatin-C; iii) the third model with eGFR replacing mGFR either by creatinine based-formulas (MDRD, EPI-CKD, CG) or cystatin-C-based-formulas (EPI-cys; EPIcys-Cr). The comparison between groups of qualitative variables was made using the χ2 test or Fisher's exact test, as appropriate, and the study of quantitative variables, with the Student's t test in case they followed a normal distribution or the Mann–Whitney test in another case. A value of p < 0.05 was considered statistically significant. We estimate the discriminative accuracy of mGFR and of each estimated formula for predicting adverse events using receiver operating curves (ROC) including 95% confidence intervals. We have also calculated the C-statistics. The C-statistics (equivalent to the area under the receiver operating characteristic curve) is a standard measure of the predictive accuracy of the logistic regression models. The variables used in logistic cox survival models were BMI, LVEF, previous coronary artery disease and NT-proBNP and each of the estimated or measured GFR. Statistical analysis was carried out with SPSS (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY) and MedCalc Statistical Software version 22.016 (MedCalc Software Ltd, Ostend, Belgium).
Data collection
A web-based online platform was designed for data collection before starting the inclusion.
Results
Patients
Ninety patients were included. Average age was 66 ± 12 years, 52 patients (58%) were male and 27 (30%) have past history of CHF (Table 1). Previous coronary artery disease was present in 35 subjects (39%), and ischemic disease was the most prevalent etiology of HF (38; 35%) followed by valvular disease (24; 27%). Most patients had diabetes (54; 60%), hypertension (68; 76%) or dyslipidemia (63; 70%). About one third of the patients had atrial fibrillation (34%) and mean LVEF was 41% ± 14.54 (Table 1). Average measured GFR was 59.7 ± 26 mL/min, whereas estimated GFR by formulas was 70.2 ± 32 mL/min (MDRD), 70.2 ± 30 mL/min (CKD-EPI), 74.8 ± 38 (Cockroft-Gault), 61.2 ± 29 (EPI-Cys) and 65.3 ± 28 (EPI-Cys_Cr) (Table 2). Cystatin-C determination was available in 60 patients with a mean value of 1.46 ± 0.69 mL/min. No differences were shown in the group with and without cystatin-C except NTproBNP level (Table 2_Supplementary material). All the patients received intravenous diuretics and doses during the first three days were recorded (Fig. 1_Supplementary material).
Table 1.
Baseline characteristics. previous treatments and laboratory variables.
| Overall | With Events | Without events | p-value | |
|---|---|---|---|---|
| N | 90 | 53 (59) | 37 (41) | |
| Age (year) | 66 ± 12 | 67 ± 13 | 65.7 ± 12 | 0.5 |
| Gender: men—n (%) | 52 (58) | 34 (64) | 18 (48%) | 0.14 |
| Body mass index kg/m2 | 29 ± 5 | 30 ± 5 | 27 ± 5 | 0.01 |
| Comorbidities | ||||
| Diabetes mellitus—n(%) | 54 (60) | 34 (64) | 20 (54) | 0.33 |
| Hypertension—n(%) | 68 (76) | 42 (79) | 26 (70) | 0.33 |
| Current smoker—n(%) | 20 (22) | 11 (21) | 9 (24) | 0.7 |
| Dyslipidemia—n(%) | 63 (70) | 38 (72) | 25 (68) | 0.67 |
| Coronary artery disease—n(%) | 35 (39) | 28 (53) | 7 (19) | 0.001 |
| Cerebrovascular diseases—n(%) | 12 (13) | 9 (17) | 3 (8) | 0.23 |
| CKD (GFR < 60 mL/min)—n(%) | 31 (34) | 24 (45) | 7 (19) | 0.009 |
| Heart failure (HF) | ||||
| De novo HF -n(%) | 63 (70) | 31 (58) | 32 (86) | 0.004 |
| Previous history of HF | 27 (30) | 22 (37) | 5 (12) | 0.006 |
| Etiology of HF | ||||
| Ischemic—n(%) | 38 (35) | 30 (59) | 8 (23%) | 0.001 |
| Idiopathic—n(%) | 16 (18) | 9 (18) | 7 (21) | 0.73 |
| Restrictive—n(%) | 2 (2) | 1 (1) | 1 (3) | 0.77 |
| Hypertensive—n(%) | 5 (6%) | 3 (6) | 2 (6) | 1 |
| Valvular—n(%) | 24 (27%) | 8 (15) | 16 (47) | 0.002 |
| Known duration of HF year | 1.7 (0–30) | 2.7 (0–30) | 0.2 (0–4) | 0.005 |
| Left ventricular diameter mm | 57 ± 8 | 59 ± 9 | 55 ± 7 | 0.017 |
| E/é ratio | 17 ± 6.5 | 17 ± 6 | 17 ± 6 | 0.54 |
| LVOT–VTI cm | 14 ± 5 | 14 ± 5 | 14 ± 4 | 0.90 |
| SPAP > 50 mmHg | 18 (20) | 10 (19) | 8 (22) | 0.73 |
| LVEF % | 41 ± 14.5 | 38 ± 14 | 43 ± 14 | 0.04 |
| Medications | ||||
| Aspirin—n(%) | 44 (49) | 30 (57) | 14 (38) | 0.08 |
| ACEi/ARAii—n(%) | 52 (61) | 32 (61) | 20 (38) | 0.34 |
| Betablockers—n(%) | 54 (60) | 34 (64) | 20 (54) | 0.34 |
| Furosemide—n(%) | 52 (58) | 40 (75) | 12 (32) | < 0.0001 |
| Statins (n-%) | 60 (67) | 41 (77) | 19 (51) | 0.10 |
| Mineralocorticoid receptor antagonists (n-%) | 21 (24) | 17 (33) | 4 (11) | 0.02 |
| Laboratory | ||||
| Hemoglobin gr/dL | 12 ± 2 | 12 ± 2 | 12.8 ± 2 | 0.09 |
| HbA1c % | 6.9 ± 1.8 | 7.1 ± 1.9 | 6.5 ± 1.5 | 0.14 |
| Triglycerides mg/dL | 119 ± 50 | 118 ± 50 | 121 ± 49 | 0.77 |
| LDL-cholesterol mg/dL | 78 ± 34 | 65 ± 28 | 95 ± 32 | 0.0001 |
| Troponin T us pg/mL | 208 ± 353 | 245 ± 372 | 156 ± 321 | 0.25 |
| NT-proBNP pg/mL | 8745 ± 8349 | 8238 ± 7949 | 9470 ± 8954 | 0.45 |
| Heart Rate bpm | 97 ± 23 | 92 ± 18 | 106 ± 26 | 0.002 |
| Systolic BP mmHg | 132 ± 29 | 127 ± 25 | 139 ± 32 | 0.06 |
| Diastolic BP mmHg | 75 ± 16.4 | 72 ± 13 | 78 ± 20 | 0.06 |
| Follow-up time year | 3 ± 2 | 2 ± 2 | 4 ± 1.5 | p < 0.001 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; LVOT-VTI, left ventricular outflow tract—velocity/time integral; SPAP, systolic pulmonary arterial pressure; LVEF, left ventricular ejection fraction; CKD, chronic kidney disease.
Table 2.
Renal function.
| Overall | With events | Without events | p value | |
|---|---|---|---|---|
| N | 90 | 53 (59) | 37 (41) | |
| Creatinine (mg/dL) | 1.2 ± 0.52 | 1.3 ± 0.5 | 1 ± 0.4 | 0.01 |
| mGFR (mL/min) | 59.74 ± 26 | 55.55 ± 22 | 65.64 ± 28 | 0.06 |
| MDRD (mL/min) | 70.18 ± 32 | 63.83 ± 28 | 79.10 ± 35 | 0.02 |
| CKD-EPI (mL/min) | 70.21 ± 30 | 66.85 ± 31 | 75.16 ± 28 | 0.20 |
| CG (mL/min) | 74.75 ± 38 | 71.96 ± 40 | 78.86 ± 36 | 0.41 |
| Cystatin-C (mL/min) *(n = 60) | 1.46 ± 0.69 | 1.52 ± 0.74 | 1.38 ± 0.63 | 0.46 |
| CKD-EPI-cys (mL/min) | 61.19 ± 29 | 60.25 ± 29 | 62.6 ± 29 | 0.76 |
| CKD-EPI-Cr-cys (mL/min) | 65.34 ± 28 | 63.5 ± 29 | 68.1 ± 29 | 0.54 |
| Bun/Cr ratio | 26.88 ± 15 | 25.47 ± 13 | 28.91 ± 17 | 0.28 |
mGFR, measured glomerular filtration rate; eGFR, estimated glomerular filtration rate; CG, Cocroft-Gault.
Baseline characteristics in patients with and without primary end-point
A total of 53 patients (59%) had a cardiovascular event during follow-up (Table 3). In 22 cases (41.5%) the event was fatal: advanced HF in 18 (82%), acute myocardial infarction in 3 cases (14%) and 1 (4.5%) fatal-stroke. Non-fatal events were observed in 31 patients (58.5%): hospitalizations due to AHF in 26 (83.8%); coronary artery disease in 3 (9.6%) and peripheral vascular disease in 2 (6.4%) cases. Non-cardiovascular fatal events occurred in 17 patients (19%): 9 sepsis and 5 oncological diseases, 1 severe intracranial bleeding, 1 due to COVID-19 and another related to advanced COPD (Table 3). During a median follow-up of 3 ± 1.9 years, 39 (43%) patients died, 22 of cardiovascular causes.
Table 3.
Patients with first-onset fatal or nonfatal major cardiovascular events (primary endpoint).
| Events | n = 53 |
|---|---|
| Coronary artery disease | |
| Unstable Angina | 0 |
| Acute Coronary Syndrome | 6 |
| New revascularization | 2 |
| Heart Failure | 43 |
| Cerebrovascular disease | |
| Stroke | 1 |
| Transient ischemic attack | 0 |
| Peripheral Vascular disease | |
| Acute ischemic event | 1 |
| Amputation | 1 |
| Revascularization | 0 |
| Fatal events | 22 |
| Non-fatal events | 31 |
| Non-cardiovascular events | 17 |
| Sepsis | 9 |
| Oncology | 5 |
| Bleeding | 1 |
| COVID | 1 |
| COPD | 1 |
COPD, chronic obstructive pulmonary disease.
Patients with CV events during follow-up were more obese, had more previous coronary artery and chronic kidney disease before admission compared with patients without events (Table 1). The presence of ischemic etiology of the HF was the most prevalent in the group with events (30% vs 8%, p 0.001), LV were more dilated and they had lower LVEF. Regarding renal function, patients with events had higher creatinine levels (1.3 vs 1 mg/dl, p = 0.012) on admission compared with the group without events, but no differences in estimated or measured GFR were observed.
Agreement between measured GFR and estimated GFR
For all the formulas evaluated, TDI averaged 73%, ranging from 54 to 81% for the CKD-EPI (creatinine + cystatin-C) and Cockcroft-Gault equations, respectively (Table 4). For example, the aMDRD formula had a TDI of 76%, meaning that 90% of estimations erred from − 76 to 76 of measured GFR. A similar TDI was observed for the other CKD-EPI formulas. CCC averaged 0.77, reflecting moderate precision and accuracy, ranging from 0.73 to 0.82, for the Cockcroft-Gault and CKD-EPI (creatinine + cystatin-C) formulas, respectively (Table 4) Finally, cp averaged 22, indicating that more than 80% of the estimations had an error greater than ± 10% (Table 4).
Table 4.
Agreement between Measured GFR and Estimated GFR.
| CCC | TDI | CP | |
|---|---|---|---|
| CockcroftGault | 0.73 (0.64) | 81.21 (95.40) | 0.20 (0.17) |
| aMDRD | 0.74 (0.67) | 75.79 (89.23) | 0.21 (0.19) |
| CKD_EPI | 0.76 (0.68) | 72.46 (85.12) | 0.22 (0.19) |
| CKD_EPI_cisc | 0.69 (0.58) | 80.60 (98.95) | 0.20 (0.18) |
| CKD_EPI_crecisc | 0.82 (0.74) | 54.07 (65.46) | 0.29 (0.24) |
The agreement between estimated by 5 creatinine and/or cystatin-C formulas and measured GFR was evaluated by specific statistics for continuous data, including the concordance correlation coefficient (CCC), total deviation index (TDI), coverage probability (CP) [X]. The CCC varies from 0 to 1 and combines meaningful components of accuracy and precision. A CCC > 0.90 reflects optimal concordance between measurements. The TDI captures a large proportion of data within a boundary for allowed differences between 2 measurements. Empirical TDI was calculated for a theoretical TDI of 10% and a CP of 90%. We defined a priori that acceptable bias between eGFR and mGFR should be at least 10%, and that 90% of the estimations should be included within these limits. This is based on previous reports and the reproducibility of measured GFR considering different methods [X]. Coverage probability varies from 0 to 1 and estimates whether a given TDI is less than a prespecified fixed percentage.
Low concordance between mGFR and eGFR
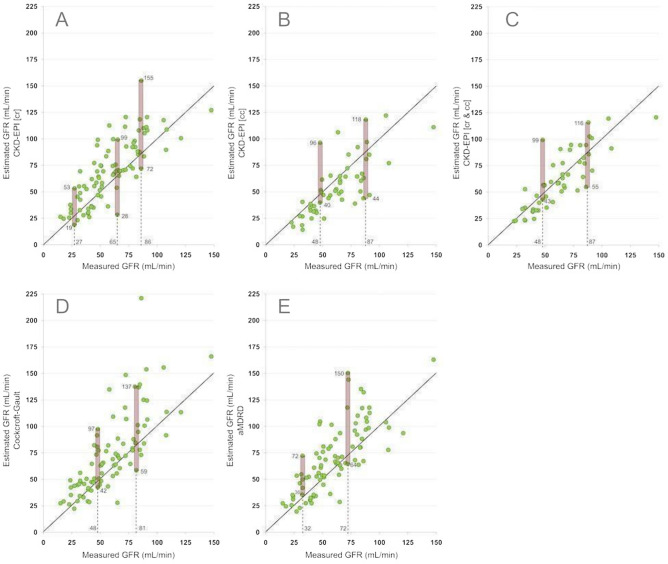
Single values of mGFR were associated with an ample range of estimations (Fig. 1). For example, in subjects with 27 mL/min of mGFR, CKD-EPI-creatinine estimated GFR from 19 to 53 mL/min (Fig. 1-A). Similar results were observed for the CKD-EPI-cystatin-C, CKD-EPI-cr-cy and MDRD equations (Fig. 1).
Fig. 1.
Plot between measured GFR and estimated GFR by five equations CKD-EPI based on creatinine (A) CKD-EPI based on cystatin-C (B), CKD-EPI based on creatinine-cystatin-C (C) or Cockroft-Gault (D); and MDRD (E).
Association between estimated or measured GFR and major CV outcomes
Simple cox survival analysis
The following variables were associated with the onset of cardiovascular events: BMI, previous coronary artery disease, chronic kidney disease and the presence of chronic heart failure, the use of furosemide and statins, creatinine, total and LDL cholesterol, LVEF, LV end-diastolic diameter (Table 5). Measured GFR or eGFR were not associated with the primary endpoint. The discriminative accuracy (AUC) of mGFR for predicting adverse events in terms of simple analysis were 0.736 for mGFR (95% Confidence interval 0.632 to 0.824). AUC of the estimated formulas are shown in Table 4 and Fig. 2_Supplementary material.
Table 5.
Simple Cox survival analysis—First onset composite event.
| First onset composite event | |||||
|---|---|---|---|---|---|
| Comorbidities | B | p-value | HR | 95% CI | |
| Lower | Upper | ||||
| Age year | 0.01 | 0.52 | 1.01 | 0.98 | 1.05 |
| Gender men | − 0.64 | 0.14 | 0.53 | 0.22 | 1.24 |
| Body mass index kg/m2 | 0.11 | 0.02 | 1.12 | 1.02 | 1.23 |
| Diabetes Mellitus | 0.42 | 0.34 | 1.52 | 0.65 | 3.58 |
| Hypertension | 0.48 | 0.33 | 1.61 | 0.61 | 4.25 |
| Current smoker | − 0.20 | 0.69 | 0.81 | 0.29 | 2.22 |
| Dyslipidemia | 0.17 | 0.67 | 1.22 | 0.49 | 3.03 |
| Previous cardiovascular disease | |||||
| Coronary artery disease | 1.57 | 0.002 | 4.8 | 1.79 | 12.84 |
| Cerebrovascular diseases | 0.84 | 0.23 | 2.32 | 0.58 | 9.22 |
| Chronic Kidney Disease | 1.27 | 0.01 | 3.55 | 1.32 | 9.45 |
| Heart Failure variables | |||||
| “De novo” Heart Failure | 1.46 | 0.009 | 0.2 | 0.07 | 0.068 |
| Duration of Heart Failure | 0.59 | 0.02 | 1.8 | 1.09 | 2.99 |
| Previous treatments | |||||
| Aspirin | 0.76 | 0.08 | 2.14 | 0.91 | 5.05 |
| BB | 0.42 | 0.34 | 1.52 | 0.65 | 3.58 |
| Furosemide | 1.86 | < 0.001 | 6.41 | 2.53 | 16.25 |
| Statins | 1.17 | 0.01 | 3.24 | 1.30 | 8.05 |
| Aldactone | − 0.08 | 0.92 | 0.92 | 0.19 | 4.4 |
| Eplerenone | 2.56 | 0.02 | 12.92 | 1.62 | 103.31 |
| Laboratory values | |||||
| Hematocrit | − 0.07 | 0.09 | 0.93 | 0.86 | 1.01 |
| Hemoglobin | − 0.18 | 0.09 | 0.83 | 0.67 | 1.03 |
| HbA1c | 0.21 | 0.15 | 1.24 | 0.92 | 1.66 |
| Uric Acid | 0.19 | 0.06 | 1.21 | 0.99 | 1.47 |
| Creatinine | 1.17 | 0.02 | 3.23 | 1.23 | 8.46 |
| mGFR | − 0.02 | 0.06 | 0.98 | 0.97 | 1.00 |
| eGFR MDRD | − 0.01 | 0.1 | 0.99 | 0.97 | 1.00 |
| eGFR CKD_EPI | − 0.01 | 0.19 | 0.99 | 0.98 | 1.00 |
| Cystatin | 0.31 | 0.45 | 1.36 | 0.60 | 3.08 |
| eGFR CKD_EPI_cys | − 0.003 | 0.76 | 0.99 | 0.98 | 1.01 |
| eGFR CKD_EPI_Cr_cys | − 0.006 | 0.54 | 0.99 | 0.98 | 1.01 |
| BUN/Cr ratio | − 0.016 | 0.20 | 0.9 | 0.95 | 1.01 |
| Troponin T us | 0.001 | 0.25 | 1.001 | 0.99 | 1.00 |
| BUN | 0.02 | 0.18 | 1.02 | 0.99 | 1.04 |
| Total Cholesterol | − 0.02 | 0.001 | 0.98 | 0.97 | 0.99 |
| Triglycerides | − 0.001 | 0.76 | 0.99 | 0.99 | 1.01 |
| LDL | − 0.03 | 0.01 | 0.97 | 0.95 | 0.98 |
| HDL | − 0.006 | 0.69 | 0.99 | 0.97 | 1.02 |
| logNT-proBNP | − 0.32 | 0.57 | 0.72 | 0.24 | 2.2 |
| HR | − 0.03 | 0.004 | 0.97 | 0.98 | 0.99 |
| Systolic blood pressure | − 0.01 | 0.06 | 0.98 | 0.97 | 1 |
| Diastolic blood pressure | − 0.02 | 0.07 | 0.98 | 0.95 | 1 |
| Atrial Fibrillation | − 0.19 | 0.67 | 0.82 | 0.34 | 1.98 |
| LV end-diastolic diameter | 0.07 | 0.02 | 1.07 | 1.01 | 1.1 |
| E/é ratio | − 0.02 | 0.54 | 0.98 | 0.92 | 1.04 |
| LVOT-VTI | − 0.005 | 0.90 | 0.99 | 0.91 | 1.09 |
| Left ventricular ejection fraction (LVEF) | − 0.03 | 0.048 | 0.97 | 0.94 | 0.99 |
Univariable regression analysis. variables associated with the first onset of a composite event.
LVOT—VTI, left ventricular outflow tract volume-time interval; CKD, chronic kidney disease.
Multiple cox survival analysis
Model 1: renal function evaluated by mGFR
The variables significantly associated with cardiovascular events were mGFR, BMI, LVEF and previous coronary artery disease. Higher levels of mGFR were associated with a lower risk for CV events. When previous coronary artery disease was replaced by previous history of chronic heart failure no major changes were observed in the model (Table 6).
Table 6.
Multiple Cox survival analysis.
| Variables | B | p-value | HR | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| CAD | 1.72 | 0.003 | 0.18 | 0.06 | 0.55 |
| logNT-proBNP | − 1.04 | 0.15 | 0.35 | 0.09 | 1.4 |
| LVEF | − 0.4 | 0.04 | 0.97 | 0.93 | 0.99 |
| BMI | 0.15 | 0.01 | 1.16 | 1.03 | 1.29 |
| mGFR | − 0.25 | 0.037 | 0.98 | 0.95 | 0.99 |
| Model 2 | |||||
| Creatinine or | 0.86 | 0.12 | 2.37 | 0.79 | 7.10 |
| Cystatin | 0.16 | 0.74 | 1.17 | 0.45 | 3.02 |
| Model 3 | |||||
| MDRD or | − 0.01 | 0.10 | 0.99 | 0.97 | 1.003 |
| CKD-EPI or | − 0.014 | 0.13 | 0.99 | 0.98 | 1.004 |
| Cockroft-Gault or | − 0.014 | 0.06 | 0.97 | 0.97 | 1.001 |
| CKD-EPI-Cys or | − 0.001 | 0.91 | 0.99 | 0.98 | 1.02 |
| CKD-EPI-cc-Cr or | − 0.005 | 0.67 | 0.99 | 0.97 | 1.02 |
All the models have been evaluated with coronary artery disease, log NT-proBNP, Left ventricular ejection fraction and Body Mass Index. In the main model, model 1, with mGFR with iohexol; In model 2, mGFR was replaced either by Creatinine and Cystatin; In model 3 mGFR has been replaced by different estimation formulas used in the study (MDRD, CKD-EPI, Cockroft-Gault, CKD-EPI-Cys, CKD-EPI-cc-Cr).
Model 2: mGFR replaced by creatinine or cystatin-C
Model 3: mGFR replaced by eGFR
None of the formulas: CKD-EPI-creatinine, CKD-EPI-cystatin-C, CKD-EPIcr-cys, MDRD or Cockrof-Gould were significantly associated with the primary endpoint (Table 6). The C-statistic of the multiple analysis model shows an AUC of 0.73 (CI 0.59–0.84) for the model with mGFR, AUC of the estimated formulas are shown in Table 5_Supplementary material.
Discussion
We analyzed the error of formulas that estimate GFR and the impact of this error in the evaluation of the association between GFR and long-term CV events in 90 patients with AHF. The main finding of this study was that mGFR outperformed eGFR in the prediction of long-term CV events in patients with AHF.
In patients with AHF, the error of formulas that estimate GFR was frequent and wide. Also, we observed that this error limited the correct evaluation of the impact of renal function as a risk factor for cardiovascular events in patients with AHF. In fact, mGFR and not eGFR was associated with the incidence of cardiovascular events.
We evaluated a group of patients with AHF defined by current European Society of Cardiology HF guidelines33. GFR was measured with a gold standard method (iohexol) and estimated with a group of formulas, the most commonly used in clinical practice. To avoid the interaction of acute haemodynamic changes with renal function, the evaluation of GFR was performed only in a clinical stable situation at least 48 h following admission. Stable diuretic doses and no inotropic or vasopressor were required, always with a SBP > 90 mmHg.
Our main finding was that eGFR was not associated with future major cardiovascular outcomes in patients admitted with AHF. The explanation of this phenomenon is not simple. Clearly, it may be considered a consequence of the error of eGFR in reflecting real GFR. Formulas that estimate renal function proved to have low precision and accuracy in the estimation of real GFR. The average variability of eGFR in our population was about ± 30%, indicating that relevant overestimation or underestimation of GFR by formulas are more frequent than expected. In general, for the creatinine-based equations, we found a 10 mL/min higher GFR than mGFR. This overestimation may falsely alter the association between eGFR and events. Previous studies comparing creatinine-based equations in HF have shown an overestimation of eGFR at lower GFR values43,44. Others observed that eGFR differed widely from mGFR in this population. However, these studies did not compare with mGFR. Smilde et al.45 showed that in CHF, eGFR equations using CG, MDRD and CrCl overestimated in the lower ranges and underestimated in the upper ranges of renal function when it was compared with mGFR with 125I-iothalamate clearance. Another study in heart transplant patients demonstrated that the level of agreement between eGFR and mGFR by iohexol was very low for creatinine-based equations (CG, MDRD, CKD-EPI), with percentage errors ranging from 93 to 157%46.
The lack of association between cystatin-C based equations and CV events may be due to factors different from the overestimation of GFR as observed for creatinine-based equations. The agreement between cystatin-C-based equations and mGFR was weak and comparable to that of creatinine-based equations. Clearly the variability was not due to the overestimation of GFR but of scatter variability. The poor agreement between formulas based on cystatin-C and mGFR has been described before. Valente et. al showed that in CHF patients with mild impairment of renal function that cystatin-C-based eGFR showed the lowest bias (− 3 ± 14 mL/min/1.73) and were more precise than creatinine-based GFR compared with measured GFR with iothalamate47.
Some studies have evaluated the capacity of diverse formulas in the prediction of future CV events following an episode of AHF. In stable and ambulatory CHF the Cockroft-Gault formula was more accurate compared with MDRD and CKD-EPI eGFR formulas to improve the risk stratification for death48. Iokfai et al.49 published that eGFR-EPI-Cys improved mortality prediction over creatinine-based, creatinine/cystatin-C-based and MDRD equations. Moreover, other studies have shown that eGFR-MDRD was an independent predictor of long-term mortality after discharge among patients with AHF treated in the coronary care unit50. On the other side, Weidmann et al.51 in a multicenter study demonstrated that the prognostic accuracy for readmission was poor for all equations, with an AUC around 0.5. Also, other studies demonstrated that the eGFR-MDRD formula showed poor prognosis in predicting adverse events in AHF patients52, although better results were demonstrated in post-cardiac transplantation53 or diabetic patients54. However, all these studies did not measure GFR simultaneously with a gold-standard procedure. The studies that performed a mGFR and eGFR showed that mGFR, either with iothalamate or iohexol, was better at predicting CV events or relevant RF decline in CHF patients. The predictive value of mGFR with iothalamate for a CV event in the following 12 months showed an area under the ROC curve of 0.83. MDRD performance (0.81; P = 0.432) was comparable, but the CG formula corrected for BSA was significantly worse (0.72; P = 0.015)45. Measured GFR by iohexol significantly declined during a 8 months follow-up period (from 52.8 to 44.4 mL/[min × 1.73 m2], P = 0.001) being undetected by eGFR equations (MDRD, CG and CKD-EPI). These results were found in CHF and are in line with our results in AHF31. In any case, considering the major role of renal dysfunction in cardiovascular disease, the poor predictive capacity of eGFR is worth investigating.
The consequences of our findings may be of clinical relevance. The error of eGFR by over or underestimation of RF might result in inappropriate continuation or discontinuation of disease-modifying heart failure therapies, such as ACEi, ARNI or MRA.
The background of the error of formulas is complex and has several causes. The serum levels of creatinine depend on several factors like meat intake, muscle mass, extra-renal clearance (gut) and particularly, renal tubular secretion and reabsorption55. By the other hand, CysC is considered largely independent of muscle wasting and sarcopenia, but the production of this molecule is associated with obesity, fat mass and inflammation20–56. Clearly, the error of these markers translates to the equations that are not able to solve the low precision and accuracy of creatinine and cystatin-C in reflecting GFR. The study was carried out on a small sample of patients and so the results must be interpreted with caution. Clearly, larger series, similar to ours, with a longer follow-up, are needed before raising definitive conclusions.
Strengths and limitations
Our study has several limitations. First, the number of patients included in the analysis may be small and so our results must be tested in larger studies; Second, cystatin-C determination was not available in all patients (n 60). Thus, this study is a generating hypothesis analysis and its results must be tested in new ad hoc designed studies. Finally, it must be considered that we worked with a sub-group of patients with AHF, excluding those unstable. Thus, our results may not apply to all subjects with AHF. Main strength is that renal function has been compared with gold-standard methods like iohexol; eGFR was determined at the same time of mGFR at 48 h of admission and in a hemodynamically stable condition.
Conclusion
In patients with AHF the error of formulas is large, frequent and random, also, mGFR and not eGFR predicted future CV events. The error of eGFR may have clinical consequences in specific subpopulations.
Supplementary Information
Acknowledgements
The authors thank the EU project DOKI –Diabetes, Obesity and the Kidney, PN: 101079207 of the call: HORIZON-WIDERA-2021-ACCESS-03, Twinning.
Author contributions
PJ and EP have contributed similarly to the design, analysis of results and revision of the manuscript. MM and MG collaborated in data collection, writing, and revision. NN and LD participated in sample determination. RP contributed to the preparation of the figures and the creation of the CRD. FG, EP, MG, FB, SL and PJ contributed to the final revision of the manuscript.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-71425-z.
References
- 1.Conrad, N. et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet391, 572–580 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heiat, A., Gross, C. P. & Krumholz, H. M. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch. Intern. Med.162, 1682–1688 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Gerber, Y. et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med.175, 996–1004 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsao, C. W. et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail.6, 678–685 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barasa, A. et al. Heart failure in young adults: 20-year trends in hospitalization, etiology, and case fatality in Sweden. Eur. Heart J.35, 25–32 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mozaffarian, D. et al. American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Executive Summary: Heart Disease and Stroke Statistics–2016 Update: A report from the American Heart Association. Circulation133(4), 447–454. 10.1161/CIR.0000000000000366 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Heidenreich, P. A. et al. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation123, 933–944 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Ahmed, A. DEFEAT heart failure: Clinical manifestations, diagnostic assessment, and etiology of geriatric heart failure. Heart Fail. Clin.3(4), 389–402 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Anand, I. S. & Gupta, P. Anemia and iron deficiency in heart failure: Current concepts and emerging therapies. Circulation138, 80–98 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Iorio, A. et al. Prevalence and prognostic impact of non-cardiac comorbidities in heart failure outpatients with preserved and reduced ejection fraction: A community-based study. Eur. J. Heart Fail.20, 1257–1266 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Mosterd, A. & Hoes, A. W. Clinical epidemiology of heart failure. Heart93, 1137–1146 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith, G. L. et al. Renal impairment and outcomes in heart failure: Systematic review and meta-analysis. J. Am. Coll. Cardiol.47(10), 1987–1996 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Damman, K. et al. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J.35, 455–469 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Hillege, H. L. et al. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation102, 203–210 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Damman, K. & Testani, J. M. The kidney in heart failure: An update. Eur. Heart J.36, 1437–1444 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Silva, R. et al. Incidence of renal dysfunction over 6 months in patients with chronic heart failure due to left ventricular systolic dysfunction: Contributing factors and relationship to prognosis. Eur. Heart J.27(5), 569–581 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Lanfear, D. E. et al. Relation of Worsened renal function during hospitalization for heart failure to long-term outcomes and rehospitalization. Am. J. Cardiol.107(1), 74–78 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damman, K. et al. Worsening renal function and prognosis in heart failure: Systematic review and meta-analysis. J. Card. Fail.13, 599–608 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Froissart, M., Rossert, J., Jacquot, C., Paillard, M. & Houillier, P. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J. Am. Soc. Nephrol.16, 763 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Stevens, L. A. et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am. J. Kidney Dis.51, 395 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaspari, F. et al. The GFR and GFR decline cannot be accurately estimated in type 2 diabetics. Kidney Int.84, 164 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Rossing, P., Rossing, K., Gaede, P., Pedersen, O. & Parving, H.-H. Monitoring kidney function in type 2 diabetic patients with incipient and overt diabetic nephropathy. Diabetes Care29, 1024 (2006). [DOI] [PubMed] [Google Scholar]
- 23.White, C. et al. Chronic kidney disease stage in renal transplantation classification using cystatin C and creatinine-based equations. Nephrol. Dial. Transplant.22, 3013 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Maillard, N. et al. Cystatin C–based equations in renal transplantation: Moving toward a better glomerular filtration rate prediction?. Transplantation.85, 1855 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Mariat, C. et al. Assessing renal graft function in clinical trials: Can tests predicting glomerular filtration rate substitute for a reference method?. Kidney Int.65, 289 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Craig, A. J., Samol, J., Heenan, S. D., Irwin, A. G. & Britten, A. Overestimation of carboplatin doses is avoided by radionuclide GFR measurement. Br. J. Cancer107, 1310 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marx, G. M. et al. Evaluation of the Cockroft-Gault, Jelliffe and Wright formulae in estimating renal function in elderly cancer patients. Ann. Oncol.15, 291 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Francoz, C. et al. Glomerular filtration rate equations for liver-kidney transplantation in patients with cirrhosis: Validation of current recommendations. Hepatology59, 1514 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Gonwa, T. A. et al. Estimation of glomerular filtration rates before and after orthotopic liver transplantation: Evaluation of current equations. Liver Transpl.10, 301 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Lim, Y.-S. et al. Serum sodium, renal function, and survival of patients with end-stage liver disease. J. Hepatol.52, 523 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cvan-Trobec, K. et al. Iohexol clearance is superior to creatinine-based renal function estimating equations in detecting short-term renal function decline in chronic heart failure. Croat Med. J.56(6), 531–541 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smilde, T. D. J., van Veldhuisen, D. J., Navis, G., Voors, A. A. & Hillege, H. L. Drawbacks and prognostic value of formulas estimating renal function in patients with chronic heart failure and systolic dysfunction. Circulation114, 1572–1580 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Theresa, A. M. et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology. European Heart Journal42(36), 3599–3726 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Luis-Lima, S. et al. Measurement of glomerular filtration rate: Internal and external validations of the iohexol plasma clearance technique by HPLC. Clin. Chim. Acta430, 84 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Bröchner-Mortensen, J. A simple method for the determination of glomerular filtration rate. Scand. J. Clin. Lab. Invest.30, 271 (1972). [DOI] [PubMed] [Google Scholar]
- 36.Cockcroft, D. W. & Gault, M. H. Prediction of creatinine clearance from serum creatinine. Nephron16, 31 (1976). [DOI] [PubMed] [Google Scholar]
- 37.Levey, A. S., Greene, T., Kusek, J. W. & Beck, G. J. for the MDRD Study Group. A simplified equation to predict glomerular filtration rate from serum creatinine (Abstract). J. Am. Soc. Nephrol.11, 155 (2000). [Google Scholar]
- 38.Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med.150, 604 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inker, L. A. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med.367, 20 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DuBois, D. & DuBois, E. F. A formula to estimate the approximate surface area of height and weight are known. Ann. Intern. Med.17, 863 (1916). [Google Scholar]
- 41.Lin, L., Hedayat, A. & Wu, W. Statistical Tools for Measuring Agreement (Springer Science+Business Media, 2012). [Google Scholar]
- 42.Lin, L., Hedayat, A., Sinha, B. & Yang, M. Statistical methods in assessing agreement: Models, issues, and tools. J. Am. Stat. Assoc.97, 257–270 (2002). [Google Scholar]
- 43.Plischke, M. et al. Renal function in heart failure: A disparity between estimating function and predicting mortality risk. Eur. J. Heart Fail.15(7), 763–770 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Delphine, K. et al. Cystatin C versus creatinine for GFR estimation in CKD due to heart failure. Am. J. Kidney Dis.69(2), 320–323 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Smilde, T. D., van Veldhuisen, D. J., Navis, G., Voors, A. A. & Hillege, H. L. Drawbacks and prognostic value of formulas estimating renal function in patients with chronic heart failure and systolic dysfunction. Circulation.114(15), 1572–1580 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Kolsrud, O. et al. Measured and not estimated glomerular filtration rate should be used to assess renal function in heart transplant recipients. Nephrol. Dial. Transplant.31(7), 1182–1189 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Valente, M. A. et al. The Chronic Kidney Disease Epidemiology Collaboration equation outperforms the Modification of Diet in Renal Disease equation for estimating glomerular filtration rate in chronic systolic heart failure. Eur. J. Heart Fail.16, 86–94 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Zamora, E. et al. Estimated glomerular filtration rate and prognosis in heart failure: Value of the Modification of Diet in Renal Disease Study-4, chronic kidney disease epidemiology collaboration, and cockroft-gault formulas. J. Am. Coll. Cardiol.59(19), 1709–1715 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Cheang, I. et al. Cystatin C-based CKD-EPI estimated glomerular filtration rate equations as a better strategy for mortality stratification in acute heart failure: A STROBE-compliant prospective observational study. Medicine99(44), e22996. 10.1097/MD.0000000000022996 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takagi, A., Iwama, Y., Yamada, A., Aihara, K. & Daida, H. Estimated glomerular filtration rate is an independent predictor for mortality of patients with acute heart failure. J. Cardiol.55(3), 317–321. 10.1016/j.jjcc.2009.12.005 (2010) (Epub 2010 Jan 19). [DOI] [PubMed] [Google Scholar]
- 51.Weidmann, Z. M. et al. Prediction of mortality using quantification of renal function in acute heart failure. Int. J. Cardiol.15(201), 650–657. 10.1016/j.ijcard.2015.08.097 (2015) (Epub 2015 Aug 11). [DOI] [PubMed] [Google Scholar]
- 52.Gardner, R. S. et al. Renal dysfunction, as measured by the modification of diet in renal disease equations, and outcome in patients with advanced heart failure. Eur. Heart J.28, 3027–3033 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Singh, T. P., Givertz, M. M. & Gauvreau, K. Risk stratification for in-hospital mortality after heart transplantation using the modification of diet in renal disease and the chronic kidney disease epidemiology collaboration equations for estimated glo-merular filtration rate. Transplantation98, 1000–1006 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Tancredi, M. et al. The relationship between three eGFR formulas and hospitalization for heart failure in 54,486 individuals with type 2 diabetes. Diabetes/Metab. Res. Rev.32, 730–735 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Valentova, M., Anker, S. D. & von Haehling, S. Cardiac cachexia revisited: The role of wasting in heart failure. Heart Fail. Clin.16, 61–69 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Stevens, L. A. et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int.75, 652–660 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.