Abstract
Pistacia lentiscus L. is an aromatic plant containing a significant percentage of essential oil (EO) used in fragrance, pharmaceuticals, cosmetics, and the food industry. The purpose of this work is focused on the optimization of Pistacia lentiscus L. oleo gum resin EO yield extracted by superheated steam extraction (SHSE) by response surface methodology, including extraction parameters of particle size (0. 5 − 1 mm), temperature (140–180 °C) and time (90–150 min). The optimum conditions for Pistacia lentiscus L. EO extracted by SHSE were found to be (particle size: 0.75 mm, time: 120 min and temperature: 160 ℃) which produced the highest EO yield of 5.7%. A regression model was developed, demonstrating a robust quadratic correlation with an R2 value of 0.9991, making it suitable for predictions. Furthermore, the yield of Pistacia lentiscus L. EO extracted by SHSE was compared with the conventional steam and hydro distillation techniques. The study revealed that SHSE yielded higher quantities of EO than other extraction methods. GC-MS analyzed the chemical composition of Pistacia lentiscus L. EO. The predominant compound of Pistacia lentiscus L. EO was determined to be α-pinene, while the other identified compounds include trans-verbenol, verbenol, cis-verbenone, camphene, β-myrcene, d-limonene, cymene, α-myrtenol, α-campholenal, α-copaene, and α-thujene, whose content differed according to different extraction techniques. Overall, superheated steam extraction is an efficient technique for extracting Pistacia lentiscus L. essential oil that enhances EO yield, requiring less time for extraction.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-74972-7.
Keywords: Chemical composition, Essential oil, Pistacia lentiscus L., Response surface methodology, Yield
Subject terms: Plant sciences, Chemistry, Analytical chemistry
Introduction
Pistacia lentiscus L. is a Mediterranean-dwelling and medicinal plant that belongs to the Anacardiaceae family and is located in areas like Island of Chios, Israel, Cyprus, Spain, and Tunisia, Italy, Iran1. Medicinal plants are used in food for their nutritional and therapeutic benefits, including antioxidant and antimicrobial properties that improve an individual’s health2. They are used to treat many diseases including arthritis, high blood pressure, toothache, headaches, rheumatism, and gastrointestinal issues3. Medicinal plants contain useful components that are used to meet the increasing demand for cost-effective and safe preservatives2 and are also used for discovering new medicines4. Pistacia lentiscus L. plant is used in perfumes, beverages, cosmetics, food, and medicine. In modern pharmacology this plant gum is used as anti-ulcer, anti-diabetic, anti-proliferative, hepatoprotective/cardioprotective, anti-inflammatory, and anti-cancer. Essential oil of this plant has antibacterial, antioxidant as well as antifungal activities5. It also treats gastrointestinal disease, asthma, kidney stones, liver and throat infections, ulcer, stomach aches, and diarrhea6. Mastic gum, mainly derived from the trunk of mastic tree Pistacia lentiscus L., is a naturally produced aromatic resin that is yellowish-white in color7. It is a secondary metabolite of plants comprising essential oil, polysaccharides and resin acids. α-pinene is the active ingredient found in the Pistacia lentiscus L. EO followed by terpinen-4-ol, verbenol, and α-terpineol. According to literature, the chemical composition of Pistacia lentiscus L. EO may changes with geological position, nature of soil, season, age of plant and environmental conditions8.
Essential oils (EOs) are complex, naturally occurring and volatile substances with a distinctive smell that are produced as secondary metabolites by aromatic plants9. Essential oils have become increasingly in demand over the last few decades, especially in the culinary, pharmaceutical, and cosmetics industries. Customers’ concerns about the harmful effects of artificial additives or product compositions are increasing daily. The various significant expansion of downstream industries has further stimulated this demand. Moreover, numerous research articles have demonstrated an efficient use of EO as a natural and safe substitute for synthetic antioxidants or additives in nutritional goods. It has been noted that over 25% of prescription drugs have active components or plant extracts derived from chemical compounds found in plants10–12. The characteristics of EOs include antimicrobial, antioxidant, antitumoral antidiabetic, and anti-inflammatory properties13,14. Atropine (anticholinergic), quinine (antiparasitic), quinidine alkaloids (analgesics), artemisinin (antimalarial), vincristine (antineoplastic), and taxol (antineoplastic) are a few examples. Research on herbal plants’ ability to improve human health and well-being is still continuing, even their long history of use for therapeutic purposes15.
Hydro distillation (HD) and steam distillation (SD) are the conventional techniques used for the extraction of essential oils (EOs). These methods are preferred for their low cost and ease of use16. Moreover, the advanced techniques are also used to extract essential oils including microwave-assisted extraction (MAE) and superheated steam extraction. Hydro distillation is an easiest, and most popular technique for extracting EO in both large and small-scale industries17. The plant material to be extracted is mixed with water and heated in this method. Alternatively, in steam distillation process, the plant material to be extracted is separated from the water, and steam is passed through the sample to extract the essential oil from oleo resin. Essential oil comes from liquid extract in this extraction method and must be separated from the separating funnel18. Different methods, such as solvent extraction, provide similar or higher-quality extracts19. Compared to steam distillation, hydro distillation has a few advantages, one of which is the lower initial equipment cost. The limitations of this technique are that it is a time-consuming method and cannot separate aromatic compounds18. Advantages of steam distillation include higher essential oil yield, minimum loss of polar components, and enhanced EO composition20. The limitations of this technique are high energy cost, long time required for extraction, and more energy use and solvent. Microwave-assisted extraction is an effective method for extracting EOs from various plant components21. In this method, solvent heating is done by microwave radiation to separate EO22. The MAE technique offers several advantages over conventional extraction techniques, including improved heating, rapid extractions, selectivity, volumetric heating, and greater yields21,23.
Superheated steam extraction (SHSE) is a relatively new technique of extraction that uses superheated steam (SHS) as an extraction agent24. Steam heated above its boiling point at a specific pressure is known as SHS25. The pressure and composition of the vessel used to generate steam, determine the SHS temperature, which ranges from 101 °C to 1000 °C25. The benefits of such steam include a lower oxygen capability, a higher extraction efficiency, and enhanced heat conductivity26,27. Non-polar compounds can be synthesized along with polar compounds since the dielectric constant of SHS and its polarity diminishes with the increase in temperature24. The rise in temperature in case of SHS helps disrupt the plant cell membrane and the subsequent liberation of EO26. The essential oils of Boswellia serrata oleo gum resin, thyme, and black pepper have been extracted by SHSE26,28.
Response surface methodology (RSM) evaluates numerous factors’ impact and interactions on responses. Therefore, RSM is a set of statistical and numerical methods utilized efficiently for developing, enhancing, and optimizing processes29. A response surface methodology based on the central composite design (CCD) was used to optimize the EO extracted by SHSE. The optimum extraction conditions for the highest essential oil yield were found by optimizing essential oil through RSM, and the correlated impacts between the various extraction parameters (time, temperature and particle size) were also observed30.
Superheated steam extraction is an emerging technique, and a literature study revealed that no methodology has been used for optimum extraction of EO from Pistacia lentiscus L oleo gum resin utilizing this technique. Therefore, this study was designed to optimize the experimental condition (extraction temperature, time and superheated steam flow rate) for maximum EO extraction by SHSE. For this purpose, response surface methodology based on central composite design was employed. The results of SHSE were also compared with conventional HD and SD techniques. Further, the effect of different extraction techniques and conditions on chemical composition of EOs was investigated by GC-MS.
Material and method
Collection of plant material
The Pistacia lentiscus L. oleo gum resin was collected using taping from fully grown Pistacia lentiscus L. trees in the rugged mountains, low rainfall, and semi-arid climate of Zhob District, Balochistan, Pakistan. Dr. Fahim Arshad, Associate Professor authenticated plant material. and voucher specimen (MPZ-004) was submitted to the Herbarium, Department of Botany, University of Okara, Pakistan. The oleo gum resin was washed with distilled water and then crushed into different mesh sizes (1 mm,0.75 mm,0.5 mm) by grinding and then stored into polyethylene bags for further use.
Hydro distillation
The hydrodistillation was carried out using a Clevenger-type apparatus that consisted of a 5000 mL round-bottom flask, a heating mantle, a dean Stark apparatus, a separating funnel and condensers. The round-bottom flask was first filled with 300 g of Pistacia lentiscus L. oleo gum resin and submerged in 3000 mL of distilled water. The volumetric flask with the plant material and water was boiled by placing it on the heating mantle. The water and Pistacia lentiscus L. oleo gum resin EO evaporated and condensed. After the condensation process, the remaining mixture was transferred to a funnel to facilitate the separation of the layer of water from the layer of oil. The yield was calculated after separating the oil layer31. The process was repeated thrice and the average yield (%) was calculated. The formula that was used to calculate EO yield is stated as follows (Eq. 1);
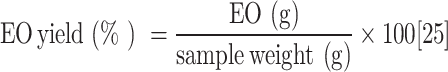 |
1 |
Steam distillation
The instrument used during the steam distillation process included a round bottom flask, a condenser, a biomass flask, heating mantle, a collection vessel and a thermometer. A sample of 100 g of ground Pistacia lentiscus L. was placed on the top of the flask with a circular base and then transferred to the biomass flask. The water encountered the plant material in the biomass flask- it was then boiled using the heating mantle, and the steam was generated. A condenser condensed the EO and water vapor solution to get liquid droplets. The EO and aqueous layers were separated in the collection vessel according to their densities. The essential oil layer was stored in a glass vial after separation. Anhydrous sodium sulfate was used to dehydrate the essential oil. The extraction procedure was conducted thrice to verify its reproducibility and the average yield was calculated (%)32.
Superheated steam extraction
The superheated steam extraction (SHSE) was carried out with SHS extraction equipment which consists of a stainless-steel extraction chamber, SHS source, the condenser, and the collection chamber for the hydrosol. SHS temperature was continuously monitored by thermocouples placed near the entry and exit points of the stainless-steel extraction vessel. The extracted chamber was loaded with 100 g of Pistacia lentiscus L. oleo gum resin (i.e., 0.5, 0.75, 1 mesh) and treated under SHS at 140, 160, 180 ℃ and constant pressure for 1 h24. The mixture was then poured into amber glass vials and passed through a micro filter for filtration before further analysis. SHSE was carried out three times to get more reliable results, and the average yield was calculated (%).
Experimental design
Response surface methodology is applied to investigate the influence of the temperature of the reaction medium, particle size, and the time required for the reaction. Therefore, the mathematical model representing the dependence of the predicted responses y values on the reaction conditions x is formulated as follows: (Eq. 2)
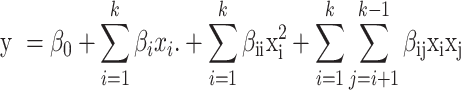 |
2 |
where β0, βi, βii, and βij are constant coefficients which remain the same throughout the process33. Analysis of variance (ANOVA) was employed to validate the mathematical model and identify which parameter is significant concerning the objective function30. This study employed a central composite design (CCD) with 18 experimental combinations (including three central points). Each experiment was performed with 3 replications to confirm the reliability of results.
Gas chromatography-mass spectrometry (GC–MS)
The chemical composition of EO samples was analyzed using gas chromatography-mass spectrometry (GC–MS) with an Agilent Technologies 6890 N Network GC system. GC-MS had a DB-5 capillary column (50 m × 0.25 mm, with a film thickness of 0.25 μm), inert XL mass selective detector and a 5975 C series auto injector. The heating rate for the column was 24 °C/min, with a temperature range of 60 °C to 240 °C. The GC column underwent heating to 60 °C for three minutes. The column’s temperature gradually rose to 240 °C at a rate of 24 °C per minute and remained there for 10 min. The carrier gas was helium, supplied at a 2 mL/min flow rate. Two microliters of essential oil were injected using a split ratio of one to twenty in split mode. An electron ionization method with an ionization energy of 70 eV was used for mass spectrometric detection. Temperatures of 220 °C and 290 °C were maintained for the injector and mass spectrometer transfer line. Compounds were identified by comparing retention values with those in the NIST mass spectrum library25. The mass spectra were acquired using the TurboMass software, version 5.4 (Perkin Elmer Inc, Shelton, CT, USA). The mass spectra were recorded in full scan mode, which detected the total ion current chromatograms. A mixture of aliphatic hydrocarbons (C9-C23; Sigma-Aldrich, USA) in n-hexane was injected under the temperature as mentioned earlier programmed chromatographic conditions to calculate the linear retention indices using the generalized equation of34.
The mass spectra and linear retention indices (LRI) relative to n-alkanes (C9-C23) of essential components were compared with those of commercial libraries (NIST; Wiley) and our GC-MS database built up from spectral data obtained from GC-MS data of standards (Sigma-Aldrich, USA). Further identification was made by matching their recorded mass spectra with the mass spectra of literature data35. The quantification of the individual EO components was expressed as total area percentages of the chromatograms (recorded by MS) mass spectra chromatograph.
Results and discussion
Yield optimization
Design of experiment (DOE) and statistical analysis for Pistacia lentiscus L. oleo gum resin EO yield
Response surface methodology was used to determine maximum EO yield and their relationship among the different extraction conditions. Central Composite design (CCD) was applied for the statistical experimental design with Design Expert Version 13 software. The independent factors were subsequently utilized at 3 various values of each component (lower (− 1), middle (0), and extreme (+ 1)) to (CCD) with an (α) value of ± 1. Table 1 displays the factors examined together with the levels and notations related to them. As a result, a design matrix was created that included four repeats at the center points and a collection of eighteen runs placed in an unplanned sequence. Table 2 presents the design matrix of observed values and their responses. A second-degree polynomial was used in Eq. (3). A regression framework was based upon the developed empirical data that connected the factors under investigation with the response achieved. A controlled set of results was obtained in the inquiry used in this framework.
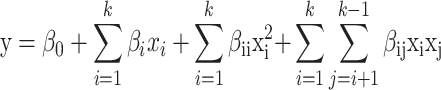 |
3 |
Table 1.
SHSE variables and their respective treatment ranges.
| Representation | Variables | Unit | Actual Value | ||
|---|---|---|---|---|---|
| (− 1) | (0) | (+ 1) | |||
| A | Temperature | °C | 140 | 160 | 180 |
| B | Time | min | 90 | 120 | 150 |
| C | Particle size | mm | 0.5 | 0.75 | 1.00 |
Table 2.
Design matrix based on CCD using Pistacia lentiscus L. experiment results for response.
| Run | A | B | C | Response |
|---|---|---|---|---|
| Extraction temperature | Extraction time | Particle size | Yield | |
| °C | min | mm | % | |
| 1 | 160 | 120 | 0.75 | 5.7 |
| 2 | 180 | 150 | 0.5 | 5.0 |
| 3 | 160 | 150 | 0.75 | 5.5 |
| 4 | 160 | 120 | 0.75 | 5.7 |
| 5 | 140 | 90 | 1 | 4.9 |
| 6 | 160 | 120 | 0.75 | 5.6 |
| 7 | 180 | 90 | 1 | 5.1 |
| 8 | 140 | 150 | 0.5 | 5.1 |
| 9 | 160 | 90 | 0.75 | 5.4 |
| 10 | 180 | 90 | 0.5 | 4.9 |
| 11 | 160 | 120 | 0.75 | 5.6 |
| 12 | 180 | 120 | 0.75 | 5.5 |
| 13 | 160 | 120 | 0.5 | 5.3 |
| 14 | 140 | 90 | 0.5 | 4.7 |
| 15 | 140 | 150 | 1 | 5.1 |
| 16 | 140 | 120 | 0.75 | 5.4 |
| 17 | 180 | 150 | 1 | 5.0 |
| 18 | 160 | 120 | 1 | 5.5 |
where the number of variables studied is k, the percentage EO yield is a response parameter (y), and the point of intercept, straight, quadratic and association coefficients for the regression are β0, βi, βii, and βij, respectively. xi and xj are the variables that are not dependent (factors) and affect the outcome values. ANOVA was used to statistically analyze the produced mathematical model’s correctness, each factor’s relevance, and the connection between all elements. The proposed model’s suitability was assessed by residual analysis. A significant threshold of 5% (p-values < 0.05) is considered in all statistical analyses.
Regression analysis and model fitting for Pistacia lentiscus L. oleo gum resin EO yield
Polynomial equations are fitted to the experimental response in regression evaluation based on least squares method (LSM), with Design-Expert software. LSM is a multiple regression method that reduces residuals, or fitting errors, to find correlations. The parameters that best fit the model describe the connection between the observed variables and their corresponding response. Fisher distribution testing (F-test) is a statistical method for investigating or evaluating the sequence model sum of squares. The regression model was selected according to a 5% level of significance indicated by the probability value (p-values or Prob > F) that is less than 0.05. Table 3 summarizes the p-values for every model using a sum of squares analysis. p-value of below 0.0001 indicates that the quadratic model fits the experimental results, and it explains the straight-line effects, dual-factor interacting effects, and quadratic impacts of superheated steam parameters (extraction time, and particle size, extraction temperature) on the examined response of EO yield at different extraction parameters. The least square method created the regression coefficients for each term while considering the smallest residuals. As a result, Eq. 3 presents a quadratic equation derived from actual data explaining the extraction of EO utilizing superheated steam components.
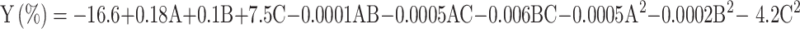 |
4 |
Table 3.
Sequential model sum of squares of polynomial models.
| Source | Mean square | Sum of squares | Df | p-value | F-value | |
|---|---|---|---|---|---|---|
| Linear vs. Mean | 0.03 | 0.09 | 3 | 0.8 | 0.3 | |
| Mean vs. Total | 501.3 | 501.3 | 1 | |||
| 2FI vs. Linear | 0.02 | 0.1 | 3 | 0.9 | 0.2 | |
| Quadratic vs. 2FI | 0.5 | 1.4 | 3 | < 0.0001 | 2784.7 | Suggested |
| Residual | 0.0001 | 0.0004 | 4 | |||
| Cubic vs. Quadratic | 0.0002 | 0.001 | 4 | 0.2 | 2.5 | Aliased |
| Total | 27.9 | 502.9 | 18 |
The model in Eq. 4 contains all the terms with matching regression coefficient values, including the quadratic factors (A2, B2, C2), interaction factors (AB, AC and BC), as well as linear factors (A, B and C). The +ve and -ve signs show the direction of each term’s impacts; to affect the output (Y). In this case, the EO extraction yield (Y) would rise with temperature increases (A), time (B), and particle size (C) for the straight terms. The negative values of A2, B2, and C2 exponential terms showed that the temperature, particle size, and time increase with the decrease in EO yield. The negative symbols on AB, BC and AC for 2 factor interaction terms indicated the antagonistic impacts on superheated steam components on the EO yield36.
ANOVA for Pistacia lentiscus L. oleo gum resin EO yield
Analysis of variance (ANOVA) was used to investigate the significances of model terms, as listed in Table 4, to determine the model’s validity, accuracy, and good fit. ANOVA is used to compare the variations resulting from random mistakes in responses that are generated with the variance due to the combined effect of factor levels (treatments), in order to determine the significance of regression utilized to evaluate response according to variables under examination. P-values assessed each term’s importance in the selected quadratic model.
Table 4.
Analysis of variance (ANOVA).
| Source | Sum of squares | df | Mean square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 1.6 | 9 | 0.2 | 1033.6 | < 0.0001 | Significant |
| B-time | 0.05 | 1 | 0.05 | 290.8 | < 0.0001 | |
| A-temperature | 0.006 | 1 | 0.006 | 37.1 | 0.0003 | |
| C-particle size | 0.04 | 1 | 0.04 | 228.1 | < 0.0001 | |
| AC | 0 | 1 | 0 | 0.3 | 0.6008 | |
| AB | 0.04 | 1 | 0.04 | 285.1 | < 0.0001 | |
| BC | 0.02 | 1 | 0.02 | 107.1 | < 0.0001 | |
| B² | 0.1 | 1 | 0.1 | 678.9 | < 0.0001 | |
| A² | 0.1 | 1 | 0.1 | 646.3 | < 0.0001 | |
| C² | 0.2 | 1 | 0.2 | 1133.3 | < 0.0001 | |
| Residual | 0.001 | 8 | 0.0002 | |||
| Pure Error | 0.0001 | 3 | 0 | |||
| Lack of Fit | 0.001 | 5 | 0.0002 | 7.5 | 0.06 | Not significant |
| Cor Total | 1.6 | 17 |
| Std. Dev. | 0.01 | R² | 0.9991 | |||
|---|---|---|---|---|---|---|
| Mean | 5.3 | Adjusted R² | 0.9982 | |||
| C.V. % | 0.2 | Predicted R² | 0.9898 | |||
| PRESS | 0.01 | Adeq Precision | 98.8 |
According to statistical evaluation, the p-value indicates the probability of denying the null hypothesis, which asserts because there is no meaningful relationship between the terms and the model and there is no meaningful relationship between the components under investigation and the study’s findings. A predefined significance level of 5% was applied to reject the null hypothesis and establish a significant association between the components and outputs. The p-values should be below 5% for validating the significance of the terms and the model. The quadratic model, containing the linear terms for extraction time (B), particle size (C), and the interaction term for extraction time and particle size (BC), showed significance with p-values below 0.05 according to the ANOVA results in Table 4. The remaining factors, on the other hand, was statistically unimportant (p-value > 0.05), whereas the exponential term A2, B2 and C2 were very important, with a p-value approaching zero (p < 0.0001). Temperature is an important aspect of this method that greatly impacts the extraction efficiency and compound stability in a superheated steam extraction method.
All other interaction effects in the superheated steam extraction method were determined to be statistically negligible, except for the interactions between extraction time and particle size (BC). They suggest that temperature, particle size, time, along with the interaction factors of time and particle size (BC) were the important variables influencing the variations in essential oil (EO) yield. The main effect was negligible because of the strong nonlinearity exhibited by the exponential effects of temperature, particle size, and time. In this case, a higher temperature of superheated steam within the investigated range will always increase EO yield. Whereas the linear curve peak indicates that the influence of extraction temperature appears to have an ideal setting within the examined range, indicating that increasing the EO yield should not be achieved by using a shorter or a longer extraction temperature. Lack of fit (LOF) trial is dependent on middle-level repetition at the central point, which was also utilized for evaluating the model’s adequacy. When a regression model is unable to sufficiently characterize the functional connection between the response variable and the experimental variables, it displays LOF. If a model exhibits both a non-significant LOF and a significant regression, it is an accurate match for the experimental data. A precise model presupposes that errors should only arise from intrinsic random fluctuations in measurements (pure errors) and not from LOF errors, resulting from differences between observed and expected values (residuals). As a result, a large LOF may impact response prediction accuracy and call for improvement. The p-value for the LOF analysis is found to be 0.06, which indicates insignificance. As a result, a large LOF may impact response prediction accuracy and call for improvement.
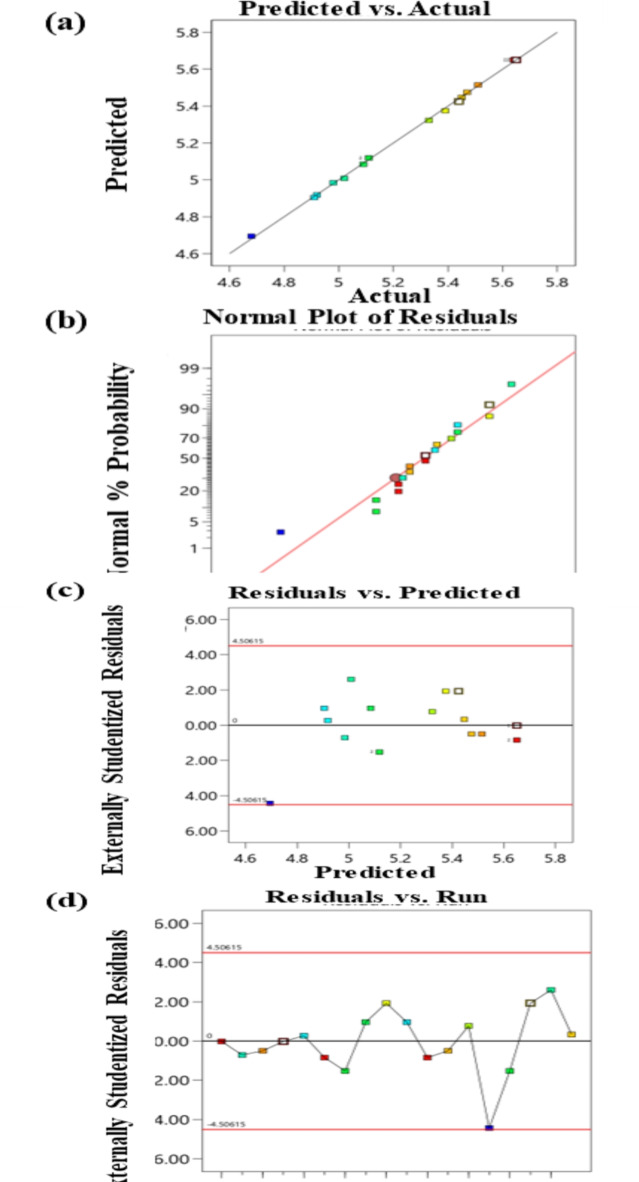
The response values (EO yield) might be predicted using the second-order model that has been given. The R-squared (R2) coefficient of determination is another critical term for assessing the model’s goodness-of-fit. R2 measures the percentage of data variation that the model can describe. It can be employed to elucidate the extent to which the correlation between one component and another might impact the variability of the former. R2 values reflect 0–100% of the model’s accuracy and range from 0 to 1. A number of 0 denotes that the model is unable to effectively represent the data, whereas a 1 denotes a beautiful match and, a very dependable model. The anticipated answers vs. observed responses illustrated in Fig. 1 provide an evaluation chart, which is typically utilized for the data visualization to assess the regression’s goodness of fit. The fitted plots display the anticipated response values that were obtained from the observed response values. Randomized distributions were used to disperse the actual and predicted responses close to the straight line, as shown in Fig. 2(a). This suggests that both values were in accord and that the generated model is appropriate for application in EO yield optimization36. The predicted model’s R2 value, as seen in Table 4, is 0.9898 (98.98%), indicating that the model fits the experimental data rather well. This shows that the influence of one or more variables (SHS factors) in the model might account for 98.98% variation in EO yields. But using the R2 alone to assess the model’s correctness might be incorrect because the value rises with the number of components, independent of their significance. Therefore, when determining the relevance of the model, predicted-R2 and adjusted-R2 are the more reliable readings. The number of important terms that might increase in the model is considered by both adjusted and predicted-R2. The adjusted R2 values will rise when significant factors are included in the model, conversely, if the factors weren’t significant, the values would fall. High adjusted-R2 values signify the inclusion of more important variables. The adjusted-R2value, which come out to be 0.9982 (99.82%), show how well the model explains the link between the variables and response. The predicted-R2statistics indicate the accuracy with which the regression model forecasts the fresh data. As a result, an elevated predicted-R2 model score will be considered highly anticipative. Model being used for this investigation produced a predicted-R2 value of 0.9898 (98.98%), which is satisfactory and indicates that the model can make plausible predictions for fresh observations. Moreover, other statistical characteristics including sufficient accuracy, coefficient of variation (CV%), and predicted residual error sum of squares (PRESS) might also be used to evaluate the model’s credibility. Enough accuracy is used to measure the ratio of signal to noise. The average prediction variance is compared to the anticipated value ranges at the design points. A ratio larger than 4 indicates that the model has sufficient discrimination, meaning it can distinguish between distinct sources of data variability. The representation is adequate for navigating in design space, as shown by the value of suitable precision achieved of 98.8. The C.V% shows the degree of data point dispersion around the mean value. A lower C.V% number (< 10) indicates that the model is highly reproducible. C.V% is 0.2%, as seen in Table 4, indicating that the model is reproducible. Meanwhile, the PRESS stands for the model’s capacity to suit every design point by fitting the model except the one that is being predicted. The value of the PRESS is then obtained by square rooting and adding the residuals. It is preferable since a smaller PRESS value results in a higher predicted-R2 value. The PRESS value is found to be 0.01, which indicates that the model is reasonably adequate36.
Fig. 1.
Diagnostic plots of the residual analysis: (a) Predicted responses vs. observed responses, (b) The normal probability plot, (c) Residuals vs. predicted responses, and (d) Residuals vs. run order.
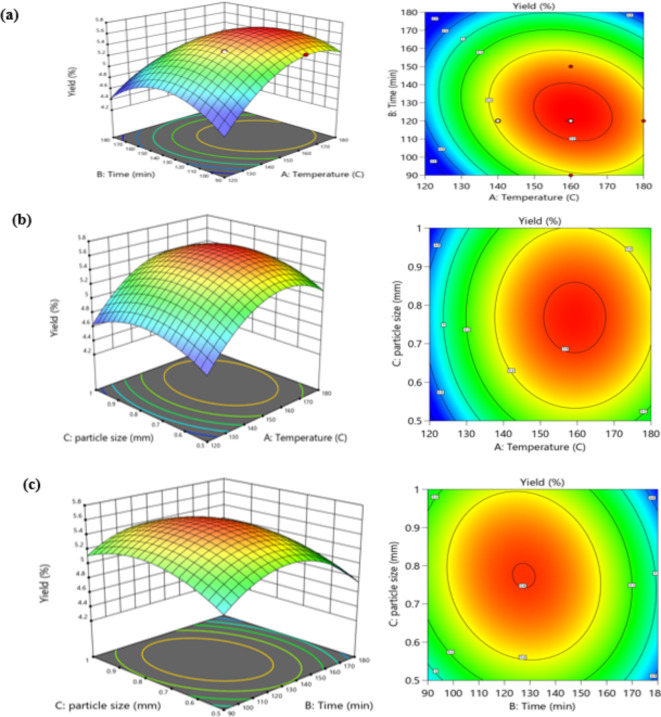
Fig. 2.
Response surface graphs; temperature and time (AB), time and particle size (BC), temperature and particle size (AC) representing the interaction of SHD parameters on Pistacia lentiscus L. EO yield.
Residual analysis for Pistacia lentiscus L. oleo gum resin EO yield
Having a significant model doesn’t always indicate that it can accurately represent the results. As a result, a residual analysis is required to comprehend the residual distribution and locate the outliers. Three diagnostic plots were analyzed: the residuals vs. run order plot Fig. 1(d), the residuals v/s predicted responses plot Fig. 1(c), and the normal probability plot Fig. 1(b). One of fundamental conditions for the validity of an ANOVA is that the residuals obey the normal (linear) distribution, as demonstrated by the plot of normal probability in Fig. 1(b). In meanwhile, as given in Fig. 1(c), plot of residuals against ascending predicted response outcomes was used to assess the homogeneity of variances. Figure 1(c) confirms the consistency of the variances by showing that the residual points were dispersed randomly with no discernible pattern on either side of the center-line. If the observations may be regarded as autonomous with regard to time sequence, it can be determined by plotting residuals against order of the experimental run. The plot of residuals vs. run order indicates that the residuals are not dependent on each other, which satisfies the ANOVA hypothesis. presented in Fig. 1(d). There was no obvious pattern in the residual points dispersed haphazardly around the midline. Additionally, neither of the plots showed the residual points since they were distributed between the top and bottom boundary lines presented in Fig. 1(c) nor (d) demonstrate any outliers. Regression analysis yielded unbiased coefficient estimates with the least amount of variation. In short, residual analysis was carried out using the abovementioned plots, demonstrating that ANOVA presumptions were satisfied37.
Effect of superheated steam variables on EO yield based on response surface plot assessment
Response surface plots, that included both two and three-dimensional (2D) contour and surface plots, may be used to visualize effects of association in the experiment area between independent variables and responses (Fig. 2). The polynomial model was utilized to create surface plots, wherein the interaction effects of 2 variables were considered simultaneously, while maintaining fixed mid-level values for the remaining parameters. The highest, intermediate, and lowest results of essential oil yield are shown by the red, green, and blue colored zones, respectively. The plot is intensely curved when the components involved have a substantial quadratic influence. Surface and contour maps of temperature (A) and time (B) with constant particle size (C) of 0.75 mm is displayed in Fig. 2(a). The highest EO yield was recorded around the middle-level temperature of 160 °C and the high-level time of 120 min. The essential oil yield first rose as the temperature rose, but at very high temperatures, it declined. However, extending the extraction period to 120 min resulted in a higher essential oil yield. On the 3D surface plot, a clear linear impact was observed, showing a trend towards increasing EO yield as extraction time increased. However, the EO yield began to decline after a given amount of time. Temperature is an important consideration in superheated steam extraction, as the water’s temperature significantly impacts the solubility of EOs. A temperature increase would change the physicochemical characteristics of water under pressure. Elevated temperatures weaken the hydrogen bonding among molecules of H2, which lowers the dielectric constant and decreases the water’s polarity. Consequently, more non-polar EO compounds become soluble. On the other hand, EO’s selectivity may be diminished by high temperatures because of co-solubilization or the generation of undesirable constituents that may degrade the quality of the EO. Given the significant increase in solubility, it can be concluded that driving force heat affects solubility more than hydrogen bonding-induced heat. The response surface plots at constant time (B) of 120 min, respectively, in relation to particle size (C) and temperature (A) are displayed in Fig. 2(b). The greatest essential oil production was produced at a middle-level temperature of 160 °C, as indicated in the preceding plot. The 3D plot, however, showed that a larger yield of EO was produced at middle level particle sizes, and a minimal yield was reached at higher level values of particle sizes, in the case of particle size. Figure 2(c) displays the response surface charts as a function of time (B) and particle size (C) with constant temperature (A)36–38. The increase in essential oil (EO) yield up to a certain temperature followed by a decline is a typical behavior observed in many extraction processes, including superheated steam extraction. At lower temperatures, the vapor pressure of the essential oil components may be insufficient to achieve effective vaporization and extraction. Higher temperatures provides more heat energy to vaporize the components of the essential oils and break the bonds between the essential oil and the plant matrix, hence the higher yields are obtained. If the temperature increases to a certain extent, heat decomposes some of the essential oil’s volatile constituents and reduces yield39.
Contour and surface maps of EO yield with reference to temperature A and time B is shown in Fig. 2. The gradual increase in the yield of EO with increase in extraction time results from time-dependent diffusion of the EO molecules from the plant matrix to the steam phase. Extended extraction periods give the steam enough time to effectively solubilize a wide range of EO constituents in the plant. Whereas, after a given period, it is often observed that the yield slows down or even decreases. This is because, the extraction efficiency decreases with time, for instance, due to steam saturation with the extracted compounds and heat sensitive EO components deterioration36. The interaction between mass transfer and particle size is found to affect the yield of the essential oil. When the particle size is small, the diffusion of steam and extraction of the plant material is increased because the steam has ample time to penetrate deeper into the plant material. The steam might not have adequate time to contact the plant material at larger particle sizes, minimizing extraction rates. Also, the plant matrices with larger particles can cause turbulent conditions that will limit the interaction of the steam with the matrix, hence limiting the extraction capability40.
The maximum value of EO yield at the middle level of temperature and time indicates that the extraction parameters have been optimized. Medium level of temperature is optimal because at this level the compounds of essential oils can easily be vaporized but at the same time the heat sensitive compounds are not deteriorate. Likewise, the middle-level time favors interaction between the steam and plant material without having a limit that causes a reduction in the efficiency of the process or a detriment to the quality of the plant material. The relationship between temperature, time, and particle size is quite intricate. However, it may be observed that these factors are interactive and that the effects they produce on extraction efficiency can be dependent on the other factors involved. For example, a longer extraction time may offset lower particle size while a higher temperature may necessitate a shorter extraction time due to increased deterioration. Existing EO extraction concepts support the discrepancies experienced in our SHSE experiments well. Extraction conditions therefore have to be optimized in a way that would maximize yield, while, at the same time minimizing degradation of the compound and the extent of the extraction process. These results accord with the relevant research on the common methods of essential oil extraction41.
Optimization and model validation for Pistacia lentiscus L. oleo gum resin EO yield
The application of numerical optimization based on the desirability function was aimed to identify the optimum conditions for EO extraction for superheated-steam extraction procedure and to evaluate the response derived from combining the components based on the optimization goal. Table 5 showed that the temperature of 159.4 °C, 126.8 min, and particle size of 0.78 mm were optimal for obtaining the maximum EO yield. Therefore, a yield of 5.8% was predicted in accordance with the desirability function of 1. Verification tests were carried out in triplicate under the recommended ideal operating parameters to confirm the developed regression model’s precision and the optimal pairing’s reliability. The experiment produced an essential oil yield of (5.71%), which is within the 95% prediction ranges and roughly corresponds to the expected value of 5.8% with a relative error of 3.04%.
Table 5.
Numerical optimization and model validation of Pistacia lentiscus L.
| Factor | Name | Optimization goal | Low Level | High Level | Prediction point |
|---|---|---|---|---|---|
| A | Temperature | In range | 140 | 180 | 159.4 |
| B | Time | In range | 90 | 150 | 126.8 |
| C | Particle size | In range | 0.5 | 1 | 0.78 |
| Response Y | EO Yield | Maximize | 4.7 | 5.7 | 5.8 |
| Predicted response | Desirability | 95% PI low | 95% PI high | Observed response | Relative error |
| 5.8 | 1 | 5.6 | 1.7 | 5.71 | 0.2% |
Comparison of Pistacia lentiscus L. oleo gum resin EO yield
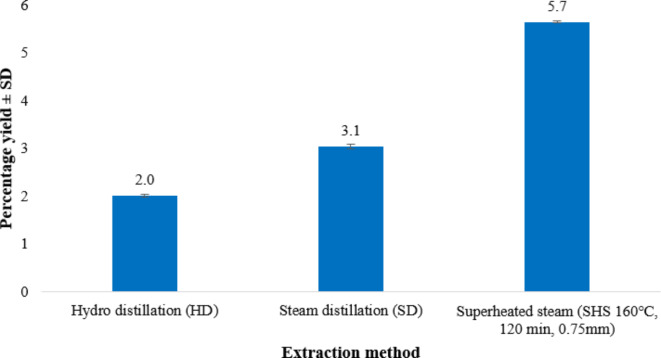
The yield of Pistacia lentiscus L. oleo gum resin EO was determined by steam distillation, hydrodistillation, and superheated steam extraction. The Pistacia lentiscus L. EO yields (%) obtained by SD, HD and SHSE are presented in Fig. 3. According to the results, the essential oil yield of Pistacia lentiscus L. extracted by HD, SD and SHSD is found to be 2.0%, 3.1% and 5.7%. Hence, the present results showed that extraction methods are important factors that modulate Pistacia lentiscus L. essential oil yields. HD observed the minimum essential oil yield. Also, the percentage yield of Pistacia lentiscus L. EO extracted by SHSE produced more yield than conventional HD and SD processes. It could be attributed to superheated steam’s high operating temperature, low polarity and viscosity, deeper penetration into pores, and higher kinetic energy value25. The EO yield of 5.7% was recorded for SHSE at 160 ℃ which is slightly higher than that from SHSE at 140 ℃, (4.7%). This may be due to different temperatures encountered during extraction and the extraction conditions30. The results showed that EO yield improved with the rise in temperature during extraction up to 160 °C in case of SHSE. Higher temperatures in the SHSE are causing higher EO vaporization from Pistacia lentiscus L. oleo gum resin; therefore, a higher essential oil yield is obtained. In another investigation, a similar observation was made by28, where the EO of black pepper and thyme leaves extracted by SHSE revealed greater yield than HD. According to previous research, the yield of Boswellia serrata Roxb. essential oil improved (8.5–10.8%) with the rise in the superheated steam temperature26. As stated in earlier studies, it was ascertained that the extraction temperature of subcritical water extraction rises with the rise in EO yield of Citrus hystrix DC. leaves30.
Fig. 3.
Percentage yields of Pistacia lentiscus L. essential oils obtained from various extraction methods.
Literature also revealed that the surface tension, polarity, viscosity, dielectric constant, and hydrogen bonds of water molecules are decreased by high temperatures and are responsible for increased diffusion power, mass transfer rate and essential oil yield30.The highest EO yield was produced at 180 °C, which was an ideal temperature. After that, a decline in yield occurs due to the component deterioration. Previous studies showed that the yield of essential oil from oleo resin decreases when it is in grinded form42. Similarly, it was reported that increasing the temperature up to 170 °C enhanced the extraction quality of anethole from Pimpinella anisum L. seeds26. According to the previous research, employment of higher temperature of subcritical water extraction (SWE) at 120 °C enhances the Curcuma longa L. EO yield43. The EO yield of Pistacia lentiscus L. oleo resin (2.4%) is higher than Pistacia vera L. (0.2%), Pistacia terebinthus L. (0.3%) and Pistacia lentiscus L. (0.3%) leaves EO yield44.
As a result, it was concluded that SHSE is an improved and novel method for extraction with a greater yield of EO comparing with conventional HD & SD.
Chemical composition of Pistacia lentiscus L. oleo gum resin EO
The chemical composition of Pistacia lentiscus L. oleo gum resin EOs isolated by HD, SD and SHE at 160 ℃ temperature, 120 min extraction time and 0.75 mm mesh size have been summarized in Table 6. The percentage chemical composition of Pistacia lentiscus L. EOs were as follows; HD (90.18%), SD (94.71%), and SHSE at 160℃ (98.41%). The main components of Pistacia lentiscus L. oleo gum resin EO are found to be α-pinene (49.5–60.3%), β-myrcene (5.48–10.33%), limonene (4.32–9.43%), cis-verbenone (2.1–5.5%), verbenol (1.5–4.1%), α-myrtenol (0.05–3.32%), cymene (0.35–2.52%), α-campholenal (0.12–2.34%), α-copaene (2.03%) and camphene (0.09–1.55%). Our results are consistent with the previous research45, in which α-pinene, limonene, caryophyllene and myrcene are found to be the major compounds of Pistacia lentiscus Var. EO. According to another report, oleo-gum resin of Pistacia lentiscus L. EO contained terpene-4-ol, α-pinene, terpineol, along with limonene as the main components46. Pistacia lentiscus L. leaves EO contained β- caryophyllene, germacrene, and γ-cadinene as the major components47. Moreover, mastic oil contained linalool, α-terpineol, and verbenone. Two new varieties of nortriterpenoid, namely polypodane and malabaricane, were discovered in the gum mastic neutral fraction48. Mainly, the EO profile of fresh mastic tree leaves showed the abundance of monoterpene hydrocarbons including myrcene, which are frequently found in parsley and thyme49.
Table 6.
Chemical composition of Pistacia lentiscus L. EO extracted by HD, SD and SHSE.
| Sr. No. | Components | LRIcalcb | LRIlitc | %Composition of essential oil | Method of identification | ||
|---|---|---|---|---|---|---|---|
| HD | SD | SHSE (160 °C) | |||||
| Monoterpene hydrocarbons | |||||||
| 1 | γ-terpinene | 1062 | 1065 | 0.67 ± 0.04a | a, b | ||
| 2 | α-phellandrene | 1005 | 1011 | 0.15 ± 0.01a | a, b | ||
| 3 | α-pinene | 938 | 939 | 49.5 ± 0.03c | 54.5 ± 0.04b | 60.3 ± 0.01a | a, b |
| 4 | Camphene | 953 | 953 | 0.09 ± 0.00c | 0.12 ± 0.01b | 1.55 ± 0.04a | a, b |
| 5 | α-thujene | 931 | 928 | 0.07 ± 0.00c | 0.16 ± 0.01b | 0.56 ± 0.00a | a, b |
| 6 | β-pinene | 980 | 980 | 0.72 ± 0.01c | 1.66 ± 0.00b | 1.76 ± 0.04a | a, b |
| 7 | 3-carene | 1001 | 1011 | 0.16 ± 0.00b | 0.21 ± 0.01a | a, b | |
| 8 | Cymene | 1020 | 1026 | 0.35 ± 0.00b | 2.52 ± 0.01a | 2.52 ± 0.02a | a, b |
| 9 | Limonene | 1031 | 1031 | 9.43 ± 0.00a | 5.79 ± 0.05b | 4.32 ± 0.03c | a, b |
| 10 | β-myrcene | 991 | 991 | 10.33 ± 0.00a | 6.2 ± 0.02b | 5.48 ± 0.01c | a, b |
| 11 | β -ocimene | 1041 | 1040 | 0.09 ± 0.00b | 0.12 ± 0.01a | a, b | |
| Oxygenated monoterpene hydrocarbons | |||||||
| 12 | Carveol | 1207 | 1208 | 0.36 ± 0.03a | a, b | ||
| 13 | β-terpineol | 1163 | 1162 | 0.12 ± 0.00b | 0.8 ± 0.02a | a, b | |
| 14 | 7-octatriene-2-ol | 1134 | 1132 | 0.12 ± 0.00c | 0.17 ± 0.03b | 1.17 ± 0.03a | a, b |
| 15 | β-linalool | 1198 | 1098 | 0.21 ± 0.00b | 0.5 ± 0.01a | a, b | |
| 16 | Artemiseole | 977 | 970 | 0.09 ± 0.00c | 1.06 ± 0.02b | 1.55 ± 0.01a | a, b |
| 17 | α-campholenal | 1127 | 1126 | 0.12 ± 0.00c | 2.34 ± 0.01a | 1.08 ± 0.03b | a, b |
| 18 | Verbenol | 1150 | 1100 | 4.1 ± 0.00a | 3.7 ± 0.01b | 1.5 ± 0.02c | a, b |
| 19 | Pinocarvone | 1162 | 1161 | 0.01 ± 0.00c | 2.98 ± 0.02a | 2.64 ± 0.04b | a, b |
| 20 | Pinocamphone | 1160 | 1160 | 0.52 ± 0.01a | a, b | ||
| 21 | α- myrtenol | 1193 | 1188 | 0.05 ± 0.00c | 3.32 ± 0.02a | 2.01 ± 0.02b | a, b |
| 22 | Cis-verbenone | 1204 | 1189 | 5.5 ± 0.01a | 2.1 ± 0.03b | 2.14 ± 0.02c | a, b |
| 23 | Cis -dihydrocarveol | 1941 | 1940 | 0.01 ± 0.00b | - | 0.63 ± 0.03a | a, b |
| 24 | Cis-thujanol | 1078 | 1076 | 0.37 ± 0.01a | a, b | ||
| 25 | Camphenol | 1105 | 1102 | 0.06 ± 0.00b | 0.75 ± 0.00a | a, b | |
| Sesquiterpene hydrocarbons | |||||||
| 26 | Germacrene D | 1477 | 1480 | 3.2 ± 0.02a | 2.5 ± 0.02b | 2.2 ± 0.01c | |
| 27 | α-copaene | 1376 | 1381 | 2.03 ± 0.01a | 0.2 ± 0.00b | a, b | |
| 28 | Caryophyllene | 1454 | 1419 | 2.9 ± 0.03c | 4.8 ± 0.00a | 3.1 ± 0.02b | a, b |
| Other hydrocarbons | |||||||
| 29 | Xylene | 877 | 875 | 0.02 ± 0.00a | 0.01 ± 0.00b | a, b | |
| 30 | 2-decyne | 1199 | 1187 | 0.36 ± 0.03a | 0.03 ± 0.01c | 0.26 ± 0.00b | a, b |
| 31 | Eugenol | 1305 | 1289 | 0.34 ± 0.01a | a, b | ||
| Total monoterpenes | 70.74 | 71.28 | 77.31 | ||||
| Total oxygenated monoterpenes | 10.39 | 16.04 | 15.65 | ||||
| Total sesquiterpenes | 8.13 | 7.3 | 5.5 | ||||
| Total other hydrocarbons | 0.92 | 0.03 | 0.27 | ||||
| Overall total concentration | 90.18 | 94.65 | 98.73 | ||||
LRIcalca = calculated linear retention index; LRIlitb = linear retention index taken from reference Both values were calculated as the mean of three GC analysis. Data shown as means ± standard deviations. Different letters in superscripts represent significant differences among Pistacia lentiscus L. oleo gum resin EO derived from different extraction methods. a = identification based on retention index. b = identification based on comparison of mass spectra”.
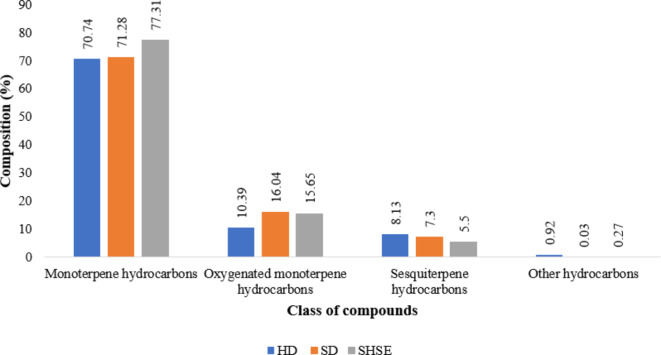
The main class of compounds present in Pistacia lentiscus L. EO is found to monoterpene hydrocarbons (70.74–77.31%), followed by oxygenated monoterpene hydrocarbons (10.39–16.04%), sesquiterpene hydrocarbons (5.5–8.13%) and other hydrocarbons (0.03–0.92%). Our results are in accordance with previous studies which indicated50, that the primary constituents in Pistacia atlantica Desf. hull essential oil are monoterpenes (75.7%), oxygenated monoterpenes (13.4%) and other components51. also reported that monoterpenes are the main class of chemical components in Pistacia lentiscus L., with α-pinene (14.5–37.5%), β-myrcene (4.7%) as well as β-pinene (2.97%) being the most abundant type. The chemical composition of Pistacia lentiscus L. EO obtained by SHSE has not been previously reported in any publication. The essential oil obtained by alternative extraction techniques was compared with the present investigation. According to another report1, the major class of compounds present in Pistacia lentiscus L. EO extracted by hydro distillation was found to be monoterpene hydrocarbons (40–78%) followed by oxygenated monoterpene hydrocarbons (7-37.6%), with α-pinene and limonene being the major components52. reported that monoterpenes (77–85%) are the main class of Pistacia lentiscus L. EO, followed by oxygenated monoterpenes (6%), sesquiterpenes (5–8%), and oxygenated sesquiterpenes (1–5%), extracted by hydro distillation and the major components were appeared as myrcene, α-pinene and β-pinene.
Fig. 4.
Variation in the chemical composition of different classes of compounds extracted by HD, SD, and SHSE.
According to the GC-MS analysis, the total number of identified components of Pistacia lentiscus L. EO extracted by HD, SD and SHSE was 26, 20 and 27. Superheated steam distillation produced more components than other extraction techniques. It may be due to the high-temperature effect of SHSE. Extreme temperature of SHSE changes the polarizability of steam. It creates a high-pressure environment that facilitates the breakdown of plant’s cell membrane, which increases the essential oil components’ ability to solubilize30. Superheated steam extraction showed the highest percentage of monoterpene hydrocarbons (77.31%) of Pistacia lentiscus L., followed by SD (71.28%) and HD 70.74%.
Similarly, the total percentage of oxygenated monoterpene hydrocarbons of Pistacia lentiscus L. extracted by HD, SD and SHSE appeared as 10.39%, 16.04% and 15.65%. The total percentage of sesquiterpene hydrocarbons extracted by HD, SD and SHSE were 8.13%, 7.3% and 5.5%. The difference in the concentration of components may be due to the different extraction conditions and climate changes30.
Limonene is a natural pesticide that is utilized as a cleaning solvent46,53 reported that the main constituents of the hydro distilled essential oil of Sardinian mastic tree leaves were caryophyllene (13.2%) and α-Germacrene (3.9%). Additionally, over 40 chemicals were found and measured in Morocco’s EO extracted from Pistacia lentiscus L. leaves. According to a previous study54Pistacia lentiscus L. leaves EO extracted by steam distillation, contained myrcene, caryophyllene, δ-cadinene, α-cadinol as well as germacrene-D as the primary components, however, Pistacia lentiscus L. twigs EO carried caryophyllene, germacrene D and myrcene as the main constituents. The previous study49 revealed that the fresh leaves of Pistacia lentiscus L. EO contained myrcene, α-germacrene, α-cadinol, trans-caryophyllene and α-cadinene as the major components. According to another report55, the main constituents of the Pistacia lentiscus L. leaves EO extracted by steam distillation were limonene, α-pinene, germacrene D and terpinen-4-ol. The main components of the Pistacia lentiscus L. fruit EO were limonene (8.1–13.0%), myrcene (6.3–11.6%), and α-pinene (7.5–11.2%), and the main constituents of the Pistacia lentiscus L. branch EO were myrcene (68.2–71.0%), limonene (9.6–19.7%), and α-pinene (34.4–46.2%)55. According to another report56, Pistacia lentiscus L. gum EO contained major percentage of monoterpene hydrocarbons (90%) and Pistacia lentiscus L. leaf EO contained major percentage of monoterpene hydrocarbons (50%), followed by oxygenated monoterpene hydrocarbons (20%) and sesquiterpene hydrocarbons (25%). Whereas, Pistacia lentiscus L. fruit EO contained 90–96% of monoterpene hydrocarbons, followed by sesquiterpene hydrocarbons (2–3%), extracted by hydro distillation and the major compounds were appeared as α-pinene and β-myrcene. Overall, α-pinene, β-myrcene, limonene, cis-verbenone, verbenol, α-myrtenol, cymene, α-campholenal, α-copaene and camphene were the major components of Pistacia lentiscus L. EO and the extraction techniques can significantly alter the chemical composition of essential oils.
Table 7.
Main Components occurring in the EOs extracted from Pistacia lentiscus L. Oleo gum resin.
| Chemical compound | Pistacia lentiscus L. oleo gum resin EO | ||
|---|---|---|---|
| HD | SD | SHSE | |
| α-pinene | 49.5 ± 0.03c | 54.5 ± 0.04b | 60.3 ± 0.01a |
| Limonene | 9.43 ± 0.00a | 5.79 ± 0.05b | 4.32 ± 0.03c |
| β -myrcene | 10.33 ± 0.00a | 6.2 ± 0.02b | 5.48 ± 0.01c |
| Germacrene D | 3.2 ± 0.02a | 2.5 ± 0.02b | 2.2 ± 0.01c |
| Caryophyllene | 2.9 ± 0.03c | 4.8 ± 0.00a | 3.1 ± 0.02b |
| Verbenol | 4.1 ± 0.00a | 3.7 ± 0.01b | 1.5 ± 0.02c |
| Cis-Verbenone | 5.5 ± 0.01a | 2.1 ± 0.03b | 2.14 ± 0.02c |
| Cymene | 0.35 ± 0.00b | 2.52 ± 0.01a | 2.52 ± 0.02a |
| α-myrtenol | 0.05 ± 0.00c | 3.32 ± 0.02a | 2.01 ± 0.02b |
| α-campholenal | 0.12 ± 0.00c | 2.34 ± 0.01a | 1.08 ± 0.03b |
| camphene | 0.09 ± 0.00c | 0.12 ± 0.01b | 1.55 ± 0.04a |
| α-copaene | 2.03 ± 0.01a | ||
Conclusion
In this study, Pistacia lentiscus L. oleoresin essential oil was extracted by superheated steam extraction, which provides a higher EO yield in a shorter extraction time. Moreover, first-time response surface methodology was used to optimize extraction conditions for SHSE. Consequently, a univariate and multivariate method was used to examine the impacts of the extraction parameters, which included temperature, time, and particle size, on the EO yields. A regression model was established and verified in accordance with the experimental design. The model-based optimization resulted in an optimal EO yield of (5.7%) under optimized extraction parameters of 120 min extraction time, 174 °C superheated steam temperature, and 0.75 mm of mesh size. This EO yield was significantly higher as than hydro distillation (2.0%) and steam distillation (3.1%). GC-MS analysis of the EO revealed that α-pinene, cis-verbenone, verbenol, trans-verbenol, myrcene and α-myrtenol were the major constituents in Pistacia lentiscus L. EO. In summary, superheated steam extraction is an effective method, for extracting EO from Pistacia lentiscus L. leading to improved EO yield. In the future, the further evaluation of the biological potential of Pistacia lentiscus L., EOs extracted by SHSE using response surface methodology is required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Nil.
Abbreviations
- ANOVA
Analysis of variance
- CCD
central composite design
- CV%
coefficient of variation
- DOE
design of experiment
- EO
essential oil
- F-test
Fisher distribution test
- GC-MS
gas chromatography-mass spectrometry
- HD
hydrodistillation
- LOF
lack of fit
- LSM
least square method
- MAE
microwave-assisted extraction
- PRESS
predicted residual error sum of squares
- RSM
response surface methodology
- SD
steam distillation
- SHS
superheated steam
- SHSE
superheated steam extraction
- SWE
subcritical water extraction
- SGML
Standard
Author contributions
Conceptualization, M.A.A (Muhammad Adnan Ayub); investigation, I.I (Iqra Iram).; validation, S.Z.I (Shahzad Zafar Iqbal), M.A.A. (Muhammad Amin Abid); project administration and methodology, Muhammad Adnan Ayub; resources, Amjad Hussain, Muhammad Amin Abid, and Iqra Ayub; review and editing, M.A.A, Iqra Iram and Rameen Waseem; writing and draft preparation, Iqra Iram and Rameen Waseem; software, Iqra Ayub; supervision, Muhammad Adnan Ayub; All authors have read and agreed to the published version of the manuscript.
Funding
This research is part of a research project (Grant No. 20-15988/NRPU/R&D/HEC/2021) funded by the Higher Education Commission (HEC), Islamabad, Pakistan.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zrira, S., Elamrani, A. & Benjilali, B. Chemical composition of the essential oil of Pistacia lentiscus L. from Morocco—a seasonal variation. Flavour Fragr. J.18(6), 475–480 (2003). [Google Scholar]
- 2.Kazeminia, M., Mehrabi, A. & Mahmoudi, R. Chemical composition, biological activities, and nutritional application of Asteraceae family herbs: A systematic review. Trends Phytochem. Res.6(3), 187–213 (2022). [Google Scholar]
- 3.El Jabboury, Z. et al. Ammi visnaga (L.) Lam.: An overview of phytochemistry and biological functionalities. Trends Phytochem. Res.7(3), 141–155 (2023). [Google Scholar]
- 4.Olaoluwa, O. et al. Ethnopharmacology, phytochemistry and biological activities of the African species of the genus Ficus L. Trends Phytochem. Res.6(1), 46–69 (2022). [Google Scholar]
- 5.Paraskevopoulou, A. & Kiosseoglou, V. Chios mastic gum and its food applications Funct. Prop. Traditional Foods. pp. 271–287 (2016).
- 6.Bozorgi, M. et al. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): A review of their traditional uses, phytochemistry, and pharmacology. The Scientific World Journal, 2013. (2013). [DOI] [PMC free article] [PubMed]
- 7.Alwadi, M. A. M. et al. Mastic (Pistacia lentiscus) gum and oral health: A state-of-the-art review of the literature. J. Nat. Med., pp. 1–16. (2023). [DOI] [PubMed]
- 8.Loolaie, M. et al. Peppermint and its functionality: A review. Arch. Clin. Microbiol.8(4), 54 (2017). [Google Scholar]
- 9.Bakkali, F. et al. Biological effects of essential oils–a review. Food Chem. Toxicol.46(2), 446–475 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Giacometti, J. et al. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int.113, 245–262 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Liu, Z. et al. Optimization of solvent-free microwave assisted extraction of essential oil from Cinnamomum camphora leaves. Ind. Crops Prod.124, 353–362 (2018). [Google Scholar]
- 12.Reyes-Jurado, F. et al. Essential oils: antimicrobial activities, extraction methods, and their modeling. Food Eng. Rev.7, 275–297 (2015). [Google Scholar]
- 13.Mohammadhosseini, M., Akbarzadeh, A. & Flamini, G. Profiling of compositions of essential oils and volatiles of Salvia limbata using traditional and advanced techniques and evaluation for biological activities of their extracts. Chem. Biodivers.14(5), e1600361 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Mohammadhosseini, M. Essential oils extracted using microwave-assisted hydrodistillation from aerial parts of eleven Artemisia species: Chemical compositions and diversities in different geographical regions of Iran. Rec. Nat. Prod.11(2), 114 (2017). [Google Scholar]
- 15.Rout, S. P. et al. Plants in traditional medicinal system-future source of new drugs. Int. J. Pharm. Pharm. Sci.1(1), 1–23 (2009). [Google Scholar]
- 16.Pheko-Ofitlhile, T. & Makhzoum, A. Impact of hydrodistillation and steam distillation on the yield and chemical composition of essential oils and their comparison with modern isolation techniques. J. Essent. Oil Res., pp. 1–11 (2024).
- 17.Rassem, H. H., Nour, A. H. & Yunus, R. M. Techniques for extraction of essential oils from plants: A review. Aust. J. Basic Appl. Sci.10(16), 117–127 (2016). [Google Scholar]
- 18.Chemat, F. & Boutekedjiret, C. Extraction//steam Distillation. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, p. 1–12 (Elsevier, 2015).
- 19.Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem.8(3), 303–313 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Gavahian, M. et al. Comparison of extraction parameters and extracted essential oils from Mentha Piperita L. using hydrodistillation and steamdistillation. Int. Food Res. J., 22(1). (2015).
- 21.Mohammadhosseini, M. Chemical composition of the essential oils and volatile fractions from flowers, stems and roots of Salvia Multicaulis Vahl. By using MAHD, SFME and HS-SPME methods. J. Essent. Oil Bearing Plants. 18(6), 1360–1371 (2015). [Google Scholar]
- 22.Eskilsson, C. S. & Björklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A. 902(1), 227–250 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Cardoso-Ugarte, G. A. et al. Microwave-assisted extraction of essential oils from herbs. J. Microw. Power Electromagn. Energy.47(1), 63–72 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Ayub, M. A. et al. Chemical composition, antioxidant, and antimicrobial activities of P. Roxburghii oleoresin essential oils extracted by steam distillation, superheated steam, and supercritical fluid CO2 extraction. J. Food Sci.88(6), 2425–2438 (2023). [DOI] [PubMed] [Google Scholar]
- 25.Ayub, M. A. et al. Comparison of conventional extraction techniques with superheated steam distillation on chemical characterization and biological activities of Syzygium aromaticum L. essential oil. Separations. 10(1), 27 (2023). [Google Scholar]
- 26.Ayub, M. A. et al. Chemical composition and biological potential of Pinus roxburghii oleoresin essential oils extracted by steam distillation, superheated steam, and supercritical fluid CO2 extraction. (2022). [DOI] [PubMed]
- 27.Jin, C. et al. Optimization of Superheated Steam Treatment Conditions for Wheat Aleurone Layer Flour 42 (Food Science and Technology, 2021).
- 28.Rouatbi, M., Duquenoy, A. & Giampaoli, P. Extraction of the essential oil of thyme and black pepper by superheated steam. J. Food Eng.78(2), 708–714 (2007). [Google Scholar]
- 29.Liyana-Pathirana, C. & Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem.93(1), 47–56 (2005). [Google Scholar]
- 30.Halim, N. A. A. et al. Optimization studies and compositional analysis of subcritical water extraction of essential oil from Citrus hystrix DC. Leaves. J. Supercrit. Fluids. 178, 105384 (2021). [Google Scholar]
- 31.Ayub, M. A. et al. Biological activity of Boswellia serrata Roxb. Oleo gum resin essential oil: Effects of extraction by supercritical carbon dioxide and traditional methods. Int. J. Food Prop.21(1), 808–820 (2018). [Google Scholar]
- 32.Azeem, M. et al. Pesticidal potential of some wild plant essential oils against grain pests Tribolium castaneum (Herbst, 1797) and aspergillus flavus (link, 1809). Arab. J. Chem.15(1), 103482 (2022). [Google Scholar]
- 33.Khiari, K. et al. Optimization of Pistacia lentiscus oil transesterification process using central composite design. Waste Biomass Valoriz.10, 2575–2581 (2019). [Google Scholar]
- 34.Van Den Dool, H. & Kratz, P. D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. (1963). [DOI] [PubMed]
- 35.Adams, R. P. Identification of essential oil components by gas chromatography/mass spectrometry. 5 online ed. Gruver, TX USA: Texensis Publishing (2017).
- 36.Ramić, M. et al. Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory. Ultrason. Sonochem.23, 360–368 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Raza, A. et al. Optimization of ultrasonic-assisted extraction of antioxidant polysaccharides from the stem of Trapa quadrispinosa using response surface methodology. Int. J. Biol. Macromol.94, 335–344 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Tekin, K., Akalın, M. K. & Şeker, M. G. Ultrasound bath-assisted extraction of essential oils from clove using central composite design. Ind. Crops Prod.77, 954–960 (2015). [Google Scholar]
- 39.Biru, M. A., Waday, Y. A. & Shumi, L. D. Optimization of essential oil extraction from bitter leaf (Vernonia Amygdalina) by using an ultrasonic method and response surface methodology. Int. J. Chem. Eng. 2022. (2022).
- 40.Yousefi, M. et al. Supercritical fluid extraction of essential oils. TRAC Trends Anal. Chem.118, 182–193 (2019). [Google Scholar]
- 41.Li, P., Tian, L. & Li, T. Study on ultrasonic-assisted extraction of essential oil from Cinnamon Bark and Preliminary Investigation of its antibacterial activity. Lecture Notes in Electrical Engineering, 332, pp. 349–360. (2015).
- 42.Rouatbi, M. & Duquenoy, A. J.J.o.f.e. Giampaoli. Extr. Essent. oil Thyme Black Pepper Superheated Steam.78(2), 708–714 (2007). [Google Scholar]
- 43.Mottahedin, P., Haghighi Asl, A. & Khajenoori, M. Extraction of curcumin and essential oil from Curcuma longa L. by subcritical water via response surface methodology. J. Food Process. Preserv.41(4), e13095 (2017). [Google Scholar]
- 44.Duru, M. et al. Chemical composition and antifungal properties of essential oils of three Pistacia species. Fitoterapia. 74(1), 170–176 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Koutsoudaki, C., Krsek, M. & Rodger, A. Chemical composition and antibacterial activity of the essential oil and the gum of Pistacia lentiscus Var. Chia. J. Agric. Food Chem.53(20), 7681–7685 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Congiu, R. et al. Extraction and isolation of Pistacia lentiscus L. essential oil by supercritical CO2. Flavour Fragr. J.17(4), 239–244 (2002). [Google Scholar]
- 47.Douissa, F. B. et al. New study of the essential oil from leaves of Pistacia lentiscus L. (Anacardiaceae) from Tunisia. Flavour Fragr. J.20(4), 410–414 (2005). [Google Scholar]
- 48.Marner, F. J., Freyer, A. & Lex, J. Triterpenoids from gum mastic, the resin of Pistacia lentiscus. Phytochemistry. 30(11), 3709–3712 (1991). [Google Scholar]
- 49.Bampouli, A. et al. Comparison of different extraction methods of Pistacia lentiscus var. Chia leaves: Yield, antioxidant activity and essential oil chemical composition. J. Appl. Res. Med. Aromatic Plants. 1(3), 81–91 (2014). [Google Scholar]
- 50.Rezaie, M. et al. Chemical composition, antioxidant and antibacterial properties of Bene (Pistacia atlantica subsp. Mutica) hull essential oil. J. Food Sci. Technol.52, 6784–6790 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fahmy, S. A. et al. Green extraction of essential oils from Pistacia lentiscus resins: Encapsulation into Niosomes showed improved preferential cytotoxic and apoptotic effects against breast and ovarian cancer cells. J. Drug Deliv. Sci. Technol.87, 104820 (2023). [Google Scholar]
- 52.Tabanca, N. et al. Chemical characterization and biological activity of the mastic gum essential oils of Pistacia lentiscus var. Chia from Turkey. Molecules. 25(9), 2136 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barra, A. et al. Characterization of the volatile constituents in the essential oil of Pistacia lentiscus L. from different origins and its antifungal and antioxidant activity. J. Agric. Food Chem.55(17), 7093–7098 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Magiatis, P. et al. Chemical composition and antimicrobial activity of the essential oils of Pistacia lentiscus var. Chia. Planta Med.65(08), 749–752 (1999). [DOI] [PubMed] [Google Scholar]
- 55.Vidrich, V. et al. Chemical composition of the essential oil of Pistacia lentiscus L. J. Essent. Oil Res.16(3), 223–226 (2004). [Google Scholar]
- 56.Boelens, M. H. & Jimenez, R. Chemical composition of the essential oils from the gum and from various parts of Pistacia lentiscus L.(mastic gum tree). Flavour Fragr. J.6(4), 271–275 (1991). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.