Abstract
Candida parapsilosis is an increasing cause of bloodstream infections (BSIs) in neonatal intensive care units (NICUs). It has been a persistent problem in the NICU of Hospital for Children and Adolescents, Helsinki University Central Hospital, Helsinki, Finland, since 1987. Fluconazole prophylaxis has been used to control the problem. The number of new infections has, however, increased markedly since September 2000. We assessed fluconazole consumption and occurrence of all Candida species in the NICU from 1991 to 2002. C. parapsilosis bloodstream isolates obtained in the NICU from 1990 to 2002 (n = 26) were genotyped and their fluconazole susceptibility was defined. A low rate of C. parapsilosis BSIs was correlated with high rates of consumption of fluconazole. No emergence of Candida species with primary resistance to fluconazole was detected. However, genotyping with a complex DNA fingerprinting probe revealed that a single strain of C. parapsilosis with decreasing susceptibility to fluconazole was responsible for cross-infections that caused BSIs in the NICU over a 12-year period. The emergence of fluconazole resistance in that strain was observed after more than 10 years of fluconazole prophylaxis.
During the last decades the incidence of candidemia in neonatal intensive care units (NICUs) has increased, and the most prevalent Candida species that cause candidemias have shifted over time from Candida albicans to Candida parapsilosis (12, 16, 23). The known risk factors for candidemia are prematurity; the use of central venous lines, intubation, parenteral nutrition, and broad-spectrum antibiotics; and prolonged hospitalization (16, 18, 20, 26, 35). In addition, in some reports colonization with Candida spp. was associated with an increased risk for candidemia, especially in very low birth weight (VLBW; <1,500 g) infants (4, 25, 26).
In VLBW infants, prevention of fungal colonization by fluconazole prophylaxis has been shown to be effective (10, 11). The efficacy of this drug in preventing infections is, however, controversial (1, 3). In addition, selection of Candida species with primary resistance to fluconazole and development of resistance to azoles are potential threats (14, 19). Two studies on fluconazole prophylaxis previously conducted in NICUs (10, 11) found no difference in the rates of fluconazole susceptibility among Candida isolates before and after a relatively short study period. Global surveillance studies also indicate that reduced susceptibility to fluconazole is extremely uncommon among bloodstream infection (BSI) isolates of C. parapsilosis (21).
The NICU of the Hospital for Children and Adolescents (HCA), Helsinki University Central Hospital, Helsinki, Finland, has had problems with nosocomial C. parapsilosis infections for years. Fluconazole prophylaxis seemed to control the problem (27), but later, the number of infected or colonized patients in the NICU started to increase again.
We describe a clonal outbreak caused by C. parapsilosis in the NICU during a 12-year period and the development of fluconazole resistance of the causative clone during the long-term use of fluconazole prophylaxis.
MATERIALS AND METHODS
Setting and surveillance.
The 18-bed NICU of HCA serves a population of 1.4 million people with 470 annual admissions, including 150 VLBW infants. The NICU consists of five rooms. Sinks are available in each room, and hand-disinfectant dispensers are available at each bed. Alcohol-based hand rub is used before and after any patient contact, and gloves are routinely used during aseptic procedures. Blood samples for culture are drawn from a peripheral vessel by venipuncture. Endotracheal aspirates are taken weekly from every patient in the NICU for surveillance culture, and other samples of other specimens are taken for culture whenever an infection is suspected. The empirical antimicrobial treatment for a suspected case of septicemia is a combination of ampicillin and netilmicin. All patients with either suspected or verified candidemia are treated with amphotericin B; since June 1999, liposomal amphotericin B has been used routinely.
An in-house system of surveillance for nosocomial infections is based on microbiology laboratory data; the infection control nurse monitors the situation weekly and visits the wards whenever nosocomial infections are suspected. The number of cultures positive for Candida spp. was obtained from laboratory reports. The numbers of admissions and patient-days in the NICU annually were obtained from the hospital administration. Fluconazole use was assessed based on the hospital pharmacy records, calculated as the number of grams delivered to the NICU annually.
Fluconazole prophylaxis policy and other control measures.
During the earlier outbreak, which occurred from 1987 to 1991 (27), fluconazole prophylaxis was first introduced with a daily intravenous dosage of 3 mg/kg of body weight for all infants with a gestational age of less than 30 weeks. This policy was continued for 9 months. The epidemic continued, and prophylaxis was thus reintroduced with a higher daily dosage (6 mg/kg) in August 1991. From December 1991 to February 1993, fluconazole was still used (6 mg/kg twice a week). After that, infants with birth weights under 1,000 g were given fluconazole prophylaxis (6 to 12 mg/kg daily). In late 2000, prophylaxis was extended to cover all infants treated in the NICU; but soon after this, the first C. parapsilosis isolates with reduced susceptibility to fluconazole were found, and the prophylaxis was ceased. In November 2002, prophylaxis was, however, reintroduced (3 mg/kg daily for all infants born before week 28 of gestation) due to the increased number of C. parapsilosis infections.
Routine control measures, most importantly, hand hygiene, were reinforced after detection of the increase in number of cases of C. parapsilosis infection in 2000. Cohorting was also used whenever possible.
Cohort study.
All patients who were treated in the NICU between September 2000 and December 2001 and who had at least one positive culture for C. parapsilosis were identified by a retrospective review of the NICU log and the microbiology laboratory reports. Demographic and clinical data for the patients were reviewed; and the length of stay in the NICU, the length of stay before the first positive culture for C. parapsilosis, the duration of mechanical ventilation and nasal continuous positive airway pressure treatment before detection of C. parapsilosis, information on exposure to indwelling devices, and information on nutrition and the use of antimicrobial agents were recorded. Data on surgical procedures performed before a positive culture for C. parapsilosis and maternal infections and antimicrobial treatments prior to delivery were also collected. The patients were categorized into three groups: (i) patients with BSIs, (ii) patients with clinical signs of superficial infections, and (iii) colonized patients.
Culture and identification of fungi.
Blood samples were incubated in pediatric BacT/Alert blood culture bottles (Organon Technica Durham, N.C.) for 6 days. Aliquots from positive bottles were Gram stained and subcultured on Sabouraud's glucose agar at 37°C. All other clinical samples were inoculated on Sabouraud's glucose agar (supplemented with penicillin and streptomycin) and incubated at 28°C and 37°C for 7 days.
Yeast isolates were subcultured on Sabouraud's glucose agar and incubated for 24 h at 35°C. All isolates were identified by standard methods, including germ tube formation, ID 32C identification panels (bioMerieux, Marcy l'Etoile, France), and morphology on Czapek-Dox Tween 80 agar and on CHROMagar plates (CHROMagar Co., Paris, France). Bloodstream isolates were preserved in 85% milk-15% glycerol tubes at −70°C for further characterization.
All stored bloodstream isolates of C. parapsilosis obtained from different patients in the NICU between November 1990 and June 2002 (n = 26) were sent to the University of Iowa, Iowa City, for genotyping and antifungal susceptibility testing. This collection contained three BSI isolates from the initial outbreak (27) obtained during 1990 and 1991 and 23 isolates obtained between 1994 and 2002.
Molecular typing.
All 26 BSI isolates were fingerprinted by Southern blot hybridization by use of the complex DNA fingerprinting probe Cp3-13 (5), according to methods described previously (28, 29). In brief, genomic DNA was extracted from cells following the protocol described by Scherer and Stevens (28). Three micrograms of the DNA preparation was digested with a combination of EcoRI and SalI. The digested DNA was electrophoresed in a 0.7% agarose gel. C. parapsilosis strain J940043 was used as a reference, and its DNA was run in the first and last lanes of the fingerprinting gels. The gels were transferred to a Hybond N+ membrane (Amersham, Piscataway, N.J.) by blotting, prehybridized with salmon sperm DNA, hybridized overnight with the 32P-labeled Cp3-13 probe, and autoradiographed.
Autoradiogram images were digitized into the DENDRON software database (31). By using this software program, migration distortions were removed with the “unwarping” option of DENDRON. The processed hybridization patterns were then automatically scanned to identify all bands and link common bands. The patterns of all test isolates were then compared in a pairwise fashion, and the similarity coefficient (SAB) between the patterns of every pair of isolates A and B was computed according to the formula 2E/(2E + a + b), where E is the number of bands common in strains A and B, a is the number of bands unique to strain A, and b is the number of bands unique to strain B. An SAB of 0.0 represents total unrelatedness (no common bands) between isolates A and B, an SAB of 1.0 represents an identical match of all bands between isolates A and B, and increasing values of SAB from 0.1 to 0.9 represent increasing levels of similarity.
Antifungal susceptibility testing.
Testing of the antifungal susceptibilities of the 26 C. parapsilosis BSI isolates to fluconazole was performed by using the CLSI (formerly the National Committee for Clinical Laboratory Standards) M27-A2 broth microdilution method (17). The range of fluconazole concentrations tested was 0.12 to 128 mg/liter, and MIC endpoints were read as the lowest concentration that resulted in prominent (∼50%) inhibition of growth relative to the growth of the drug-free control following a 48-h incubation at 35°C.
Environmental studies.
Samples of inanimate surfaces for culture were taken twice in April 2001 (48 samples from 48 sites) to identify potential environmental reservoirs, such as incubator ports and interior surfaces, water taps, sinks, ventilatory equipment, and computer keyboards. Air sampling was carried out in two rooms by use of a SAS Super 100 sampler (pbi Spa, Milan, Italy), in which 1 m3 of air was impacted onto glucose-agar plates.
Environmental swabs were cultured on glucose-peptone agar plates for 96 h at 30°C. Yeasts were identified by a standard methodology (6).
Statistical analysis.
Data were analyzed by using Epi Info software (version 6.04b; Centers for Disease Control and Prevention, Atlanta, Ga.). For categorical variables, proportions were compared by the chi-square test with Yates' correction or Fisher's exact test, as appropriate. The continuous variables were analyzed by Student's t test or the Mann-Whitney test, depending on the sample distribution. Pearson correlation coefficients were calculated to assess the relationship between the rates of C. parapsilosis BSIs and fluconazole consumption in the NICU over time.
RESULTS
Patients.
After the earlier outbreak (1987 to 1991) (27), there were constantly new cases of C. parapsilosis infections or colonizations in the NICU during the years from 1994 to 2000, with 54 patients, 9 of whom had C. parapsilosis BSIs (Fig. 1). During the following 16-month period, from September 2000 to December 2001, 56 patients (10 with BSIs) with C. parapsilosis infections or colonizations were recorded; and during the year 2002, 54 patients (6 with BSIs) with C. parapsilosis infections or colonizations were recorded.
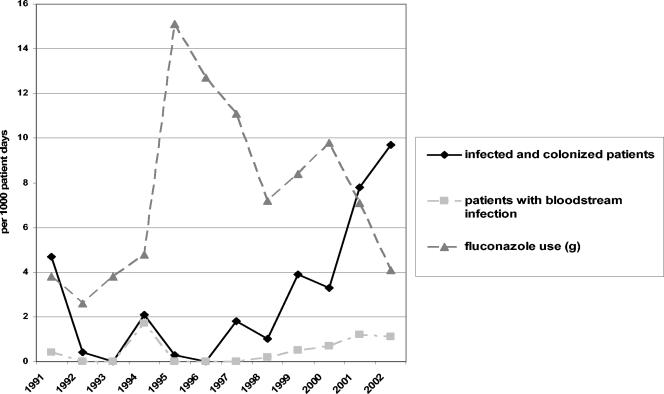
FIG. 1.
Fluconazole use and rates of patients with Candida parapsilosis infection or colonization in the neonatal intensive care unit from 1991 to 2002. Fluconazole use was calculated as the number of grams delivered to the unit annually. The number of patients with bloodstream infection is included in the number of infected and colonized patients.
Fluconazole use and positive Candida cultures.
After May 1992 and until July 1994, no C. parapsilosis infections were detected. Since then, the rate of fluconazole use in the NICU increased for the next 3 years, and during this time period the number of infections was low (Fig. 1). Later, when fluconazole use was reduced, an increased rate of C. parapsilosis infections was seen. From 1994 to 2001, a negative correlation between fluconazole consumption and the rate of C. parapsilosis BSIs was seen (r = −0.79; P = 0.009).
During the 12-year period (1991 to 2002), only a few patients were positive by culture for fungi other than C. parapsilosis or C. albicans (Table 1). C. parapsilosis was also the most prevalent Candida species in the NICU that caused BSIs. Only sporadic cases of C. albicans BSIs (zero to one per year from 1990 to 2000 and two in 2001) were seen before 2002, when six C. albicans BSIs were detected. No C. krusei or C. glabrata infections were seen.
TABLE 1.
Annual distribution of Candida species in the neonatal intensive care unit from 1991 to 2002
| Yr | No. of cultures obtained/1,000 patient days | % Cultures positive for Candida spp. | No. of patients (no. of bloodstream infections)
|
|||
|---|---|---|---|---|---|---|
| C. parapsilosis | C. albicans | C. glabrata | Other Candida spp. | |||
| 1991 | —a | — | 21 (2) | 29 (1) | 3 (0) | 0 |
| 1992 | 63 | 2.0 | 2 (0) | 0 | 1 (0) | 0 |
| 1993 | 94 | 1.2 | 0 | 2 (1) | 0 | 0 |
| 1994 | 64 | 8.6 | 6 (5) | 6 (1) | 0 | 0 |
| 1995 | 86 | 1.5 | 1 (0) | 3 (1) | 0 | 0 |
| 1996 | 85 | 1.0 | 0 | 3 (1) | 0 | 0 |
| 1997 | 114 | 6.1 | 6 (0) | 11 (0) | 0 | 0 |
| 1998 | 103 | 2.4 | 6 (1) | 6 (1) | 0 | 0 |
| 1999 | 95 | 13.1 | 24 (3) | 14 (1) | 0 | 0 |
| 2000 | 94 | 8.3 | 20 (4) | 5 (0) | 0 | 0 |
| 2001 | 251 | 19.6 | 45 (7) | 11 (2) | 1 (0) | 0 |
| 2002 | 276 | 24.5 | 54 (6) | 29 (6) | 0 | 2 (0) |
—, the data were not available.
Cohort study.
There were 624 admissions, including 155 VLBW infants, in the NICU during the 16-month study period. A total of 56 patients were infected or colonized with C. parapsilosis, representing 9% of all infants treated in the NICU. The cohort included 10 patients with BSIs, 15 patients with superficial infections (6 with conjunctivitis, 6 with wound infections, 1 with a skin infection, and 2 with urinary tract infections), and 24 patients who were only colonized. Sixty percent (6 of 10) of the patients with BSIs and 27% (4 of 15) of the patients with superficial infections were colonized with C. parapsilosis before the infection.
The analysis showed no differences between the groups by gender, type of delivery, or mortality (Table 2). Prematurity and low birth weight, as well as prolonged umbilical catheterization, were identified as risk factors for C. parapsilosis BSIs. Clear increases in the length of stay and the duration of mechanical ventilation were seen in the patients with C. parapsilosis BSIs. Half (5 of 10) of these patients received fluconazole at the time of the first positive blood culture, as did 27% (4 of 15) of the patients with other infections at the time of the positive culture. Of the colonized patients, 33% (8 of 24) received fluconazole at the time of the first positive culture. Thus, fluconazole failed to prevent both colonization and BSIs caused by C. parapsilosis in these patients.
TABLE 2.
Demographic and clinical characteristics of the patients with positive cultures for C. parapsilosis in the NICU from September 2000 to December 2001
| Variable | Patients with BSIs (cases; n = 10)a | Patients without BSIs (noncases; n = 39)a
|
P valueb | |
|---|---|---|---|---|
| Patients with superficial infections (n = 15) | Patients with colonization (n = 24) | |||
| Baseline characteristics | ||||
| Birth weight (g [range]) | 665 (480-830) | 810 (360-1,200) | 895 (440-1,410) | <0.01 |
| Gestational age (wk [range]) | 25 (24-27) | 27 (25-29) | 27 (23-32) | <0.001 |
| Male sex (no. [%] of patients) | 7 (70) | 8 (53) | 15 (63) | 0.72 |
| Antenatal antibiotics (no. [%] of patients) | 5 (50) | 5 (33) | 8 (33) | 0.46 |
| Vaginal delivery (no. [%] of patients) | 5 (50) | 5 (33) | 8 (33) | 0.46 |
| Apgar score (range) at 5 min of age | 6 (3-8) | 7 (2-10) | 7 (3-9) | 0.20 |
| Factors associated with hospitalization | ||||
| Length of stay in the NICU (days [range]) | ||||
| Total | 86 (16-111) | 58 (3-110) | 42 (10-87) | <0.01 |
| Before first CP isolationc | 13 (9-33) | 17 (4-45) | 14 (4-36) | 0.8 |
| Duration of mechanical ventilation (days [range]) | ||||
| Total | 48 (15-68) | 12 (3-29) | 14.5 (1-38) | <0.001 |
| Before first CP isolationc | 13 (9-33) | 11 (3-28) | 10.5 (1-33) | 0.09 |
| Duration of PNd (days [range]) | 13 (9-33) | 16 (3-23) | 14 (4-30) | 0.5 |
| Duration (days [range]) of vascular accessd | ||||
| Umbilical catheter | 4 (0-14) | 0 (0-5) | 0 (0-7) | <0.01 |
| Arterial catheter | 12.5 (6-17) | 11 (0-17) | 10 (3-16) | 0.10 |
| Central venous catheter | 9 (4-20) | 10 (0-17) | 6.5 (0-15) | 0.15 |
| Treatment with antibioticsd (no. [%] of patients) | ||||
| Ampicillin-netilmicin | 10 (100) | 14 (97) | 24 (100) | 1.0 |
| Vancomycin and/or cephalosporins | 10 (100) | 13 (87) | 22 (92) | 0.57 |
| Fluconazole | 5 (50) | 4 (27) | 9 (38) | 0.48 |
| Amphotericin B | 1 (10) | 0 (0) | 1 (4) | 1.0 |
| Surgical proceduresd (no. [%] of patients) | 5 (50) | 2 (13) | 8 (33) | 0.25 |
| Mortalitye (no. [%] of patients) | 1 (10) | 0 (0) | 3 (13) | 1.0 |
Data on continuous variables are presented as medians (ranges).
Cases versus noncases, i.e., patients with BSI versus patients without BSIs.
From blood cultures (cases) or other cultures (noncases). CP, Candida parapsilosis.
Before first positive blood cultures (cases) or other cultures (noncases). PN, parenteral nutrition with lipids.
Within 7 days after a positive culture for C. parapsilosis.
Molecular typing.
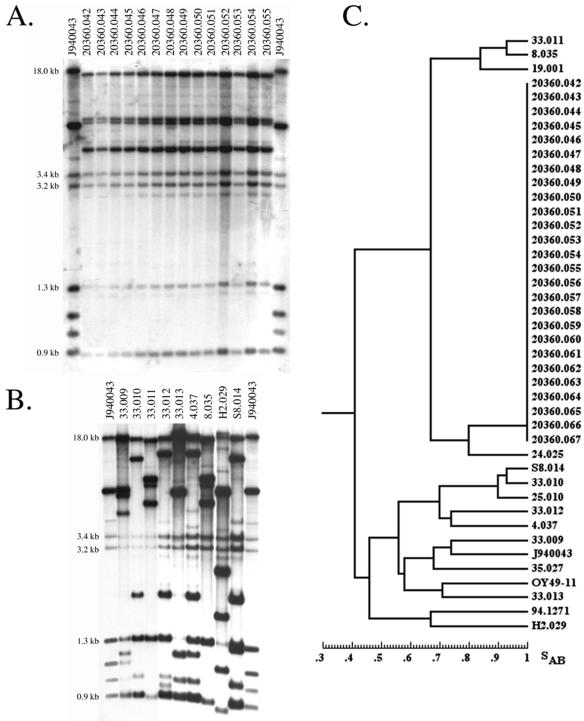
DNA fingerprinting analysis revealed that all 26 BSI isolates tested represented a single strain of C. parapsilosis (Fig. 2). The 26 hybridization patterns were identical (Fig. 2A). This strain pattern was distinct from that of reference strain J940043 and those of nine additional C. parapsilosis isolates randomly selected (Fig. 2B). When a dendrogram, based on the SAB values, that included the 26 isolates from the NICU of HCA and 16 randomly selected C. parapsilosis isolates from a worldwide collection at the University of Iowa was generated, the 26 HCA isolates clustered at an SAB of 1.00, demonstrating identical fingerprinting patterns (Fig. 2C).
FIG. 2.
DNA fingerprinting analysis. Southern blot hybridization patterns of 14 nosocomial C. parapsilosis isolates probed with the Cp3-13 probe (A) and 9 unrelated isolates (B). Samples of C. parapsilosis DNA were digested with EcoRI and SalI and electrophoresed in a 0.75% agarose gel. The molecular sizes of selected bands are indicated to the left of the gels. The reference strain, strain J940043, was analyzed in the outer two lanes to normalize the gel during computer-assisted analysis. (C) Dendrogram generated from similarity coefficients (SAB) computed for pairwise comparisons of 26 nosocomial isolates and a collection of 16 unrelated isolates, including reference strain J940043, fingerprinted with probe Cp3-13.
Antifungal susceptibility testing.
Overall, 19 of 26 of the BSI isolates were susceptible (MIC ≤ 8 mg/liter), 5 of 26 were susceptible dose dependent (MIC = 16 to 32 mg/liter), and 2 of 26 were resistant (MIC ≥ 64 mg/liter) to fluconazole (Table 3). Over time the initially susceptible isolates derived from a single strain became progressively less susceptible to fluconazole until resistant isolates became observed in the last 2 years. The proportion of isolates with the less susceptible phenotype was significantly higher among the isolates collected in 2001 and 2002 than among those collected from 1990 to 2000 (P = 0.02).
TABLE 3.
Changes in fluconazole susceptibility over time among bloodstream isolates of C. parapsilosis from the neonatal intensive care unit
| Time period | No. of isolates | MIC (mg/liter)a
|
% of isolates at MIC (mg/liter) of:
|
||||
|---|---|---|---|---|---|---|---|
| Range | 50% | 90% | ≤8 | 16-32 | ≥64 | ||
| 1990-1994 | 7 | 0.5-2 | 1 | 100 | 0 | 0 | |
| 1999-2000 | 7 | 1-16 | 2 | 86 | 14 | 0 | |
| 2001-2002 | 12 | 2-64 | 8 | 64 | 50 | 33 | 17 |
| Total | 26 | 0.5-64 | 2 | 16 | 73 | 19 | 8 |
50%, MIC at which 50% of the isolates are inhibited; 90%, MIC at which 90% of the isolates are inhibited.
Environmental samples.
Of the 48 environmental samples cultured, only 1 was positive for C. parapsilosis; this sample was obtained from the base of a tap in a room where an infant with a C. parapsilosis BSI had been nursed.
DISCUSSION
Our study shows that the long-term use of fluconazole might lead to the emergence of resistance in C. parapsilosis. Such findings in a NICU setting have not been published previously, but similar findings were recently reported in an animal model (37).
The emergence of fluconazole resistance and the increase in the proportions of intrinsically fluconazole-resistant Candida isolates are of concern when prophylaxis is used. Prophylaxis studies conducted in NICUs have shown no signs of fluconazole resistance or reduced susceptibility regarding C. parapsilosis isolates (10, 11). Kaufman et al. (10) hypothesized that this could be due to the low total fluconazole doses received. In these studies the study period has, however, been relatively short (14 to 30 months). Recent large surveillance studies have also found no evidence for an overall increase in the rates of fluconazole resistance in populations of C. parapsilosis (7, 21, 22); only one study showed a slight decrease in the percentage of fluconazole-susceptible C. parapsilosis isolates (8). The data from those studies differ from ours: they were collected from multiple centers and, in most of the studies, from several continents over time periods that varied from 2 to 10 years. However, in the study with the longest surveillance period (21), the C. parapsilosis isolates also stayed highly susceptible to fluconazole. Our findings suggest that reduced susceptibility to fluconazole may nevertheless develop over time in some strains. Given the fact that the 26 BSI isolates represented a single genotype, it is apparent that secondary resistance to fluconazole developed following continued exposure to fluconazole. Development of resistance has major clinical implications, because more toxic or more expensive drugs, such as amphotericin B, need to be used.
Among the 10 patients with C. parapsilosis BSIs included in the cohort study (2000 to 2001), 5 were receiving fluconazole at the time of the first positive blood culture, and 2 had isolates with reduced susceptibility to fluconazole. Nguyen et al. (19) reported that C. parapsilosis is the most common non-C. albicans species (in 5 of 13 of their cases) that causes candidemia in adult patients while receiving fluconazole. Two of these C. parapsilosis strains had reduced susceptibility to fluconazole. Krcmery et al. (13) studied breakthrough candidemias during fluconazole therapy in neonates and infants; the only Candida species that they identified to have caused infections were C. albicans and C. parapsilosis. No resistant strains were reported, but mean fluconazole MICs were slightly higher among isolates obtained from patients, whose fungemia developed 6 days or later after the introduction of fluconazole treatment.
A BSI-associated infectious clone of C. parapsilosis appears to have persisted for at least 12 years in the HCA NICU. Such a finding suggests either a common reservoir or horizontal transmission of the pathogen. In some NICU outbreaks a common source for candidemia was found, such as contaminated medical equipment (30) or multidose medications (36); but often, the attempts to identify such common sources have failed (9, 15, 27, 34). Our study was also unable to identify any common source or reservoir for C. parapsilosis other than the infected and colonized infants themselves, and this suggests that the pathogen was transmitted horizontally in the NICU. Horizontal transmission most likely occurs via the hands of staff and thus emphasizes the role of hand hygiene. According to Clark et al. (2), improved hand hygiene compliance was crucial in controlling a large outbreak of C. parapsilosis BSIs in adults. Horizontal transmission seems to be especially important for C. parapsilosis, in contrast to C. albicans, which is usually acquired vertically from the mother (24, 33). This might be important when implementation of fluconazole prophylaxis is considered. Our data support the hypothesis of Kaufman et al. (10) that prophylaxis might be more effective against C. albicans than against C. parapsilosis. The nosocomial acquisition of the latter may more often involve strains that are endemic to the NICU and that possess reduced susceptibility to the prophylactic drug used.
In contrast to the earlier outbreak (27), the present study found no increased rates of mortality in association with C. parapsilosis BSIs. Many studies have reported high rates of mortality among neonates with candidemia: a large multicenter study of 151 fungemic VLBW infants reported that 32% of them died due to candidemia (32). The case-fatality ratio from candidemia, according to Kossoff et al. (12) was 14%. In both studies the rate of mortality from C. albicans infection was, however, significantly higher than from C. parapsilosis infection (36% versus 7% [32] and 26% versus 4% [12]).
Our study has several limitations. First, our study focused on bloodstream isolates, and only those isolates underwent subtyping and susceptibility testing. This research frame cannot reject the possibility that other clonal lines of C. parapsilosis may have been circulating in the NICU at the same time or that subpopulations of C. parapsilosis with reduced susceptibility may have already existed earlier but were not detected until 1999 and after. Second, the environmental investigations during the study period were scarce. No systematic sampling of the hands of the staff for culture was performed. Thus, a breakdown in hand hygiene possibly had an effect on the clonal expansion process detected. Third, it is difficult to evaluate retrospectively the actual culturing activities in the NICU over such a long period. The frequency of cultures increased markedly in 2001 and 2002, which most likely led to the increased detection of colonized patients. However, this was unlikely to have an effect on the number of BSIs detected, because blood samples for culture were taken only when an infection was suspected. We know that the strain responsible for BSIs caused the large majority of BSIs in the NICU; but the possibility exists that this strain was not unique, because a few C. parapsilosis BSI strains were not available for subtyping. The fluconazole prophylaxis policy in the NICU was also modified over time. Patient-based data on fluconazole use were available only for a limited time period, i.e., for the cohort study; and the information on fluconazole use in the NICU was based on pharmacy delivery reports. The retrospective cohort study was designed only to identify risk factors for invasive C. parapsilosis infections, i.e., BSIs. Thus, the risk factors for C. parapsilosis colonization remained unknown. Furthermore, our approach was not designed to study the efficacy of fluconazole prophylaxis, and therefore, no conclusions on it can be drawn.
Our data demonstrate that during long-term fluconazole use, (i) a single strain of C. parapsilosis was maintained in the NICU over a period of more than 12 years, (ii) it was the major C. parapsilosis strain in the NICU causing BSIs, (iii) subclones of this strain displayed different fluconazole susceptibilities, and (iv) with time, a larger proportion of the subclones became less susceptible to fluconazole. Our study thus suggests that the use of fluconazole prophylaxis contributed to the emergence of subclones of C. parapsilosis with decreased susceptibility among the isolates responsible for BSIs. The role of fluconazole prophylaxis in NICUs in general still remains controversial, but the role of basic hygiene measures should never be underestimated.
Acknowledgments
We are grateful to Richard J. Hollis and the personnel in the Molecular Epidemiology and Fungus Testing Laboratory of the University of Iowa for collaboration. We also thank Tuula Salomaa and Tarja Komulainen for help with collection of the data.
This work was supported by a personal grant to E.S. from the Foundation for Pediatric Research in Finland to enable work full time as a Ph.D. student in 2002 and 2003. DNA fingerprinting was funded by NIH grants DE014219 and AI2393 to D.R.S.
REFERENCES
- 1.Casalaz, D. 2002. Fluconazole prophylaxis against fungal infection in preterm infants. N. Engl. J. Med. 346:1913-1914. (Author reply, 346:1913-1914.) [PubMed] [Google Scholar]
- 2.Clark, T. A., S. A. Slavinski, J. Morgan, T. Lott, B. A. Arthington-Skaggs, M. E. Brandt, R. M. Webb, M. Currier, R. H. Flowers, S. K. Fridkin, and R. A. Hajjeh. 2004. Epidemiologic and molecular characterization of an outbreak of Candida parapsilosis bloodstream infections in a community hospital. J. Clin. Microbiol. 42:4468-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dani, C., G. Bertini, and M. Pezzati. 2002. Fluconazole prophylaxis against fungal infection in preterm infants. N. Engl. J. Med. 346:1913-1914. (Author reply, 346:1913-1914.) [DOI] [PubMed] [Google Scholar]
- 4.El-Masry, F. A., T. J. Neal, and N. V. Subhedar. 2002. Risk factors for invasive fungal infection in neonates. Acta Paediatr. 91:198-202. [DOI] [PubMed] [Google Scholar]
- 5.Enger, L., S. Joly, C. Pujol, P. Simonson, M. Pfaller, and D. R. Soll. 2001. Cloning and characterization of a complex DNA fingerprinting probe for Candida parapsilosis. J. Clin. Microbiol. 39:658-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans, E. G. V., and M. D. Richardson. 1989. Medical mycology: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 7.Hajjeh, R. A., A. N. Sofair, L. H. Harrison, G. M. Lyon, B. A. Arthington-Skaggs, S. A. Mirza, M. Phelan, J. Morgan, W. Lee-Yang, M. A. Ciblak, L. E. Benjamin, L. T. Sanza, S. Huie, S. F. Yeo, M. E. Brandt, and D. W. Warnock. 2004. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 42:1519-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazen, K. C., E. J. Baron, A. L. Colombo, C. Girmenia, A. Sanchez-Sousa, A. del Palacio, C. de Bedout, and D. L. Gibbs. 2003. Comparison of the susceptibilities of Candida spp. to fluconazole and voriconazole in a 4-year global evaluation using disk diffusion. J. Clin. Microbiol. 41:5623-5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang, Y. C., T. Y. Lin, H. S. Leu, H. L. Peng, J. H. Wu, and H. Y. Chang. 1999. Outbreak of Candida parapsilosis fungemia in neonatal intensive care units: clinical implications and genotyping analysis. Infection 27:97-102. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman, D., R. Boyle, K. C. Hazen, J. T. Patrie, M. Robinson, and L. G. Donowitz. 2001. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N. Engl. J. Med. 345:1660-1666. [DOI] [PubMed] [Google Scholar]
- 11.Kicklighter, S. D., S. C. Springer, T. Cox, T. C. Hulsey, and R. B. Turner. 2001. Fluconazole for prophylaxis against candidal rectal colonization in the very low birth weight infant. Pediatrics 107:293-298. [DOI] [PubMed] [Google Scholar]
- 12.Kossoff, E. H., E. S. Buescher, and M. G. Karlowicz. 1998. Candidemia in a neonatal intensive care unit: trends during fifteen years and clinical features of 111 cases. Pediatr. Infect. Dis. J. 17:504-508. [DOI] [PubMed] [Google Scholar]
- 13.Krcmery, V., M. Huttova, F. Mateicka, L. Laho, L. Jurga, A. Ondrusova, Z. Tarekova, K. Kralinsky, J. Hanzen, A. Liskova, M. Mrazova, A. Sabo, M. Pisarcikova, G. Kovacicova, D. Chovancova, and Z. Szovenyiova. 2001. Breakthrough fungaemia in neonates and infants caused by Candida albicans and Candida parapsilosis susceptible to fluconazole in vitro. J. Antimicrob. Chemother. 48:521-525. [DOI] [PubMed] [Google Scholar]
- 14.Lewis, R. E., and M. E. Klepser. 1999. The changing face of nosocomial candidemia: epidemiology, resistance, and drug therapy. Am. J. Health Syst. Pharm. 56:525-533. [PubMed] [Google Scholar]
- 15.Lupetti, A., A. Tavanti, P. Davini, E. Ghelardi, V. Corsini, I. Merusi, A. Boldrini, M. Campa, and S. Senesi. 2002. Horizontal transmission of Candida parapsilosis candidemia in a neonatal intensive care unit. J. Clin. Microbiol. 40:2363-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacDonald, L., C. Baker, and C. Chenoweth. 1998. Risk factors for candidemia in a children's hospital. Clin. Infect. Dis. 26:642-645. [DOI] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution testing of yeasts. Approved standard, 2nd ed. M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Ng, P. C. 1994. Systemic fungal infections in neonates. Arch. Dis. Child. 71:F130-F135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen, M. H., J. E. Peacock, Jr., A. J. Morris, D. C. Tanner, M. L. Nguyen, D. R. Snydman, M. M. Wagener, M. G. Rinaldi, and V. L. Yu. 1996. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am. J. Med. 100:617-623. [DOI] [PubMed] [Google Scholar]
- 20.Pfaller, M. A. 1996. Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin. Infect. Dis. 22:S89-S94. [DOI] [PubMed] [Google Scholar]
- 21.Pfaller, M. A., and D. J. Diekema. 2004. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin. Microbiol. Infect. 10:11-23. [DOI] [PubMed] [Google Scholar]
- 22.Pfaller, M. A., D. J. Diekema, S. A. Messer, L. Boyken, and R. J. Hollis. 2003. Activities of fluconazole and voriconazole against 1,586 recent clinical isolates of Candida species determined by broth microdilution, disk diffusion, and Etest methods: report from the ARTEMIS Global Antifungal Susceptibility Program, 2001. J. Clin. Microbiol. 41:1440-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rangel-Frausto, M. S., T. Wiblin, H. M. Blumberg, L. Saiman, J. Patterson, M. Rinaldi, M. Pfaller, J. E. Edwards, Jr., W. Jarvis, J. Dawson, and R. P. Wenzel. 1999. National epidemiology of mycoses survey (NEMIS): variations in rates of bloodstream infections due to Candida species in seven surgical intensive care units and six neonatal intensive care units. Clin. Infect. Dis. 29:253-258. [DOI] [PubMed] [Google Scholar]
- 24.Reef, S. E., B. A. Lasker, D. S. Butcher, M. M. McNeil, R. Pruitt, H. Keyserling, and W. R. Jarvis. 1998. Nonperinatal nosocomial transmission of Candida albicans in a neonatal intensive care unit: prospective study. J. Clin. Microbiol. 36:1255-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowen, J. L., M. A. Rench, C. A. Kozinetz, J. M. Adams, Jr., and C. J. Baker. 1994. Endotracheal colonization with Candida enhances risk of systemic candidiasis in very low birth weight neonates. J. Pediatr. 124:789-794. [DOI] [PubMed] [Google Scholar]
- 26.Saiman, L., E. Ludington, M. Pfaller, S. Rangel-Frausto, R. T. Wiblin, J. Dawson, H. M. Blumberg, J. E. Patterson, M. Rinaldi, J. E. Edwards, R. P. Wenzel, W. Jarvis, et al. 2000. Risk factors for candidemia in neonatal intensive care unit patients. Pediatr. Infect. Dis. J. 19:319-324. [DOI] [PubMed] [Google Scholar]
- 27.Saxen, H., M. Virtanen, P. Carlson, K. Hoppu, M. Pohjavuori, M. Vaara, J. Vuopio-Varkila, and H. Peltola. 1995. Neonatal Candida parapsilosis outbreak with a high case fatality rate. Pediatr. Infect. Dis. J. 14:776-781. [DOI] [PubMed] [Google Scholar]
- 28.Scherer, S., and D. A. Stevens. 1987. Application of DNA typing methods to epidemiology and taxonomy of Candida species. J. Clin. Microbiol. 25:675-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmid, J., E. Voss, and D. R. Soll. 1990. Computer-assisted methods for assessing strain relatedness in Candida albicans by fingerprinting with the moderately repetitive sequence Ca3. J. Clin. Microbiol. 28:1236-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherertz, R. J., K. S. Gledhill, K. D. Hampton, M. A. Pfaller, L. B. Givner, J. S. Abramson, and R. G. Dillard. 1992. Outbreak of Candida bloodstream infections associated with retrograde medication administration in a neonatal intensive care unit. J. Pediatr. 120:455-461. [DOI] [PubMed] [Google Scholar]
- 31.Soll, D. R. 2000. The ins and outs of DNA fingerprinting the infectious fungi. Clin. Microbiol. Rev. 13:332-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stoll, B. J., N. Hansen, A. A. Fanaroff, L. L. Wright, W. A. Carlo, R. A. Ehrenkranz, J. A. Lemons, E. F. Donovan, A. R. Stark, J. E. Tyson, W. Oh, C. R. Bauer, S. B. Korones, S. Shankaran, A. R. Laptook, D. K. Stevenson, L. A. Papile, and W. K. Poole. 2002. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 110:285-291. [DOI] [PubMed] [Google Scholar]
- 33.Waggoner-Fountain, L. A., M. W. Walker, R. J. Hollis, M. A. Pfaller, J. E. Ferguson II, R. P. Wenzel, and L. G. Donowitz. 1996. Vertical and horizontal transmission of unique Candida species to premature newborns. Clin. Infect. Dis. 22:803-808. [DOI] [PubMed] [Google Scholar]
- 34.Vazquez, J. A., D. Boikov, S. G. Boikov, and A. S. Dajani. 1997. Use of electrophoretic karyotyping in the evaluation of Candida infections in a neonatal intensive-care unit. Infect. Control Hosp. Epidemiol. 18:32-37. [DOI] [PubMed] [Google Scholar]
- 35.Weese-Mayer, D. E., D. W. Fondriest, R. T. Brouillette, and S. T. Shulman. 1987. Risk factors associated with candidemia in the neonatal intensive care unit: a case-control study. Pediatr. Infect. Dis. J. 6:190-196. [DOI] [PubMed] [Google Scholar]
- 36.Welbel, S. F., M. M. McNeil, R. J. Kuykendall, T. J. Lott, A. Pramanik, R. Silberman, A. D. Oberle, L. A. Bland, S. Aguero, M. Arduino, S. Crow, and W. R. Jarvis. 1996. Candida parapsilosis bloodstream infections in neonatal intensive care unit patients: epidemiologic and laboratory confirmation of a common source outbreak. Pediatr. Infect. Dis. J. 15:998-1002. [DOI] [PubMed] [Google Scholar]
- 37.Yoder, B. A., D. A. Sutton, V. Winter, and J. J. Coalson. 2004. Resistant Candida parapsilosis associated with long term fluconazole prophylaxis in an animal model. Pediatr. Infect. Dis. J. 23:687-688. [DOI] [PubMed] [Google Scholar]