Abstract
Chlamydophila abortus is one of the major causes of infectious abortion in pregnant sheep (enzootic abortion of ewes or EAE) worldwide. Organisms shed in infected placentas and uterine discharges at lambing time are the main sources of environmental contamination, responsible for transmission to susceptible animals and possible human contacts. In the present study, a recently developed test, based on a recombinant fragment of the polymorphic outer membrane protein POMP90 (rOMP90-4 indirect enzyme-linked immunosorbent assay [iELISA]) and one based on the variable segment 2 (VS2) region of the major outer membrane protein (MOMP) (MOMP VS2 iELISA) were compared using sera from C. abortus-infected ewes at different stages throughout pregnancy. The rOMP90 iELISA detected antibody much earlier in pregnancy than the MOMP iELISA, which, like the complement fixation test, detected antibody only at the time of abortion or lambing. No anti-MOMP antibody response could be detected in three of seven experimentally infected ewes. Furthermore, the rOMP90 iELISA detected antibody in an animal that seroconverted during the course of the study, which the MOMP iELISA failed to detect. Overall, the results show that the rOMP90-4 iELISA is considerably more sensitive than the MOMP VS2 iELISA for identifying animals infected with C. abortus. Earlier detection of infection will allow appropriate control measures to be taken to reduce environmental contamination, thus limiting the spread of infection, financial losses, and the possible risks of zoonotic transmission to humans.
Chlamydiaceae species cause a wide variety of acute and chronic diseases in animals and humans, including reproductive disorders (e.g., Chlamydophila abortus, Chlamydophila pecorum, Chlamydia trachomatis, and Chlamydia suis), respiratory disease (e.g., Chlamydophila pneumoniae, Chlamydophila psittaci, and C. suis), conjunctivitis (e.g., C. trachomatis, Chlamydophila felis, and C. pecorum), and arthritis and enteric infections (C. pecorum) (21). The animal pathogens C. abortus and C. psittaci are also known to cause zoonotic infections in humans and so are of considerable public health significance (21).
C. abortus is the most common cause of infectious abortion in sheep (enzootic abortion of ewes [EAE]) and goats in the United Kingdom, resulting in major economic losses (1). The disease is characterized by acute placentitis, and abortion usually occurs 2 to 3 weeks before the end of gestation, resulting in the expulsion of dead or weak lambs. Excreted organisms shed in infected placentae and uterine discharges contaminate the environment, and susceptible sheep most probably contract the disease through ingestion or inhalation of C. abortus-infected material at this time (5, 38). In nonpregnant animals the organism can exist in a latent or silent form, possibly in lymphoid tissue (12, 18), where it remains until at least the onset of pregnancy. The infection exists at a subclinical level throughout pregnancy, although pathological changes start to develop in the placenta after day 90 of gestation (9). Infection cannot be detected either serologically or by direct detection of the pathogen (e.g., modified Ziehl-Neelsen staining [36], PCR, etc.) until the time of abortion, when infectious organisms are excreted and maternal titers of antibody to C. abortus rapidly increase, which coincides with the development of protective immunity. Although ewes develop immunity and do not experience further C. abortus-induced abortions, they may continue to excrete infectious organisms during estrus or subsequent lambings (29), thereby continuing to contaminate the environment and spread infection.
The most widely accepted serological test for diagnosing EAE is the complement fixation test (CFT) (35). In the United Kingdom and elsewhere, the CFT is used by national veterinary laboratories to screen and accredit sheep and goats as being EAE free. However, the CFT can be insensitive and lacks specificity (20, 26). In particular, antigenic cross-reactivity with C. pecorum, as well as with other gram-negative bacteria, such as Acinetobacter spp., complicates the interpretation of results. Other serological tests, based on chlamydial antigen preparations (2, 10, 11, 26) and purified chlamydial lipopolysaccharide (15, 36) have been reported but lack specificity because they are based on cross-reactive antigens. Other more sensitive and specific tests based on a C. abortus major outer membrane protein (MOMP)-specific monoclonal antibody (31), and recombinant protein fragments of the MOMP (variable segment 2 [VS2] MOMP indirect enzyme-linked immunosorbent assay [iELISA]) (16) and polymorphic outer membrane proteins (ELISAr-Chlamydia and rOMP91B iELISA) (7, 24) have been developed. More recently, we have reported the development of an iELISA based on a recombinant protein fragment of POMP90 (rOMP90-4 iELISA) (22). The rOMP90-4 test was found to be more sensitive and specific than the CFT or rOMP91B iELISA for differentiating between animals infected with C. abortus and C. pecorum.
The aim of this study was to compare the sensitivities of the rOMP90-4 and MOMP VS2 iELISAs for detecting chlamydial antibodies in C. abortus-infected pregnant ewes at different stages throughout pregnancy. This was achieved by conducting a retrospective analysis of serum samples derived from a pregnant-ewe challenge experiment to characterize the inflammatory immune response to C. abortus infection in the placenta (8). The potential of each antigen for use as a diagnostic reagent for detecting EAE is discussed.
MATERIALS AND METHODS
Experimental infection of pregnant ewes.
C. abortus isolate S26/3 (28) was grown in McCoy cells in complete RPMI medium (Life Technologies Ltd., Paisley, United Kingdom) and stored in Chlamydia transport medium (SPG [0.25 M sucrose, 10 mM sodium phosphate, 5 mM glutamic acid, pH 7.2]) (33) at −70°C. The inoculum was diluted in phosphate-buffered saline prior to inoculation of pregnant ewes as described previously (8).
Fourteen 5- to 6-year-old Scottish Blackface ewes were group mated with Suffolk rams. All ewes were seronegative to C. abortus by the CFT and by immunoblot analysis against whole chlamydial elementary bodies (EBs). Gestational age was estimated to the nearest week. At 10 weeks of gestation (wg), seven pregnant ewes were each inoculated by subcutaneous injection over the left prefemoral lymph node with 2 × 106 inclusion forming units of C. abortus (group A). The remaining seven ewes served as uninfected controls and were each inoculated with a similar volume of uninfected McCoy cell lysate in RPMI medium (group B). Both the challenged and control animals were each divided into two groups and housed in separate pens. Ewes were allowed to continue through to abortion or lambing. Placentas were collected and macroscopically assessed for EAE lesions, and representative cotyledons were removed for bacteriological and pathological analysis. Sheep were bled before, during, and after pregnancy for serological analysis. The care and use of experimental animals were approved by the Institute's Experiments and Ethical Review Committee and complied with both Home Office Regulations and all local animal welfare policies.
Bacteriological analysis.
Following abortion or lambing, smears of placental membranes were prepared, stained by the modified Ziehl-Neelsen method, and examined under high-power microscopy for the presence of EBs (34). To attempt growth of chlamydial organisms in cell culture, placental cotyledons were aseptically ground in SPG and dilutions (1/60) prepared in complete RPMI medium containing 1 μg/ml cycloheximide (Sigma-Aldrich Company Ltd., Poole, United Kingdom). Diluted material was inoculated onto confluent McCoy cell monolayers grown in complete RPMI medium on coverslips in trac bottles (Bibby Sterilin Ltd., Stone, United Kingdom). The bottles were centrifuged at 3,000 × g at room temperature for 2 × 15 min and incubated at 37°C in 5% CO2. After 72 h, coverslips were fixed in methanol, stained by Giemsa Gurr (Merck Ltd., Poole, United Kingdom), and examined for the presence of chlamydial inclusions by light microscopy.
Antigen production.
The rOMP90-4 antigen fragment was cloned into expression vector pGEX-4T-1, expressed as a soluble glutathione S-transferase (GST) fusion protein, and purified as described previously (22). Variable segment 1 (VS1) to VS4 of the MOMP-encoding gene were cloned into expression vector pGEX-2T (16), expressed as GST fusion proteins, and purified as described for rOMP90-4. Primers were engineered with BamHI/SmaI and EcoRI restriction enzyme sites (underlined; see below) to allow cloning in frame into the expression vector. The primer pairs used to generate MOMP VS1 were 5′-GATGGATCCAAAACTATCACCGGCATGGGT-3′ and 5′-GCAGAATTCAACCATTCGGCGTCTTGTAAG-3′; those used to generate MOMP VS2 were 5′-AGCCCCGGGTGCGGCATTCAACCTCGTTG-3′ and 5′-ACAGAATTCGAGAATGTTGTATCTGTATAAAATTC-3′; those used to generate MOMP VS3 were 5′-GCGGGATCCGGAGCAGAGTTCCAATACGC-3′ and 5′-TGCGAATTCTGGTATTTAATTGTAGCCGACT-3′; and those used to generate MOMP VS4 were 5′-GTAGGATCCTCACGAGCAACTTTTGATGC-3′ and 5′-TTCGAATTCTTGATCTGAATCGAAGCAAT-3′. GST alone was also expressed from the empty vector (i.e., no insert) for use as a GST background control in the iELISAs. Protein concentrations were determined by densitometry of sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis gels with bovine serum albumin standards using a GS-670 imaging densitometer (Bio-Rad Laboratories Ltd., Hemel Hempstead, United Kingdom).
Immunoblotting.
C. abortus S26/3 EBs, purified from infected McCoy cells as described previously (28), and recombinant MOMP and POMP antigens were subjected to SDS-polyacrylamide gel electrophoresis after boiling in sample buffer containing 2% SDS and 5% 2-mercaptoethanol. Protein was transferred to nitrocellulose by semidry blotting and blocked with 5% nonfat dried milk (NFDM)-Tris-buffered saline, pH 7.6, overnight at 4°C. Blots were incubated with sheep sera diluted 1/100 in Tris-buffered saline-0.1% Tween 20 (TBST) and with monoclonal antibodies (MAb) to MOMP (MAb 4/11) (23, 27) and POMP (MAb 181) (23, 37), both diluted 1/1,000 in TBST, at room temperature for 1 h. Bound antibody was detected using horse radish peroxidase-conjugated donkey anti-sheep immunoglobulin g (IgG) or goat anti-mouse IgG (Sigma-Aldrich Company Ltd., Poole, United Kingdom) diluted 1/1,000 in TBST with 3,3′-diaminobenzidine tetrahydrochloride (Sigma ‘Fast’ DAB tablets) as the substrate.
iELISAs.
Both the MOMP VS2 and rOMP90-4 iELISAs were performed as described previously (22). Briefly, 96-well microtiter plates (Immulon; Dynex Technologies, Ashford, United Kingdom) were coated overnight at 4°C with antigen at a concentration of 0.1 μg of protein/well in 0.1 M sodium carbonate-bicarbonate buffer (pH 9.6). Odd-numbered rows were coated with GST, and even-numbered rows were coated with GST-rOMP90-4 or GST-MOMP VS2. Following three washes with phosphate-buffered saline (pH 7.4)-0.05% Tween 20 (PBST), the plates were blocked with 10% NFDM in bicarbonate buffer for 60 min at 37°C. The plates were washed as before, and then sheep sera (diluted 1:100 in 5% NFDM-PBST) were added to the appropriate wells and the plates incubated for 60 min at 37°C. After further washing, horseradish peroxidase-conjugated donkey anti-sheep IgG (diluted 1:1,000 in 5% NFDM-PBST) or horseradish peroxidase-conjugated rabbit anti-sheep IgM (μ) (Kirkegaard and Perry Laboratories) was added and incubated for 60 min at 37°C. Bound antibody was detected with an o-phenylenediamine peroxidase substrate set (Sigma Fast o-phenylenediamine dihydrochloride tablet set) according to the manufacturer's instructions (Sigma-Aldrich Company Ltd., Poole, United Kingdom). The reaction was stopped after 10 min by the addition of 3 M sulfuric acid, and the absorbance at 492 nm measured on a Labsystems iEMS MF microplate reader (Thermo Life Sciences, Basingstoke, United Kingdom). Net optical density values were obtained by subtracting the absorbance values of the GST wells from the GST-rOMP90-4 or GST-VS2 wells. The cutoff value for both iELISAs was previously determined to be 0.2.
CFT.
The CFT was performed essentially as previously described (35), with some modifications (2). Samples were tested at twofold dilutions from 1/32 to 1/512. CFT titers were expressed as the highest serum dilution giving 50% or less hemolysis graded as 2+ and 0% as 4+. A titer of 4+ at 1/32 or greater was assumed to be positive.
Statistical analysis.
Data were analyzed using linear mixed models (6), typically incorporating pen and animal as random effects and the animal-by-week interaction as a random effect, where the week component in the within-animal stratum was modeled using a power model of order 1, based on the time between observations. Thus, the data were modeled in a way that takes account of the lack of independence of successive observations from the same animal while also allowing for the highly unbalanced nature of the data set. Various explanatory factors were also fitted as fixed effects, such as factors to indicate whether the animal had been mated, whether the animal had given birth to live young, and whether the animal had aborted. Week was also included in the model as a fixed effect, to model any temporal structure in the data which was not explained by the other explanatory factors. The data were log transformed before analysis to ensure that the distributional properties were those required for this statistical analysis. Significance of effects was assessed using Wald tests (6). The small sample size at the animal stratum and the large autocorrelation observed in the within-animal stratum necessitated the use of a scaled Wald statistic assessed against an F distribution, rather than the simpler chi-square test. In each case, the denominator degrees of freedom for the test was estimated conservatively to avoid inflation of the type 1 error rate. In many cases, this will have produced an inflated type 2 error; a less stringent testing criterion would have consistently produced smaller test P values. The data were analyzed using the Genstat 7th edition (30).
RESULTS
Clinical outcome and bacteriological results.
Experimental infection of the seven pregnant ewes in group A with C. abortus resulted in the birth of six stillborn lambs from three of the ewes at 3 weeks prior to full term (Table 1). The placentas recovered from these ewes had macroscopically visible lesions typical of those seen in chlamydial abortion (21), with 50 to 100% of the area of the placenta affected. Chlamydial bodies were identified in placental smears and following recovery in cell culture. The remaining four ewes gave birth to live lambs around 1 week preterm. In the six placentas recovered from these animals, most of the placental membranes and cotyledons appeared normal, although two had visible lesions covering 5 to 20% of the intercotyledonary membrane area. The presence of chlamydial bodies was confirmed in all tissue samples taken, other than those from ewe A7, by modified Ziehl-Neelsen staining of placental smears or by Giemsa staining of infectious organisms recovered in cell culture.
TABLE 1.
Clinical and bacteriological results for infected and control ewes
| Sheep no.a | Treatment group | Length of gestation (wk) | No. of lambs born | No. of fetuses aborted | Proportion of placenta affected (%)
|
Smear or cell culture result
|
||
|---|---|---|---|---|---|---|---|---|
| Placenta 1 | Placenta 2 | Placenta 1 | Placenta 2 | |||||
| A1 | Infected | 18 | 2 | 100 | 100 | + | + | |
| A2 | Infected | 18 | 2 | 100 | 100 | + | + | |
| A3 | Infected | 18 | 2 | 50 | 90 | + | + | |
| A4 | Infected | 19 | 1 | 20 | NAb | + | NA | |
| A5 | Infected | 20 | 1 | 5 | NA | + | NA | |
| A6 | Infected | 20 | 2 | 0 | 0 | + | + | |
| A7 | Infected | 20 | 2 | 0 | 0 | − | − | |
| B1 | Control | 18 | 2 | 100 | NFc | + | NA | |
| B2 | Control | 20 | 1 | 0 | NA | − | NA | |
| B3 | Control | 20 | 2 | 0 | 0 | − | − | |
| B4 | Control | 21 | 2 | 0 | 0 | − | − | |
| B5 | Control | 21 | 2 | 0 | 0 | − | − | |
| B6 | Control | 21 | 2 | 0 | 0 | − | − | |
| B7 | Control | 22 | 1 | 0 | NA | − | NA | |
The two groups of experimentally infected (group A) and control (group B) ewes were housed into the following subgroups: A1 to A4, A5 to A7, B1 to B4, and B5 to B7.
NA, not applicable.
NF, not found.
One of the control ewes in group B (B1), which was housed with three other animals (Table 1), aborted two fetuses at 18 wg. Macroscopic lesions typical of C. abortus infection were observed in the one placenta that was recovered, and cell culture confirmed the presence of chlamydial organisms. The other control ewes produced live healthy lambs at full term, and all of the placentas recovered appeared normal, with no chlamydial organisms detectable in tissue smears or following recovery in cell culture.
Serological analysis of the infected ewes by iELISA.
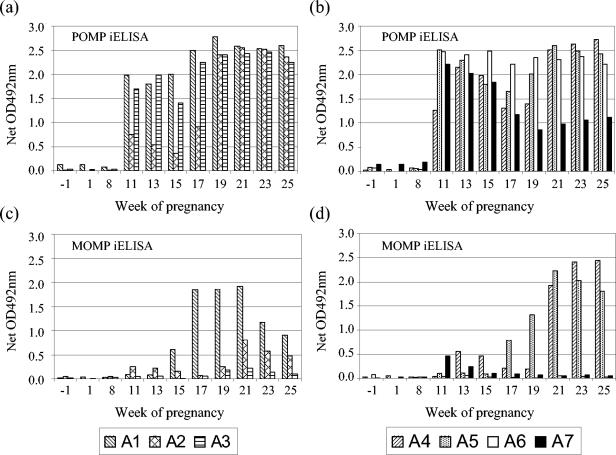
The results of the serological analysis of the serum samples collected from each of the infected ewes prior to, throughout, and after pregnancy with both the recombinant MOMP VS2 and POMP90-4 iELISAs are shown in Fig. 1. There was a statistically significant increase in the mean antibody response (total IgG) to POMP90-4, compared to premating baseline levels, as early as 1 week after experimental infection (P < 0.001). Comparing anti-POMP responses postinfection but prelambing or preabortion, weak statistical evidence suggested that the mean response in the animals that aborted was lower than that observed in the animals that lambed normally (P = 0.06). In contrast to there being no significant difference between the mean pre- and postlambing anti-POMP responses (P = 0.35), there was a highly significant increase in mean postabortion responses compared to preabortion levels (P = 0.005), consistent with a reaction to abortion. Following either birth or abortion, there was no evidence of any significant difference between the mean responses in the two groups (P = 0.94).
FIG. 1.
Comparison of POMP and MOMP iELISA results for the experimentally infected ewes. Sera obtained from C. abortus-infected ewes (infected at 10 wg) throughout pregnancy were tested by POMP90 (a and b) and MOMP VS2 (c and d) iELISAs. Ewes A1, A2, and A3 aborted at 18 wg. Ewe A4 lambed at 19 wg, while A5-A7 lambed at 20 wg. For experimental details, see Materials and Methods. OD, optical density.
In contrast to the anti-POMP responses, there was no immediate statistically significant change in the mean anti-MOMP responses detectable in infected animals. Rather, there was evidence of significant differences in the mean response in different weeks of the experiment (P = 0.01), where the estimated means consistently increased from week 11 to week 21. This trend manifested itself as a statistically significant (P = 0.02) increase in the mean response relative to the mean week −1 baseline from week 17 onward, 7 weeks after experimental infection, where the mean response was above the cutoff threshold for MOMP. The prebirth or preabortion mean responses in the animals that gave birth were marginally higher than those in the animals that aborted, but the difference lacked statistical significance (P = 0.50). There was weak statistical evidence that the ewes that gave birth exhibited higher mean MOMP responses after birth than before (P = 0.06), but the observed increase in the mean anti-VS2 MOMP response in observations post- versus preabortion lacked statistical significance (P = 0.21). Responses in animals postabortion were lower than those in animals which gave birth, but again this difference was not formally statistically significant (P = 0.39). The lack of significance of the results obtained with the MOMP VS2 iELISA compared to the POMP90-4 iELISA was a consequence of the fact that two of the ewes that aborted (A2 and A3) and two of the ewes that gave birth (A6 and A7) had very little or no anti-VS2 MOMP response (Fig. 1c and d).
Serological analysis of control ewes by iELISA.
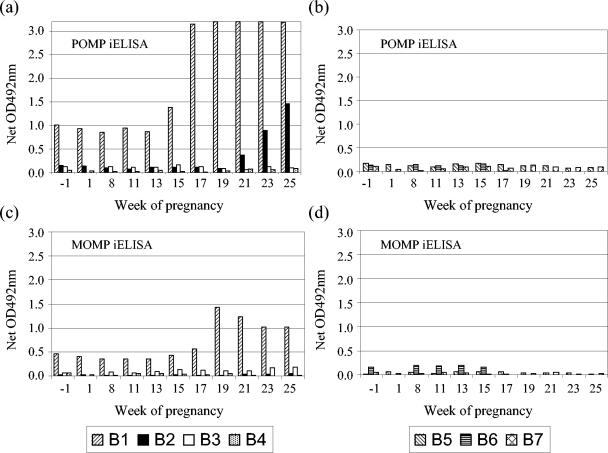
The pen containing the ewe that aborted (B1) showed a marked antibody response to the POMP antigen prior to abortion, around which time the antibody levels rose considerably, but also prior to mating (Fig. 2a). This was in contrast to the anti-VS2 MOMP response (Fig. 2c), which was low prior to abortion and which did not increase to the same extent as the anti-POMP response following abortion. When observations from all animals were used to parameterize statistical models for baseline (week −1) anti-VS2 MOMP and anti-POMP responses, the observations from animal B1 provided extreme outliers in both models. Hence, observations from animal B1 were anomalous from the initial stages of the experiment, justifying its removal from all subsequent statistical analyses. An increasing anti-POMP antibody titer was detected in another ewe (B2) that was housed in the same pen, following its lambing at 20 wg (Fig. 2a). No anti-VS2 MOMP response was detected in this animal (Fig. 2c). Data from this animal were incorporated into subsequent analyses, since no consistent pattern of lack of fit was observed. All other control ewes were negative by both ELISAs (Fig. 2b and d), with no statistically significant differences from the baseline occurring in any week for either test.
FIG. 2.
Comparison of POMP and MOMP iELISA results for the control ewes. Sera obtained from the uninfected control ewes throughout pregnancy were tested by POMP90 (a and b) and MOMP VS2 (c and d) iELISAs. Ewes B1 to B4 were housed separately from ewes B5 to B7. Ewe B1 aborted at 18 wg, whereas ewe B2 lambed at 20 wg. All other control ewes lambed at 20 to 22 wg (see Table 1). For experimental details, see Materials and Methods. OD, optical density.
Detection of IgM antibodies by POMP iELISA.
Serum samples from the challenged ewes, and seroresponsive control ewe B1, were also tested for the presence of IgM antibodies using the POMP iELISA (results not shown). POMP-specific antibodies could be detected in ewes A3, A5, A6, and A7 1 week following infection, although this response was considerably reduced compared to the IgG response and was short-lived, returning to prechallenge levels by 2 to 3 weeks postchallenge. No IgM response could be detected in the other ewes.
EB immunoblot analysis of serum samples.
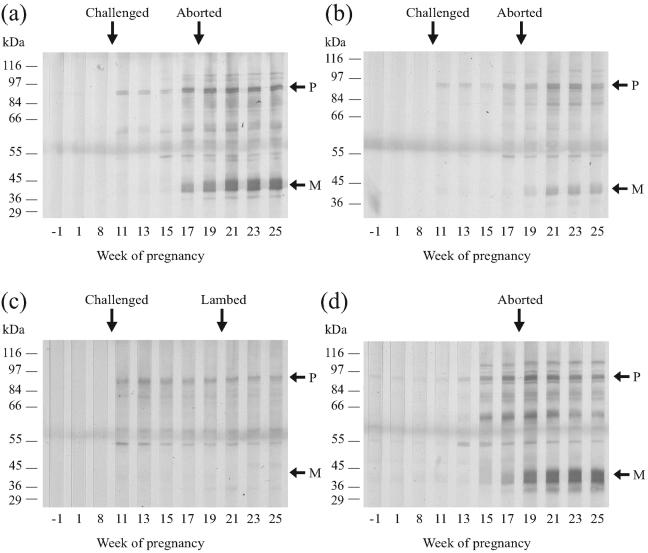
Immunoblotting with serum samples from the infected ewes against purified EB lysates demonstrated the presence of anti-POMP antibodies from 1 week post experimental infection with C. abortus (Fig. 3a to c; Table 2), showing consistency with the POMP90-4 iELISA results. Similarly, the anti-MOMP reactivity observed on immunoblots generally was consistent with the anti-VS2 MOMP iELISA results (Table 2), where anti-MOMP antibodies could only be detected from the time of abortion or lambing. Two (A6 and A7) of the three ewes that were negative by MOMP VS2 iELISA were also negative by immunoblot analysis (Fig. 3c; Table 2), while the third (A3) was positive by immunoblotting following abortion (Fig. 3b). Serum from ewe A2, which had a poor anti-VS2 MOMP response, only exhibited weak reactivity upon immunoblotting (not shown).
FIG. 3.
Immunoblot analysis of serum samples from C. abortus-infected ewes. Serum samples from experimentally infected ewes A1 (a), A3 (b), and A6 (c) and the seroresponsive control ewe B1 (d) obtained throughout pregnancy were immunoblotted against whole EBs run on a 12% SDS-polyacrylamide gel. Ewes were challenged at 10 wg (a to c) and either aborted at 18 wg (a, b, and d) or lambed at 20 wg (c). For experimental details, see Materials and Methods. Molecular masses are indicated on the left. P and M indicate the 90-kDa POMP and 40-kDa MOMP antigens, respectively (25, 27). This figure was prepared from digital photographic images using CorelDRAW version 9.
TABLE 2.
Summary of EB and recombinant MOMP immunoblot results
| Sheep no. | Treatment group | EB reactivity to:
|
rMOMP reactivity to:
|
||||
|---|---|---|---|---|---|---|---|
| MOMP | POMP | VS1 | VS2 | VS3 | VS4 | ||
| A1 | Infected | + | + | + | + | − | − |
| A2 | Infected | Wa | + | − | − | − | W |
| A3 | Infected | + | + | + | − | − | + |
| A4 | Infected | + | + | + | + | − | W |
| A5 | Infected | + | + | + | + | − | − |
| A6 | Infected | − | + | − | − | − | − |
| A7 | Infected | − | + | − | − | − | − |
| B1 | Control | + | + | + | W | − | − |
| B2 | Control | − | + | − | − | − | − |
W, weak reactivity.
Immunoblotting of EBs with serum samples from the control ewe that aborted (B1) showed weak anti-POMP reactivity up to 13 weeks into pregnancy but increasing reactivity after that date, whereas anti-MOMP reactivity was only observed from around the time of abortion (Fig. 3d). Immunoblot assay results for control ewe B2, which was positive by anti-POMP (Fig. 2a) but not anti-VS2 MOMP (Fig. 2c) iELISA following lambing, showed increasing anti-POMP reactivity from 21 wg, but no anti-MOMP response could be detected (Table 2). All remaining control animals were negative by both iELISA and immunoblotting (results not shown).
Immunoblot analysis with recombinant MOMP fragments.
Immunoblotting of the serum samples against each of the four recombinant MOMP variable segments showed that the anti-MOMP reactivity of sera observed following immunoblotting against whole EB lysate was principally directed against the VS1 and VS2 regions of the protein (Table 2). Only the MOMP-positive sera from ewe A3 showed no anti-VS2 reactivity by immunoblotting; instead, antibodies were directed against the VS1 and VS4 regions, confirming the lack of positivity by VS2 iELISA.
DISCUSSION
In this study we have compared antibody responses throughout pregnancy, in C. abortus-infected pregnant sheep, to two of the principal immunodominant antigens, MOMP and POMP90, present on the surface of the pathogen (23, 25) using previously developed ELISAs based on recombinant protein fragments of these proteins (16, 22). The VS2 MOMP iELISA was shown to behave similarly to the CFT in only detecting a rise in antibody titer at the time of abortion and lambing. Furthermore, and again like the CFT, the test lacked sensitivity as it detected no response or only a very weak response in four of the seven ewes that were experimentally infected. However, further analysis showed that for one of these four animals the antibody response was directed against two of the other surface-exposed variable amino acid sequences or variable segments of MOMP, namely, VS1 and VS4 (3, 17, 39), rather than VS2. In addition, one of the other MOMP-negative ewes (A7) was possible genuinely EAE negative, having cleared the initial infection, as no organisms could be detected or recovered from the placenta.
Overall, the humoral responses in the seven animals were directed principally against VS1 and VS2, with only one animal having a strong anti-VS4 response. None of the animals had any anti-VS3 response, perhaps reflecting the identification of serotype-, subspecies-, and serogroup-specific antigenic determinants in only the VS1, VS2, and VS4 regions of C. trachomatis (3, 4). The results of this study imply that any recombinant MOMP serological test should be based on both VS1 and VS2 to maximize the identification of true-positive samples, although this should be confirmed by testing a larger number of infected animals. Indeed, a competitive ELISA for diagnosing EAE has also been developed using monoclonal antibodies to the VS1 and VS2 fragments of C. abortus MOMP (31). However, even taking this into consideration, three of the animals had little or no anti-MOMP response. Such variability in the response to MOMP following abortion has been previously observed in immunoblotting experiments (19, 25) and implies that this antigen is not appropriate for screening animals on an individual basis.
On the other hand, POMP proteins have been suggested to be serologically important antigens with regard to EAE diagnosis as they have been shown to react strongly with sera from C. abortus-infected sheep but not to sera from C. pecorum-infected animals (14, 32). Furthermore, in contrast to MOMP, we have observed consistent antibody responses to the highly immunogenic 90-kDa POMP antigens in all convalescent sheep serum samples that have been tested, including those in this study. In addition, and again in contrast to results obtained with the MOMP iELISA, the POMP iELISA was also found to detect a humoral response prior to abortion or lambing. Indeed, the POMP ELISA detected significantly elevated mean antibody levels as early as 1 week post experimental infection, corresponding to approximately 77 days of gestation. To our knowledge, this is the earliest that any serological test has detected antichlamydial antibodies in pregnant ewes harboring a primary latent infection. Overall, these results are important as early diagnosis of a primary infection and the detection of persistently infected animals, combined with control measures such as vaccination or antibiotic treatment and improved management practices at the time of lambing, will reduce the environmental spread of infection to other susceptible animals. Ultimately, this will limit financial losses and the possible risks of zoonotic transmission to humans.
Although the POMP test clearly detects antibody prelambing or preabortion, this does not necessarily reflect an active infection. It is possible that the test detects circulating antibody resulting from the initial infection, which could explain the results obtained for ewe A7, which was infected but neither aborted nor had any organisms detectable by smear or culture. On the other hand, the test does detect variation in the anti-POMP responses preabortion or prelambing and detects increases in the responses postabortion, which suggests that it does detect a current infection. Indeed, the antibody response in ewe A7 steadily declined following initial infection until the time of lambing and then steadily increased over the remaining sampling period of the study, suggesting a response to lambing and reflecting a current infection. Thus, this animal appears to have had a low-level infection that was not detected in the small areas of the placenta sampled. However, in general, while a high or persisting antibody titer does not necessarily reflect a current or persistent infection, the identification of positive animals will allow all possible control measures to be considered. This will be particularly important for screening replacements prior to introducing them into flocks with no clinical histories of abortion.
The abortion that occurred in the control group in this study was unexpected since all animals were prescreened by the CFT and Western blot analysis before commencing the experiment and found to be seronegative. This again highlights the lack of sensitivity of the CFT and the problems that can occur when using this test to confirm EAE-free status. To eliminate the possibility of cross-infection, the control ewes were housed separately from the infected ewes, and both clothing and footwear were changed before entering pens. It is therefore concluded that this ewe was naturally infected prior to selection for the experiment, possibly during the previous lambing season. Interestingly, a good anti-POMP response could be detected in this ewe prior to and throughout the first 13 weeks of pregnancy, in contrast to the very low anti-MOMP response, suggesting that the POMP test is capable of detecting latently infected animals. However, this will require further investigation as these are results from only one animal. In contrast, the anti-MOMP response was much poorer, only increasing at around the time of abortion and then gradually declining over the remaining period of the study. Furthermore, the POMP ELISA also detected an increasing antibody titer in another control ewe, which the MOMP ELISA failed to identify. This ewe must have picked up infection from the ewe (with which it was housed) that aborted, but this was too late into pregnancy to result in abortion or in any macroscopic or bacteriological evidence of infection in the placenta. Seroconversion was also demonstrated in this ewe by immunoblot analysis following lambing, but only antibodies to POMP antigens could be detected, consistent with the ELISA results. These results again highlight the difference in sensitivity between the two ELISAs.
As well as examining the IgG antibody response, we also investigated the IgM response following primary infection. The latter, as determined by the POMP90 iELISA, was relatively weak and rapidly decreased by 2 to 3 weeks postinfection. In some ewes, no anti-POMP IgM response was detectable. Fuensalida-Draper and Rodolakis (13) suggested that the lack of a detectable IgM response may be attributable to preexposure to intestinal chlamydiae. While this is possible, it seems unlikely, as reinfection would be with a heterologous rather than a homologous strain. However, as suggested by Papp et al. (29), the lack of an IgM response may explain why the CFT, which has an IgM bias, does not reliably detect seropositive animals.
Thus, in summary, the results presented in this study show that the POMP90-4 iELISA is more sensitive than either the CFT or MOMP VS2 iELISA in detecting C. abortus-infected animals during pregnancy and may also detect antibody in latently infected animals. These findings, combined with the previously described results demonstrating the specificity of the POMP test (22), illustrate the advantages and utility of the test for controlling the spread of EAE and also limiting the risk of zoonotic transmission.
Acknowledgments
This work was funded by the Scottish Executive Environment and Rural Affairs Department.
REFERENCES
- 1.Aitken, I. D. 2000. Chlamydial abortion, p. 81-86. In W. B. Martin and I. D. Aitken (ed.), Diseases of sheep. Blackwell Science, Oxford, England.
- 2.Anderson, I. E., A. J. Herring, G. E. Jones, J. C. Low, and A. Greig. 1995. Development and evaluation of an indirect ELISA to detect antibodies to abortion strains of Chlamydia psittaci in sheep sera. Vet. Microbiol. 43:1-12. [DOI] [PubMed] [Google Scholar]
- 3.Baehr, W., Y. X. Zhang, T. Joseph, H. Su, F. E. Nano, K. D. Everett, and H. D. Caldwell. 1988. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc. Natl. Acad. Sci. USA 85:4000-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batteiger, B. E. 1996. The major outer membrane protein of a single Chlamydia trachomatis serovar can possess more than one serovar-specific epitope. Infect. Immun. 64:542-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blewett, D. A., F. Gisemba, J. K. Miller, F. W. A. Johnson, and M. J. Clarkson. 1982. Ovine enzootic abortion: the acquisition of infection and consequent abortion within a single lambing season. Vet. Rec. 111:499-501. [DOI] [PubMed] [Google Scholar]
- 6.Brown, H., and R. Prescott. 1999. Applied mixed models in medicine. John Wiley, Chichester, United Kingdom.
- 7.Buendia, A. J., F. Cuello, L. Del Rio, M. C. Gallego, M. R. Caro, and J. Salinas. 2001. Field evaluation of a new commercially available ELISA based on a recombinant antigen for diagnosing Chlamydophila abortus (Chlamydia psittaci serotype 1) infection. Vet. Microbiol. 78:229-239. [DOI] [PubMed] [Google Scholar]
- 8.Buxton, D., I. E. Anderson, D. Longbottom, M. Livingstone, S. Wattegedera, and G. Entrican. 2002. Ovine chlamydial abortion: characterisation of the inflammatory immune response in placental tissues. J. Comp. Pathol. 127:133-141. [DOI] [PubMed] [Google Scholar]
- 9.Buxton, D., R. M. Barlow, J. Finlayson, I. E. Anderson, and A. Mackellar. 1990. Observations on the pathogenesis of Chlamydia psittaci infection of pregnant sheep. J. Comp. Pathol. 102:221-237. [PubMed] [Google Scholar]
- 10.Cevenini, R., A. Moroni, V. Sambri, S. Perini, and M. La Placa. 1989. Serological response to chlamydial infection in sheep, studied by enzyme-linked immunosorbent assay and immunoblotting. FEMS Microbiol. Immunol. 1:459-464. [DOI] [PubMed] [Google Scholar]
- 11.Donn, A., G. E. Jones, A. Ruiu, M. Ladu, J. Machell, and A. Stancanelli. 1997. Serological diagnosis of chlamydial abortion in sheep and goats: comparison of the complement fixation test and an enzyme-linked immunosorbent assay employing solubilised proteins as antigen. Vet. Microbiol. 59:27-36. [DOI] [PubMed] [Google Scholar]
- 12.Entrican, G. 2002. Immune regulation during pregnancy and host-pathogen interactions in infectious abortion. J. Comp. Pathol. 126:79-94. [DOI] [PubMed] [Google Scholar]
- 13.Fuensalida-Draper, E., and A. Rodolakis. 1978. Kinetics of the complement fixing and immunofluorescent antibody response in experimental chlamydiosis in ewes. Ann. Rech. Vet. 9:505-516. [PubMed] [Google Scholar]
- 14.Griffiths, P. C., H. L. Philips, M. Dawson, and M. J. Clarkson. 1992. Antigenic and morphological differentiation of placental and intestinal isolates of Chlamydia psittaci of ovine origin. Vet. Microbiol. 30:165-177. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths, P. C., J. M. Plater, M. W. Horigan, M. P. M. Rose, C. Venables, and M. Dawson. 1996. Serological diagnosis of ovine enzootic abortion by comparative inclusion immunofluorescence assay, recombinant lipopolysaccharide enzyme-linked immunosorbent assay, and complement fixation test. J. Clin. Microbiol. 34:1512-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herring, A. J., G. E. Jones, S. M. Dunbar, P. F. Nettleton, T. A. Fitzgerald, I. E. Anderson, S. N. Chapman, and T. M. A. Wilson. 1998. Recombinant vaccines against Chlamydia psittaci—an overview of results using bacterial expression and a new approach using a plant virus ‘overcoat’ system, p. 434-437. In R. S. Stephens, G. I. Byrne, G. Christiansen, I. N. Clarke, J. T. Grayston, R. G. Rank, G. L. Ridgway, P. Saikku, J. Schachter, and W. E. Stamm (ed.), Chlamydial infections. Proceedings of the Ninth International Symposium on Human Chlamydial Infection. International Chlamydian Symposium, San Francisco, Calif.
- 17.Herring, A. J., T. W. Tan, S. Baxter, N. F. Inglis, and S. Dunbar. 1989. Sequence analysis of the major outer membrane protein gene of an ovine abortion strain of Chlamydia psittaci. FEMS Microbiol. Lett. 53:153-158. [DOI] [PubMed] [Google Scholar]
- 18.Huang, H. S., D. Buxton, and I. E. Anderson. 1990. The ovine immune response to Chlamydia psittaci; histopathology of the lymph node. J. Comp. Pathol. 102:89-97. [DOI] [PubMed] [Google Scholar]
- 19.Huang, H. S., T. W. Tan, D. Buxton, I. E. Anderson, and A. J. Herring. 1990. Antibody response of the ovine lymph node to experimental infection with an ovine abortion strain of Chlamydia psittaci. Vet. Microbiol. 21:345-351. [DOI] [PubMed] [Google Scholar]
- 20.Jones, G. E., J. C. Low, J. Machell, and K. Armstrong. 1997. Comparison of five tests for the detection of antibodies against chlamydial (enzootic) abortion of ewes. Vet. Rec. 141:164-168. [DOI] [PubMed] [Google Scholar]
- 21.Longbottom, D., and L. J. Coulter. 2003. Animal chlamydioses and zoonotic implications. J. Comp. Pathol. 128:217-244. [DOI] [PubMed] [Google Scholar]
- 22.Longbottom, D., S. Fairley, S. Chapman, E. Psarrou, E. Vretou, and M. Livingstone. 2002. Serological diagnosis of ovine enzootic abortion by enzyme-linked immunosorbent assay with a recombinant protein fragment of the polymorphic outer membrane protein POMP90 of Chlamydophila abortus. J. Clin. Microbiol. 40:4235-4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longbottom, D., J. Findlay, E. Vretou, and S. M. Dunbar. 2001. Immunoelectron microscopic localisation of the OMP90 family on the outer membrane surface of Chlamydia psittaci. FEMS Microbiol. Lett. 164:111-117. [DOI] [PubMed] [Google Scholar]
- 24.Longbottom, D., E. Psarrou, M. Livingstone, and E. Vretou. 2001. Diagnosis of ovine enzootic abortion using an indirect ELISA (rOMP91B iELISA) based on a recombinant protein fragment of the polymorphic outer membrane protein POMP91B of Chlamydophila abortus. FEMS Microbiol. Lett. 195:157-161. [DOI] [PubMed] [Google Scholar]
- 25.Longbottom, D., M. Russell, S. M. Dunbar, G. E. Jones, and A. J. Herring. 1998. Molecular cloning and characterization of the genes coding for the highly immunogenic cluster of 90-kilodalton envelope proteins from the Chlamydia psittaci subtype that causes abortion in sheep. Infect. Immun. 66:1317-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markey, B. K., M. S. McNulty, and D. Todd. 1993. Comparison of serological tests for the diagnosis of Chlamydia psittaci infection of sheep. Vet. Microbiol. 36:233-252. [DOI] [PubMed] [Google Scholar]
- 27.McCafferty, M. C., A. J. Herring, A. A. Andersen, and G. E. Jones. 1995. Electrophoretic analysis of the major outer membrane protein of Chlamydia psittaci reveals multimers which are recognized protective monoclonal antibodies. Infect. Immun. 63:2387-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClenaghan, M., A. J. Herring, and I. D. Aitken. 1984. Comparison of Chlamydia psittaci isolates by DNA restriction endonuclease analysis. Infect. Immun. 45:384-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papp, J. R., P. E. Shewen, and C. J. Gartley. 1994. Abortion and subsequent excretion of chlamydiae from the reproductive tract of sheep during estrus. Infect. Immun. 62:3786-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Payne, R. W. 2000. REML analysis of mixed models, p. 413-503. In R. W. Payne (ed.), The guide to Genstat, part 2: statistics. Lawes Agricultural Trust, Rothamsted, United Kingdom.
- 31.Salti-Montesanto, V., E. Tsoli, P. Papavassiliou, E. Psarrou, B. K. Markey, G. E. Jones, and E. Vretou. 1997. Diagnosis of ovine enzootic abortion, using a competitive ELISA based on monoclonal antibodies against variable segments 1 and 2 of the major outer membrane protein of Chlamydia psittaci serotype 1. Am. J. Vet. Res. 58:228-235. [PubMed] [Google Scholar]
- 32.Souriau, A., J. Salinas, C. De Sa, K. Layachi, and A. Rodolakis. 1994. Identification of subspecies- and serotype 1-specific epitopes on the 80- to 90-kilodalton protein region of Chlamydia psittaci that may be useful for diagnosis of chlamydial induced abortion. Am. J. Vet. Res. 55:510-514. [PubMed] [Google Scholar]
- 33.Spencer, W. N., and F. W. A. Johnson. 1983. Simple transport medium for the isolation of Chlamydia psittaci from clinical material. Vet. Rec. 113:535-536. [PubMed] [Google Scholar]
- 34.Stamp, J. T., A. D. McEwen, J. A. A. Watt, and D. I. Nisbet. 1950. Enzootic abortion in ewes. I. Transmission of the disease. Vet. Rec. 62:251-254. [DOI] [PubMed] [Google Scholar]
- 35.Stamp, J. T., J. A. A. Watt, and R. B. Cockburn. 1952. Enzootic abortion in ewes: complement fixation test. J. Comp. Pathol. 62:93-101. [DOI] [PubMed] [Google Scholar]
- 36.Sting, R., and H. M. Hafez. 1992. Purification of Chlamydia psittaci antigen by affinity chromatography on polymyxin B agarose for use in the enzyme-linked immunosorbent assay (ELISA). Zentbl. Bakteriol. 277:436-445. [DOI] [PubMed] [Google Scholar]
- 37.Vretou, E., H. Loutrari, L. Mariani, K. Costelidou, P. Eliades, G. Conidou, S. Karamanou, O. Mangana, V. Siarkou, and O. Papadopoulos. 1996. Diversity among abortion strains of Chlamydia psittaci demonstrated by inclusion morphology, polypeptide profiles and monoclonal antibodies. Vet. Microbiol. 51:275-289. [DOI] [PubMed] [Google Scholar]
- 38.Wilsmore, A. J., V. Parsons, and M. Dawson. 1984. Experiments to demonstrate routes of transmission of ovine enzootic abortion. Br. Vet. J. 140:380-391. [DOI] [PubMed] [Google Scholar]
- 39.Yuan, Y., Y. X. Zhang, N. G. Watkins, and H. D. Caldwell. 1989. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect. Immun. 57:1040-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]