Abstract
Recipients of licensed anthrax vaccine (AVA; Biothrax) could serve as a source of hyperimmune plasma and immunoglobulin for therapy and prophylaxis. We measured serum antibodies during serial weekly to biweekly plasmapheresis in 38 individuals previously vaccinated with 4 to 27 doses of AVA. Immunoglobulin G (IgG) to protective antigen (PA) and toxin neutralization assay (TNA) antibody levels were highly correlated (r = 0.86930 and P < 0.0001 for anti-PA concentration versus TNA concentration). Significant decreases in antibody titer and concentration were observed over time when compared for the number of days from the last AVA injection (P < 0.0001 for both anti-PA and TNA concentration) and for the number of days from the first plasmapheresis (P = 0.0007 for anti-PA concentration and P = 0.0025 for TNA concentration). The rate of the decrease in total IgG concentration (half-life [t1/2] = 198.90 days after first plasmapheresis) was significantly less than the decrease in anti-PA IgG (t1/2 = 63.53 days) (P < 0.0001), indicating that the reduction in anti-PA IgG was more likely due to natural decay than plasmapheresis. The time since the last injection and the time after initial plasmapheresis are important elements in considering an optimal schedule for collecting anthrax hyperimmune plasma. Good correlation between IgG to PA and TNA antibodies suggests that the anti-PA enzyme-linked immunosorbent assay can be used as a high-throughput screen for functional immune reactivity in donor plasma units.
In 2001, bioterrorism attacks in the United States resulted in 11 cases of inhalation anthrax and 11 cases of cutaneous anthrax (7, 17, 18). Among these, five deaths from inhalation anthrax occurred, despite the use of appropriate antibiotics and intensive supportive care. A critical need exists to develop adjunctive therapies for managing patients with systemic infection with Bacillus anthracis.
At a time when the threat to large populations of susceptible individuals from biological weapons has evolved from the hypothetical to the real, immune plasma and/or immune globulins have emerged as potentially important complements to vaccine and antibiotic countermeasures (4). In the preantibiotic era, passive immunotherapy formed a mainstay for managing many infectious conditions (5). Today, immune plasma or purified immune globulins derived from hyperimmunized animals and previously vaccinated humans continue to be used as therapy and/or prophylaxis for a variety of viral (2, 10, 30, 37, 41) and bacterial (1, 6, 15, 42) diseases or intoxications.
The lethality of anthrax is primarily due to the effects of toxins (lethal toxin and/or edema toxin) elaborated by B. anthracis throughout the infectious cycle. Immunotherapy, in conjunction with antibiotics, represents one option for addressing the extremely high case fatality rates associated with systemic anthrax by interfering with toxin activity at multiple stages in the pathogenic process. In 1965, as a result of observing the successful treatment of inhalation anthrax in nonhuman primates using a combined regimen of penicillin, anthrax immune plasma of equine origin, and vaccine, Lincoln et al. proposed that antiserum be administered concurrently with antibiotics to counter toxin release as bacterial cells were lysed by antibiotics (21). In recent years, our understanding of toxin expression, the mechanisms of toxin activity, and the role of anthrax toxins in the various stages of infection have been greatly enhanced. With these insights, a role for specific antibody therapy in neutralizing anthrax toxins and recovery from intoxication has emerged (32, 39).
Recipients of anthrax vaccine are a potential source of hyperimmune plasma and fractionated immunoglobulin for therapy and prophylaxis. In 2002, a collaborative program involving the U.S. Centers for Disease Control and Prevention (CDC), the Department of Defense, and the National Institutes of Health (NIH) was established to procure anthrax immune plasma for assessing efficacy in animal models and to make available a therapeutic agent for contingency use in humans under an investigational protocol. We sought to characterize levels of neutralizing and immunoglobulin G (IgG) to protective antigen (PA) antibodies in this group of donors to better inform current and future collection and testing strategies for this potentially promising therapeutic modality.
(This research was presented in part at the 42nd Annual Meeting of the Infectious Diseases Society of America, Boston, Mass., 30 September to 3 October 2004 [abstract 1016].)
MATERIALS AND METHODS
Study design.
A protocol to collect plasma from individuals who had received at least four doses of anthrax vaccine (AVA) and were within 3 to 12 weeks of their last vaccination if they had received four to six inoculations or within 6 months of their last vaccination if they had received seven or more AVA inoculations was reviewed and approved by institutional review boards at the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), the NIH, and the CDC, as well as the Human Subjects Research Review Board in the Office of the U.S. Army Surgeon General. Volunteers were recruited from the ranks of USAMRIID personnel at risk of laboratory exposure to anthrax. Prospective donors provided informed consent and then were screened and enrolled if they met allogeneic donor eligibility criteria in compliance with American Association of Blood Bank standards and U.S. Food and Drug Administration regulations. Weekly to biweekly plasmapheresis was performed at the NIH Clinical Center Department of Transfusion Medicine. Volunteers were asked to provide between 600 ml and 800 ml of plasma per donation, depending upon body weight. Serum samples for antibody measures were collected at the time of plasmapheresis. Informed consent was obtained from each individual before any procedure was performed. The study was performed in accordance with International Committee on Harmonisation guidelines for good clinical practice and with the Declaration of Helsinki.
Laboratory studies.
Antibodies to B. anthracis PA were measured using a modification of a previously described indirect enzyme-linked immunosorbent assay (ELISA) (34). In brief, twofold serial dilutions of serum from 1:800 to 1:102,400 were made in predefined regions of 96-well plates coated with recombinant protective antigen (rPA) (Science Applications International Corp, Frederick, MD). Twofold dilutions of an anti-AVA standard human reference serum (AVR414; CDC, Atlanta, GA) (40) from 1:200 to 1:12,800 were made in different wells of each coated plate. Positive serum controls with known high, medium, and low concentrations of anti-rPA IgG and a negative serum control were also included on each plate. Plates were incubated at 37°C for 60 min and washed three times. A peroxidase-conjugated goat anti-human IgG antibody (Kirkegaard and Perry, Gaithersburg, MD) was then added to detect bound antigen colorimetrically. Color development was stopped after a 30-min incubation by adding peroxidase stop solution (Kirkegaard and Perry). Optical density (OD) values were read within 30 min by using a Bio-Tek ELx808 plate with 405-nm and 490-nm filters and KC4 software (BIO-TEK Instruments, Inc., Winooski, VT). OD values were converted to immunoglobulin concentration (in μg/ml) by using a standard curve calibration factor (36). Antibody concentration was determined at the dilution nearest the inflection point of a standard serum curve. Titers were expressed as the reciprocal of the highest dilution of the test serum yielding a mean OD value equal to or greater than the cutoff value (0.253) (as described in reference 36) for the assay.
B. anthracis lethal toxin neutralization activity was measured using a validated colorimetric toxin neutralization assay (TNA) (14, 34). In brief, confluent monolayers of J774A.1 (mouse macrophage) cells were grown in 96-well plates and used after overnight incubation. Twofold dilutions of test (from 1:50 to 1:102,400) and anti-AVA reference standard (AVR414) (from 1:100 to 1:6,400; initial dilution determined from antibody levels measured by ELISA) sera made in 96-well titration plates were combined with recombinant B. anthracis PA (rPA) (Science Applications International Corp) and lethal factor (List Biologicals, Campbell, CA). After a 1-h incubation at 37°C, the serum-lethal toxin mixtures were then added to the cell monolayers and incubated at 37°C for 4 h. Cell viability after exposure to the serum-toxin mixtures was determined by adding thiazolyl blue (MTT) (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; Sigma, St. Louis, MO) in 50% dimethyl formamide (Sigma) to each plate. After incubation at 37°C overnight, OD values were read at 570 nm by using a Bio-Tek ELx808 reader and KC4 software (BIO-TEK Instruments, Inc.).
A four-parameter logistic fit of the OD data was used to determine serum TNA antibody titers (the dilution of serum resulting in 50% neutralization of anthrax lethal toxin, or ED50). The neutralization capacity of each test serum in relation to that of the reference serum (50% neutralization factor, or NF50, also known as the neutralization ratio) (14) was calculated from the quotient of the ED50 of the reference serum and the ED50 of the test serum. The concentration of immunoglobulin (in μg/ml) was determined by using a standard curve calibration factor.
Total IgG antibody concentrations were measured by nephelometry under commercial contract (Esoterix, Inc., Austin, TX).
Statistical analysis.
No outliers were identified in ELISA or TNA titer or concentration variables. After log10 transformations were applied, the dependent variables met assumptions of normality and homogeneity of variance. Pearson product-moment correlation was used to compute correlation coefficients between ELISA and TNA values. Repeated measures mixed model analysis of variance (ANOVA) was used to compare values over time and between IgG measures. Days from last anthrax vaccine injection and days from first plasmapheresis were both used as measures of time. The rate of antibody decay was derived from the negative slopes of the regression lines for total IgG, IgG to PA, and TNA antibody concentrations versus time in days. Half-life (t1/2) was calculated as the time required for antibody concentrations to decrease by 50% from the initial value. All analyses were conducted using SAS version 8.2 (SAS OnlineDoc, version 8, 2000, SAS Institute Inc., Cary, NC).
RESULTS
Donor characteristics.
Serum samples were collected during serial weekly to biweekly plasmapheresis of 38 subjects who met protocol inclusion criteria. The majority of donors were Caucasian (87%) and male (84%) (Table 1). The median age was 37 years (range = 20 to 59 years). The median number of AVA doses received by donors was seven (range = 4 to 27); 42.1% had received four to six doses and 31.6% had received seven to nine doses, while the remainder (26.3%) had received 10 or more vaccinations. The median number of plasma donations per study volunteer was six (6 donors donated one to three times, 7 donated four to five times, and the remaining 25 donated six times each); a total of 196 procedures were performed. The time interval from the last AVA dose received to the initial plasmapheresis in donors ranged from 5 to 144 days (median = 29 days), and the time from the last AVA dose received to the final plasmapheresis in donors ranged from 28 to 197 days (median = 88 days). The length of time from initial plasmapheresis to last plasmapheresis was 0 (for one-time donors) to 118 days (median = 53 days). The median time between successive donations was 7 days (range = 6 to 63 days).
TABLE 1.
Characteristics of plasma donors
| Characteristic | Value |
|---|---|
| Age (years) | |
| Range | 20-59 |
| Mean | 37.60 |
| Median | 37 |
| Gender | |
| Male | 32 (84%) |
| Female | 6 (16%) |
| Race/ethnicity | |
| Caucasian | 33 (87%) |
| African American | 2 (5%) |
| Hispanic | 3 (8%) |
| AVA dose no.a | |
| Range | 4-27 |
| Mean | 8.68 |
| Median | 7 |
AVA is anthrax vaccine absorbed (Biothrax).
Antibody levels.
IgG to PA and TNA antibody levels in serum from study subjects varied widely. IgG to PA dilutional titers ranged from 1:3,200 to 1:204,800 (geometric mean titer = 1:19,184), while IgG to PA concentrations ranged from 38 μg/ml to 1,991 μg/ml (geometric mean concentration = 186 μg/ml) (Table 2). The distribution of TNA antibody titers was 1:86 to 1:15,049 (geometric mean titer = 1:844), and that of TNA antibody concentrations was 13 μg/ml to 1,917 μg/ml (geometric mean concentration = 126 μg/ml) (Table 2). Among individual samples, TNA NF50 correlated closely with both TNA concentration and TNA titer (r = 0.90101 and P < 0.0001 for concentration versus NF50; r = 0.93726 and P < 0.0001 for titer versus NF50).
TABLE 2.
Consolidated IgG to PA and TNA antibody levels
| Antibody | Maximum | Minimum | Median | Geometric mean | 95% Confidence interval |
|---|---|---|---|---|---|
| IgG to PA | |||||
| Reciprocal titer | 204,200 | 3,200 | 12,800 | 19,184 | 16,722-22,009 |
| Concentration (μg/ml) | 1,991 | 38 | 170 | 186 | 167-207 |
| TNA | |||||
| Reciprocal titer (ED50) | 15,049 | 84 | 897 | 844 | 747-954 |
| Concentration (μg/ml) | 1,917 | 13 | 140 | 126 | 110-145 |
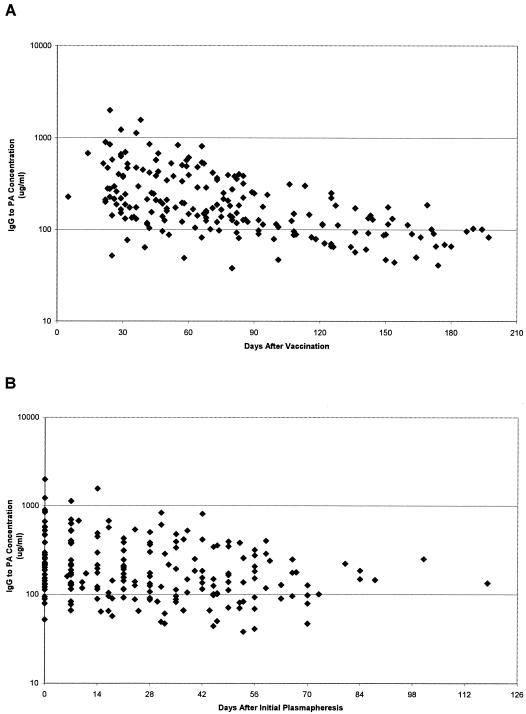
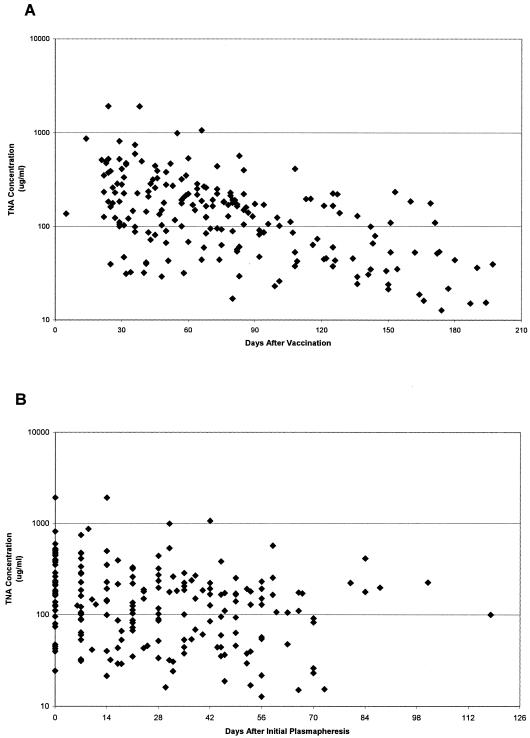
By repeated measures ANOVA, significant trends toward decreasing antibody concentration over time were observed for IgG to PA (P < 0.0001 for days from last AVA injection and P = 0.0007 for days from first plasmapheresis) (Fig. 1) and for TNA (P < 0.0001 for days from last AVA injection and P = 0.0025 for days from first plasmapheresis) (Fig. 2) antibodies. Similar trends toward decreasing dilutional titer over time were observed for both IgG to PA (P < 0.0001) and TNA (P < 0.0001) antibodies when compared for days since last AVA injection and for days from first plasmapheresis (P = 0.0073 for IgG to PA; P = 0.0004 for TNA) (data not shown).
FIG. 1.
IgG antibody to B. anthracis PA concentrations in sera from plasma donors. Antibody levels declined significantly over time for days from last AVA vaccination (P < 0.0001) (A) and for days from initial plasmapheresis (P < 0.0007) (B).
FIG. 2.
B. anthracis TNA antibody concentrations in sera from plasma donors. Antibody levels declined significantly over time for days from last AVA vaccination (P < 0.0001) (A) and for days from initial plasmapheresis (P < 0.0001) (B).
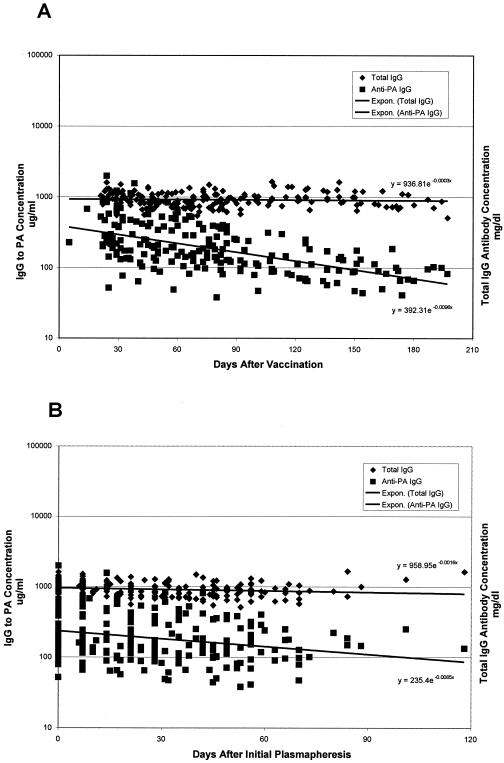
The rate of decay of total IgG concentrations in the days after subjects' initial plasmapheresis procedures was measured at 0.0015 log10 mg/dl/day (antibody t1/2 was 198.90 days), a significant decrease by repeated measures ANOVA (P = 0.0001). However, the rate of change was significantly less than the rate of decrease in IgG to PA (decay rate = 0.0047 log10 μg/ml/day; t1/2 = 63.53 days) (P < 0.0001 by repeated measures ANOVA) (Fig. 3). Decreases in total IgG concentration after the last AVA injection were not significant (P = 0.6878). However, there was a significant interaction effect of time and total IgG and IgG to PA concentrations; the rate of decrease in IgG to PA after the last AVA injection (decay rate = 0.0044 log10 μg/ml/day; t1/2 = 67.58 days) was significantly greater than that of total IgG (decay rate = 0.0001 log10 mg/dl/day; t1/2 = 4,387.92 days) (P < 0.0001) (Fig. 3). Decay kinetics of TNA concentration were similar to that observed for IgG to PA (data not shown).
FIG. 3.
Comparative rates of decline between IgG antibody to B. anthracis PA concentrations and total IgG antibody concentrations in sera from plasma donors. The rate of decrease in total IgG concentration was significantly less than that for the IgG to PA concentration over the interval from the last AVA injection (P < 0.0001) (A) and over the interval from initial plasmapheresis (P < 0.0001) (B). Regression lines and respective equations are included.
Correlations between IgG to PA and TNA antibodies.
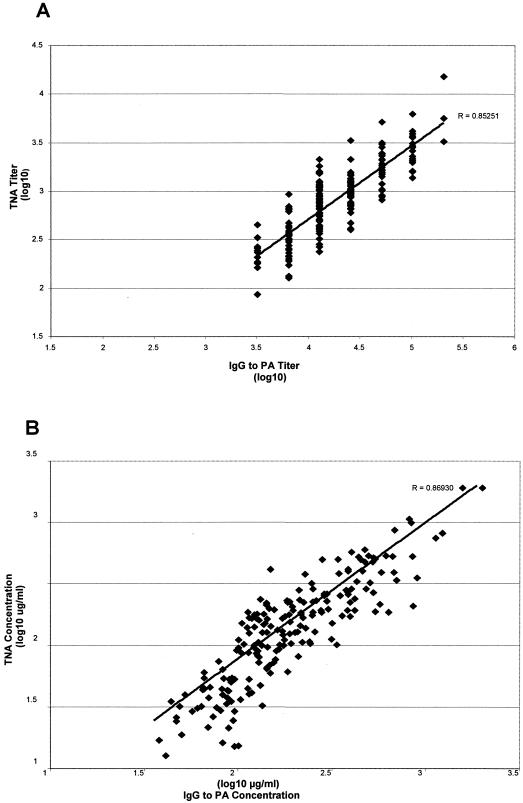
While levels of antibody varied widely by plasma donor and over time, strong correlations for individual samples were observed with IgG to PA and TNA antibody titers (r = 0.85251, P < 0.0001) as well as IgG to PA and TNA antibody concentrations (r = 0.86930, P < 0.0001) (Fig. 4). There was no correlation between total IgG and IgG to PA concentrations (r = −0.03, P = 0.6722) (data not shown).
FIG. 4.
Correlations between IgG to B. anthracis PA and TNA antibody titers (A) and concentrations (B). Regression lines and correlation coefficients (R) are included for each plot.
DISCUSSION
Immunotherapy for anthrax has a long-standing historical precedent. During the first half of the 20th century, parenteral administration of equine hyperimmune anthrax antiserum was used successfully to cure cutaneous disease in humans (12, 16, 19, 27, 28, 29). Therapeutic response was dose dependent, but no controlled studies were performed, and other than volume, details of the antibody preparations used (concentration, potency, etc.) were not reported. Although largely supplanted by antimicrobial agents, in some parts of the world equine antibody preparations continue to be used in managing this condition (9).
The utility of immune antiserum in therapy of inhalation anthrax has been studied for many years in animal systems. Polyclonal equine antiserum raised against the B. anthracis Sterne strain proved effective in treating inhalation anthrax in nonhuman primates when administered intramuscularly once (day 1) after aerosol exposure or twice (days 1 and 6) after exposure; 40 to 45% survival (compared with 90% lethality in controls) was achieved, and mean time to death was delayed from 5 to more than 20 days (13). In later studies, 69% cure rates were achieved in lethally challenged rhesus macaques treated with equine antiserum in conjunction with penicillin (22), and 84% cure rates were achieved when antiserum was combined with antibiotics and vaccine (21).
Improved survival and delays in time to death in guinea pigs were demonstrated after administration of polyclonal rabbit serum raised against B. anthracis PA up to 96 h after lethal anthrax spore challenge (20, 23). Protective effects appeared to correlate with toxin neutralization activity in this model (20, 38). Of interest, similar success with murine monoclonal antibodies to PA selected for high toxin neutralization activity was not achieved in guinea pigs (20, 23). Murine monoclonal anti-PA and anti-lethal factor did prove effective in neutralizing lethal toxin in an in vivo rat model, however (25, 26). Recently, recombinant antibody fragments engineered by fusing toxin-neutralizing, monoclonal, single-chain variable fragments to a human constant κ domain (31) and by generating phage display libraries bearing Fab fragments derived from blood or bone marrow of AVA-vaccinated donors (44) were shown to be capable of protecting rats from lethal toxin challenge.
We examined the dynamics of polyclonal IgG antibodies to PA in humans previously vaccinated with anthrax vaccine adsorbed who subsequently underwent serial plasmapheresis. Although wide (>10-fold) variations in IgG to PA and TNA antibody titers and concentrations were observed among plasma donors in this study, we were able to discern patterns in antibody dynamics that should prove useful in designing future plasma collection strategies.
Total IgG and IgG subclass composition for the standard reference serum used in this study, AVR414, has been determined and published (40). While measurement of IgG subclass reactivity in samples from our study population may have allowed us to determine the degree to which subclass compositional differences influenced variability in antibody concentrations, we were, unfortunately, unable to perform these analyses. In addition, we were unable to assess the contribution of IgM to our study findings. It is noteworthy, however, that in the study by Quinn et al. of anthrax antibodies in victims of bioterrorism-associated anthrax, strong correlations between IgG to PA and TNA antibodies were observed in both early (<4 weeks after infection) and late (>4 weeks after infection) serum samples, suggesting that any IgM to PA present did not make a significant contribution to neutralizing activity (35).
Concerns over the potential impact of serial plasmapheresis on immune competence and overall health have prompted critical assessments by regulatory agencies to ensure the well-being of donors. Studies have shown that weekly plasma donors may experience declines in total IgG, IgA, and IgM concentrations during the initial 4 to 6 months of serial plasmapheresis, with subsequent stabilization (8, 11). Considerable individual variability exists, however (3), and no significant impact on humoral and cellular immunity has been documented (43). We observed a small but consistent and statistically significant reduction in total IgG and IgG to PA antibody levels during the period after initial plasmapheresis in this study cohort. Comparing IgG to PA and TNA with total IgG concentrations in the same study subjects revealed differential rates of decline, indicating that the observed temporal changes in anti-PA and TNA levels were due primarily to natural antibody decay rather than plasmapheresis. We were unable to assess the impact of weekly versus biweekly plasmapheresis due to limited sample size and the uncontrolled nature of the study. Nevertheless, our findings that IgG to PA and TNA antibody concentrations decreased relatively slowly after initial plasmapheresis (t1/2 = 63.35 days and 57.30 days, respectively) and that geometric mean antibody concentrations after repeated donations generally remained above levels correlating with protection against aerosol challenge in the rabbit model (24, 33) suggest that plasmapheresis conducted at weekly intervals is not likely to influence the degree of humoral immune protection afforded vaccinees.
Initial measures of total IgG concentrations in our study subjects were in the normal range (784 mg/dl to 1,610 mg/dl; mean = 1,032.19 mg/dl), and the prolonged rate of decay observed (t1/2 = 198.90 days) was not unexpected because of continual stimulation of new and different antibody-producing cells over time. Similarly, the t1/2 observed for IgG to PA (63.53 days) was considerably longer than that reported for human IgG (7 to 23 days, depending upon subclass). While the continued production of antibodies specific for PA following vaccination would be expected to result in prolonged decay rates compared with measures of decay under static (e.g., postinfusion) conditions, additional factors may also have contributed to this finding. Possibilities include the depot effect of the vaccine's alhydrogel adjuvant, which resulted in low-level release of antigen and thus prolonged immune stimulation; promotion of a Th2-type response by alhydrogel which, with multiple vaccinations, would be expected to manifest as a significant increase in IgG4 subclass and apparent prolongation of the response; IgG subclass switching as a consequence of repetitive immunization (57.9% of plasma donors had received seven or more AVA injections), resulting in antibodies with high functional affinity for PA that may have extended half-lives; or other, as yet undefined, factors.
Caution must be exercised in extrapolating findings from this sample to other populations. The relatively limited sample size (n = 38 plasma donors), the wide variability in donor characteristics, and the absence of a control group are factors that may have biased our results and limit our ability to predict an optimal time for collecting plasma from immunized vaccinees.
We found significant correlations between IgG to PA and TNA antibody titers and concentrations. Similar correlations were reported among vaccinees participating in a dose reduction and route change study of AVA (34) and for survivors of natural cutaneous and inhalation anthrax (35). These observations provide evidence that, for AVA recipients and B. anthracis infection, a relatively simple biochemical assay (i.e., enzyme immunoassay) can serve as a valid surrogate for functional immune reactivity (i.e., lethal toxin neutralization). It should be possible, therefore, to apply the anti-PA enzyme immunoassay as a high-throughput screen in assessing functional antibody activity contained in donor plasma.
The efficacy and potency of anthrax immune plasma and/or immune globulin in treating systemic anthrax disease in humans have not yet been demonstrated in controlled studies. Additional efforts to more completely understand correlates and determinants of immunity against anthrax, as well as controlled studies to define anti-PA and TNA antibody kinetics, are needed to inform use of hyperimmune plasma and fractionated immune globulin for therapy and/or prophylaxis of this deadly infection.
Acknowledgments
We gratefully acknowledge Gladys Sanders, Cynthia Mann, and the nursing staff of the Dowling Apheresis Clinic, Department of Transfusion Medicine, NIH, for apheresis expertise; Marc Fischer, Special Pathogens Branch, CDC, for his scientific contributions, guidance in human subjects considerations, and protocol development; and Vincent Fulton and the staff of the Anthrax Vaccine Immunization Program at USAMRIID for recruiting and volunteer management support. We extend special thanks to Conrad Quinn, CDC, for insightful review and comment on the manuscript.
The research described herein was sponsored by the Centers for Disease Control and Prevention (02FED097 10-00) and the U.S. Army Medical Research and Materiel Command (AVIP project 03-0-VP-003).
The opinions expressed are those of the authors and should not be construed to represent those of the Department of the Army, the Department of Defense, Camber Corporation, Goldbelt-Raven LLC, the Centers for Disease Control and Prevention, the National Institutes of Health, or the Department of Health and Human Services.
REFERENCES
- 1.Arnon, S. S., R. Schechter, T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, J. Hauer, M. Layton, S. Lillibridge, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, D. L. Swerdlow, K. Tonat, and the Working Group on Civilian Biodefense. 2001. Botulinum toxin as a biological weapon: medical and public health management. JAMA 285:1059-1070. [DOI] [PubMed] [Google Scholar]
- 2.Brunell, P. A., A. A. Gershon, W. T. Hughes, H. D. Riley, Jr., and J. Smith. 1972. Prevention of varicella in high risk children: a collaborative study. Pediatrics 50:18-22. [PubMed] [Google Scholar]
- 3.Burgin, M., G. Hopkins, B. Moore, J. Nasser, A. Richardson, and R. Minchinton. 1992. Serum IgG and IgM levels in new and regular long-term plasmapheresis donors. Med. Lab. Sci. 49:265-270. [PubMed] [Google Scholar]
- 4.Casadevall, A. 2002. Passive antibody administration (immediate immunity) as a specific defense against biological weapons. Emerg. Infect. Dis. 8:833-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadevall, A., and M. D. Scharff. 1995. Return to the past: the case for antibody-based therapies in infectious diseases. Clin. Infect. Dis. 21:150-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1985. Diphtheria, tetanus, and pertussis: guidelines for vaccine prophylaxis and other preventive measures. Morb. Mortal. Wkly. Rep. 34:895-900. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 2001. Investigation of bioterrorism-related anthrax: Connecticut, 2001. Morb. Mortal. Wkly. Rep. 50:1077-1079. [PubMed] [Google Scholar]
- 8.Ciszewski, T. S., S. Ralston, D. Acteson, S. Wasi, and S. J. Strong. 1993. Protein levels and plasmapheresis intensity. Transfus. Med. 3:59-65. [DOI] [PubMed] [Google Scholar]
- 9.Dong, S. L. 1990. Progress in the control and research of anthrax in China. Salisbury Med. Bull. 68:104-105. [Google Scholar]
- 10.Ferry, B. J. 1976. The efficacy of vaccinial immune globulin. Vox Sang. 31:68-76. [DOI] [PubMed] [Google Scholar]
- 11.Friedman, B. A., M. A. Schork, J. L. Mocniak, and H. A. Oberman. 1975. Short-term and long-term effects of plasmapheresis on serum proteins and immunoglobulins. Transfusion 15:467-472. [DOI] [PubMed] [Google Scholar]
- 12.Gold, H. 1936. Studies on anthrax. Clinical report of ten human cases. J. Lab. Clin. Med. 4:134-152. [Google Scholar]
- 13.Henderson, D. W., S. Peacock, and F. C. Belton. 1956. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. J. Hyg. 54:28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hering, D., W. Thompson, J. Hewetson, S. Little, S. Norris, and J. Pace-Templeton. 2004. Validation of the anthrax lethal toxin neutralization assay. Biologicals 1:17-27. [DOI] [PubMed] [Google Scholar]
- 15.Hibbs, R. G., J. T. Weber, A. Corwin, B. M. Allos, M. S. Abd el Rehim, S. E. Sharkawy, J. E. Sarn, and K. T. McKee, Jr. 1996. Experience with the use of an investigational F(ab′)2 heptavalent botulism immune globulin of equine origin during an outbreak of type E botulism in Egypt. Clin. Infect. Dis. 23:337-340. [DOI] [PubMed] [Google Scholar]
- 16.Hodgson, A. E. 1928. The serum treatment of cutaneous anthrax. Lancet 215:594-595. [Google Scholar]
- 17.Inglesby, T. V., T. O'Toole, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. M. Friedlander, J. Gerberding, J. Hauer, J. Hughes, J. McDade, M. T. Osterholm, G. Parker, T. M. Perl, P. K. Russell, K. Tonat, and the Working Group on Civilian Biodefense. 2002. Anthrax as a biological weapon, 2002. Updated recommendations for management. JAMA 287:2236-2252. [DOI] [PubMed] [Google Scholar]
- 18.Jernigan, D. B., P. L. Raghunathan, B. P. Bell, R. Brechner, E. A. Bresnitz, J. C. Butler, M. Cetron, M. Cohen, T. Doyle, M. Fischer, C. Greene, K. S. Griffith, J. Guarner, J. L. Hadler, J. A. Hayslett, R. Meyer, L. R. Petersen, M. Phillips, R. Pinner, T. Popovic, C. P. Quinn, J. Reefhuis, D. Reissman, N. Rosenstein, A. Schuchat, W. J. Shieh, L. Siegal, D. L. Swerdlow, F. C. Tenover, M. Traeger, J. W. Ward, I. Weisfuse, S. Wiersma, K. Yeskey, S. Zaki, D. A. Ashford, B. A. Perkins, S. Ostroff, J. Hughes, D. Fleming, J. P. Koplan, J. L. Gerberding, and the National Anthrax Epidemiologic Investigation Team. 2002. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg. Infect. Dis. 8:1019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knudson, G. B. 1986. Treatment of anthrax in man: history and current concepts. Mil. Med. 151:71-77. [PubMed] [Google Scholar]
- 20.Kobiler, D., Y. Gozes, H. Rosenberg, D. Marcus, S. Reuveny, and Z. Altboum. 2002. Efficiency of protection of guinea pigs against infection with Bacillus anthracis spores by passive immunization. Infect. Immun. 70:544-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lincoln, R. E., F. Klein, J. S. Walker, B. W. Haines, W. L. Jones, B. G. Mahlandt, and R. H. Friedman. 1965. Successful treatment of rhesus monkeys for septicemic anthrax. Antimicrob. Agents Chemother. 4:759-763. [PubMed] [Google Scholar]
- 22.Lincoln, R. E., J. S. Walker, F. Klein, and B. W. Haines. 1964. Anthrax. Adv. Vet. Sci. 9:327-368. [Google Scholar]
- 23.Little, S. F., B. E. Ivins, P. F. Fellows, and A. M. Friedlander. 1997. Passive protection by polyclonal antibodies against Bacillus anthracis infection in guinea pigs. Infect. Immun. 65:5171-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Little, S. F., B. E. Ivins, P. F. Fellows, M. L. M. Pitt, S. L. W. Norris, and G. P. Andrews. 2004. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine 22:422-430. [DOI] [PubMed] [Google Scholar]
- 25.Little, S. F., S. H. Leppla, and E. Cora. 1988. Production and characterization of monoclonal antibodies to the protective antigen component of Bacillus anthracis toxin. Infect. Immun. 56:1807-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Little, S. F., S. H. Leppla, and A. M. Friedlander. 1990. Production and characterization of monoclonal antibodies against the lethal factor component of Bacillus anthracis lethal toxin. Infect. Immun. 58:1606-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lockwood, C. B., and F. W. Andrewes. 1905. A case of anthrax successfully treated by Sclavo's serum. Br. Med. J. 1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucchesi, P. 1932. Serum treatment of 19 cases of anthrax including one of external, internal and bacteremic type. Am. J. Med. Sci. 183:795-802. [Google Scholar]
- 29.Lucchesi, P. F., and N. Gildersleeve. 1941. The treatment of anthrax. JAMA 16:1506-1508. [Google Scholar]
- 30.Maiztegui, J. I., N. J. Fernandez, and A. J. De Damilano. 1979. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurologic syndrome. Lancet 2:1216-1217. [DOI] [PubMed] [Google Scholar]
- 31.Maynard, J. A., C. B. Maassen, S. H. Leppla, K. Brasky, J. L. Patterson, B. L. Iverson, and G. Georgiou. 2002. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat. Biotechnol. 20:597-601. [DOI] [PubMed] [Google Scholar]
- 32.Moayeri, M., and S. H. Leppla. 2004. The roles of anthrax toxin in pathogenesis. Curr. Opin. Microbiol. 1:19-24. [DOI] [PubMed] [Google Scholar]
- 33.Pitt, M. L. M., S. F. Little, B. E. Ivins, P. Fellows, J. Barth, J. Hewetson, P. Gibbs, M. Dertzbaugh, and A. M. Friedlander. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19:4768-4773. [DOI] [PubMed] [Google Scholar]
- 34.Pittman, P. R., G. Kim-Ahn, D. Y. Pifat, K. Coonan, P. Gibbs, S. Little, J. G. Pace-Templeton, R. Myers, G. W. Parker, and A. M. Friedlander. 2002. Anthrax vaccine: immunogenicity and safety of a dose-reduction, route-change comparison study in humans. Vaccine 20:1412-1420. [DOI] [PubMed] [Google Scholar]
- 35.Quinn, C. P., P. M. Dull, V. Semenova, H. Li, S. Crotty, T. H. Taylor, E. Steward-Clark, K. L. Stamey, D. S. Schmidt, K. W. Stinson, A. E. Freeman, C. M. Elie, S. K. Martin, C. Greene, R. D. Aubert, J. Glidewell, B. A. Perkins, R. Ahmed, and D. S. Stephens. 2004. Immune responses to Bacillus anthracis protective antigen in patients with bioterrorism-related cutaneous or inhalation anthrax. J. Infect. Dis. 190:1228-1236. [DOI] [PubMed] [Google Scholar]
- 36.Quinn, C. P., V. A. Semenova, C. M. Elie, S. Romero-Steiner, C. Greene, H. Li, K. Stamey, E. Steward-Clark, D. S. Schmidt, E. Mothershed, J. Pruckler, S. Schwartz, R. F. Benson, L. O. Helsel, P. F. Holder, S. E. Johnson, M. Kellum, T. Messmer, W. L. Thacker, L. Besser, B. D. Plikaytis, T. H. Taylor, Jr., A. E. Freeman, K. J. Wallace, P. Dull, J. Sejvar, E. Bruce, R. Moreno, A. Schuchat, J. R. Lingappa, S. K. Martin, J. Walls, M. Bronsdon, G. M. Carlone, M. Bajani-Ari, D. A. Ashford, D. S. Stephens, and B. A. Perkins. 2002. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen. Emerg. Infect. Dis. 10:1103-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reed, E. C., R. A. Bowden, P. S. Dandliker, K. E. Lilleby, and J. D. Meyers. 1988. Treatment of cytomegalovirus pneumonia with ganciclovir and intravenous cytomegalovirus immunoglobulin in patients with bone marrow transplants. Ann. Intern. Med. 109:783-788. [DOI] [PubMed] [Google Scholar]
- 38.Reuveny, S., M. D. White, Y. Y. Adar, Y. Kafri, Z. Altboum, Y. Gozes, D. Kobiler, A. Shafferman, and B. Velan. 2001. Search for correlates of protective immunity conferred by anthrax vaccine. Infect. Immun. 69:2888-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawada-Hirai, R., I. Jiang, F. Wang, S. M. Sun, R. Nedellec, P. Ruther, A. Alvarez, D. Millis, P. R. Morrow, and A. S. Kang. 2004. Human anti-anthrax protective antigen neutralizing monoclonal antibodies derived from donors vaccinated with anthrax vaccine adsorbed. J. Immune Based Ther. Vaccines 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semenova, V. A., E. Steward-Clark, K. L. Stamey, T. H. Taylor, Jr., D. S. Schmidt, S. K. Martin, N. Marano, and C. P. Quinn. 2004. Mass value assignment of total and subclass-specific immunoglobulin G in a human standard anthrax reference serum. Clin. Diagn. Lab. Immunol. 11:919-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szmuness, W., A. M. Prince, M. Goodman, C. Ehrich, R. Pick, and M. B. Ansari. 1974. Hepatitis B immune serum globulin in prevention of nonparenterally transmitted hepatitis. N. Engl. J. Med. 290:701-706. [DOI] [PubMed] [Google Scholar]
- 42.Tacket, C. O., W. X. Shandera, J. M. Mann, N. T. Hargrett, and P. A. Blake. 1984. Equine antitoxin use and other factors that predict outcome in type A foodborne botulism. Am. J. Med. 76:794-798. [DOI] [PubMed] [Google Scholar]
- 43.Tran-Mi, B., H. Stroch, K. Seidel, T. Schulzki, H. Haubelt, C. Anders, D. Nagel, K. E. Siegler, A. Vogt, D. Seller, and B. P. Hellstern. 2004. The impact of different intensities of regular donor plasmapheresis on humoral and cellular immunity, red cell and iron metabolism, and cardiovascular risk markers. Vox Sang. 86:189-197. [DOI] [PubMed] [Google Scholar]
- 44.Wild, M. A., H. Xin, T. Maruyama, M. J. Nolan, P. M. Calveley, J. D. Malone, M. R. Wallace, and K. S. Bowdish. 2003. Human antibodies from immunized donors are protective against anthrax toxin in vivo. Nat. Biotechnol. 21:1305-1306. [DOI] [PubMed] [Google Scholar]