Summary
Chagas disease, the most common form of nonischaemic cardiomyopathy globally, is one of the leading causes of morbidity and mortality in Latin America. Chagas cardiomyopathy has a wide clinical spectrum and prognosis, which is primarily determined by the severity of left ventricular dysfunction. Chagas disease also affects the brain, particularly manifesting as cardioembolic strokes and cognitive impairments. Disease progression is influenced by various factors such as anti-parasite treatments, host–parasite interactions, and other determinants.
This review explores Chagas disease, covering clinical presentations, the range of severity of Chagas cardiomyopathy, and neurological manifestations. We investigate factors that influence the progression of cardiomyopathy, including anti-parasitic treatments, interactions between hosts and parasites, and the influence of social determinants on the course of the disease. This review analyses key prognostic factors associated with the progression and mortality of Chagas cardiomyopathy, offering insights into this potentially fatal illness.
Keywords: Chagas disease, Chagas cardiomyopathy, Progression, Severity
Search strategy and selection criteria.
The material covered was sourced from searches conducted in PubMed, Scopus, and Google Scholar. The search utilised combinations of MeSH terms such as “Chagas Disease,” “Trypanosoma cruzi infection,” “Disease Progression,” and “Chagas cardiomyopathy,” alongside free text terms like “Neurological manifestations,” “Risk factors progression,” and “Social determinants of progression.” Filters applied included language restrictions to English, Portuguese, and Spanish and study types including reviews, clinical trials, cohort studies, and case–control studies. Additionally, reference lists of selected key articles were reviewed to identify further relevant studies.
Overview of Chagas disease
Cardiovascular clinical presentation and severity
Chagas disease is the leading cause of infectious cardiomyopathy worldwide,1 representing the most frequent and severe manifestation of this condition.2,3 Despite the actual disease control, current estimates indicate a significant burden, with approximately 1.2 million patients affected by Chagas cardiomyopathy, which is the major cause of mortality.4 While Chagas disease remains endemic in Latin America, its epidemiology landscape has been influenced by continuous migratory patterns, elevating it to a global health concern.2
Chagas disease is caused by the protozoan parasite Trypanosoma cruzi, which has a wide genetic variety, including six distinct typing units (DTUs) known as TcI-TcVI. These DTUs are closely related to several epidemiological, ecological, and clinical aspects of Chagas disease throughout the Americas.5
The disease usually presents in an acute and a chronic phase.2 The acute phase has various clinical manifestations, the most common are nonspecific viral-like signs and symptoms including fever, malaise, and lymphadenopathy. The acute phase usually resolves spontaneously after which patients remain chronically infected if untreated, in a clinically asymptomatic chronic phase called indeterminate form.3,6 The acute phase resulting from oral transmission differs significantly from the vector-transmitted acute phase, characterised by more severe myocarditis and a higher risk of death.7
In the indeterminate form, sensitive diagnostic techniques including radionuclide oesophageal scintigraphy, speckle tracking echocardiography, and cardiac magnetic resonance imaging can reveal subtle alterations in which prognosis value remains to be defined.6 Although this form generally carries a good prognosis, T.cruzi infected individuals are at risk of progression to heart or digestive disorders years after the acute phase.5 The earliest sign of progression is the development of electrocardiographic (ECG) abnormalities, which provides the initial evidence of cardiac involvement.6,8
Chagas cardiomyopathy presents with a wide variety of ECG abnormalities, ranging from nonspecific ST- and T-wave abnormalities to advanced atrioventricular block and ventricular tachyarrhythmias.9 The most common abnormalities are right bundle branch block, left anterior fascicular block, and diffuse ST-T changes.9 A greater number of typical ECG alterations are associated with the severity of the Chagas cardiomyopathy.10 Although several ECG changes have been reported in Chagas cardiomyopathy, some are more likely to be associated with left ventricular dilation and dysfunction. Artificial Intelligence (AI) can now understand the ECG's digital information, allowing for the creation of AI-powered systems capable of diagnosing left ventricular systolic dysfunction. A previous study using the AI-ECG algorithm to identify left ventricular dysfunction in 1304 patients with Chagas disease showed that the AI algorithm presented a high-level accuracy in recognising ventricular dysfunction. Moreover, during the course of the disease, the ECG shows progressive abnormalities that indicate worsening myocardial damage and adverse outcomes.11 Even in elderly patients with comorbidities, the appearance of a new major ECG abnormality predicts a higher risk of death.12
Chagas cardiomyopathy can present in a preclinical phase characterised by asymptomatic ECG abnormalities with preserved global left ventricular function.2,6,13 Subtle changes in left ventricular segmental contractility were detected either by conventional or speckle-tracking echocardiography.14 Patients then progress to left ventricular dilation with dysfunction before the appearance of heart failure symptoms. In some patients, right-sided heart failure may be prominent. However, when clinically apparent, right ventricular dysfunction is typically linked to advanced left ventricular dysfunction.13,15
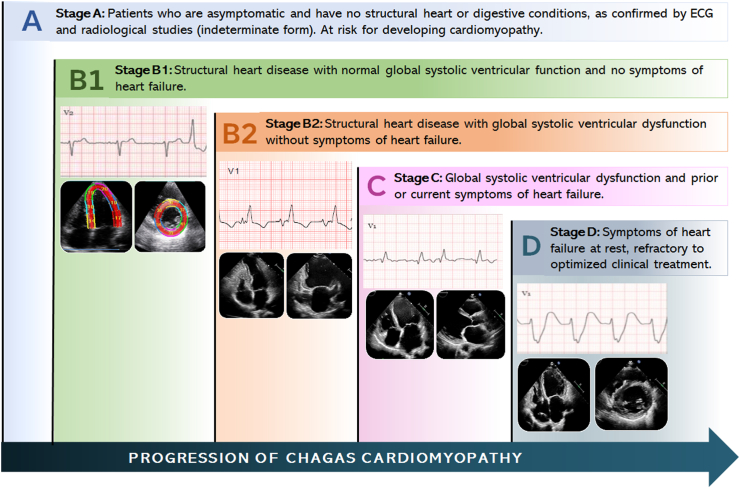
A classification for heart failure in Chagas cardiomyopathy taking into account the presence of general functional and structural changes, and specifically assessing left ventricular systolic function, facilitates the identification of subgroups with distinct prognoses.13 This classification proves valuable in guiding patient management strategies (Fig. 1). The incorporation of other cardiac imaging modalities, especially cardiac magnetic resonance (CMR), provides relevant insights into left ventricular function and the extent of myocardial fibrosis.
Fig. 1.
Clinical features, electrocardiogram (ECG), and echocardiographic findings based on the stage of Chagas disease.
Chagas cardiomyopathy generally develops gradually and steadily, although in some cases, it may have a more sudden course.3 The clinical presentations of this condition span a range of severity, from cases without symptoms, known as “silent” cardiopathy, to thromboembolic events, heart failure, and arrhythmias, which are the three primary clinical syndromes.13
Chagas cardiomyopathy typically manifests with heart failure symptoms, including exertional dyspnoea leading to a gradual reduction in physical activity. As the condition progresses, even mild exercise can induce dyspnoea, and patients may experience orthopnoea or paroxysmal nocturnal dyspnea.13,15 Additionally, dyspnoea may be accompanied by fatigue, abdominal discomfort in response to hepatic congestion, oedema, particularly in the lower limbs, nausea, anorexia, and eventually, cachexia.
Cardiac arrhythmia is a common feature in Chagas cardiomyopathy, with ventricular ectopic activity being predominant from the early stages of its natural course.3,6 The involvement of the sinus node and atrioventricular conduction system is also very common. Arrhythmias can be asymptomatic or result in palpitations, dizziness, shortness of breath, weakness, pre-syncope, syncope, or cardiac arrest. Sudden death is the most threatening manifestation of Chagas cardiomyopathy, frequently caused by physical activity and can be linked with ventricular tachycardia (VT), ventricular fibrillation (VF), and, less frequently, asystole or total heart block (THB).16 Usually, the severity of ventricular arrhythmias correlates with the degree of ventricular dysfunction.
Thromboembolic events are commonly observed in Chagas cardiomyopathy, and they are a significant contributor to mortality, ranking as the third most common cause of death in this population.3 In a clinical context, the main manifestation consists of thromboembolic events affecting the brain, followed by embolism to other systemic organs and limbs, as well as pulmonary embolism. The prevalence of risk factors for thromboembolic events can vary depending on the phase of the natural history of the disease.17
Patients with Chagas cardiomyopathy frequently report chest pain, often presenting as atypical angina, which is triggered by physical exertion in the absence of abnormalities in the epicardial coronary arteries. Patients with this condition may have abnormal myocardial perfusion, indicating an underlying issue with the regulation of myocardial blood flow at the microvascular level.18
The clinical presentation and prognosis of Chagas cardiomyopathy have recently changed significantly due to increased life expectancy, improved access to healthcare, and advances in heart failure treatment.13,15 Comorbidities become more common as people age, posing new challenges for the management and risk stratification of Chagas cardiomyopathy patients. Furthermore, efforts have been directed toward early disease diagnosis, to detect Chagas cardiomyopathy before symptoms and complications.
Neurological manifestations of Chagas disease
Since his initial studies, Carlos Chagas recognised that T. cruzi could affect the brain both acutely and chronically. As for other organs, the clinical manifestations of acute cerebral infection are rare. Symptomatic presentations usually affect children under 2 years of age and involve signs of meningoencephalitis (e.g., delirium, seizures, focal neurological signs). While these cases have high morbimortality, most patients enter into the chronic phase without any neurological problems.
The brain is a preferential site for Chagas disease reactivation during immunosuppression, for example, in patients with HIV infection or undergoing an immunosuppressant regimen for organ transplantation.19,20 In this context, the most common presentation is a tumour-like supratentorial lesion (also referred to as brain ‘chagoma’) with seizure and focal neurological signs.20 This presentation is clinically indistinguishable from other tumour-like lesions, such as neurotoxoplasmosis, that are frequently associated with HIV infection and other immunosuppressive conditions. Therefore, a high level of clinical suspicion is warranted when addressing people from endemic regions to consider a reactivated acute form of Chagas' disease as a potential differential diagnosis.
Cardioembolic strokes are a major concern when managing people with chronic forms of Chagas disease. Most patients present with an abrupt onset of partial anterior cerebral circulation syndrome that includes varying degrees of motor and/or sensory deficits involving the face, and limbs; homonymous hemianopia; and cortical dysfunction, such as aphasia.21 Anatomopathological studies have reported incidence of cerebral infarction in up to 35% of cases.19 In a cohort of 213 patients with Chagas disease cardiomyopathy, Nunes et al. reported an incidence of 2.67 ischemic cerebrovascular events (transient ischemic attack or stroke) per 100 patients/year.17 A similar number was found in a recent independent cohort by Cerqueira-Silva et al.22 These authors also reported that stroke incidence was higher in Chagas disease cardiomyopathy when compared to non-Chagas patients with heart failure (20.2 vs. 13.9 events per 1000 patient-years).22 Risk factors for cardioembolic strokes in Chagas disease include atrial fibrillation, left ventricle systolic dysfunction, apical aneurysm, and left ventricle thrombus.23
In endemic areas, Chagas disease is a relevant risk factor for ischemic stroke independent of the severity of the underlying cardiomyopathy, suggesting that factors other than thromboembolism are involved. Other causes, such as small vessel disease and atherosclerosis, have been identified in these patients.24 Some authors have also postulated that infection-driven microvascular changes and autoimmune mechanisms may contribute to the increased risk of stroke in this population, a hypothesis that remains to be confirmed.25 An important gap in the literature refers to the limited evidence to specifically guide primary and secondary prevention of cardioembolic stroke in Chagas disease. A scoring system to guide the decision of anticoagulation in this population has been proposed but not externally validated and implemented.26,27 Thus, general cardiology guidelines have been applied to the management of Chagas disease patients in clinical practice.21
The existence of a ‘chronic nervous form’ of the disease, characterised by chronic behavioural and cognitive symptoms, was originally proposed by Carlos Chagas.19,28 This form could represent the sequelae of the acute phase meningoencephalitis and/or infection-related progressive neurodegeneration.19,28 However, clinical and anatomopathological evidence has not confirmed its existence. For most authors, the neuronal loss and brain atrophy described, respectively, in pathological and neuroimaging studies represent the consequence of hypoxemia resulting from Chagas disease cardiomyopathy.29 Despite the highly controversial nature of this ‘chronic nervous form’, it is indisputable that patients with Chagas disease, especially those with cardiomyopathy, display a high frequency of behavioural (e.g., anxiety and depression) and cognitive impairments.30,31 Finally, it is important to acknowledge peripheral dysautonomia as a possible neurological manifestation of Chagas disease leading to cardiovascular, gastrointestinal, among other symptoms.32
Chagas disease progression
Impact of the anti-parasite treatment on progression
Treatment of T. cruzi infection still relies on drugs licensed more than 50 years ago: nifurtimox (launched by Bayer in 1965) and benznidazole (launched by Roche in 1971). The safety profile of both drugs is far from ideal, with frequent adverse events and high rates of drug discontinuation, mainly in adults. Although in the acute phase of the disease and in cases of reactivation, there is consensus on the indication for treatment, in the chronic phase there is still a high degree of uncertainty. Currently, parasiticidal treatment is offered to patients with chronic Chagas disease in the indeterminate phase, especially patients under 18 years of age.13 Serological cure is achieved in up to 100% of patients with congenital disease treated during the first year of life and in 76% of patients with acute disease. Moreover, the efficacy of treatment seems to decrease as time passes since the incidence of primary infection and is very poor when visceral involvement is established (60–93% in children aged up to 13 years and 2–40% in adults with late chronic disease.33
There is little evidence on the potential impact of treatment on the course of Chagas cardiomyopathy. In patients with cardiomyopathy, treatment should be offered to those with mild and moderate involvement.
Observational studies have demonstrated clinical benefit in both survival and disease progression.34, 35, 36 A recent meta-analysis of these observational studies found a modest effect of treatment with benznidazole on disease progression.37 The odds ratio (OR) for the progression of cardiac disease in patients treated with benznidazole versus placebo was OR 0.49 (95% CI: 0.2–1.2). Only one prospective observational study found a significant reduction of the risk of clinical progression in those treated with benznidazole (OR: 0.27; 95% CI: 0.1–0.5).38 In contrast, the single randomised placebo-controlled clinical trial designed to assess the effect of benznidazole on clinical progression failed to demonstrate any effect in reducing the progression of heart disease in a large adult population with moderate to severe cardiomyopathy followed during 5 years.39 Although the conclusions of this study are not generalisable to all patients in the chronic phase of the disease, it does suggest that treatment with benznidazole is not beneficial and should be contraindicated in advanced stages of the disease.
Risk factors related to host and parasite
Over the years to decades, about 30–40% of these patients progress to the determinate phase of the disease, characterised by cardiac and/or gastrointestinal manifestations.6 The progression from the indeterminate form to the cardiac form of the disease is due to the sum of several factors dependent on both the host and the parasite.
There is increasing evidence on predisposing factors to organ damage in patients with chronic Chagas disease; especially a pivotal role of the host's genetic background in regulating the innate and specific immune response against the parasite, which leads to tissue damage when it is improperly regulated.40 Different HLA class I and II polymorphisms have been identified as protective or predisposing factors to the development of chagasic cardiomyopathy in different Latin-American populations. The highly polymorphic HLA class I and II molecules determine the efficiency of presentation of the T. cruzi epitopes to CD8+ and CD4+ T cells, respectively. This modulation of the immune response could have an impact on the clinical expression of the disease.
The production of cytokines is under genetic control and is influenced by polymorphisms in several cytokine genes. Cytokines polymorphisms have also been associated with Chagas disease and its progression, such as TNF, lymphotoxin-α, IL6, IL1B, IL-10, IL-4, TGF-β, IL-12, macrophage migration inhibitory factor and chemokine ligand 9.41, 42, 43 Despite the data heterogeneity and the geographical variations in the findings, it is known that patients’ genetic background influences the production of TH1/TH2/T regulatory cytokines, thereby modulating the clinical progression of the Chagas disease since an immune response dysregulation leads to the development of the cardiac form.
Recent sequencing techniques such as GWAS have provided new data on the genetic determinants of the disease and immunological traits. These studies have identified new loci associated with a higher risk for developing Chagas cardiomyopathy (locus tagged by rs34238187 and located on chromosome 18 near C18orf42 and rs2458298 near SAC3D1 NAALADL1 on chromosome 11).44,45 Subsequently, it has been observed that these regions had higher methylation levels in patients with cardiomyopathy compared to controls. Moreover, other regions such as the CCDC88B gene, an inflammation-related gene, were also hypermethylated. This confirms the regulation of gene expression in an area already related to Chagas cardiomyopathy and reveals the regulation of gene expression of an inflammation.46,47 Interestingly, the genetic background has not only been associated with disease expression. A recent study has associated the variant of the PPP3CA gene with a protective effect against acquiring Chagas disease in Amazonian populations in the past, which probably led to a reduction in the prevalence of carriers.48
The diversity of T. cruzi strains and the multiplicity of their genotypes and phenotypes have long been recognised. Currently, these strains are grouped into seven discrete typing units (DTUs), TcI-TcVI, and Tcbat. This genetic diversity has been related to geographical distribution, pathogenesis, clinical features, and response to serological tests and therapy.49 Since the discovery of this genetic diversity, attempts have been made to correlate genotypic variability with phenotypic expression. Despite some progress, our ability to draw definitive conclusions remains somewhat constrained. Moreover, some DTUs seem to contribute to increased risk of certain morbidities. The alignment of the distribution of T. cruzi DTUs and disease severity has led to a general, though not absolute, association between TcI and acute and/or milder chronic cardiac disease and TcII, TcV, and TcVI and more severe chronic cardiac.50
Despite the persistence of the parasite in the tissue is well recognised as the underlying cause of the progression to myocardiopathy, it is still controversial whether the parasitaemia is associated with the cardiac form.51 A recent meta-analysis failed to demonstrate a correlation between parasitaemia through PCR and CCC.52 In only two studies, the proportion of PCR positivity was significantly greater within Chagas cardiomyopathy. Both studies were performed in endemic regions, suggesting the potential role of reinfections as a relevant factor in the progression of the disease.32
Social determinants of progression
Chagas disease is strongly associated with poor housing conditions in rural Latin American subsistence-level agricultural communities, providing the ideal domestic and peridomestic habitat for the triatomine vectors.6 Thus, Chagas cardiomyopathy, including severe forms, is more frequent among older men from endemic regions and specifically among those who spent their childhood and youth living in rural impoverished conditions compared to those who grew up under more favourable socioeconomic circumstances.53 Contemporary medical management of arrhythmias and congestive heart failure, pacemaker and implantable cardioverter-defibrillators (ICD), and cardiac transplantation have all greatly improved quality of life and prolonged survival among patients with Chagas cardiomyopathy.15 However, limited or lack of access to health resources translates to a poorer prognosis.54
Studies comparing quality of life indicators between groups of people with and without Chagas disease show worse scores for people with Chagas disease, particularly those who are living alone, obese, and with low educational attainment.55 It is less clear whether socioeconomic vulnerability independently affects the occurrence and progression of cardiomyopathy within the T. cruzi-infected population. The decades-long delay between initial infection and the earliest manifestations of Chagas cardiomyopathy complicates analyses that attempt to establish a causal link between social determinants and progression risk. By the time cardiac manifestations are detected, the individual may be living under radically different conditions and/or in an entirely different location from the one in which infection occurred. Theoretical mechanisms through which socioeconomic disadvantage could mediate the risk of cardiac progression include repeated exposure to the parasite in persistently infested housing (a surrogate for which could be the length of time living in a highly endemic/impoverished area), access to quality healthcare (specifically, access to anti-trypanosomal treatment early in life), nutritional factors, and intense physical activity based on occupation. Human data relating to these factors are sparse, and no published data are available describing the impact of food insecurity, unemployment, unstable housing, or structural conflict on the progression of Chagas disease.
Repeated exposure to T. cruzi
Since the 1920s, Chagas disease experts have hypothesised that repeated exposure to the parasite is a key factor driving the risk of Chagas cardiomyopathy based on clinical observations in highly affected villages before and after the institution of vector control. For example, typical village homes near the highly endemic Bolivian and Argentine Chaco are often constructed of natural materials (such as mud, wood, and thatch), creating many crevices that represent an ideal habitat for the Triatomine vector. 56,57 Thus, people living in such homes are likely repeatedly exposed to infected Triatomines, even after vector control programs are instituted in these areas of high endemicity.58,59
Elimination of parasitaemia in mouse models of chronic infection prevents progression to end-organ disease60 while repeated infections with the same or diverse T. cruzi strains increase the risk of cardiac progression.61 Detection of parasitaemia in humans with chronic Chagas disease is a risk factor for progression to Chagas cardiomyopathy in some studies.51,62
Reinfection
While it is plausible that repeated T. cruzi infection could play a role in the progression to Chagas cardiomyopathy,63 reinfection cannot be directly measured in humans. Detection of multiple circulating T. cruzi strains by molecular techniques has been proposed as a proxy, but these could have been acquired simultaneously from a vector with a mixed infection. Therefore, direct human evidence for an association between repeated infections and cardiac progression is lacking.
Vector control programs
Residence in poor housing (which facilitates human exposure to the Triatomine vector) may be an independent predictor of Chagas cardiomyopathy severity.53 Many studies show a shift in the pattern of age-specific prevalence of cardiac findings attributable to Chagas disease as vector control programs have become widespread and effective at eliminating house infestation.9 In a community survey after a successful vector control program in Venezuela, severe Chagas cardiomyopathy was observed to be less prevalent and to occur at older ages.64 Nevertheless, the post-vector control survey showed no difference in age-specific prevalence of ECG abnormalities among infected individuals, compared to historical surveys prior to institution of vector control.
Length of residence in an endemic area
A Brazilian case–control study of people with Chagas disease and characteristic ECG changes versus those with normal ECG65 did not show an association between cardiomyopathy and length of residence in an endemic area. However, an association was found between cardiomyopathy risk and having a sibling with one of the following factors: heart disease, death due to heart disease, sudden death or pacemaker, suggesting a possible genetic link. A study in Bolivia compared people with Chagas disease and a low versus normal left ventricular ejection fraction; the final multivariable model demonstrated that more severe disease was associated with male sex, older age, and decades living in housing with earthen walls and floor; no association was found with overweight, hypertension, coronary artery disease, diabetes or positive results by PCR.53 In a more recent analysis conducted as part of a congenital Chagas disease study, years of living in a house infested with triatomes was used as a proxy for the likelihood of repeated exposure.66 The study was conducted in the Bolivian Chaco, where intense house infestation was still widespread in rural villages and >20% of children had T. cruzi infection in a survey conducted around the same time,67 as well as in the city of Santa Cruz. Among women with Chagas disease aged 13–46 years, 28 (9.3%) had ECG abnormalities consistent with Chagas cardiomyopathy. The risk of cardiomyopathy rose by 6% per year of living in an infested house with a strong dose–response relationship, supporting the hypothesis that chronic exposure to the parasite may play a role in pathogenesis.
Access to healthcare
Adequate access to health services, specifically to anti-trypanosomal treatment, is necessary to reduce the risk of progression to Chagas cardiomyopathy. Unfortunately, healthcare access is challenging for a variety of reasons in many endemic regions. A Brazilian cohort study of 1637 people with Chagas disease suggested that those residing in areas with fewer physicians per thousand inhabitants, those living in municipalities with lower Primary Health Care coverage, and (with borderline significance) those who had never used benznidazole had a higher likelihood of developing cardiac complications.68 An Argentine cohort study of 801 Chagas disease patients found that those with medical insurance and more years of education were less likely to progress to Chagas cardiomyopathy.69
Nutritional factors
The typical diet of people living in rural highly endemic parts of the Chaco typically comprises primarily complex carbohydrates with occasional meat (ranging from game to goats to beef), though inclusion of cheap processed foods is gradually becoming more common.70,71 While nutritional factors have not been extensively studied as potential factors in Chagas cardiomyopathy, diet and lipid metabolism may be important factors that influence progression from indeterminate form to Chagas cardiomyopathy.72 These factors are challenging to interpret as determinants of cardiac progression given that significant variability exists in the diet and nutritional status of people from different socio-economic backgrounds and those who have migrated from rural to urban communities.
Diabetes risk factors
Hyperglycaemia appears to be more common in people with Chagas cardiomyopathy compared to age, sex, and BMI-matched groups with indeterminate chronic Chagas disease and people without Chagas disease.73 This observation could be related to diet and/or food insecurity. Importantly, a simple carbohydrate-rich diet is often associated with both poverty74 and cardiovascular disease.75
Additional evidence supports other metabolic risk factors for Chagas-related heart disease. In some studies, patients with Chagas cardiomyopathy are reported to have increased myocardial lipid levels.76 Murine models indicate that consumption of a high-fat diet during indeterminate chronic Chagas disease may alter lipid metabolism, facilitating progression to Chagas cardiomyopathy.77 A Brazilian cross-sectional study of 361 people with chronic Chagas disease reported a high prevalence of metabolic syndrome.72
Trace minerals, specifically selenium
The association of deficiency of the essential trace element selenium with other heart diseases led researchers to investigate its potential role in the progression of Chagas cardiomyopathy. Major dietary sources of selenium include meat, fish, and eggs. Poverty is therefore likely to be associated with lower selenium intake, although this connection has not been explicitly evaluated in studies of Chagas disease. In an observational study in Brazil, Chagas cardiomyopathy patients with NYHA class II-IV had significantly lower selenium levels than those in NYHA class I-II and healthy controls.78 However, a subsequent clinical trial among 66 patients in Chagas cardiac class B1 and B2 failed to demonstrate a significant impact of selenium supplementation on maintenance of LVEF at 1 year.79
Alcohol, tobacco, and recreational drug use
The influence of alcohol, tobacco, and recreational drug use on progression to Chagas cardiomyopathy have been evaluated by few studies68,80; no independent association has yet been identified.
Physical activity
Physical exercise has been hypothesised to prevent progression to Chagas cardiomyopathy, and this hypothesis is supported by murine studies such as one comparing T. cruzi infected sedentary mice to mice undergoing an exercise program; while both groups showed similar cardiac parasite loads, the exercised mice developed less cardiac fibrosis.81 Limited available human studies variably support physical activity as a risk factor for progression to Chagas cardiomyopathy.
While data on the social determinants impacting the progression of Chagas disease are sparse, important goals for clinicians and public health officials caring for populations at risk for Chagas disease should include (1) improving housing quality to prevent infection/reinfection, (2) strengthening vector control programs, and (3) facilitating healthcare access.
Chagas cardiomyopathy risk stratification
Chagas cardiomyopathy exhibits significant variability in its clinical course and prognosis. The classification, based on the severity of ventricular dysfunction and adapted from the American Heart Association (AHA), is employed to assess prognosis (Table 1). While many patients remain asymptomatic in the indeterminate form throughout their lives, there is a risk of progression to cardiomyopathy at a mean annual rate of 1.9%.82 Individuals who experienced the acute phase of Chagas disease have a higher risk of developing chronic cardiomyopathy, with a global estimate of 4.6% annually.82
Table 1.
Chagas disease classification based on clinical stages.
| Indeterminate form |
Chagas cardiomyopathy |
||||
|---|---|---|---|---|---|
| Stage Aa | Stage B1 | Stage B2 | Stage C | Stage D | |
| NYHA functional class | I | I | I | I, II, III or IV | IV |
| Electrocardiogram | Normal | Abnormal | Abnormal | Abnormal | Abnormal |
| Cardiomegaly on radiography | Absent | Absent | May be present | Usually present | Present |
| Segmental contractility abnormalities | Usually absent | May be present | May be present | May be present | May be present |
| Left ventricular ejection fraction (%) | ≥55% | ≥55% | <55% (41%–54%) | <55% (≤40%) | Usually ≤25% |
| Complex ventricular arrythmiab | Usually absent | May be present | Usually present | Present | Present |
| Myocardial fibrosis on CMR | Can be detected | Usually detected | Usually detected | Detected | Detected |
Abbreviation: CMR, Cardiac magnetic resonance.
At risk of developing cardiomyopathy in the absence of structural heart and digestive disease.
Ventricular premature beats occurring in pairs or runs on a 24-h Holter monitor.
In recent decades, the occurrence of cardiomyopathy linked to Chagas disease has been consistently decreasing due to successful vector control. A study with 499 blood donors with Chagas disease found that the annual progression to cardiomyopathy was 0.92%.83 Nevertheless, if the patient develops cardiomyopathy, the prognosis worsens with an increased likelihood of mortality. An estimated annual mortality rate of 7.9% was found in a meta-analysis of 52 studies on patients with cardiomyopathy.84 There was significant variation in mortality rates based on the specific characteristics of the populations in each study. Patients in stages C and D with left ventricular systolic dysfunction and overt heart failure have a mortality rate of 22.4% per year.84
Prognostic factors in Chagas cardiomyopathy have been increasingly recognised, allowing to identify patients at different risk levels. Several variables derived from different methods including clinical examination, ECG, Holter monitoring, stress testing, cardiopulmonary testing, chest X-ray, echocardiogram, and CMR are used to assess the severity of cardiomyopathy and guide mortality risk assessment (Table 2). Three key pathophysiologic characteristics of the disease should be considered. These risk factors consist of overt heart failure, NYHA functional class III/IV, left ventricular systolic dysfunction, and ventricular electrical instability, mainly determined by the presence of non-sustained ventricular tachycardia (NSVT) on 24-h Holter monitoring.13
Table 2.
Risk factors reported to be associated with mortality in Chagas cardiomyopathy.
| Clinical-ECG | Imaging | Other methods |
|---|---|---|
| Male gender | Reduced LVEFa | Cardiomegaly |
| Advanced age | RV dysfunction | Anti-T. cruzi antibody titer |
| NYHA functional class III/IVa | LV diastolic dysfunction | Increased BNP/NT-ProBNP |
| High heart rate | Myocardial fibrosis | T wave amplitude variability |
| Atrial fibrillation | Low speckle tracking strain | Non-sustained VTa |
| Pathological Q waves | Mechanical dispersion | Decreased exercise time |
| Increased QRS duration | Perfusion defects | Reduction of maximal O2 consume |
| Increased QT dispersion | Impaired sympathetic innervation | VT at the EF study |
Abbreviations: EF, electrophysiological study; LV, left ventricular; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; RV, right ventricular; VT, ventricular tachycardia.
Three key risk factors are NYHA functional class III/IV, left ventricular systolic dysfunction, and ventricular electrical instability, mainly determined by the presence of non-sustained ventricular tachycardia (NSVT) on 24-h Holter.
Several studies have examined the prognosis of Chagas cardiomyopathy, identifying different risk factors, such as reduced left ventricular ejection fraction, right ventricular systolic dysfunction, left ventricular diastolic dysfunction, increased left atrial volume, and alterations in myocardial strain indices. Certain alterations in the electrocardiogram were also indicative of adverse outcomes, particularly variability in T-wave amplitude, QT interval dispersion, reduced heart rate variability, and prolonged QRS complex duration. The Selvester QRS score is a useful clinical tool for assessing changes in ventricular depolarization recorded by a 12-lead ECG, which are associated with myocardial scarring and prognosis.85
Cardiac magnetic resonance (CMR) imaging plays a pivotal role in risk stratification for patients with Chagas cardiomyopathy. The presence and extent of myocardial fibrosis were correlated with poor prognosis. Similarly, elevated plasma levels of B-type natriuretic peptides (BNP and NT-proBNP) were significant predictors of mortality. When considered individually, variables associated with a worse prognosis generally exhibit low positive predictive value, limiting their use. Therefore, there has been a shift towards investigating risk scores prognostic models constructed from diverse combinations of demographic, clinical, and laboratory parameters. However, these scores can be challenging to integrate into daily practice.
Rassi et al.86 developed and validated a widely used risk score for predicting all-cause mortality in Chagas cardiomyopathy. In the original cohort of 424 patients followed for an average of 7.9 years, the overall mortality rate was 31%. Patients were divided into low, medium, and high-risk groups, with 10-year mortality rates of 10%, 44%, and 84%, respectively. Another risk score has been developed to predict death during 2 years in patients with Chagas cardiomyopathy.87 This score includes five variables that can be easily assessed at public health primary care units, making it a reliable tool for risk stratification in community-based patients. However, the proposed scores have limitations to use in routine medical care as complementary exams are not readily available in the limited-resource setting. Thus, diagnosis at a subclinical stage is the best approach to therapeutic decision-making in patients with Chagas cardiomyopathy to prevent disease progression.
Summary and conclusions
We provide an overview of the clinical manifestations of Chagas disease, with a particular focus on cardiovascular and neurological presentations. This aims to improve understanding of the wide range of clinical aspects of the disease. Moreover, we meticulously outline the main factors that impact the disease progression and severity. Through the identification of these determinants, healthcare providers can accurately categorise risk and tailor therapies accordingly, leading to improved patient care and overall prognosis.
Contributors
MCPN = conceptualisation, writing—original draft, and writing—review & editing.
CB = supervision, writing—original draft, and writing—review & editing.
EHC = writing—original draft, and writing—review & editing.
ALT = methodology, writing—original draft, and writing—review & editing.
IM = supervision, writing—original draft, and writing—review &editing.
Declaration of interests
C.B. has received royalties for Chagas disease topics from UpToDate (Wolters Kluwer Health).
M.C.P.N., E.H.P., A.L.T., and I.M. have no conflicts of interest to declare.
Acknowledgements
Dr Nunes is supported in part by National Council for Scientific and Technological Development (CNPq) and TMRC-grant number U01 AI168383/AI.
References
- 1.Moolani Y., Bukhman G., Hotez P.J. Neglected tropical diseases as hidden causes of cardiovascular disease. PLoS Negl Trop Dis. 2012;6(6) doi: 10.1371/journal.pntd.0001499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bern C. Chagas' disease. N Engl J Med. 2015;373(5):456–466. doi: 10.1056/NEJMra1410150. [DOI] [PubMed] [Google Scholar]
- 3.Nunes M.C., Dones W., Morillo C.A., Encina J.J., Ribeiro A.L., Council on Chagas Disease of the Interamerican Society of C Chagas disease: an overview of clinical and epidemiological aspects. J Am Coll Cardiol. 2013;62(9):767–776. doi: 10.1016/j.jacc.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 4.Organization WH . World Health Organization website; 2018. Chagas disease (American trypanosomiasis)http://www.who.int/mediacentre/factsheets/fs340/en.2019 [PMC free article] [PubMed] [Google Scholar]
- 5.Carrasco H.J., Segovia M., Llewellyn M.S., et al. Geographical distribution of Trypanosoma cruzi genotypes in Venezuela. PLoS Negl Trop Dis. 2012;6(6) doi: 10.1371/journal.pntd.0001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pérez-Molina J.A., Molina I. Chagas disease. Lancet. 2018;391(10115):82–94. doi: 10.1016/S0140-6736(17)31612-4. [DOI] [PubMed] [Google Scholar]
- 7.Torres R.M., Correia D., Nunes MdCP., et al. Prognosis of chronic Chagas heart disease and other pending clinical challenges. Mem Inst Oswaldo Cruz. 2022;117 doi: 10.1590/0074-02760210172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marin-Neto J.A., Almeida Filho O.C., Pazin-Filho A., Maciel B.C. [Indeterminate form of Chagas' disease. Proposal of new diagnostic criteria and perspectives for early treatment of cardiomyopathy] Arq Bras Cardiol. 2002;79(6):623–627. doi: 10.1590/s0066-782x2002001500008. [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro A.L., Sabino E.C., Marcolino M.S., et al. Electrocardiographic abnormalities in Trypanosoma cruzi seropositive and seronegative former blood donors. PLoS Negl Trop Dis. 2013;7(2) doi: 10.1371/journal.pntd.0002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Figueiredo Brito B.O., Pinto-Filho M.M., Cardoso C.S., et al. Association between typical electrocardiographic abnormalities and NT-proBNP elevation in a large cohort of patients with Chagas disease from endemic area. J Electrocardiol. 2018;51(6):1039–1043. doi: 10.1016/j.jelectrocard.2018.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brito BOdF., Attia Z.I., Martins L.N.A., et al. Left ventricular systolic dysfunction predicted by artificial intelligence using the electrocardiogram in Chagas disease patients–the SaMi-Trop cohort. PLoS Negl Trop Dis. 2021;15(12) doi: 10.1371/journal.pntd.0009974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brito B.O.F., Lima E.M., Soliman E.Z., Silva E.F., Lima-Costa M.F., Ribeiro A.L.P. The evolution of electrocardiographic abnormalities in the elderly with Chagas disease during 14 years of follow-up: the Bambui cohort study of aging. PLoS Negl Trop Dis. 2023;17(6) doi: 10.1371/journal.pntd.0011419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marin-Neto J.A., Rassi Jr A., Oliveira G.M.M., et al. Diretriz da SBC sobre Diagnóstico e Tratamento de Pacientes com Cardiomiopatia da Doença de Chagas–2023. Arq Bras Cardiol. 2023;120 doi: 10.36660/abc.20230269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbosa M.M., Rocha M.O.C., Vidigal D.F., et al. Early detection of left ventricular contractility abnormalities by two-dimensional speckle tracking strain in Chagas' disease. Echocardiography. 2014;31(5):623–630. doi: 10.1111/echo.12426. [DOI] [PubMed] [Google Scholar]
- 15.Nunes M.C.P. Chagas cardiomyopathy: an update of current clinical knowledge and, a scientific statement from the American heart association. Circulation. 2018;138(12):e169–e209. doi: 10.1161/CIR.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 16.Rassi A., Jr., Rassi S.G., Rassi A. Sudden death in Chagas' disease. Arq Bras Cardiol. 2001;76(1):75–96. doi: 10.1590/s0066-782x2001000100008. [DOI] [PubMed] [Google Scholar]
- 17.Nunes M.C.P., Barbosa M.M., Ribeiro A.L.P., Barbosa F.B.L., Rocha M.O. Ischemic cerebrovascular events in patients with Chagas cardiomyopathy: a prospective follow-up study. J Neurol Sci. 2009;278(1-2):96–101. doi: 10.1016/j.jns.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Hiss F.C., Lascala T.F., Maciel B.C., Marin-Neto J.A., Simoes M.V. Changes in myocardial perfusion correlate with deterioration of left ventricular systolic function in chronic Chagas' cardiomyopathy. JACC Cardiovasc Imaging. 2009;2(2):164–172. doi: 10.1016/j.jcmg.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Pittella J.E. Central nervous system involvement in Chagas disease: a hundred-year-old history. Trans R Soc Trop Med Hyg. 2009;103(10):973–978. doi: 10.1016/j.trstmh.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Shelton W.J., Gonzalez J.M. Outcomes of patients in Chagas disease of the central nervous system: a systematic review. Parasitology. 2024;151(1):15–23. doi: 10.1017/S0031182023001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lage T.A.R., Tupinambas J.T., Padua L.B., et al. Stroke in Chagas disease: from pathophysiology to clinical practice. Rev Soc Bras Med Trop. 2022;55 doi: 10.1590/0037-8682-0575-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerqueira-Silva T., Goncalves B.M., Pereira C.B., et al. Chagas disease is an independent predictor of stroke and death in a cohort of heart failure patients. Int J Stroke. 2022;17(2):180–188. doi: 10.1177/17474930211006284. [DOI] [PubMed] [Google Scholar]
- 23.Nunes M.C., Kreuser L.J., Ribeiro A.L., et al. Prevalence and risk factors of embolic cerebrovascular events associated with Chagas heart disease. Glob Heart. 2015;10(3):151–157. doi: 10.1016/j.gheart.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Montanaro V.V.A., Silva G.S., Oliveira Filho J., et al. Exploring the chagas disease-stroke ‘connection’: findings from a large multicenter study. Cerebrovasc Dis. 2024:1. doi: 10.1159/000536068. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira-Filho J. Stroke and brain atrophy in chronic Chagas disease patients: a new theory proposition. Dement Neuropsychol. 2009;3(1):22–26. doi: 10.1590/S1980-57642009DN30100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sousa A.S., Xavier S.S., Freitas G.R., Hasslocher-Moreno A. Prevention strategies of cardioembolic ischemic stroke in Chagas' disease. Arq Bras Cardiol. 2008;91(5):306–310. doi: 10.1590/s0066-782x2008001700004. [DOI] [PubMed] [Google Scholar]
- 27.Mendes F., Mediano M.F.F., Silva R.S., et al. Discussing the score of cardioembolic ischemic stroke in chagas disease. Trop Med Infect Dis. 2020;5(2):82. doi: 10.3390/tropicalmed5020082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lannes-Vieira J., Vilar-Pereira G., Barrios L.C., Silva A.A. Anxiety, depression, and memory loss in Chagas disease: a puzzle far beyond neuroinflammation to be unpicked and solved. Mem Inst Oswaldo Cruz. 2023;118 doi: 10.1590/0074-02760220287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveira-Filho J., Vieira-de-Melo R.M., Reis P.S., et al. Chagas disease is independently associated with brain atrophy. J Neurol. 2009;256:1363–1365. doi: 10.1007/s00415-009-5105-7. [DOI] [PubMed] [Google Scholar]
- 30.de Souza A.C., da Costa Rocha M.O., Teixeira A.L., Júnior J.O.D., de Sousa L.A.P., Nunes M.C.P. Depressive symptoms and disability in chagasic stroke patients: impact on functionality and quality of life. J Neurol Sci. 2013;324(1-2):34–37. doi: 10.1016/j.jns.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Dias J.S., Lacerda A.M., Vieira-de-Melo R.M., et al. Cognitive dysfunction in chronic Chagas disease cardiomyopathy. Dement Neuropsychol. 2009;3:27–33. doi: 10.1590/S1980-57642009DN30100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olivo Freites C., Sy H., Gharamti A., et al. Chronic chagas disease—the potential role of reinfections in cardiomyopathy pathogenesis. Curr Heart Fail Rep. 2022;19(5):279–289. doi: 10.1007/s11897-022-00568-9. [DOI] [PubMed] [Google Scholar]
- 33.Pérez-Molina J.A., Crespillo-Andújar C., Bosch-Nicolau P., Molina I. Trypanocidal treatment of Chagas disease. Enferm Infecc Microbiol Clín. 2021;39(9):458–470. doi: 10.1016/j.eimce.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Viotti R., Vigliano C., Lococo B., et al. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med. 2006;144(10):724–734. doi: 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]
- 35.Cardoso C.S., Ribeiro A.L.P., Oliveira C.D.L., et al. Beneficial effects of benznidazole in Chagas disease: NIH SaMi-Trop cohort study. PLoS Negl Trop Dis. 2018;12(11) doi: 10.1371/journal.pntd.0006814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasslocher-Moreno A.M., Saraiva R.M., Sangenis L.H., et al. Benznidazole decreases the risk of chronic Chagas disease progression and cardiovascular events: a long-term follow up study. eClinicalMedicine. 2021;31:100694. doi: 10.1016/j.eclinm.2020.100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crespillo-Andújar C., Comeche B., Hamer D.H., et al. Use of benznidazole to treat chronic Chagas disease: an updated systematic review with a meta-analysis. PLoS Negl Trop Dis. 2022;16(5) doi: 10.1371/journal.pntd.0010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crespillo-Andújar C., Venanzi-Rullo E., López-Vélez R., et al. Safety profile of benznidazole in the treatment of chronic Chagas disease: experience of a referral centre and systematic literature review with meta-analysis. Drug Saf. 2018;41:1035–1048. doi: 10.1007/s40264-018-0696-5. [DOI] [PubMed] [Google Scholar]
- 39.Morillo C.A., Marin-Neto J.A., Avezum A., et al. Randomized trial of benznidazole for chronic Chagas' cardiomyopathy. N Engl J Med. 2015;373(14):1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- 40.Ayo C.M., Dalalio MMdO., Visentainer J.E.L., et al. Genetic susceptibility to Chagas disease: an overview about the infection and about the association between disease and the immune response genes. Biomed Res Int. 2013;2013:284729. doi: 10.1155/2013/284729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flórez O., Martín J., González C. Interleukin 4, interleukin 4 receptor-α and interleukin 10 gene polymorphisms in Chagas disease. Parasite Immunol. 2011;33(9):506–511. doi: 10.1111/j.1365-3024.2011.01314.x. [DOI] [PubMed] [Google Scholar]
- 42.Araújo-Jorge T.C., Waghabi M., Bailly S., Feige J.J. The TGF-β pathway as an emerging target for chagas disease therapy. Clin Pharmacol Ther. 2012;92(5):613–621. doi: 10.1038/clpt.2012.102. [DOI] [PubMed] [Google Scholar]
- 43.Cunha-Neto E., Chevillard C. Chagas disease cardiomyopathy: immunopathology and genetics. Mediators Inflamm. 2014;2014:683230. doi: 10.1155/2014/683230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casares-Marfil D., Strauss M., Bosch-Nicolau P., et al. A genome-wide association study identifies novel susceptibility loci in chronic Chagas cardiomyopathy. Clin Infect Dis. 2021;73(4):672–679. doi: 10.1093/cid/ciab090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabino E.C., Franco L.A.M., Venturini G., et al. Genome-wide association study for Chagas Cardiomyopathy identify a new risk locus on chromosome 18 associated with an immune-related protein and transcriptional signature. PLoS Negl Trop Dis. 2022;16(10) doi: 10.1371/journal.pntd.0010725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Casares-Marfil D., Kerick M., Andrés-León E., et al. GWAS loci associated with Chagas cardiomyopathy influences DNA methylation levels. PLoS Negl Trop Dis. 2021;15(10) doi: 10.1371/journal.pntd.0009874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brochet P., Ianni B., Nunes J.P., et al. Blood DNA methylation marks discriminate Chagas cardiomyopathy disease clinical forms. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1020572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Couto-Silva C.M., Nunes K., Venturini G., et al. Indigenous people from Amazon show genetic signatures of pathogen-driven selection. Sci Adv. 2023;9(10) doi: 10.1126/sciadv.abo0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zingales B. Trypanosoma cruzi genetic diversity: something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018;184:38–52. doi: 10.1016/j.actatropica.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 50.Tavares de Oliveira M., Fuzo C.A., da Silva M.C., et al. Correlation of TcII discrete typing units with severe chronic Chagas cardiomyopathy in patients from various Brazilian geographic regions. PLoS Negl Trop Dis. 2022;16(12) doi: 10.1371/journal.pntd.0010713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabino E., Ribeiro A., Lee T., et al. Detection of Trypanosoma cruzi DNA in blood by PCR is associated with Chagas cardiomyopathy and disease severity. Eur J Heart Fail. 2015;17(4):416–423. doi: 10.1002/ejhf.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosch-Nicolau P., Espinosa-Pereiro J., Salvador F., Sánchez-Montalvá A., Molina I. Association between trypanosoma cruzi DNA in peripheral blood and chronic chagasic cardiomyopathy: a systematic review. Front Cardiovasc Med. 2022;8 doi: 10.3389/fcvm.2021.787214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hidron A., Gilman R., Justiniano J., et al. Chagas cardiomyopathy in the context of the chronic disease transition. PLoS Negl Trop Dis. 2010;4(5) doi: 10.1371/journal.pntd.0000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clark E.H., Sherbuk J., Okamoto E., et al. Hyperendemic Chagas disease and the unmet need for pacemakers in the Bolivian Chaco. PLoS Negl Trop Dis. 2014;8(6) doi: 10.1371/journal.pntd.0002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olivera M.J., Fory J.A., Buitrago G. Comparison of health-related quality of life in outpatients with chagas and matched non-chagas chronic heart failure in Colombia: a cross-sectional analysis. Am J Trop Med Hyg. 2021;104(3):951–958. doi: 10.4269/ajtmh.20-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salm A., Gertsch J. Cultural perception of triatomine bugs and Chagas disease in Bolivia: a cross-sectional field study. Parasit Vectors. 2019;12(1):291. doi: 10.1186/s13071-019-3546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goncalves R., Landivar D., Grover Sanez Liendo E., et al. Improving houses in the Bolivian Chaco increases effectiveness of residual insecticide spraying against infestation with Triatoma infestans, vector of Chagas disease. Trop Med Int Health. 2021;26(9):1127–1138. doi: 10.1111/tmi.13640. [DOI] [PubMed] [Google Scholar]
- 58.Perez-Cascales E., Sossa-Soruco V.M., Breniere S.F., Depickere S. Reinfestation with Triatoma infestans despite vigilance efforts in the municipality of Saipina, Santa Cruz, Bolivia: situational description two months after fumigation. Acta Trop. 2020;203 doi: 10.1016/j.actatropica.2019.105292. [DOI] [PubMed] [Google Scholar]
- 59.Gaspe M.S., Cardinal M.V., Fernandez M.D.P., et al. Improved vector control of Triatoma infestans limited by emerging pyrethroid resistance across an urban-to-rural gradient in the Argentine Chaco. Parasit Vectors. 2021;14(1):437. doi: 10.1186/s13071-021-04942-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang L., Tarleton R.L. Parasite persistence correlates with disease severity and localization in chronic Chagas' disease. J Infect Dis. 1999;180(2):480–486. doi: 10.1086/314889. [DOI] [PubMed] [Google Scholar]
- 61.Bustamante J.M., Novarese M., Rivarola H.W., et al. Reinfections and Trypanosoma cruzi strains can determine the prognosis of the chronic chagasic cardiopathy in mice. Parasitol Res. 2007;100:1407–1410. doi: 10.1007/s00436-006-0425-3. [DOI] [PubMed] [Google Scholar]
- 62.Basquiera A.L., Sembaj A., Aguerri A.M., et al. Risk progression to chronic Chagas cardiomyopathy: influence of male sex and of parasitaemia detected by polymerase chain reaction. Heart. 2003;89(10):1186–1190. doi: 10.1136/heart.89.10.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dias J.C.P. Chagas disease control and the natural history of human Chagas disease: a possible interaction? Mem Inst Oswaldo Cruz. 2000;95(Suppl II):14–22. [Google Scholar]
- 64.Acquatella H., Catalioti F., Gomez-Mancebo J.R., Davalos V., Villalobos L. Long-term control of Chagas disease in Venezuela: effects on serologic findings, electrocardiographic abnormalities, and clinical outcome. Circulation. 1987;76(3):556–562. doi: 10.1161/01.cir.76.3.556. [DOI] [PubMed] [Google Scholar]
- 65.Zicker F., Smith P.G., Netto J.C., Oliveira R.M., Zicker E.M. Physical activity, opportunity for reinfection, and sibling history of heart disease as risk factors for Chagas' cardiopathy. Am J Trop Med Hyg. 1990;43(5):498–505. doi: 10.4269/ajtmh.1990.43.498. [DOI] [PubMed] [Google Scholar]
- 66.Kaplinski M., Jois M., Galdos-Cardenas G., et al. Sustained domestic vector exposure is associated with increased Chagas cardiomyopathy risk but decreased parasitemia and congenital transmission risk among young women in Bolivia. Clin Infect Dis. 2015;61(6):918–926. doi: 10.1093/cid/civ446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hopkins T., Goncalves R., Mamani J., Courtenay O., Bern C. Chagas disease in the Bolivian Chaco: persistent transmission indicated by childhood seroscreening study. Int J Infect Dis. 2019;86:175–177. doi: 10.1016/j.ijid.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 68.Ferreira A.M., Sabino E.C., Oliveira L.C., et al. Impact of the social context on the prognosis of Chagas disease patients: multilevel analysis of a Brazilian cohort. PLoS Negl Trop Dis. 2020;14(6) doi: 10.1371/journal.pntd.0008399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Viotti R., Vigliano C.A., Alvarez M.G., et al. The impact of socioeconomic conditions on chronic Chagas disease progression. Rev Esp Cardiol. 2009;62(11):1224–1232. doi: 10.1016/s1885-5857(09)73349-3. [DOI] [PubMed] [Google Scholar]
- 70.Jones A.D. The production diversity of subsistence farms in the Bolivian Andes is associated with the quality of child feeding practices as measured by a validated summary feeding index. Public Health Nutr. 2015;18(2):329–342. doi: 10.1017/S1368980014000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Valeggia C.R., Burke K.M., Fernandez-Duque E. Nutritional status and socioeconomic change among Toba and Wichi populations of the Argentinean Chaco. Econ Hum Biol. 2010;8(1):100–110. doi: 10.1016/j.ehb.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xavier I.G.G., Vieira M.C., Rodrigues Junior L.F., et al. Prevalence of metabolic syndrome and associated factors among patients with chronic Chagas disease. PLoS One. 2021;16(4) doi: 10.1371/journal.pone.0249116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.dos Santos V.M., da Cunha S.F., Teixeira Vde P., et al. [Frequency of diabetes mellitus and hyperglycemia in chagasic and non-chagasic women] Rev Soc Bras Med Trop. 1999;32(5):489–496. doi: 10.1590/s0037-86821999000500004. [DOI] [PubMed] [Google Scholar]
- 74.Duncan B.B., Franca E.B., Passos V.M.A., et al. The burden of diabetes and hyperglycemia in Brazil and its states: findings from the global burden of disease study 2015. Rev Bras Epidemiol. 2017;20Suppl 1(Suppl 01):90–101. doi: 10.1590/1980-5497201700050008. [DOI] [PubMed] [Google Scholar]
- 75.Jenkins D.J.A., Dehghan M., Mente A., et al. Glycemic index, glycemic load, and cardiovascular disease and mortality. N Engl J Med. 2021;384(14):1312–1322. doi: 10.1056/NEJMoa2007123. [DOI] [PubMed] [Google Scholar]
- 76.Johndrow C., Nelson R., Tanowitz H., Weiss L.M., Nagajyothi F. Trypanosoma cruzi infection results in an increase in intracellular cholesterol. Microbes Infect. 2014;16(4):337–344. doi: 10.1016/j.micinf.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lizardo K., Ayyappan J.P., Cui M.H., Balasubramanya R., Jelicks L.A., Nagajyothi J.F. High fat diet aggravates cardiomyopathy in murine chronic Chagas disease. Microbes Infect. 2019;21(1):63–71. doi: 10.1016/j.micinf.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rivera M.T., de Souza A.P., Hasslocher-Moreno A.M., et al. Progressive Chagas' cardiomyopathy is associated with low selenium levels. Am J Trop Med Hyg. 2002;66(6):706–712. doi: 10.4269/ajtmh.2002.66.706. [DOI] [PubMed] [Google Scholar]
- 79.Holanda M.T., Mediano M.F.F., Hasslocher-Moreno A.M., et al. Effects of Selenium treatment on cardiac function in Chagas heart disease: results from the STCC randomized Trial. eClinicalMedicine. 2021;40 doi: 10.1016/j.eclinm.2021.101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silva Sde A., Gontijo E.D., Amaral C.F. Case-control study of factors associated with chronic Chagas heart disease in patients over 50 years of age. Mem Inst Oswaldo Cruz. 2007;102(7):845–851. doi: 10.1590/s0074-02762007000700010. [DOI] [PubMed] [Google Scholar]
- 81.Pedra-Rezende Y., Barbosa J.M.C., Bombaca A.C.S., et al. Physical exercise promotes a reduction in cardiac fibrosis in the chronic indeterminate form of experimental chagas disease. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.712034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chadalawada S., Sillau S., Archuleta S., et al. Risk of chronic cardiomyopathy among patients with the acute phase or indeterminate form of chagas disease: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(8):e2015072. doi: 10.1001/jamanetworkopen.2020.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nunes M.C.P., Buss L.F., Silva J.L.P., et al. Incidence and predictors of progression to Chagas cardiomyopathy: long-term follow-up of trypanosoma cruzi–seropositive individuals. Circulation. 2021;144(19):1553–1566. doi: 10.1161/CIRCULATIONAHA.121.055112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chadalawada S., Rassi Jr A., Samara O., et al. Mortality risk in chronic Chagas cardiomyopathy: a systematic review and meta-analysis. ESC Heart Fail. 2021;8(6):5466–5481. doi: 10.1002/ehf2.13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Loring Z., Chelliah S., Selvester R.H., Wagner G., Strauss D.G. A detailed guide for quantification of myocardial scar with the Selvester QRS score in the presence of electrocardiogram confounders. J Electrocardiol. 2011;44(5):544–554. doi: 10.1016/j.jelectrocard.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rassi A., Jr., Rassi A., Little W.C., et al. Development and validation of a risk score for predicting death in Chagas' heart disease. N Engl J Med. 2006;355(8):799–808. doi: 10.1056/NEJMoa053241. [DOI] [PubMed] [Google Scholar]
- 87.Oliveira C.D.L., Nunes M.C.P., Colosimo E.A., et al. Risk score for predicting 2-year mortality in patients with chagas cardiomyopathy from endemic areas: sami-trop cohort study (vol 9, e014176, 2020) J Am Heart Assoc. 2021;9(6) doi: 10.1161/JAHA.119.014176. [DOI] [PMC free article] [PubMed] [Google Scholar]