Abstract
Norepinephrine (NE) modulates synaptic transmission and long-term plasticity through distinct subtype adrenergic receptor (AR)-mediated-intracellular signaling cascades. However, the role of NE modulates glutamatergic long-term potentiation (LTP) in the hypothalamic paraventricular nucleus (PVN) magnocellular neuroendocrine cells (MNCs) is unclear. We here investigate the effect of NE on high frequency stimulation (HFS)-induced glutamatergic LTP in rat hypothalamic PVN MNCs in vitro, by whole-cell patch-clamp recording, biocytin staining and pharmacological methods. Delivery of HFS induced glutamatergic LTP with a decrease in N2/N1 ratio in the PVN MNCs, which was enhanced by application of NE (100 nM). HFS-induced LTP was abolished by the blockade of N-methyl-D-aspartate receptors (NMDAR) with D-APV, but it was rescued by the application of NE. NE failed to rescue HFS-induced LTP of MNCs in the presence of a selective β1-AR antagonist, CGP 20712. However, application of β1-AR agonist, dobutamine HCl rescued HFS-induced LTP of MNCs in the absence of NMDAR activity. In the absence of NMDAR activity, NE failed to rescue HFS-induced MNC LTP when protein kinase A (PKA) was inhibited by extracellular applying KT5720 or intracellular administration of PKI. These results indicate that NE activates β1-AR and triggers HFS to induce a novel glutamatergic LTP of hypothalamic PVN NMCs via the postsynaptic PKA signaling pathway in vitro in rats.
Keywords: Adrenergic receptor, Long-term potentiation, Magnocellular neuroendocrine neuron, Paraventricular hypothalamic nucleus, Synaptic transmission
INTRODUCTION
In hypothalamic paraventricular nucleus (PVN), the excitatory glutamatergic inputs and inhibitory gamma-aminobutyric acid (GABA) afferents from other regions of the brain modulate the activity of magnocellular neuroendocrine cells (MNCs), such as oxytocin (OT) and vasopressin (VP) cells [1-4]. Activation of glutamatergic inputs induces glutamate release, evokes excitatory postsynaptic currents (EPSCs) and produces long-term synaptic change in PVN MNCs [1,5,6].
Long-term plasticity of the excitatory glutamatergic synaptic transmission in the hypothalamic MNCs plays important roles in controlling physiological and pathophysiological responses [1,7,8]. Under in vitro conditions, high frequency stimulation (HFS) of the excitatory inputs has been shown to induce an N-methyl-D-aspartate receptors (NMDAR)/nitric oxide (NO) cascades-dependent presynaptic glutamatergic long-term potentiation (LTP) in PVN MNCs of rats through the protein kinase A (PKA) signaling pathway [1]. The induction of glutamatergic LTP in the hypothalamic PVN OT-ergic neurons is dependent on NMDAR [9]. Importantly, the long-term changes of glutamatergic and GABAergic synaptic transmission of the PVN neuronal circuitry contribute to various physiological response [1,9-11]. Numerous evidences have shown that stressors produce long-term changes in the glutamatergic and GABAergic synaptic inputs of PVN MNCs, and alternates the neuronal secretion of OT, VP and other hormones [12-14]. In addition, acute stress decreases the threshold of induction of a late-onset LTP at excitatory CA1 to subicular burst-spiking neuronal synapses via activation of NMDAR and β-adrenergic receptors (AR) [15].
Norepinephrine (NE) distinctly modulates neuronal activity, synaptic transmission and long-term plasticity via various subtypes of AR-mediated intracellular signaling cascades [16]. Under in vitro conditions, NE was shown to increase the frequency of spontaneous inhibitory synaptic currents (sIPSCs) via somatic or dendritic α1-ARs, but decreases the frequency of sIPSCs via presynaptic GABAergic neuronal α2-ARs in PVN parvocellular cells in vitro [17]. In cerebellar cortex, activation of β-AR by NE did not directly alter parallel fiber-Purkinje cell synaptic transmission in the flocculus, but decreased the threshold for induction of long-term depression through PKA signaling pathway [18]. Furthermore, NE was shown to decrease spontaneous complex spikes activity and the facial stimulation-evoked molecular interneuron-Purkinje cell synaptic transmission via AR/PKA signaling pathway in mouse cerebellar cortex in vitro [19,20]. Notably, both α- and β-AR subtypes are abundantly expressed in the hypothalamic PVN, and contributes to modulation the neuronal activity and synaptic transmission during various stress response [21,22], suggesting that NE modulates the long-term synaptic plasticity of the excitatory glutamatergic inputs in hypothalamic PVN MNCs. However, the role of NE during induction of the glutamatergic LTP in hypothalamic PVN MNCs is unknown. Therefore, we here investigated the mechanism of NE modulated HFS-induced glutamatergic LTP in the hypothalamic PVN MNCs in vitro in rats, employing whole-cell patch-clamp recording technique and pharmacological methods.
METHODS
Animal preparation
The procedures of experiments were approved by the Animal Care and Use Committee of Yanbian University and were in accordance with the animal welfare guidelines of the U.S. National Institutes of Health. The permit number is SYXK (Ji) 2011-006. Male Wistar rats (postnatal day 28–35) were group-housed on a 12-h light: 12-h dark cycle with free access to food and water, under constant temperature (24°C ± 1°C) and humidity (50% ± 5%).
Hypothalamic slice preparation
A total of 61 Wistar rats were deeply anaesthetized with isoflurane and decapitated. The brain was quickly removed and immerged in ice-cold oxygenated artificial cerebrospinal fluid (ACSF) containing the following: (in mM) 125 NaCl, 3 KCl, 1 MgSO4, 2 CaCl2, 1 NaH2PO4, 25 NaHCO3, and 10 D-glucose bubbled with 95% O2/5% CO2 (pH 7.3; 295–300 mOsm). Coronal hypothalamic slices were sectioned to 350 µm by a vibratome (VT 1200s; Leica), and recovered for at least 1 h in a chamber filled with 95% O2/5% CO2 equilibrated ACSF at room temperature (24°C–26°C) before whole-cell patch-clamp recordings were performed [1,23].
Whole-cell recording, electric stimulation and biocytin histochemistry
The hypothalamic slices were visualized using a Nikon microscopy (Eclipse FN1; Nikon Corp.) fitted with infrared differential interference contrast optics. Recording electrodes were pulled by a puller (P100; Narishiga), and filled with a solution containing (in mM): potassium gluconate 120, HEPES 10, EGTA 1, KCl 5, MgCl2 3.5, NaCl 4, biocytin 8, Na2ATP 4, and Na2GTP 0.2 (pH 7.3 with KOH, osmolarity adjusted to 300 mOsm).
Whole-cell patch-clamp recordings from the PVN MNCs were identified by location, morphology, and exhibited a transient outward rectification in response to a series of depolarizing current pulses delivered at a hyperpolarized membrane [1,23]. Membrane currents and potentials were monitored with an Axopatch 200B amplifier (Molecular Devices), low-pass filtered at 5 kHz, and acquired through a Digidata 1440 series analog-to-digital interface on a personal computer using Clampex 10.4 software (Molecular Devices). The resistance of recording electrodes was 4–6 MΩ in the bath, with series resistances in the range of 10–20 MΩ. Neurons were held in voltage-clamp mode at –70 mV. Series resistance was monitored by applying voltage pulses (10 ms, –5 mV), and only neurons with stable series resistance were include in the analysis. For electrical stimulation of excitatory glutamatergic inputs, a glass electrode containing ACSF (0.1–0.5 MΩ) was placed in the PVN around recorded neuron of the slice. Paired-current pulses (0.2 ms, 10–100 µA; duration: 50 ms) at 0.05 Hz were delivered through the glass electrode mounted on remote-control manipulators (MP-385; Sutter Instrument Company). The paired-pulse ratio (N2/N1 ratio) was calculated as the amplitude of second EPSC (N2) over the amplitude of first EPSC (N1). HFS (100 Hz, 100 pulses, three times; 10 sec interval) to induce long-term plasticity of excitatory inputs in the PVN neurons [1]. The time epoch used for calculate average value of baseline was 10 min before HFS delivering, while the time epoch used for calculate post-HFS were 30–40 min after HFS. The amplitude of N1 was normalized by the mean value of baseline. The ACSF included picrotoxin (50 µM) during all recordings to prevent GABAA receptor-mediated inhibitory. In some experiments, D-APV was added in ACSF for blockade of the NMDAR-dependent glutamatergic LTP of PVN MNCs. For inhibiting postsynaptic PKA in some experiments, protein kinase inhibitor-(6-22) amide (PKI; 5 µM) was added in pipette internal solution.
After electrophysiology recording, the slices were removed and fixed in 4% paraformaldehyde in 0.1 phosphate buffer (PH 7.4). The slices were incubated over night with the avidin–biotin complex (ABC Elite kit; Vector Laboratories) at room temperature. Biocytin was detected by 3,3′-diaminobenzidine tetrahydrochloride histochemistry.
Chemicals
The reagents, which included picrotoxin (50 µM), NE CGP 20712 (1-AR antagonist), dobutamine (β1-AR agonist), and D-(-)-2-Amino-5-phosphonopentanoic acid (D-APV) were purchased from Sigma-Aldrich. KT5720 (a specific PKA inhibitor) and protein kinase inhibitor-(6-22) amide (PKI) were bought from Tocris Cookson. In order to completely inhibit PKA, KT5720 was perfused in the slice at least 20 min before whole-cell recording and continue throughout the entire experimental process.
Data analysis
Electrophysiological data were analyzed using Clampfit 10.4 software (Molecular Devices). All data are expressed as the mean ± SEM. One-way ANOVA (post-hoc multiple comparison) and Mann–Whitney–Wilcoxon test (SPSS software) were used to determine the level of statistical significance between groups of data. p-values less than 0.05 were considered to indicate a statistically significant difference between experimental groups.
RESULTS
NE enhanced HFS-induced glutamatergic LTP of PVN MNCs
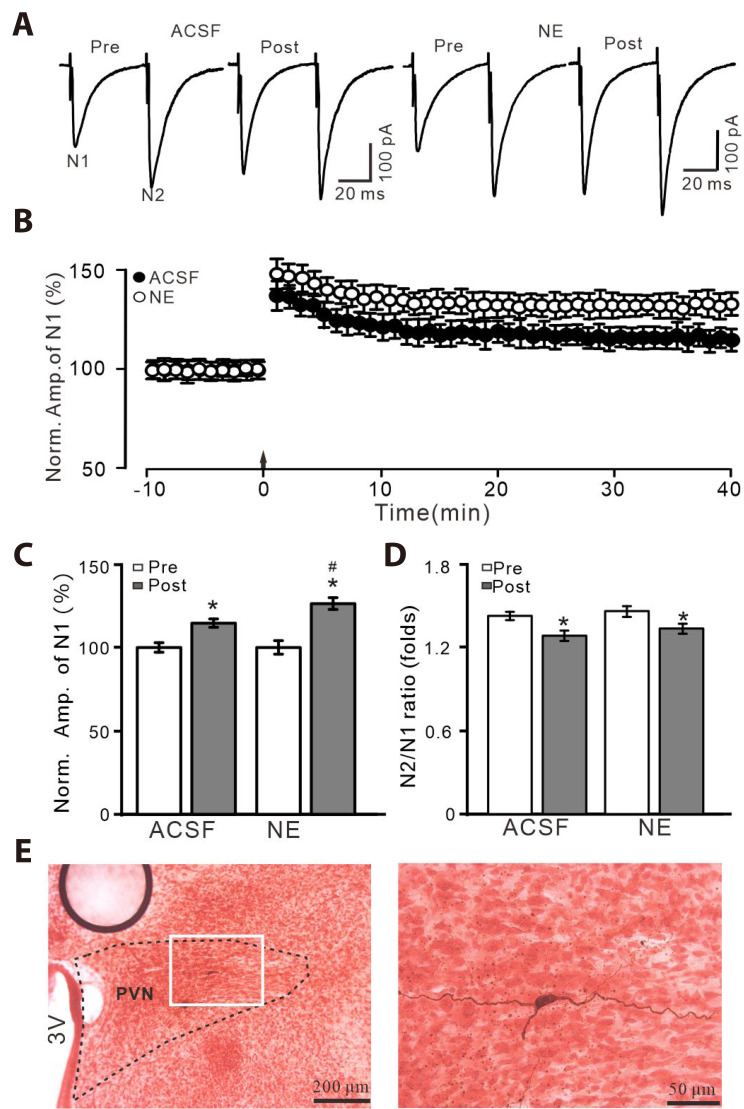
A total of 82 PVN neurons from 61 rats were identified as PVN MNCs by whole-cell patch clamp recording technique and biocytin staining method [1,23] (Fig. 1E). Blockade of GABAA receptor with picrotoxin (50 µM), paired-pulse stimulation (0.2 ms, 10–100 µA; interval, 50 ms) evoked a couple of EPSCs, N1 and N2 in PVN MNCs (Fig. 1A). Delivery of HFS (100 Hz, 100 pulses, 3 times, 10 sec interval) induced long-term increase in the amplitude of EPSCs under control conditions (ACSF), which was significantly enhanced by a non-selective ARs agonist, NE (100 nM; Fig. 1B). During 35–40 min after the delivery of HFS, the normalized amplitude of N1 (N1 of post HFS/N1 of pre HFS) in the presence of NE was 128.7 ± 2.6% of the baseline (100.0 ± 3.1%; p < 0.001, n = 7 cells from 5 mice), which was significantly higher than control (ACSF: 116.7 ± 2.5%; Fig. 1C). During 35–40 min after the delivery of HFS, the mean N2/N1 ratio was 1.21 ± 0.026 (ACSF) and 1.26 ± 0.025 (NE), each of which was significantly lower than baseline (ACSF: 1.42 ± 0.022 and NE: 1.46 ± 0.026, respectively; p < 0.001, n = 7 cells; Fig. 1D). Biocytin staining illustrated the location and morphological characteristics of these putative MNCs (Fig. 1E). These results indicate that application of NE produces an enhancement of HFS-induced LTP of PVN MNCs, accompanied with a decrease in N2/N1 ratio.
Fig. 1. NE enhanced HFS-induced glutamatergic LTP of PVN MNCs.
(A) Representative whole-cell recording traces showing paired-pulse stimulation (duration: 0.2 msec, interval: 50 msec) evoked EPSCs in the PVN MNCs before (pre), after (post) delivering HFS (100 pulses, 3 times, 10 sec interval) in treatment with ACSF (left) and NE (100 nM; right). (B) Summary of data showing the normalized amplitude of N1 before and after the delivery of HFS (arrow head) in each treatment. (C, D) Bar graphs show normalized amplitude of N1 (C) and N2/N1 ratio (D) before (pre) and after (post) the delivery of HFS. (E) Biocytin staining showing microscopic image (left, ×4) and enlarged microphotograph (right, ×40) illustrated the morphology of recorded MNC. NE, norepinephrine; HFS, high frequency stimulation; LTP, long-term potentiation; PVN, paraventricular nucleus; MNCs, magnocellular neuroendocrine cells; EPSCs, excitatory postsynaptic currents; ACSF, artificial cerebrospinal fluid. *p < 0.05 vs. pre; #p < 0.05 vs. post of control; n = 7 cells from 5 mice in each group.
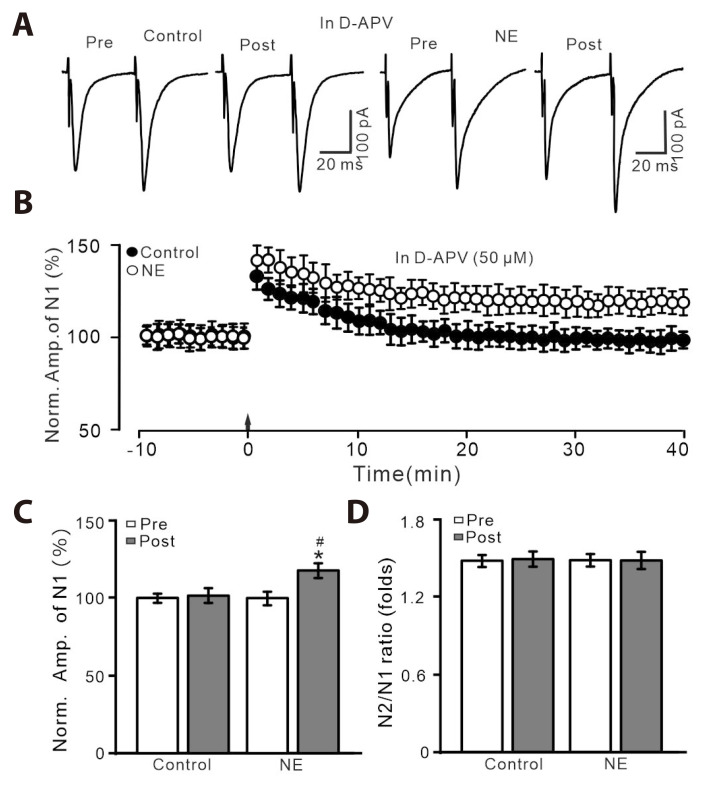
Previous study demonstrated that HFS-induced glutamatergic LTP in PVN MNCs through activation of NMDAR in vitro in rats [1], we then employed a specific NMDAR antagonist, D-APV (50 µM) to test whether HFS-induced glutamatergic LTP of PVN MNCs through the activation of NMDAR. Blockade of NMDAR with D-APV, LTP of PVN MNCs was abolished in ACSF, which was persisted in the presence of NE (100 nM; Fig. 2A-C). In the presence of D-APV and NE, the normalized amplitude of N1 was 115.4 ± 3.4% of baseline (100.0 ± 2.6%; p < 0.001, n = 7 cells), which was significantly higher than control (ACSF: 99.6 ± 3.3%; p < 0.05; Fig. 2B, C). The N2/N1 ratio during 35–40 min after the delivery of HFS in ACSF or NE was no significantly different from baseline (p > 0.05, n = 8 cells; Fig. 2D). These results indicate that blockade of NMDAR abolished HFS-induced LTP of PVN NMCs in ACSF, but the LTP was rescued by the application of NE.
Fig. 2. HFS-induced LTP was abolished by an NMDAR blocker, D-APV, but it was rescued by the application of NE.
(A) In the presence of D-APV (50 µM), representative whole-cell recording traces showing paired-pulse stimulation (duration: 0.2 msec, interval: 50 msec) evoked EPSCs in PVN MNCs before (pre), after (post) delivering HFS in treatment with ACSF (control; left) and NE (100 nM; right). (B) Pooled data showing the time course of the normalized amplitude of N1 before and after delivery of HFS (arrow head) during treatment with ACSF and NE. (C, D) Bar graphs show the normalized amplitude of N1 (C) and N2/N1 ratio (D) in each group, before (pre) and after (post) delivery of HFS. HFS, high frequency stimulation; LTP, long-term potentiation; NMDAR, N-methyl-D-aspartate receptors; D-APV, D-(-)-2-Amino-5-phosphonopentanoic acid; NE, norepinephrine; EPSCs, excitatory postsynaptic currents; PVN, paraventricular nucleus; MNCs, magnocellular neuroendocrine cells; ACSF, artificial cerebrospinal fluid. *p < 0.05 vs. pre; #p < 0.05 vs. post of control; n = 7 cells from 6 mice in each group.
NE enhanced HFS-induced LTP of PVN MNCs via the activation of β-AR
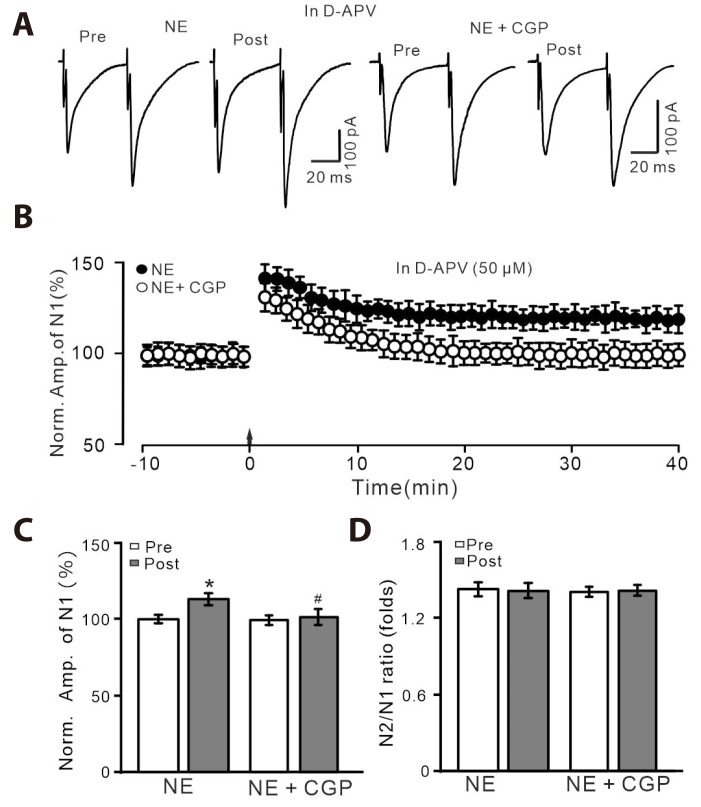
AR subtypes are abundantly expressed in the hypothalamic PVN, and modulates PVN neuronal activity and synaptic transmission [21,22]. We then tested which subtype AR mediated the HFS-induced LTP of PVN MNCs. Under the blockade of NMDAR-dependent LTP with D-APV (50 µM), NE rescued HFS-induced LTP of PVN MNCs (Fig. 3A-C), without a change in N2/N1 ratio (Fig. 3D). Application of a selective β1-AR antagonist, CGP 20712 (1 µM) completely prevented NE to rescue the LTP of PVN MNCs (Fig. 3A-C). These results indicate that NE rescues the HFS-induced PVN MNCs LTP through activation of β1-AR.
Fig. 3. In the presence of D-APV, the NE-rescued LTP was abolished by.
β1-AR antagonist. (A) In the presence of D-APV (50 µM), representative whole-cell recording traces showing paired-pulse stimulation (duration: 0.2 msec, interval: 50 msec) evoked EPSCs in PVN MNCs before (pre), after (post) delivering HFS during treatment with NE (100 nM; left) and a mixture of NE (100 nM) + CGP 20712 (1 µM). (B) In the presence of D-APV, pooled data showing the time course of normalized amplitude of N1 before and after the delivery of HFS (arrow head) during treatment with NE and a mixture of NE + CGP 20712. (C, D) Bar graphs show the normalized amplitude of N1 (C) and N2/N1 ratio (D) in each group, before (pre) and after (post) the delivery of HFS. D-APV, D-(-)-2-Amino-5-phosphonopentanoic acid; NE, norepinephrine; LTP, long-term potentiation; AR, adrenergic receptor; EPSCs, excitatory postsynaptic currents; PVN, paraventricular nucleus; MNCs, magnocellular neuroendocrine cells. *p < 0.05 vs. pre; #p < 0.05 vs. post of control; n = 8 cells from 6 mice in each group.
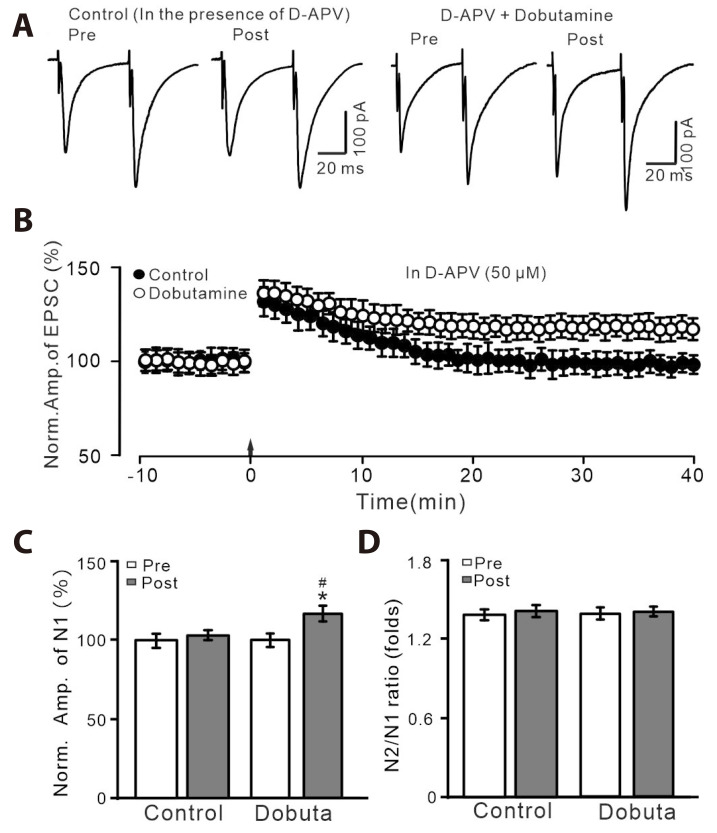
We further observed whether delivery of HFS during the activation of β1-AR could induce PVN MNCs LTP in the absence of NMDAR-dependent LTP. In the presence of D-APV and β1-AR agonist, dobutamine (1 µM), delivery of HFS induced a significant increase in the amplitude of N1 for over 40 min (Fig. 4A, B). During 35–40 min after the delivery of HFS, the normalized amplitude of N1 was 116.7 ± 3.4% of baseline (p < 0.05, n = 7 cells), which was significantly higher than that of control (D-APV alone; p < 0.05; Fig. 4C). The mean N2/N1 ratio was not significantly different from baseline (p > 0.05, n = 7 cells; Fig. 4D). These results indicate that pharmacological activation of β1-AR could rescue HFS-induced PVN MNCs LTP in the absence of NMDAR-dependent LTP.
Fig. 4. HFS-induced LTP was abolished by D-APV, but it was reversed by.
β1-AR agonist. (A) In the presence of D-APV (50 µM), representative whole-cell recording traces showing paired-pulse stimulation (duration: 0.2 msec, interval: 50 msec) evoked EPSCs in PVN MNCs before (pre), after (post) delivering HFS during treatment with NE (100 nM; left) and a mixture of NE + dobutamine (1 µM; right). (B) Pooled data showing the time course of normalized amplitude of N1 before and after the delivery of HFS (arrow head) during treatment with D-APV (50 µM) and a mixture of D-APV + dobutamine. (C, D) Bar graphs show the normalized amplitude of N1 (C) and N2/N1 ratio (D) in each group, before (pre) and after (post) delivery of HFS. HFS, high frequency stimulation; LTP, long-term potentiation; D-APV, D-(-)-2-Amino-5-phosphonopentanoic acid; AR, adrenergic receptor; EPSCs, excitatory postsynaptic currents; PVN, paraventricular nucleus; MNCs, magnocellular neuroendocrine cells. *p < 0.05 vs. pre; #p < 0.05 vs. post of control; n = 7 cells from 5 mice in each group.
Blockade of NMDAR, NE reversed LTP through PKA signaling pathway
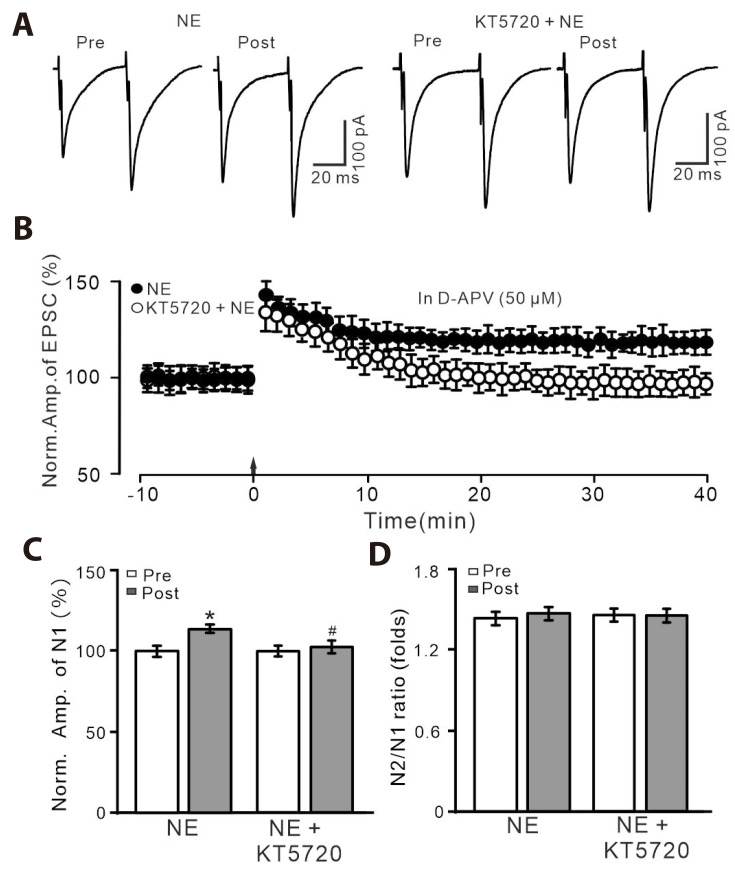
We further employed a specific PKA inhibitor, KT5720 (100 nM) to examine whether NE rescued PVN MNCs LTP through PKA signaling pathway. Slices were pre-incubated with KT5720 in the bath for about 20 min before recordings and were perfused continuously throughout the experiments to inhibit PKA. NE failed to rescue the HFS-induced LTP of PVN MNCs in the presence of extracellular PKA inhibitor (Fig. 5A, B). In the presence of D-APV, KT5720 and NE, the normalized N1 amplitude between 35–40 min after the HFS was 99.5 ± 2.8% of the baseline (100.0 ± 2.4%; p > 0.05, n = 9 cells), which was significantly lower than that of NE alone (p < 0.05; Fig. 5C). The mean value of N2/N1 ratio during 35–40 min after the delivery of HFS was similar to that of baseline (p > 0.05, n = 9 cells; Fig. 5D). These results indicate that NE rescues HFS-induced PVN MNCs LTP through PKA signaling pathway.
Fig. 5. In the presence of D-APV and KT5720, NE could not rescue LTP of PVN MNCs.
(A) In the presence of D-APV (50 µM), representative whole-cell recording traces showing paired-pulse stimulation (duration: 0.2 msec, interval: 50 msec) evoked EPSCs in PVN MNCs before (pre), after (post) delivering HFS during treatment with NE (100 nM; left) and a mixture of NE (100 nM) + KT5720 (100 nM; right). (B) Pooled data showing the time course of the normalized amplitude of N1 before and after delivery of HFS (arrow head) during treatment with NE and a mixture of NE + KT5720. (C, D) Bar graphs show the normalized amplitude of N1 (C) and N2/N1 ratio (D) in each group, before (pre) and after (post) delivery of HFS. D-APV, D-(-)-2-Amino-5-phosphonopentanoic acid; NE, norepinephrine; LTP, long-term potentiation; PVN, paraventricular nucleus; MNCs, magnocellular neuroendocrine cells; EPSCs, excitatory postsynaptic currents; HFS, high frequency stimulation. *p < 0.05 vs. pre; #p < 0.05 vs. post of control; n = 9 cells from 6 mice in each group.
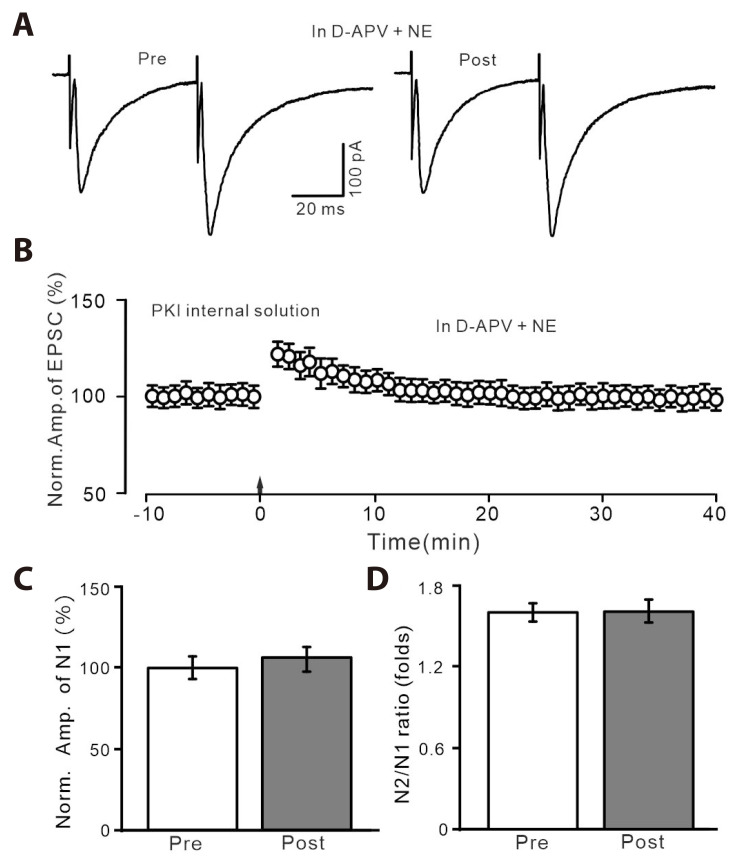
We then inhibited postsynaptic PKA by introducing intracellular protein kinase inhibitor-(6-22) amide (PKI) in the pipette solution, to observe whether NE rescued the HFS-induced PVN MNCs LTP via postsynaptic PKA signaling pathway. With PKI in pipette solution, delivery of HFS in the presence of D-APV and NE failed to induce PVN MNCs LTP (Fig. 6A, B). The normalized amplitude of N1 between 35–40 min after the HFS was 102.7 ± 3.8% of baseline (100.0 ± 3.2%; p > 0.05, n = 6 cells; Fig. 6C). The mean value of N2/N1 ratio during 35–40 min after the delivery of HFS was not significantly different from baseline (p > 0.05, n = 6 cells; Fig. 6D). These results indicate that NE rescues the HFS-induced LTP of PVN MNCs through postsynaptic PKA signaling pathway.
Fig. 6. In the presence of D-APV and recording with PKI internal solution, NE could not rescue LTP of PVN MNCs.
(A) Representative current traces showing the evoked EPSCs recorded with PKI internal solution from a PVN MNCs before (pre) and after (post) delivering HFS during treatment with D-APV (50 µM) + NE (100 nM). (B) Pooled data showing the time course of normalized amplitude of N1 before and after delivery of HFS (arrow head) during treatment with D-APV + NE. (C, D) Bar graphs show the normalized amplitude of N1 (C) and N2/N1 ratio (D) before (pre) and after (post) delivery of HFS. D-APV, D-(-)-2-Amino-5-phosphonopentanoic acid; PKI, protein kinase inhibitor; NE, norepinephrine; LTP, long-term potentiation; PVN, paraventricular nucleus; MNCs, magnocellular neuroendocrine cells; EPSCs, excitatory postsynaptic currents; HFS, high frequency stimulation. *p < 0.05 vs. pre; #p < 0.05 vs. post of control; n = 6 cells from 5 mice in each group.
DISCUSSION
We here demonstrated that HFS induced a glutamatergic LTP with a decrease in N2/N1 ratio in the PVN MNCs, which was enhanced by NE. The HFS-induced LTP was abolished by the blockade of NMDAR with D-APV, but it was rescued by application of NE or a selective β1-AR agonist. Blockade of NMDAR and β1-AR, NE failed to rescue the PVN MNCs LTP. In the absence of NMDAR activity, NE failed to rescue HFS-induced MNC LTP when PKA was inhibited by extracellular applying KT5720 or intracellular administration PKI. These results indicate that NE activates β1-AR in the hypothalamic PVN MNCs, induces novel glutamatergic LTP via the postsynaptic PKA signaling pathway in vitro in rats.
Previous studies have shown that LTP of the excitatory glutamatergic synaptic transmission in the hypothalamic PVN MNCs plays a critical role in controlling physiological and pathophysiological responses [1,7-9]. We found previously that HFS of the glutamatergic inputs produced an NMDAR/NO cascades-dependent presynaptic glutamatergic PVN MNCs LTP via the PKA signaling pathway in vitro in rats [1]. Consistent with our previous results [1], the present study showed that the delivery of HFS under control conditions induced LTP of PVN MNCs accompanied with a decrease of N2/N1 ratio, which was abolished by extracellular blockade of NMDAR [1]. Notably, HFS-induced MNCs LTP was enhanced by the application of NE, suggesting that NE augmented the MNCs LTP through activation of ARs. Furthermore, NE rescued the HFS-induced PVN NMCs LTP in absence of NMDAR activity, suggesting that NE activated ARs and triggered HFS to induce novel glutamatergic LTP of PVN MNCs. The results indicate that NE modulates long-term synaptic plasticity of PVN MNCs via distinct ARs mediated-intracellular signaling [16].
Various AR subtypes are abundantly expressed in the hypothalamic PVN, and contribute to modulation the neuronal activity and synaptic transmission [21,22]. The present results showed that blockade of β1-AR, NE failed to rescue the HFS-induced LTP of PVN NMCs, whereas pharmacological activation of β1-AR could trigger HFS-induced LTP of PVN MNCs. These results indicate that NE rescued the HFS-induced LTP of PVN NMCs through activation of β1-AR. Activation of β-AR modulated long-term synaptic plasticity has been demonstrated in various regions of the brain [18,24-27]. In cerebellar cortex, activation of β-AR by a specific agonist or NE decreases the threshold for induction of long-term depression of parallel fiber-Purkinje cell synaptic transmission [18]. In addition, activation of β-ARs produces enhancement of memory consolidation and the amygdala–perirhinal pathway LTP in the perirhinal cortex [26], and NE enhances memory formation and has powerful effects on induction of the excitatory synaptic LTP by activation of β-ARs in mammalian hippocampus [25,27]. Consistent with the previous reports, our results suggest that NE rescues the HFS-induced LTP of PVN MNCs through activation of β1-AR.
PKA signaling plays a critical role in long-term glutamatergic plasticity of PVN MNCs, and activation of β-AR modulates neurotransmitter release, synaptic transmission and plasticity in the central nervous system through cAMP/PKA signaling pathway [1,19,23,25,26]. Our data showed that extracellular inhibition of PKA, NE failed to rescue the HFS-induced PVN MNCs LTP, which indicated that NE rescued the HFS-induced PVN MNCs LTP through the PKA signaling pathway. Furthermore, we added PKI in pipette solution to inhibit the postsynaptic PKA, and observed whether NE rescued the HFS-induced PVN MNCs LTP through the postsynaptic PKA signaling cascade. The results showed that blockade the postsynaptic PKA, NE failed to rescue the LTP of PVN MNCs. These results indicate that NE rescues the LTP of PVN MNCs through the postsynaptic PKA signaling pathway. Our previous study has demonstrated that the glutamatergic LTP of PVN MNCs accompanied with a decrease in paired-pulse ratio, which suggested that the HFS-induced LTP under control conditions through a presynaptic mechanism [1]. Consistent with previous study, our present results showed that HFS of the excitatory glutamate inputs induced an NMDAR/NO cascade- and PKA signaling-dependent presynaptic LTP of the PVN MNCs in vitro in rats. These results suggests that the NO-dependent presynaptic glutamatergic LTP of MNCs might be the predominant LTP under physiological conditions and might play important roles in physiological responses, such as dehydration or lactation [1,7]. However, activation AR triggered HFS to produce a novel postsynaptic LTP of the PVN MNCs through β1-AR/PKA signaling pathway, suggested that the AR-dependent postsynaptic LTP induced during some specific physiological response, such as acute stress stimulation. Stress stimulation induces excitation of corticotropin-releasing hormone (CRH) neurons via excitatory glutamatergic inputs, which produces an increase in CRH level, and subsequently improves the secretion of NE [8]. The increase of NE would activate β-AR and mediates the HFS-induced postsynaptic LTP of the PVN MNCs. These results suggest that AR-mediated postsynaptic LTP together with NO-mediated presynaptic LTP may contribute to upregulation of MNCs activity during stress responses. It is known that β-ARs are G-protein coupled receptors, which signal through activation of Gs type G proteins, followed by stimulation of adenylyl cyclase and increased production of intracellular cAMP, as well activates cAMP-dependent PKA [25]. The activation of PKA produces an inhibition of protein phosphatase 1 thereby facilitating protein phosphorylation required for LTP induction [25,27]. Therefore, delivery of HFS in the presence of NE may cause a significant increase in PKA-mediated phosphorylation and the membrane surface expression of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs) in PVN MNCs, resulting an increase in AMPARS recruitment on the membrane surface [28]. Therefore, the postsynaptic LTP could be expressed by an increase either in the number of AMPARs or in their single channel conductance [29]. Taken together, our present results suggest that NE activates β1-AR of the hypothalamic PVN MNCs, triggers LTP of PVN MNCs through the postsynaptic β1-AR/PKA signaling pathway, resulting in an enhancement of the HFS-induced glutamatergic LTP in vitro in rats.
ACKNOWLEDGEMENTS
None.
Footnotes
FUNDING
This work was supported by the Major Projects of the Ministry of Science and Technology of China (2021ZD0202300) and the National Natural Science Foundations of China (32070986, 32171005, 82160152).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Zhang BB, Jin H, Bing YH, Zhang XY, Chu CP, Li YZ, Qiu DL. A nitric oxide-dependent presynaptic LTP at glutamatergic synapses of the PVN magnocellular neurosecretory cells in vitro in rats. Front Cell Neurosci. 2019;13:283. doi: 10.3389/fncel.2019.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Csáki A, Kocsis K, Halász B, Kiss J. Localization of glutamatergic/aspartatergic neurons projecting to the hypothalamic paraventricular nucleus studied by retrograde transport of [3H]D-aspartate autoradiography. Neuroscience. 2000;101:637–655. doi: 10.1016/S0306-4522(00)00411-5. [DOI] [PubMed] [Google Scholar]
- 3.van den Pol AN, Wuarin JP, Dudek FE. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science. 1990;250:1276–1278. doi: 10.1126/science.1978759. [DOI] [PubMed] [Google Scholar]
- 4.Decavel C, Van den Pol AN. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- 5.Wuarin JP, Dudek FE. Patch-clamp analysis of spontaneous synaptic currents in supraoptic neuroendocrine cells of the rat hypothalamus. J Neurosci. 1993;13:2323–2331. doi: 10.1523/JNEUROSCI.13-06-02323.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stern JE, Galarreta M, Foehring RC, Hestrin S, Armstrong WE. Differences in the properties of ionotropic glutamate synaptic currents in oxytocin and vasopressin neuroendocrine neurons. J Neurosci. 1999;19:3367–3375. doi: 10.1523/JNEUROSCI.19-09-03367.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panatier A, Gentles SJ, Bourque CW, Oliet SH. Activity-dependent synaptic plasticity in the supraoptic nucleus of the rat hypothalamus. J Physiol. 2006;573:711–721. doi: 10.1113/jphysiol.2006.109447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salter EW, Sunstrum JK, Matovic S, Inoue W. Chronic stress dampens excitatory synaptic gain in the paraventricular nucleus of the hypothalamus. J Physiol. 2018;596:4157–4172. doi: 10.1113/JP275669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuura T, Kawasaki M, Suzuki H, Fujitani T, Baba K, Nishimura H, Ikeda N, Yamanaka Y, Tsukamoto M, Yoshimi Y, Ohnishi H, Ueta Y, Sakai A. Nitric oxide synthase contributes to the maintenance of LTP in the oxytocin-mRFP1 neuron of the rat hypothalamus. J Neuroendocrinol. 2023;35:e13340. doi: 10.1111/jne.13340. [DOI] [PubMed] [Google Scholar]
- 10.Maguire J. Stress-induced plasticity of GABAergic inhibition. Front Cell Neurosci. 2014;8:157. doi: 10.3389/fncel.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanton LM, Price AJ, Manning EE. Hypothalamic corticotrophin releasing hormone neurons in stress-induced psychopathology: revaluation of synaptic contributions. J Neuroendocrinol. 2023;35:e13268. doi: 10.1111/jne.13268. [DOI] [PubMed] [Google Scholar]
- 12.Herman JP, Tasker JG. Paraventricular hypothalamic mechanisms of chronic stress adaptation. Front Endocrinol (Lausanne) 2016;7:137. doi: 10.3389/fendo.2016.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, Nemeroff CB. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci. 1986;6:2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma XM, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 1999;140:3623–3632. doi: 10.1210/endo.140.8.6943. [DOI] [PubMed] [Google Scholar]
- 15.Bartsch JC, von Cramon M, Gruber D, Heinemann U, Behr J. Stress-induced enhanced long-term potentiation and reduced threshold for N-methyl-D-aspartate receptor- and β-adrenergic receptor-mediated synaptic plasticity in rodent ventral subiculum. Front Mol Neurosci. 2021;14:658465. doi: 10.3389/fnmol.2021.658465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chay A, Zamparo I, Koschinski A, Zaccolo M, Blackwell KT. Control of βAR- and N-methyl-D-aspartate (NMDA) receptor-dependent cAMP dynamics in hippocampal neurons. PLoS Comput Biol. 2016;12:e1004735. doi: 10.1371/journal.pcbi.1004735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han SK, Chong W, Li LH, Lee IS, Murase K, Ryu PD. Noradrenaline excites and inhibits GABAergic transmission in parvocellular neurons of rat hypothalamic paraventricular nucleus. J Neurophysiol. 2002;87:2287–2296. doi: 10.1152/jn.2002.87.5.2287. [DOI] [PubMed] [Google Scholar]
- 18.Inoshita T, Hirano T. Norepinephrine facilitates induction of long-term depression through β-adrenergic receptor at parallel fiber-to-Purkinje cell synapses in the flocculus. Neuroscience. 2021;462:141–150. doi: 10.1016/j.neuroscience.2020.05.037. [DOI] [PubMed] [Google Scholar]
- 19.Cui LN, Sun N, Li BX, Wang LF, Zhang XY, Qiu DL, Chu CP. Noradrenaline inhibits complex spikes activity via the presynaptic PKA signaling pathway in mouse cerebellar slices. Neurosci Lett. 2020;729:135008. doi: 10.1016/j.neulet.2020.135008. [DOI] [PubMed] [Google Scholar]
- 20.Wang JY, Weng WC, Wang TQ, Liu Y, Qiu DL, Wu MC, Chu CP. Noradrenaline depresses facial stimulation-evoked cerebellar MLI-PC synaptic transmission via α2-AR/PKA signaling cascade in vivo in mice. Sci Rep. 2023;13:15908. doi: 10.1038/s41598-023-42975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaguchi N, Mimura K, Okada S. GABAB receptors in the hypothalamic paraventricular nucleus mediate β-adrenoceptor-induced elevations of plasma noradrenaline in rats. Eur J Pharmacol. 2019;848:88–95. doi: 10.1016/j.ejphar.2019.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Domingos-Souza G, Martinez D, Sinkler S, Heesch CM, Kline DD. Alpha adrenergic receptor signaling in the hypothalamic paraventricular nucleus is diminished by the chronic intermittent hypoxia model of sleep apnea. Exp Neurol. 2021;335:113517. doi: 10.1016/j.expneurol.2020.113517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu CP, Jin WZ, Bing YH, Jin QH, Kannan H, Qiu DL. Effects of stresscopin on rat hypothalamic paraventricular nucleus neurons in vitro. PLoS One. 2013;8:e53863. doi: 10.1371/journal.pone.0053863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tasker JG, Dudek FE. Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1991;434:271–293. doi: 10.1113/jphysiol.1991.sp018469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Dell TJ, Connor SA, Guglietta R, Nguyen PV. β-Adrenergic receptor signaling and modulation of long-term potentiation in the mammalian hippocampus. Learn Mem. 2015;22:461–471. doi: 10.1101/lm.031088.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laing M, Bashir ZI. β-Adrenoceptors and synaptic plasticity in the perirhinal cortex. Neuroscience. 2014;273:163–173. doi: 10.1016/j.neuroscience.2014.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Dell TJ, Connor SA, Gelinas JN, Nguyen PV. Viagra for your synapses: enhancement of hippocampal long-term potentiation by activation of beta-adrenergic receptors. Cell Signal. 2010;22:728–736. doi: 10.1016/j.cellsig.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M, Ramasamy VS, Kang HK, Jo J. Oleuropein promotes hippocampal LTP via intracellular calcium mobilization and Ca2+-permeable AMPA receptor surface recruitment. Neuropharmacology. 2020;176:108196. doi: 10.1016/j.neuropharm.2020.108196. [DOI] [PubMed] [Google Scholar]
- 29.Park P, Georgiou J, Sanderson TM, Ko KH, Kang H, Kim JI, Bradley CA, Bortolotto ZA, Zhuo M, Kaang BK, Collingridge GL. PKA drives an increase in AMPA receptor unitary conductance during LTP in the hippocampus. Nat Commun. 2021;12:413. doi: 10.1038/s41467-020-20523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]