Abstract
Despite having a very low incidence of disease, reindeer (Rangifer tarandus) are subject to tuberculosis (TB) testing requirements for interstate shipment and herd accreditation in the United States. Improved TB tests are desperately needed, as many reindeer are falsely classified as reactors by current testing procedures. Sera collected sequentially from 11 (experimentally) Mycobacterium bovis-infected reindeer and 4 noninfected reindeer were evaluated by enzyme-linked immunosorbent assay (ELISA), immunoblotting, and multiantigen print immunoassay (MAPIA) for antibody specific to M. bovis antigens. Specific antibody was detected as early as 4 weeks after challenge with M. bovis. By MAPIA, sera were tested with 12 native and recombinant antigens, which were used to coat nitrocellulose. All M. bovis-infected reindeer developed responses to MPB83 and a fusion protein, Acr1/MPB83, and 9/11 had responses to MPB70. Other antigens less commonly recognized included MPB59, ESAT-6, and CFP10. Administration of purified protein derivatives for skin testing boosted serum antibody responses, as detected by each of the assays. Of the noninfected reindeer, 2/4 had responses that were detectable immediately following skin testing, which correlated with pathological findings (i.e., presence of granulomatous lesions yet the absence of acid-fast bacteria). The levels of specific antibody produced by infected reindeer appeared to be associated with disease progression but not with cell-mediated immunity. These findings indicate that M. bovis infection of reindeer elicits an antibody response to multiple antigens that can be boosted by skin testing. Serological tests using carefully selected specific antigens have potential for early detection of infections in reindeer.
Mycobacterium bovis infection of reindeer (Rangifer tarandus) is rare, especially in North America, where there are no published reports of the occurrence of tuberculosis (TB) in this species. Despite the low incidence of disease, reindeer are subject to regulations in the United States Department of Agriculture (USDA) Bovine Tuberculosis Eradication Uniform Methods and Rules (29), requiring testing for interstate movement and herd accreditation. For TB surveillance of reindeer within the United States, a single cervical test (a measure of delayed-type hypersensitivity) is the primary test and the comparative cervical skin test (CCT) is used to confirm infection. Although exact numbers are difficult to ascertain, many reindeer have tested positive by skin testing for TB surveillance. Strains of Mycobacterium bovis or other Mycobacterium spp. within the Mycobacterium tuberculosis complex, however, have not been isolated from these reindeer upon necropsy. Reasons for the high rate of false-positive reactions elicited by skin testing are unclear, although it may be due to unusual exposure to nontuberculous Mycobacterium spp. or an exaggerated cellular immune response to mycobacterial antigens. Therefore, improved TB tests are urgently needed to avoid the unnecessary slaughter of reindeer falsely identified as TB reactors.
For TB surveillance of captive wildlife (e.g., zoos or game farms) and nontraditional livestock, blood-based TB assays are appealing, as they require a single handling event, thereby minimizing capture-associated injuries. Blood-based assays are also more readily used in capture surveillance programs with free-ranging wildlife (e.g., white-tailed deer [Odocoileus virginianus] in Michigan or Cape buffalo in Africa). While in vitro cell-based assays appear promising, they require processing of the blood sample within 24 h and are subject to complications inherent with overnight delivery (e.g., temperature fluctuations and delays in setup). For these reasons, serological tests are particularly attractive for use in TB surveillance of nontraditional livestock and wildlife. A major limiting factor for the development of serological TB assays, particularly for reindeer, is the lack of specific information about antigens recognized by antibodies that are produced during disease. Thus, experimental M. bovis infection studies are necessary to characterize the humoral immune response and to identify the most reactive antigens that could be employed in serodiagnostic tests.
Previous studies with cattle and white-tailed deer have revealed both similarities and differences in the antibody responses against M. bovis infection of these two species (17, 18, 34). In particular, antigen recognition patterns appear to differ from animal to animal in both species, and antibody levels are significantly elevated shortly after the intradermal tuberculin injection(s) for skin testing. Little, if anything, is known concerning antibody responses of reindeer to M. bovis infection.
The present study describes the humoral response of reindeer to experimental infection with M. bovis. Specific objectives were to determine (i) antigen recognition patterns by serum antibodies, (ii) relationships of the humoral responses with cell-mediated immunity and disease progression, (iii) the effect of tuberculin skin testing on the antibody response, and (iv) the potential for serological tests in TB surveillance programs for reindeer.
MATERIALS AND METHODS
Animals, challenge, and necropsy.
Thirty-four castrated male reindeer (Rangifer tarandus) of approximately 9 months of age were obtained from a TB-free herd in Michigan and housed at the National Animal Disease Center, Ames, Iowa, according to institutional guidelines and approved animal care and use protocols. Eleven animals were experimentally infected with M. bovis, and four animals were kept noninoculated as a control group. For M. bovis infection, the challenge inoculum (105 CFU in 0.2 ml of phosphate buffered saline [PBS], pH 7.2) was instilled directly into the tonsillar crypts of anesthetized reindeer (n = 11) as described for the inoculation of white-tailed deer (21). The strain of M. bovis used for the challenge inoculum (95-1315 [USDA, Animal Plant and Health Inspection Service {APHIS} designation]) was originally isolated from a white-tailed deer in Michigan (24). Inoculum consisted of mid-log-phase M. bovis cells grown in Middlebrook's 7H9 medium supplemented with 10% oleic acid-albumin-dextrose complex (Difco, Detroit, Michigan) plus 0.05% Tween 80 (Sigma Chemical Co., St. Louis, Missouri). At the time of inoculation, reindeer were moved from an outdoor pen into climate-controlled rooms (two to three animals/room) within a biosafety level 3 confinement facility. Negative airflow exited the building through HEPA filters, ensuring that air from animal pens was pulled towards a central corridor and through HEPA filters before exiting the building. The airflow velocity was 10.4 air changes/h. Four noninoculated reindeer were housed in a climate-controlled room in a building (biosafety level 2 facility) separate from the building in which the infected reindeer were housed. Additionally, serum samples from 19 reindeer housed outdoors at the National Animal Disease Center were obtained.
Thirteen months after inoculation, reindeer in the infected (n = 11) and noninoculated (n = 4) groups were euthanized by an intravenous injection of sodium pentobarbital (Fort Dodge Animal Health, Fort Dodge, Iowa) and examined. Various tissues were collected for bacteriologic culture and microscopic examination. Detailed descriptions of cellular immune responses (35), bacteriologic culture, histopathology, and gross necropsy results are presented elsewhere (23).
CCT.
Ninety days after M. bovis inoculation, reindeer were tested for in vivo responsiveness to mycobacterial antigens by a modified CCT technique enabling the collection of biopsy specimens for which the dermal reactions to purified protein derivatives (PPDs) at 24, 48, and 72 h postinjection could be analyzed (23, 33). Briefly, the cervical region was clipped and animals injected intradermally in three separate locations with M. bovis PPD and a single location with Mycobacterium avium PPD (PPDs obtained from National Veterinary Services Laboratory, Ames, Iowa). A standard CCT (i.e., single intradermal injection each of M. avium PPD and M. bovis PPD) was performed 8 months after M. bovis inoculation (22, 29). For each of the skin tests, 100 μg of M. bovis PPD and 40 μg of M. avium PPD were administered at each respective location according to guidelines described in USDA, APHIS circular 91-45-011 (29). Skin thickness at each injection site was measured prior to injection of PPDs and 72 h after administration. Changes in skin thickness were calculated and responses plotted on a scattergram developed by USDA for the interpretation of the CCT for bison, cattle, and cervidae (Form VS-6-22D). Responses by individual reindeer within both infected and noninfected groups are presented elsewhere (23).
Enzyme-linked immunosorbent assay (ELISA).
Lipoarabinomannan (LAM)-enriched mycobacterial antigen was prepared from M. bovis strain 95-1315 as described previously (31). Briefly, bacilli harvested from 4-week cultures were sonicated in PBS, further disrupted with 0.1- to 0.15-mm glass beads (Biospec Products, Bartlesville, Oklahoma) in a bead beater (Biospec Products), centrifuged, filtered (0.22-μm-pore-size filter), and digested in a 1-mg/ml proteinase K (Roche Molecular Biochemicals, Indianapolis, Indiana) solution (50 mM Tris, 1 mM CaCl2 buffer, pH 8.0) for 1 h at 50°C. Protein concentrations were determined (Bio-Rad, Hercules, California) and antigen stored at −20°C.
Immulon II 96-well microtiter plates (Dynatech, Chantilly, Virginia) were coated with 100 μl/well (4 μg) antigen diluted in carbonate/bicarbonate coating buffer (pH 9.6). Antigen-coated plates, including control wells containing coating buffer alone, were incubated for 15 h at 4°C. Plates were washed three times with 200 μl/well PBS containing 0.05% Tween 20 (PBST) (Sigma), and blocked with 200 μl/well of a commercial milk diluent/blocking solution (Kirkegaard and Perry Laboratories, Gaithersburg, Maryland). After incubation for 1 h at 37°C in the blocking solution, wells were washed nine times with 200 μl/well PBST and test sera added to wells (100 μl/well). Test and control sera were diluted 1:100 in PBS containing 0.1% gelatin. After incubation for 20 h at 4°C with diluted test sera, wells were washed nine times with 200 μl/well PBST and incubated for 1 h at 37°C with 100 μl/well of biotin-protein G (Sigma) diluted 1:5,000 in PBS plus 0.1% gelatin. Wells were washed nine times with 200 μl/well PBST and incubated for 1 h at 37°C with 100 μl/well of peroxidase-streptavidin (Sigma) diluted 1:2,000 in PBS plus 0.1% gelatin. Wells were washed nine times with 200 μl/well PBST and incubated for 4 min at room temperature with 100 μl/well of SureBlue 3,3′,5,5′-tetramethyl benzidine nsrsid6847181\delrsid6847181\charrsid6847181 microwell peroxidase substrate (Kirkegaard and Perry Laboratories). The reaction was stopped by the addition of 100 μl/well of 0.18 M sulfuric acid and the absorbances (450 nm) of individual wells measured using an automated ELISA plate reader (Molecular Devices, Menlo Park, California). Data are presented as the changes in optical density readings calculated by subtracting the mean optical density readings for wells receiving coating buffer alone (two replicates) from the mean optical density readings for antigen-coated wells (two replicates) receiving the same serum sample.
Immunoblot assay.
Electrophoresis and immunoblot assays were performed using previously described procedures (2) with the following modifications. The antigen for immunoblotting was a whole-cell sonicate (WCS) of M. bovis strain 95-1315. Following standard culture, mycobacteria were pelleted (10,000 × g for 20 min) and washed twice with cold PBS. Pellets were resuspended in PBS and sonicated on ice with a probe sonicator. Sonication consisted of three 10-min bursts at 18 W on ice, with 10-min chilling periods between sonications. Debris was removed by centrifugation (12,000 × g for 5 min) and supernatants harvested and stored at −20°C. Protein concentrations were determined by using a protein assay (Bio-Rad, Richmond, CA). Comparisons of the reactivities of serial serum samples against WCS antigen were conducted using a slot-blotting device (Bio-Rad, Richmond, CA). Antigen (200 μg) was electrophoresed through preparative 12% (wt/vol) polyacrylamide gels and transferred to nitrocellulose filters. These filters were placed in a blocking solution consisting of PBST and 2% (wt/vol) bovine serum albumin (PBST-BSA). After the blocking, the filters were placed into the slot blot device, and individual sera, diluted 1:200 in PBST-BSA, were added to independent slots. After a 2-h incubation with gentle rocking, blots were washed three times with PBST and incubated with horseradish peroxidase-conjugated protein G (Sigma) diluted 1:40,000 in PBST-BSA for 1.5 h. Blots were again washed three times with PBST and developed for chemiluminescence in SuperSignal detection reagent (Pierce Chemical Co.).
Multiantigen print immunoassay (MAPIA).
The following recombinant antigens of M. bovis were purified to near homogeneity as polyhistidine-tagged proteins (Rv numbers according to the classification of Cole et al. [3] in parentheses): ESAT-6 (Rv3875) and CFP10 (Rv3874) produced at the Statens Serum Institut, Copenhagen, Denmark; MPB59 (Rv1886c), MPB64 (Rv1980c), MPB70 (Rv2875), and MPB83 (Rv2873) (produced at the Veterinary Sciences Division, Stormont, Belfast [15]). Alpha-crystallin (Acr1) (Rv3391) and the 38-kDa protein PstS1 (Rv0934) were purchased from Standard Diagnostics, Seoul, South Korea. Polyprotein fusions CFP10/ESAT-6 and Acr1/MPB83 were constructed by overlapping PCR using gene-specific oligonucleotides to amplify the genes from M. tuberculosis H37Rv chromosomal DNA. The fused polygene PCR products were cloned into the pMCT6 Escherichia coli expression vector by using SmaI/BamHI restriction enzymes. The polyproteins were purified to near homogeneity by exploiting the polyhistidine tag encoded by the vector. M. bovis culture filtrate (MBCF) was obtained from a field strain of M. bovis (T/91/1378; Veterinary Sciences Division, Belfast, United Kingdom) cultured in synthetic Sauton's medium. MAPIA was performed as described previously (19). Briefly, antigens were immobilized on nitrocellulose membrane (Schleicher & Schuell, Keene, NH) at a protein concentration of 0.05 mg/ml by using a semiautomated airbrush-printing device (Linomat IV; Camag Scientific Inc., Wilmington, DE). The membrane was cut, perpendicular to the antigen bands, into 4-mm-wide strips. Strips were blocked for 1 h with 1% nonfat skim milk in PBS with 0.05% Tween 20 and then incubated for 1 h with serum samples diluted 1:40 in blocking solution. After being washed, strips were incubated overnight with horseradish peroxidase-conjugated protein G (Sigma, St. Louis, MO) diluted 1:1,000, followed by another washing step. Deer antibodies bound to printed antigens were visualized with 3,3′,5,5′-tetramethyl benzidine (Kirkegaard and Perry Laboratories, Gaithersburg, MD).
Statistics.
Data were analyzed by repeated measures (one-way analysis of variance followed by a Tukey-Kramer multiple comparisons test, using a commercially available statistics program [InStat 2.00; GraphPAD Software, San Diego, CA]). Pearson's product-moment correlations between MAPIA and pathology scores were computed.
RESULTS
Infection status.
Inoculation of M. bovis into tonsillar crypts of reindeer resulted in gross and microscopic lesions in all inoculated animals (23). Tuberculous lesions were most prominent in medial retropharyngeal lymph nodes and were also detected in tonsils, mesenteric lymph nodes, lungs, and lung-associated lymph nodes. Infected reindeer did not have clinical signs of TB or disseminated disease after 1 year of colonization, suggesting low-grade chronic infection. M. bovis cultures were isolated from tissues of 6/11 infected animals but not from control reindeer; however, granulomatous lesions with no acid-fast bacteria were detected within the tracheobronchial lymph nodes of 2/4 noninoculated reindeer (Table 1).
TABLE 1.
Comparison of pathologies and serologies from noninoculated reindeer
| Animal | ELISA antibody response (ΔOD)a
|
Pathologyb | |
|---|---|---|---|
| Day −7 | Post-CCT | ||
| 123 | −0.021 | 0.891 | g, m |
| 124 | −0.626 | 0.023 | — |
| 129 | −0.066 | 0.478 | g, m |
| 132 | 0.023 | −0.013 | — |
Change in optical density (ΔOD) (response to M. bovis-derived lipoarabinomannan-enriched antigen minus the response to no antigen) by sera from noninoculated reindeer at the initiation of the study (day −7) and 2 weeks after injection of purified protein derivative for the initial comparative cervical test (post-CCT) 16 weeks later.
g, gross lesions consistent with tuberculosis; m, caseonecrotic granuloma(s) seen upon microscopic examination (Mycobacterium bovis was not identified by culture or in situ PCR and no acid-fast bacteria present in lesions); —, gross or microscopic lesions not detected.
Serological response to LAM-enriched antigens and WCS.
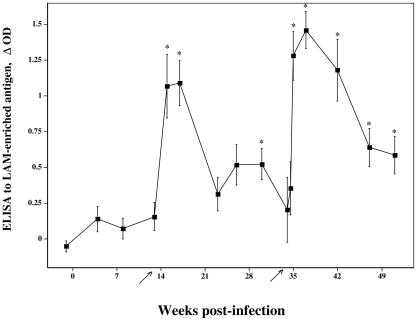
Antibodies to M. bovis LAM were detected by ELISA in sera taken 15 weeks after challenge with M. bovis, 2 weeks after the injection of PPDs for skin testing (Fig. 1). By 22 weeks postchallenge, responses to LAM had waned and were again boosted upon the injection of PPDs for the second skin test. The two noninoculated reindeer that had granulomatous lesions also had responses to M. bovis LAM that were detectable after the skin test (Table 1).
FIG. 1.
Response kinetics of serum antibody specific for LAM-enriched antigen. Sera from M. bovis-infected reindeer (▪; n = 11) were analyzed for reactivity to M. bovis-derived LAM by ELISA. Data are presented as mean (± standard errors of the mean) changes in optical density (ΔOD). Arrows on the x axis indicate time points when purified protein derivatives were injected for skin testing (CCT). Asterisks (*) indicate responses that exceed (P < 0.05) preinfection responses (i.e., week −1).
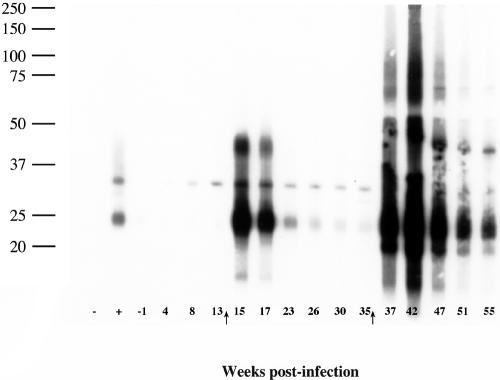
By the immunoblotting of M. bovis WCS, responses by infected reindeer were detected as early as 8 weeks postchallenge (Fig. 2). Specific bands of reactivity at <15, ∼25, ∼32, ∼42, ∼75, and ∼100 kDa were detected. Responses were boosted by the injection of PPDs for the skin test. As was the case with the LAM ELISA, injection of PPDs for the skin test elicited a response detectable by the immunoblotting of samples from two of the four noninoculated reindeer (animals 123 and 129), although the response was detectable only after the second skin test for animal 123. Immunoblot responses (i.e., intensity and number of bands) by 10/11 infected reindeer greatly exceeded the detected responses in samples from the two responding noninoculated reindeer. The two noninoculated reindeer had only a single, light band of reactivity at ∼25 kDa, whereas sera from infected reindeer elicited multiple, heavy bands of reactivity upon immunoblotting.
FIG. 2.
Preparative immunoblots of M. bovis strain 95-1315 WCS antigen probed with sera from a reindeer experimentally infected with M. bovis. Molecular mass markers are indicated in the left margin (in kDa) and weeks postinfection in the lower margin. Arrows in the lower margin indicate time points when purified protein derivatives were injected for skin testing (CCT). Symbols in the lower left margin refer to sera from known noninfected (−) and infected (+) white-tailed deer used as controls for the assay. Numbers in the lower margin refer to weeks relative to infection. Immunoblots were performed on samples from each reindeer at all time points indicated. Responses presented in this figure are representative of 10/11 of the infected reindeer. One infected reindeer (animal 125) had only minimal responses detectable by immunoblot analysis.
Serological response to purified proteins.
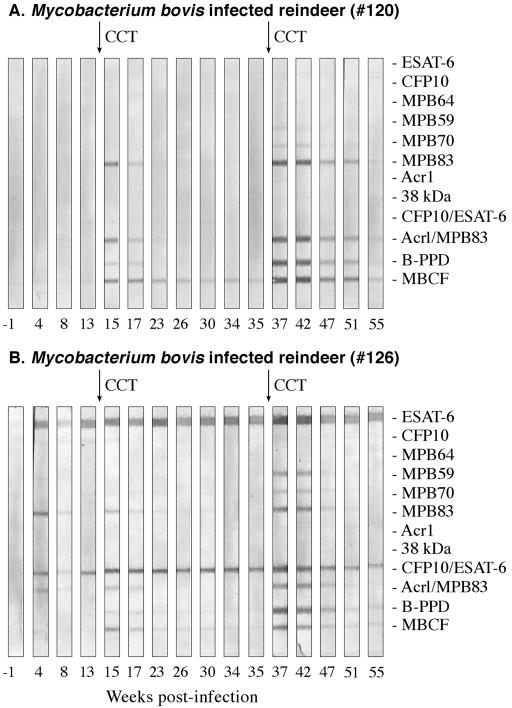
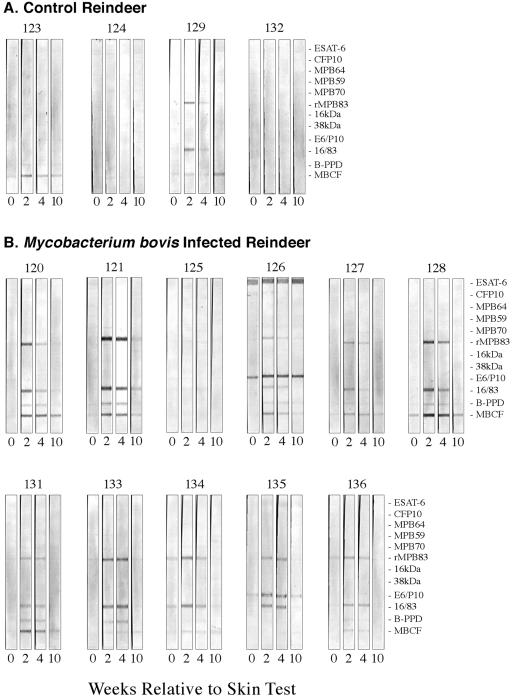
To further characterize the humoral immune response, the MAPIA was used to determine responses to a panel of recombinant M. tuberculosis/M. bovis proteins. Antigens recognized by sera from M. bovis-infected reindeer included MPB83, MPB70, MPB59, Acr1, ESAT-6, CFP10, and fusion proteins ESAT-6/CFP10 and Acr1/MPB83, although not all antigens were recognized at each time point and some antigens were not recognized by sera from each animal (Table 2; Fig. 3). For comparison, two complex antigens (M. bovis PPD and MBCF) were included in the assay and were recognized, at least at some time point, by each M. bovis-infected animal. Responses by infected reindeer were detected as early as 4 weeks postchallenge by MAPIA (Fig. 3B). With animal 126, MPB83 and ESAT-6 were both early antigenic targets with reactivities to each of the single proteins and their respective fusion proteins (Fig. 3B). As with the ELISA and immunoblotting, the intradermal tuberculin injection for skin testing boosted responses of infected reindeer to several antigens (Fig. 4). Serum antibodies against a limited number of antigens (MPB83 and/or MBCF) were elicited by skin testing in 2/4 noninoculated reindeer (animals 123 and 129) (Fig. 4A). However, the detected antibody levels in samples from the experimentally infected reindeer consistently exceeded the detected antibody levels in samples from the two responding noninoculated reindeer (Fig. 4).
TABLE 2.
Rates of antibody responses to protein antigens detected by MAPIA in reindeer infected with Mycobacterium bovis
| Antigen | No. of reactorsa (n = 11) | Sensitivity (%) |
|---|---|---|
| MPB83 | 11 | 100 |
| Acr1/MPB83 | 11 | 100 |
| MPB70 | 9 | 82 |
| MPB59 | 6 | 55 |
| Acr1 | 5 | 45 |
| ESAT-6 | 4 | 36 |
| CFP10/ESAT-6 | 3 | 27 |
| CFP10 | 1 | 9 |
| 38-kDa antigen | 0 | 0 |
| MPB64 | 0 | 0 |
| MBCF | 11 | 100 |
| PPDbb | 11 | 100 |
Responses are indicative of rates of antigen recognition for each animal measured at various time points postinfection.
PPDb, M. bovis purified protein derivative.
FIG. 3.
Antibody responses to recombinant antigens detected by MAPIA in reindeer experimentally infected with M. bovis. Arrows in the upper margin indicate time points when purified protein derivatives were injected for skin testing (CCT). Antigens printed are shown in the right margin. Strips represent different time points during infection when serum samples were collected (shown in weeks in the lower margin). Representative responses by two different M. bovis-infected animals, animal 120 (A) and animal 126 (B), are provided to demonstrate the variability in antigen recognition patterns.
FIG. 4.
Effects of injection of PPDs for skin testing on antibody responses (as measured by MAPIA) by noninfected (A) and infected (B) reindeer to M. bovis antigens. Reindeer were injected with PPDs for skin testing 3 months after challenge with M. bovis and sera collected at indicated time points for analysis of antibody. Numbers in the upper margins indicate animal identification numbers. Numbers in lower margins indicate weeks relative to skin test. Antigens are indicated in the right margin.
Specificity of MAPIA.
As indicated, responses in samples from 2/4 noninoculated reindeer after the skin test were detected, indicating prior sensitivity to cross-reactive antigens. To evaluate the specificity of MAPIA, samples from noninfected reindeer were evaluated. These samples included sera obtained from reindeer in the infected group (n = 11) and the noninoculated group (n = 4) at the preinoculation time point as well as samples collected from 19 reindeer that were part of another study. Serum antibodies against a limited number of antigens (5/34 responded to MPB83, 2/34 responded to ESAT-6, and 1/34 responded to MBCF) were detected and indicative of relative specificities of 85%, 91%, and 97% for MPB83, ESAT-6, and MBCF, respectively. Further studies to evaluate a larger sample size (i.e., >200 reindeer serum samples) from a wide range of locations (i.e., Alaska to Tennessee) within the United States to better estimate the specificity of MAPIA are under way.
Association of antibody responses with pathology and bacteriology findings.
Individual serological responses characterized by MAPIA appeared to correlate with pathological and bacteriologic results. To provide this comparison, infected reindeer were divided into three subgroups, strong, moderate, and weak antibody reactors, based on magnitude of the humoral responses and number of antigens recognized in MAPIA (Table 3). Additionally, MAPIA and pathology scores were calculated based upon MAPIA band intensities/numbers and necropsy findings, respectively. By use of linear correlation analysis, a positive correlation between MAPIA and pathology scores was detected (r = 0.68; P = 0.02) (Table 3). All infected animals had positive histopathology results (i.e., presence of granulomatous lesions with acid-fast organisms present), and most of them had gross lesions consistent with TB found in at least one tissue specimen. Each subgroup of antibody responders had one animal without gross lesions. Among the strong antibody responders, animal 128 did not have gross lesions; however, this animal was euthanized 7 months postinfection, or 6 months earlier than the other infected reindeer. Further, M. bovis culture was recovered from tissues of 3/3 strong antibody responders, 0/5 moderate antibody responders, and 1/3 weak antibody responders. Intriguingly, there were only two animals with M. bovis strains isolated from the lungs, and both were found among the group of strong antibody responders (i.e., animals 121 and 128). Also, the subgroups clearly differed by extent of lesions and M. bovis replication in various tissues (Table 3) so that the strong antibody responders had a greater number of tissues with lesions and/or M. bovis than did the other two subgroups.
TABLE 3.
Association of the tuberculin-boosted antibody responses to M. bovis antigens with pathology/bacteriology findings in experimentally infected reindeer
| Animal | Results for antibodies in MAPIA
|
Pathology and bacteriology results
|
|||||
|---|---|---|---|---|---|---|---|
| Band intensitya | No. of bands | MAPIA scoreb | Gross lesions | Culture from tissues | No. of positive tissuesc | Pathology scored | |
| 121 | +++ | 4 | 7 | Positive | Positive | 3 | 5 |
| 126 | +++ | 6 | 9 | Positive | Positive | 5 | 7 |
| 128e | +++ | 4 | 7 | Positive | Positive | 4 | 6 |
| 120 | ++ | 4 | 6 | Negative | Negative | 1 | 1 |
| 131 | ++ | 4 | 6 | Negative | Negative | 1 | 1 |
| 133 | ++ | 4 | 6 | Negative | Negative | 1 | 1 |
| 134 | ++ | 3 | 5 | Negative | Negative | 3 | 3 |
| 135 | ++ | 3 | 5 | Negative | Negative | 0 | 0 |
| 125 | +/− | 2 | 2.5 | Negative | Negative | 2 | 2 |
| 127 | + | 4 | 5 | Positive | Positive | 1 | 3 |
| 136 | + | 3 | 4 | Negative | Negative | 0 | 0 |
Magnitude of antibody response to most seroreactive antigen(s) detected by MAPIA 2 to 4 weeks after skin testing performed 3 months postinfection was scored visually as strong (+++), moderate (++), weak (+), or very weak (+/−).
MAPIA score is the band intensity (e.g., +++ represents 3 and +/− represents 0.5) plus number of bands.
At necropsy (13 months postinfection), 15 tissue specimens, including tonsil, lung, spleen, liver, kidney, and 10 different lymph nodes, were examined for gross lesions consistent with tuberculosis and histopathology (caseonecrotic granuloma with acid-fast bacilli); the tissues were also used to isolate M. bovis by bacteriologic culture; disease status was confirmed for all 11 infected reindeer by positive histopathology findings in one or more tissue specimens (not shown). Numbers of tissues with positive gross pathology and/or bacteriology results are shown.
The pathology score is the value for gross lesions plus the value for culture from tissues plus the number of positive tissues. Positive and negative findings were assigned values of 1 and 0, respectively. Pearson's correlation coefficient was 0.68 (P = 0.02) for the comparison of MAPIA scores to pathology scores.
Euthanized 7 months postinfection due to complications unrelated to M. bovis inoculation.
Antibody responses and cell-mediated immunity.
The serological findings obtained for each animal in the present study were compared with skin test results and gamma interferon (IFN-γ) responses that are described in detail elsewhere (23, 35). All 11 infected reindeer tested positive by CCT (APHIS Form VS-6-22D) for the interpretation of CCT for bison, cattle, and cervidae, using either possible interpretation (23), performed 3 and 8 months after M. bovis inoculation. No correlation between the skin test reactions obtained with M. bovis PPD and the levels of serum immunoglobulin responses detected by ELISA or MAPIA was found. In particular, at 3 months postinfection, the strongest MAPIA responders (animals 121, 126, and 128) produced skin test reactions (changes in skin thickness) ranging from 17.5 mm to 24.9 mm, while the weakest MAPIA responders (animals 125, 127, and 136) produced skin test reactions ranging from 12.9 mm to 19.3 mm, demonstrating no significant difference between the extreme subgroups. Further, the weakest CCT reactor, animal 135 (3.0 mm), and the strongest CCT reactor, animal 131 (25.0 mm), had essentially the same magnitude of serological response (Fig. 4). Similarly, there was no correlation between humoral responses and in vitro IFN-γ production to M. bovis PPD stimulation (data not shown). Interestingly, animal 126, the only strong and early ESAT-6 antibody responder (Fig. 3), did not have a prominent in vitro IFN-γ response to this antigen. Instead, the highest levels of IFN-γ under ESAT-6 stimulation were produced by animal 120, which showed no antibody response against this antigen throughout the course of infection (Fig. 3).
DISCUSSION
The present study demonstrates that experimental infection of reindeer with M. bovis elicits antibody responses detectable by several immunoassays using various mycobacterial antigens. It is the first study, as far as we know, to evaluate antibody responses and antigen recognition patterns by reindeer to M. bovis infection. In MAPIA, the most frequently recognized proteins were MPB83, MPB70, and MPB59. Other antigens, including ESAT-6 and CFP10, had moderate individual seroreactivity (36% and 9%, respectively) and potential for detection of early infection (reindeer 126) (Fig. 3B). Sera from two of four noninoculated reindeer exhibited weak reactivities to mycobacterial antigens, and these responses correlated with pathological findings. Lesions found in lung-associated lymph nodes from these two reindeer were granulomatous with no isolation of M. bovis and no detection of acid-fast bacteria. Infection with M. tuberculosis complex mycobacteria was further ruled out by in situ PCR for IS6110. In a concurrent study evaluating cellular immune responses, each of these two reindeer had intermittent IFN-γ responses to M. bovis PPD considered positive, yet these two reindeer did not exhibit positive IFN-γ responses to rESAT6/CFP10 (35). Interestingly, IFN-γ responses to M. bovis PPD by these two reindeer exceeded concurrent responses to M. avium PPD. One of the two noninfected reindeer exhibiting positive antibody responses also tested positive by skin test (second CCT only) (23). It should be noted, however, that both IFN-γ and skin test responses to M. bovis PPD by infected reindeer greatly exceeded those of noninfected reindeer (23, 35). Together, these findings indicate that lymphocytes (both B and T cells) from nontuberculous reindeer react to certain antigens within M. bovis PPD, likely due to exposure to cross-reactive epitopes of other nontuberculous species of bacteria, highlighting the unique response of this host to mycobacterial antigens.
Reindeer are susceptible to infection with other mycobacterial agents, such as M. avium subsp. paratuberculosis and Mycobacterium kansasii (13). Lesions in M. kansasii-infected reindeer closely resemble those seen from M. bovis-inoculated reindeer (13). Antigens traditionally considered specific for the M. tuberculosis complex (e.g., ESAT-6, CFP10, and MPB83) are also produced by M. kansasii and have been shown to elicit an immune response in M. kansasii-sensitized/infected cattle, humans, nonhuman primates, and guinea pigs (6, 7, 8, 9, 30; W. R. Waters and K. P. Lyashchenko, unpublished observations). In the present study, the only other mycobacterium isolated was Mycobacterium duvalii from a sample of kidney. Mycobacterium duvalii is commonly associated with soil and is not known to be a pathogen of animals. While this is speculation, it is likely that responses detected by noninoculated reindeer resulted from infection/sensitization with nontuberculous mycobacteria or closely related bacteria, resulting in a cross-reactive response.
In earlier studies, LAM has proven useful in antibody-based tests of mycobacterial infections in cattle and white-tailed deer and provides a tool to objectively evaluate antibody response kinetics upon experimental inoculation (5, 12, 14, 25-28, 31, 32, 34). However, this antigen is broadly cross-reactive with nontuberculous mycobacteria. The use of purified proteins could improve the specificities of antibody-based TB tests, but the sensitivities might be insufficient. Previous serological studies on tuberculous cattle, Eurasian badgers (Meles meles), and white-tailed deer have demonstrated that antibody responses of these species to M. bovis are characterized by recognition of variable patterns of multiple proteins (1, 10, 17, 18, 20, 34). MPB70 and MPB83 proteins are predominantly reactive in cattle, whereas MPB83 is the most serodominant antigen in white-tailed deer and, particularly, in European badgers (4, 10, 11, 20, 34). Sera from M. bovis-infected cattle, white-tailed deer, and badgers also react with other specific proteins (e.g., ESAT-6 and CFP10), but the set of most frequently recognized antigens may vary among the host species (10, 17, 18, 20, 34). Thus, it is crucial to determine antigen recognition patterns over the course of M. bovis infection for each of the various hosts. Utilizing several of the most immunodominant antigens as defined for reindeer in the present study, a simple serological test can be developed using advanced immunoassay technologies, such as the lateral-flow format suitable for rapid field testing (10).
The present study also demonstrated that humoral responses did not correlate with T-cell immunity of infected reindeer. However, the extent of pathology developed in individuals correlated well with the magnitude of their antibody responses. These findings are in agreement with recent observations on antibody responses of M. bovis-infected cattle and white-tailed deer (19, 34, 36), thus indicating that the serological approach may have not only diagnostic value but also prognostic potential. As is the case with cattle (36), it appears that antibody levels are positively correlated with disease progression and may be indicative of host susceptibility among cervid species. Disease progression in white-tailed deer is more rapid than that in reindeer (23); likewise, antibody responses by M. bovis-infected white-tailed deer (34) are more vigorous and develop more rapidly than those of reindeer (present study). Specific IFN-γ responses by inoculated reindeer were robust, detected early, and maintained throughout the duration of the study (35), indicative of a prominent cellular response. The results indicate that antibody-based tests provide a convenient and inexpensive antemortem tool to evaluate disease progression (positive correlation with antibody) and/or protective immunity (negative correlation with antibody) in animals used in trials for new vaccines. To serve this purpose, the serological assays must differentiate antibody responses produced against vaccine antigens from those induced by M. bovis infection.
Studies with cattle and white-tailed deer have also demonstrated that antibody responses of infected animals can be boosted significantly by the intradermal tuberculin injection administered for routine skin testing (11, 15). These anamnestic responses are mediated mostly by IgG1 in cattle and may involve multiple antigens (16, 17). In the present study, we found a similar boosting effect of tuberculin skin testing on humoral responses to M. bovis in experimentally infected reindeer. The sharply elevated antibody levels appeared 2 weeks after skin testing and gradually declined a few weeks later, suggesting that B-cell memory primed by M. bovis inoculation played a role. This phenomenon requires further investigation and, if proven diagnostically specific, may be a powerful approach to enhance the potential of TB serodiagnosis. For instance, a serological TB test could be used in conjunction with standard skin testing procedures (i.e., as a complementary or confirmatory test).
Ultimately, it would be advantageous to develop and implement a stand-alone blood-based assay for TB surveillance of reindeer and other species. Another option is for antibody-based tests to be used as confirmatory tests performed several weeks after skin testing procedures. This approach could prove useful in decreasing the number of reindeer slaughtered due to false-positive skin test reactions to the complex cocktail of antigens contained within M. bovis PPD. Future studies will determine the specificity of such a test, particularly given the likelihood of confounding cross-reactive responses probably elicited by exposure to nontuberculous mycobacteria.
Acknowledgments
USDA, APHIS, in part, provided funds for these studies.
We thank Peter Lasley, Rebecca Lyon, Jessica Pollock, and Shelly Zimmerman for excellent technical support. We also thank Richard Auwerda, Gary Buck, Doug Ewing, Andrew Gibson, Don Hackbarth, Todd Holtz, Terry Krausman, David Panthen, Brian Pottebaum, Don Robinson, Jay Steffen, Johann Thiel, Wayne Varland, and Larry Wright for excellent animal care.
REFERENCES
- 1.Amadori, M., K. P. Lyashchenko, M. L. Gennaro, J. M. Pollock, and I. Zerbini. 2002. Use of recombinant proteins in antibody tests for bovine tuberculosis. Vet. Microbiol. 85:379-389. [DOI] [PubMed] [Google Scholar]
- 2.Bannantine, J., and J. R. Stabel. 2000. HspX is present within Mycobacterium paratuberculosis-infected macrophages and is recognized by sera from some infected cattle. Vet. Microbiol. 76:343-358. [DOI] [PubMed] [Google Scholar]
- 3.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 4.Fifis, T., C. Costopoulos, L. A. Corner, and P. R. Wood. 1992. Serological reactivity to Mycobacterium bovis protein antigens in cattle. Vet. Microbiol. 30:343-354. [DOI] [PubMed] [Google Scholar]
- 5.Gaborick, C., M. D. Salman, R. P. Ellis, and J. Triantis. 1996. Evaluation of a five-antigen ELISA for diagnosis of tuberculosis in cattle and cervidae. J. Am. Vet. Med. Assoc. 209:962-966. [PubMed] [Google Scholar]
- 6.Geluk, A., K. E. Van Meijgaarden, K. L. Franken, B. Wieles, S. M. Arend, W. R. Faber, B. Naafs, and T. H. Ottenhoff. 2004. Immunological crossreactivity of the Mycobacterium leprae CFP-10 with its homologue in Mycobacterium tuberculosis. Scand. J. Immunol. 59:66-70. [DOI] [PubMed] [Google Scholar]
- 7.Geluk, A., K. E. van Meijgaarden, K. L. Franken, Y. W. Subronto, B. Wieles, S. M. Arend, E. P. Sampaio, T. de Boer, W. R. Faber, B. Naafs, and T. H. Ottenhoff. 2002. Identification and characterization of the ESAT-6 homologue of Mycobacterium leprae and T-cell cross-reactivity with Mycobacterium tuberculosis. Infect. Immun. 70:2544-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gey van Pittius, N. C., R. M. Warren, and P. D. van Helden. 2002. ESAT-6 and CFP-10: what is the diagnosis? Infect. Immun. 70:6509-6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gey Van Pittius, N. C., J. Gamieldien, W. Hide, G. D. Brown, R. J. Siezen, and A. D. Beyers. 2001. The ESAT-6 gene cluster of Mycobacterium tuberculosis and other high G+C Gram-positive bacteria. Genome Biol. 2:0044-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenwald, R., J. Esfandiari, S. Lesellier, R. Houghton, J. Pollock, C. Aagaard, P. Andersen, R. G. Hewinson, M. Chambers, and K. Lyashchenko. 2003. Improved serodetection of Mycobacterium bovis infection in badgers (Meles meles) using multiantigen test formats. Diagn. Microbiol. Infect. Dis. 46:197-203. [DOI] [PubMed] [Google Scholar]
- 11.Harboe, M., H. G. Wiker, J. R. Duncan, M. M. Garcia, T. W. Dukes, B. W. Brooks, C. Turcotte, and S. Nagai. 1990. Protein G-based enzyme-linked immunosorbent assay for anti-MPB70 antibodies in bovine tuberculosis. J. Clin. Microbiol. 28:913-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jark, U., I. Ringena, B. Franz, G. F. Gerlach, M. Beyerbach, and B. Franz. 1997. Development of an ELISA technique for serodiagnosis of bovine paratuberculosis. Vet. Microbiol. 51:189-198. [DOI] [PubMed] [Google Scholar]
- 13.Kiupel, M., H. Simmon, D. Berry, and C. Bolin. 2003. Mycobacterium kansasii in reindeer (Rangifer tarandus), p. 120. In Proceedings of the American Association of Veterinary Laboratory Diagnosticians.
- 14.Koets, A. P., V. P. Rutten, M. de Boer, D. Bakker, P. Valentin-Weigand, and W. van Eden. 2001. Differential changes in heat shock protein-, lipoarabinomannan-, and purified protein derivative-specific immunoglobulin G1 and G2 isotype responses during bovine Mycobacterium avium subsp. paratuberculosis infection. Infect. Immun. 69:1492-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lightbody, K. A., R. A. Skuce, S. D. Neill, and J. M. Pollock. 1998. Mycobacterial antigen-specific antibody responses in bovine tuberculosis: an ELISA with potential to confirm disease status. Vet. Rec. 142:295-300. [DOI] [PubMed] [Google Scholar]
- 16.Lightbody, K. A., J. McNair, S. D. Neill, and J. M. Pollock. 2000. IgG isotype antibody responses to epitopes of the Mycobacterium bovis protein MPB70 in immunised and in tuberculin skin test-reactor cattle. Vet. Microbiol. 75:177-188. [DOI] [PubMed] [Google Scholar]
- 17.Lyashchenko, K., A. O. Whelan, R. Greenwald, J. M. Pollock, P. Andersen, R. G. Hewinson, and H. M. Vordermeier. 2004. Association of tuberculin-boosted antibody responses with pathology and cell-mediated immunity in cattle vaccinated with Mycobacterium bovis BCG and infected with M. bovis. Infect. Immun. 72:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyashchenko, K. P., J. M. Pollock, R. Colangeli, and M. L. Gennaro. 1998. Diversity of antigen recognition by serum antibodies in experimental bovine tuberculosis. Infect. Immun. 66:5344-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyashchenko, K. P., M. Singh, R. Colangeli, and M. L. Gennaro. 2000. A multi-antigen print immunoassay for the development of serological diagnosis of infectious diseases. J. Immunol. Methods 242:91-100. [DOI] [PubMed] [Google Scholar]
- 20.McNair, J., D. M. Corbett, R. M. Girvin, D. P. Mackie, and J. M. Pollock. 2001. Characterization of the early antibody response in bovine tuberculosis: MPB83 is an early target with diagnostic potential. Scand. J. Immunol. 53:365-371. [DOI] [PubMed] [Google Scholar]
- 21.Palmer, M. V., D. L. Whipple, and S. C. Olsen. 1999. Development of a model of natural infection with Mycobacterium bovis in white-tailed deer. J. Wildl. Dis. 35:450-457. [DOI] [PubMed] [Google Scholar]
- 22.Palmer, M. V., D. L. Whipple, and W. R. Waters. 2001. Tuberculin skin testing in white-tailed deer (Odocoileus virginianus). J. Vet. Diagn. Investig. 13:530-533. [DOI] [PubMed] [Google Scholar]
- 23.Palmer, M. V., W. R. Waters, T. C. Thacker, and B. V. Thomsen. Experimental infection of reindeer (Rangifer tarandus) with Mycobacterium bovis. Submitted for publication. [DOI] [PubMed]
- 24.Schmitt, S. M., S. D. Fitzgerald, T. M. Cooley, C. S. Bruning-Fann, L. Sullivan, D. Berry, T. Carlson, R. B. Minnis, J. B. Payeur, and J. Sikarskie. 1997. Bovine tuberculosis in free-ranging white-tailed deer from Michigan. J. Wildl. Dis. 33:749-758. [DOI] [PubMed] [Google Scholar]
- 25.Sugden, E. A., B. S. Samagh, D. R. Bundle, and J. R. Duncan. 1987. Lipoarabinomannan and lipid-free arabinomannan antigens of Mycobacterium paratuberculosis. Infect. Immun. 55:762-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugden, E. A., A. H. Corner, B. S. Samagh, B. W. Brooks, C. Turcotte, K. H. Nielsen, R. B. Stewart, and J. R. Duncan. 1989. Serodiagnosis of ovine paratuberculosis, using lipoarabinomannan in an enzyme-linked immunosorbent assay. Am. J. Vet. Res. 6:850-854. [PubMed] [Google Scholar]
- 27.Sugden, E. A., K. Stilwell, E. B. Rohonczy, and P. Martineau. 1997. Competitive and indirect enzyme-linked immunosorbent assays for Mycobacterium bovis infections based on MPB70 and lipoarabinomannan antigens. Can. J. Vet. Res. 61:8-14. [PMC free article] [PubMed] [Google Scholar]
- 28.Sugden, E. A., K. Stilwell, and A. Michaelides. 1997. A comparison of lipoarabinomannan with other antigens used in the absorbed enzyme immunoassays for the serological detection of cattle infected with Mycobacterium paratuberculosis. J. Vet. Diagn. Investig. 9:413-417. [DOI] [PubMed] [Google Scholar]
- 29.United States Department of Agriculture. 1999. Bovine tuberculosis eradication uniform methods and rules. APHIS 91-45-011. U.S. Government Printing Office, Washington, D.C.
- 30.Vosloo, W., P. Tippoo, J. E. Hughes, N. Harriman, M. Emms, D. W. Beatty, H. Zappe, and L. M. Steyn. 1997. Characterisation of a lipoprotein in Mycobacterium bovis (BCG) with sequence similarity to the secreted protein MPB70. Gene 188:123-128. [DOI] [PubMed] [Google Scholar]
- 31.Waters, W. R., M. V. Palmer, and D. L. Whipple. 2002. Mycobacterium bovis-infected white-tailed deer (Odocoileus virginianus): detection of immunoglobulin specific to crude mycobacterial antigens by ELISA. J. Vet. Diag. Investig. 14:470-475. [DOI] [PubMed] [Google Scholar]
- 32.Waters, W. R., J. M. Miller, M. V. Palmer, J. R. Stabel, D. E. Jones, K. A. Koistinen, E. M. Steadham, M. J. Hamilton, W. C. Davis, and J. P. Bannantine. 2003. Early induction of a humoral and cellular immune response during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 71:5130-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waters, W. R., M. V. Palmer, S. C. Olsen, R. E. Sacco, and D. L. Whipple. 2003. Immune responses of elk to Mycobacterium bovis bacille Calmette Guerin vaccination. Vaccine 21:1518-1526. [DOI] [PubMed] [Google Scholar]
- 34.Waters, W. R., M. V. Palmer, J. P. Bannantine, D. L. Whipple, R. Greenwald, J. Esfandiari, P. Andersen, J. McNair, J. M. Pollock, and K. P. Lyashchenko. 2004. Antigen recognition by serum antibodies in white-tailed deer (Odocoileus virginianus) experimentally infected with Mycobacterium bovis. Clin. Diagn. Lab. Immunol. 11:849-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waters, W. R., M. V. Palmer, R. E. Slaughter, S. L. Jones, J. E. Pitzer, and F. C. Minion. A blood-based gamma interferon assay for the detection of Mycobacterium bovis-infected reindeer (Rangifer tarandus). Submitted for publication. [DOI] [PMC free article] [PubMed]
- 36.Welsh, M. D., R. T. Cunningham, D. M. Corbett, R. M. Girvin, J. McNair, R. A. Skuce, D. G. Bryson, and J. M. Pollock. 2005. Influence of pathological progression on the balance between cellular and humoral immune responses in bovine tuberculosis. Immunology 114:101-111. [DOI] [PMC free article] [PubMed] [Google Scholar]