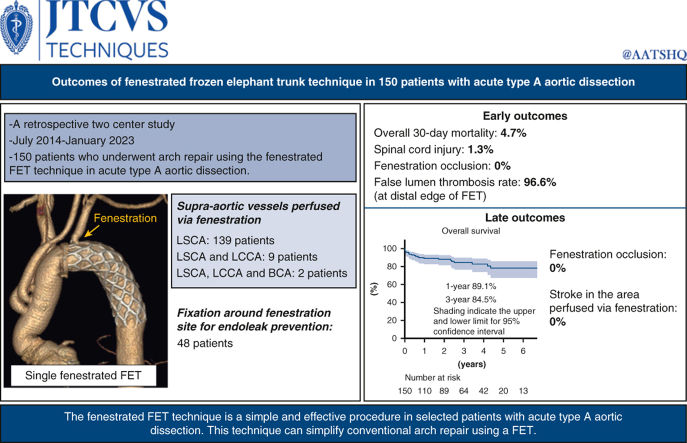
Abstract
Objective
The fenestrated frozen elephant trunk (FET) technique provides proximalization of distal anastomosis and antegrade blood flow into supra-aortic vessels through fenestration in a FET. We investigated the outcomes of the fenestrated FET technique in acute type A aortic dissection.
Methods
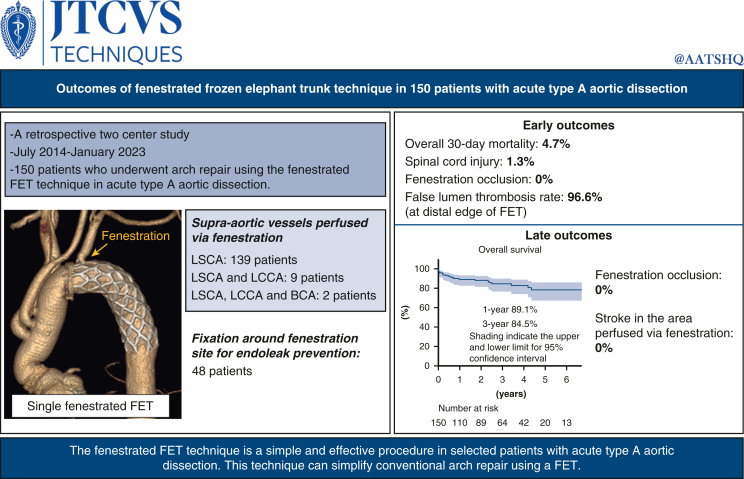
We evaluated 150 patients who underwent arch repair using the fenestrated FET technique for acute type A aortic dissection between July 2014 and January 2023. FET was deployed under hypothermic circulatory arrest and manually fenestrated under direct vision on the supra-aortic vessel aspect. Fenestration was performed for the left subclavian artery alone in 139 patients, 2 supra-aortic vessels in 9 patients, and total supra-aortic vessels in 2 patients. Fixation around fenestration site for endoleak prevention was performed in 48 patients.
Results
The overall 30-day mortality rate was 4.7% (7 out of 150). Two patients developed paraparesis. Adequate blood flow into the supra-aortic vessels through fenestrations were confirmed in all patients at discharge. The false lumen thrombosis rate at the distal edge of FET was 96.6%. The median follow-up period was 28 months. The 1-year and 3-year overall survival rate was 89.1% and 84.5%, respectively. During the follow-up period, neither fenestration occlusion nor stroke was noted in the cerebral area perfused via the fenestration. Distal stent graft-induced new entry was noted in 2 patients.
Conclusions
The fenestrated FET technique is a straightforward and secure procedure for selected patients with acute type A aortic dissection. This technique can facilitate arch repair.
Key Words: aortic dissection, arch repair, frozen elephant trunk, fenestration
Graphical Abstract
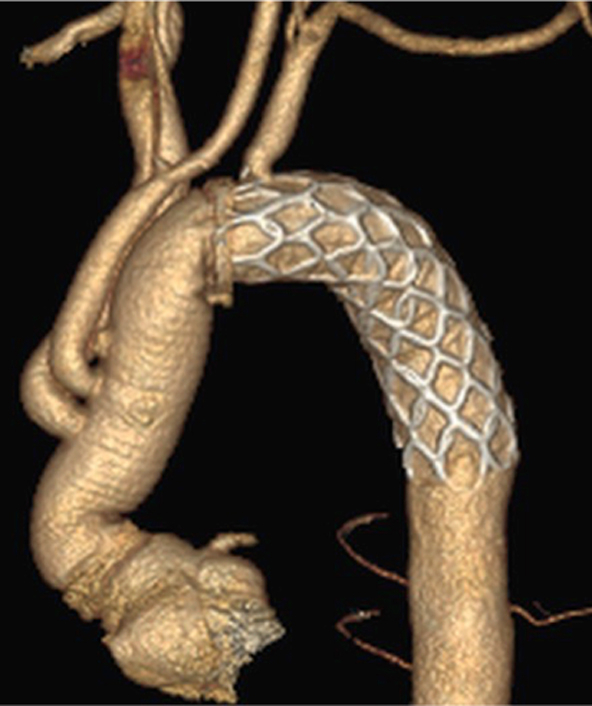
Three-dimensional computed tomography image of the fenestrated frozen elephant trunk technique.
Central Message.
The fenestrated frozen elephant trunk technique is a feasible procedure in patients with acute type A aortic dissection.
Perspective.
Arch repair using a FET allows for better remodeling of the distal aorta; however, it is technically demanding. The fenestrated FET technique in which reconstruction of 1 or more supra-aortic vessels is not necessary can simplify arch repair and potentially improve the outcomes. Further studies are warranted regarding the long-term outcomes of this technique.
The frozen elephant trunk (FET) technique is commonly performed as a valuable adjunct to total arch replacement (TAR) for acute type A aortic dissection (ATAAD). FET provides better postoperative remodeling in the distal aorta compared with conventional elephant trunk.1 The advantages of FET in ATAAD include proximalization of distal anastomosis, entry tear closure and remodeling in the descending aorta, expansion of the compressed true lumen, and landing platform for future endovascular aortic repair. However, expanding the indications for TAR using FET remains controversial because some investigators have reported that extensive aortic repair is associated with higher early mortality rates compared with hemiarch replacement.2,3
Among the difficulties in TAR is the reconstruction of supra-aortic vessels, especially the left subclavian artery (LSCA), which is located deep in the operative field. Generally, the LSCA is reconstructed either in situ within the mediastinum or using an extra-anatomical bypass into the left axillary artery. The fenestrated FET technique consists of fenestration of FET and antegrade blood flow into 1 or more supra-aortic vessels via the fenestration site.4, 5, 6, 7 Advantage of the fenestrated FET technique is the lack of need for the reconstruction of supra-aortic vessels. We previously reported the outcomes of this technique in 22 patients.8 However, endoleaks via the fenestration sites remain a concern of this technique. The long-term effects of endoleaks as well as the safety of antegrade blood flow into the supra-aortic vessels via fenestration remain unknown. Furthermore, it is unclear whether this technique provides excellent remodeling of the distal aorta and if it is comparable to that of the conventional FET technique. Thus, in this study, we investigated the early and late outcomes of the fenestrated FET technique in 150 patients.
Patients and Methods
Patient Population and Study Design
This retrospective study was based on data obtained from our institutional database. Between July 2014 and January 2023, 341 patients underwent surgical repair for ATAAD at Nerima Hikarigaoka Hospital and Kyoto Katsura Hospital. Of them, 150 consecutive patients who underwent arch repair with the fenestrated FET technique were included in this study.
Following were the indications for extended aortic repair: entry tear in the aortic arch or the proximal descending aorta; arch aneurysm; or distal malperfusion. Patient age younger than 75 years was also considered a relative indication for extended repair. Indications for the fenestrated FET technique were similar to those of extended aortic repair as discussed above. In the early study period, the fenestrated FET technique was not deemed suitable in patients with an entry tear located within 2 cm from the origin of the LSCA or with an obviously dissected LSCA. However, since the introduction of suture fixation around the fenestration site in June 2019, the fenestrated FET technique was performed regardless of these 2 conditions.
The number of the supra-aortic vessels perfused via the fenestration can be classified into single, double, and total groups. The single group includes fenestration for only the LSCA, whereas the double group includes fenestration for LSCA and the left common carotid artery (LCCA), and the total group includes fenestration for all the supra-aortic vessels. Although 3 types of fenestrations were performed, we primarily performed single fenestration because suture fixation around the fenestration site is technically difficult in double and total fenestration groups and reconstructions of LCCA and the brachiocephalic artery are relatively simple. This 2-center study was approved by the Institutional Review Board of Nerima Hikarigaoka Hospital (23020902; March 13, 2023), and the requirement for individual consent was waived for the analysis of anonymized data.
Surgical Procedure
The surgery was performed via a median sternotomy. Cardiopulmonary bypass was established via cannulation of the ascending aorta using ultrasound (114 patients), the axillary artery (31 patients), the femoral artery (12 patients), the apex of the heart (1 patient), the right atrium, and a left ventricular vent. Double arterial cannulation was required in 8 patients.
Lower body hypothermic circulatory arrest was achieved for a target rectal temperature of 25 to 28 °C. Retrograde and antegrade cold blood cardioplegia were intermittently administered. Antegrade selective cerebral perfusion for the brachiocephalic artery, LCCA and LSCA was initiated. When LSCA cannula was an obstacle during fenestration, LSCA perfusion was temporarily paused. The aortic arch was transected, and a commercially available FET (J Graft FROZENIX; Japan Lifeline) was deployed into the true lumen of the distal aorta. The size of the stent graft was selected based on 90% of the diameter of the descending aorta at the level of distal end of the FET, which was determined using preoperative computed tomography (CT). A FET of the optimal length was selected to ensure that the distal end of the FET was placed in the straight segment of the descending aorta using preoperative CT. Subsequently, an approximately 10-mm hole was created in the FET graft using scissors under direct vision on the supra-aortic vessel aspect although the size of fenestration depends on number of vessels perfused via fenestration (Video 1). A single fenestration was made in the FET in 139 patients, 2 fenestrations were performed in 9 patients, and the total fenestrated FET technique was used in 2 patients according to the distal anastomosis level. Number of fenestrations was determined by entry location and presence of the dissected supra-aortic vessel during the early study period. However, during the later study period, single fenestration became the routine procedure to comfortably perform LSCA fenestration site fixation.
The fenestration site was fixed to prevent endoleaks via the fenestration in consecutive 48 patients who underwent single-fenestration FET at Nerima Hikarigaka Hospital later than June 2019. Fixation was performed using the following technique: the LSCA was encircled with a U-shaped piece of a 4-branched graft, and 4-0 polypropylene running sutures were placed around the fenestration site.5 Subsequently, the distal end was reinforced with a felt strip, and a 4-branched J graft (Japan Lifeline) was anastomosed using 4-0 polypropylene running sutures. Antegrade distal perfusion via the branch graft and rewarming were initiated, and a proximal anastomosis was performed using continuous 4-0 polypropylene sutures. The supra-aortic vessels were reconstructed using continuous 5-0 polypropylene sutures. Intraoperatively, we measured the blood pressure in both upper extremities and used a near-infrared spectroscopy sensor attached to the forehead to confirm adequate antegrade blood flow to the upper extremities and the head via the fenestrations. Preoperative cerebrospinal fluid drainage was not performed.
Statistical Analysis
Data were expressed as mean ± SD for continuous variables and frequencies and percentages for categorical variables. An endoleak was defined as pooling of the contrast medium continuously with the fenestration site between FET and the aortic intima. Overall survival was analyzed using the Kaplan-Meier method. Statistical analyses were performed using SPSS software version 25 (IBM Corp).
Results
Patient Characteristics
Preoperative patient characteristics are shown in Table 1. Overall, 33 patients (22%) developed shock. Preoperative malperfusion was observed in 48 patients (32%). In 123 patients (82%), the operation was performed within 48 hours of onset of ATAAD. Preoperative CT revealed that the false lumen in the aorta was patent or partially thrombosed in 117 patients (78%). Preoperative CT data, including anatomy of the dissected arch, are shown in Table 2. The LSCA was dissected in 19 patients (13%).
Table 1.
Preoperative patient characteristics (N = 150)
| Variable | Result |
|---|---|
| Age (y) | 67.5 ± 14.7 |
| Female sex | 66 (44.0) |
| Hypertension | 108 (72.0) |
| Diabetes | 7 (4.7) |
| Dyslipidemia | 26 (17.3) |
| Ischemic heart disease | 8 (5.3) |
| Atrial fibrillation | 11 (7.3) |
| Cerebrovascular disease | 21 (14.0) |
| Chronic kidney disease∗ | 11 (7.3) |
| Hemodialysis | 1 (0.7) |
| Chronic obstructive pulmonary disease | 9 (6.0) |
| Marfan syndrome | 4 (2.7) |
| Bicuspid aortic valve | 3 (2.0) |
| Shock | 33 (22.0) |
| Preoperative cardiac arrest | 6 (4.0) |
| Severe aortic regurgitation | 12 (8.0) |
| Malperfusion | 48 (32.0) |
| Onset to surgery within 48 h | 123 (82.0) |
| Prior cardiac surgery | 5 (3.3) |
Values for continuous variables are expressed as mean ± SD and values for categorical variables are expressed as n (%).
Serum creatinine >1.5 mg/dL.
Table 2.
Preoperative computed tomography data (N = 150)
| Variable | Result |
|---|---|
| Diameter of the arch (mm) | 33.0 ± 4.4 |
| Patent false lumen at the arch | 106 (70.7) |
| Diameter of the LSCA (mm) | 10.5 ± 2.3 |
| Distance between the origin of the LSCA and left vertebral artery (mm) | 40.4 ± 8.6 |
| Distance between the LCCA and LSCA (mm) | 9.1 ± 4.3 |
| Dissected left subclavian artery | 19 (12.7) |
| Entry tear location | |
| Ascending aorta | 64 (42.7) |
| Aortic arch | 62 (41.3) |
| Descending aorta | 21 (14.0) |
| Unknown | 3 (2.0) |
Values for continuous variables are presented as mean ± SD and values for categorical variables are presented as n (%). LCCA, Left common carotid artery; LSCA, left subclavian artery.
Operative Data
Intraoperative patient characteristics are shown in Table 3. The arterial cannulation site was the ascending aorta in 114 patients (76%) followed by the axillary artery in 31 patients (21%). Primary entry tear was located in the ascending aorta or arch in 126 patients (84%), descending aorta in 21 patients (14%), and unknown in 3 patients (2%). The distal anastomosis levels were in zone 2 in 139 patients, zone 1 in 9 patients and zone 0 in 2 patients. Regarding the size of the FET, the 27-mm stent graft was the most commonly used (67 patients [45%]) followed by the 29-mm stent graft (40 patients [27%]). Regarding the length of the FET, 90 mm (74 patients) or 120 mm (73 patients) FETs were mainly used. Suture fixation around the fenestration site for endoleak prevention was performed in 48 patients (32%). In 48 patients who underwent suture fixation technique, an entry tear was located at the ostia of the LSCA in 6 patients, and the LSCA was preoperatively dissected in 12 patients.
Table 3.
Intraoperative data (N = 150)
| Variable | Result |
|---|---|
| Cardiopulmonary bypass time (min) | 224 ± 63 |
| Cardiac ischemic time (min) | 132 ± 44 |
| Lower body hypothermic circulatory arrest (min) | 59 ± 23 |
| Concomitant procedure | 35 (23.3) |
| Suture fixation around fenestration site | 48 (32.0) |
| Distal anastomosis level | |
| Zone 2 | 139 (92.7) |
| Zone 1 | 9 (6.0) |
| Zone 0 | 2 (1.3) |
| Length of FET (mm) | |
| 60 | 1 (0.7) |
| 90 | 74 (49.3) |
| 120 | 73 (48.7) |
| 150 | 2 (1.3) |
| Thoracic vertebra level of distal end of FET | |
| 5 | 6 (4.0) |
| 6 | 52 (34.7) |
| 7 | 88 (58.7) |
| 8 | 4 (2.7) |
Values for continuous variables are presented as mean ± SD and values for categorical variables are presented as n (%). FET, Frozen elephant trunk.
Early Outcomes
The early outcomes are presented in Table 4. The overall 30-day mortality rate was 4.7%. Postoperative paraparesis was noted in 2 patients in whom we used a 9–cm-long FET with the distal end of the FET at the thoracic vertebra 6 level. These patients were treated with cerebrospinal fluid drainage postoperatively and completely recovered at discharge. Three patients (2.0%) who developed FET kinking between the graft and the stent portions were successfully treated with thoracic endovascular aortic repair. Fenestration site occlusion was not noted in any patient. Acute kidney injury requiring dialysis was noted in 12 patients (8.0%).
Table 4.
In-hospital outcomes (N = 150)
| Variable | Result |
|---|---|
| Overall 30-d mortality | 7 (4.7) |
| Overall in-hospital mortality | 11 (7.3) |
| Stroke | 14 (9.3) |
| Paraplegia | 0 |
| Paraparesis | 2 (1.3) |
| Occlusion of fenestration site | 0 |
| Re-exploration for bleeding | 2 (1.3) |
| Tracheostomy | 3 (2.0) |
| Acute kidney injury requiring dialysis | 12 (8.0) |
Values for categorical variables are presented as n (%).
Postoperative CT Findings
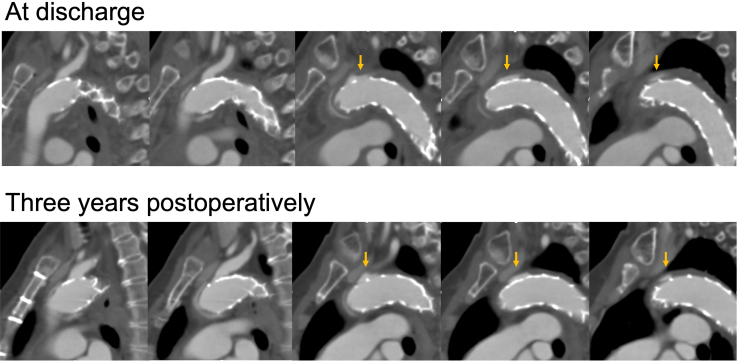
Postoperative CT revealed that the distal end of the FET was located at the thoracic vertebra 5 or 6 level in 58 patients, thoracic vertebra 7 in 88 patients, and thoracic vertebra 8 in 4 patients. Contrast-enhanced CT was performed before discharge in 146 patients (97.3%). Endoleak via fenestration, pooling of contrast medium between a FET and the intima, was noted in 14 patients (Figure E1). Among them, 12 patients were with a single fenestration and 2 patients with 2 fenestrations. In patients who underwent suture fixation around the fenestration site, no endoleak was detected postoperatively (Figure 1). The false lumen was thrombosed at the distal end of the FET in 141 patients (96.6%), at the level of the diaphragm in 97 patients (66.4%) and at the level of the celiac artery in 80 patients (54.8%).
Figure E1.
Computed tomography scans at discharge and 3 years postoperatively in a patient with endoleak. Postoperative endoleak via fenestration remained unchanged during the follow-up period. Arrows indicate endoleak.
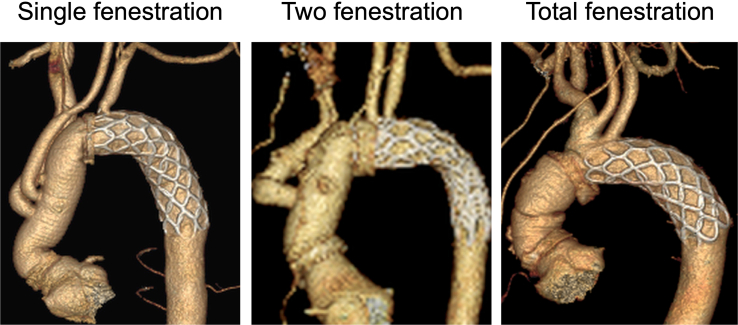
Figure 1.
Postoperative three-dimensional computed tomography of single, 2, and total fenestration techniques. The supra-aortic vessels were perfused via the fenestration on the frozen elephant trunk.
Mid-Term Outcomes
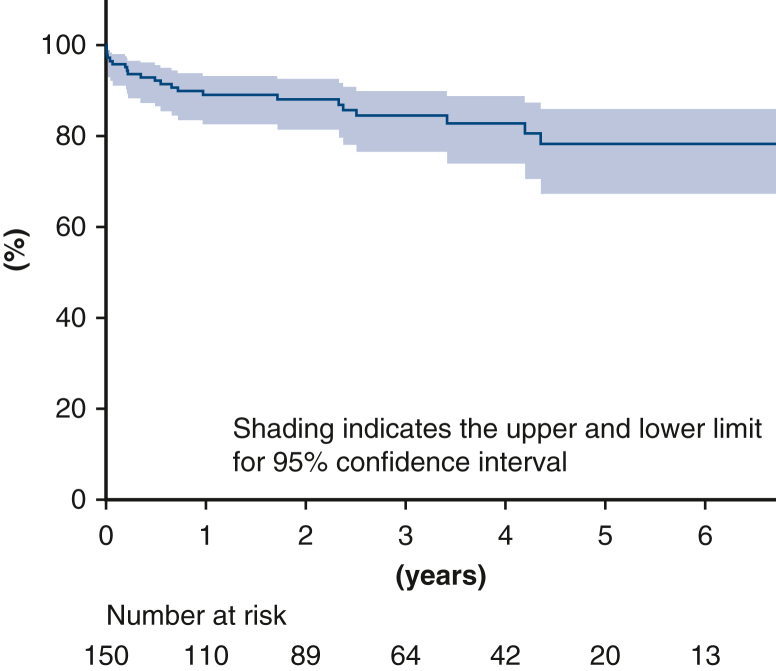
The median follow-up period was 28 months (0-111 months). During the follow-up, 12 patients died. However, deaths due to aortic rupture or dissection were not noted. Kaplan-Meier analysis revealed that the 1-year and 3-year overall survival rates were 89.1% and 84.5%, respectively (Figure 2). A distal stent graft-induced new entry was noted in 2 patients and successfully treated using thoracic endovascular aortic repair. Fenestration site occlusion or stroke in the cerebral area perfused via the fenestration were not noted. In 14 patients in whom endoleak was detected at discharge, no endoleak-related aneurysmal formations were noted on follow-up CT (median follow-up period, 49 months). Figure E1 shows CT scans of a patient with endoleak at discharge and follow-up. The endoleak was not changed over 3 years.
Figure 2.
Kaplan-Meier curve of the overall survival with 95% CI.
Discussion
This study highlights that the fenestrated FET technique can be performed safely in selected patients with ATAAD. Antegrade blood flow via fenestration was securely maintained and no stroke associated with the fenestration site was observed during the follow-up. Additionally, aneurysms caused by endoleaks were not noted. Suture fixation around the fenestration was a reliable measure to prevent endoleaks via the fenestrations.
Compared with hemiarch replacement, TAR requires distal anastomosis in the deep operative field and reconstruction of the supra-aortic vessels. Proximalization of the distal anastomosis is possible since the introduction of FET. However, it is still controversial how supra-aortic vessels, especially LSCA, are reconstructed during TAR. Bypass to the left axillary artery is a useful option. However, this reconstruction method is time-consuming and can cause nerve injury to the axillary plexus and infections, although the incidences of these adverse events are not high. Reconstruction of the LSCA within the mediastinum is technically challenging, especially in patients with large statue. Bleeding at the anastomosis site of LSCA can be critical. The fenestrated FET technique can potentially solve the above challenges.
Advantages of the fenestrated FET technique include proximalization of distal anastomosis and no need for reconstruction of the supra-aortic vessels. Vocal cord palsy affects patients’ quality of life and reportedly occurs in as many as 20% of patients following TAR.9 It is obvious that distal anastomosis at zone 2 or more proximally can help avoid recurrent nerve injury.10 Low spinal cord injury rate in our study might be also due to proximalization of distal anastomosis. The lack of the need for reconstruction of the LSCA considerably simplifies TAR. Our technique provides both proximalization of distal anastomosis and avoidance of LSCA reconstruction, which can be technically challenging in some patients. Fenestration is created on a FET under direct vision and requires neither specific instruments nor techniques. Even when adding suture fixation around the fenestration, suturing can be performed roughly because this is only for the prevention of endoleaks and not for hemostasis. Unlike meticulous anastomosis of the LSCA in the deep operative field, we believe that suture fixation around the fenestration is technically easy, does not require hemostasis, and more comfortable for surgeons.
Fenestration alone can cause endoleaks around an FET. However, it is unclear whether endoleaks require reinterventions. Unlike endoleaks following endovascular aortic repair for aneurysms, endoleaks via fenestrations in aortic dissection do not immediately result in aortic rupture. In our cohort, aneurysm formation or reintervention due to endoleaks were not noted. In patients with a single fenestration, 1 debranching TEVAR can be reserved for possible fenestration-related problems, such as endoleaks and aneurysmal formations. Nonetheless, endoleaks should be prevented in specific circumstances, such as an entry tear in the proximity of the fenestration. Our suture fixation around the fenestrations were secure enough to prevent endoleaks although it is controversial whether suture fixation should be performed in all patients. We believe that the fenestrated FET technique is feasible even in true aneurysms if endoleaks can be prevented.
Another method to prevent endoleaks is a direct stent-insertion into the supra-aortic vessels via a fenestration. Roselli and colleagues4 reported excellent outcomes of fenestration of FET by placing a stent into the LSCA via fenestration. Hashizume and colleagues7 demonstrated that fenestrated FET combined with stent-insertion via the fenestration significantly shortened the operation time compared with conventional TAR. However, endoleaks and persistent blood flow into the false lumen of the descending aorta were reported even with the addition of the stent-insertion via fenestration.11
In cases of dissected LSCA, a fenestration alone leaves the communication patent between the false lumen in the LSCA and the descending aorta, thus resulting in a patent false lumen in the distal aorta. Suture fixation around it can interrupt the communication and contribute to thrombosis in the false lumen of distal aorta. When the LSCA is preoperatively dissected, suture fixation should be added or the fenestrated FET technique should be avoided.
Better remodeling of the false lumen is an advantage of FET compared with the conventional elephant trunk technique. Inoue and colleagues1 reported that false lumen thrombosis rates following TAR using a FET were 93% at the distal edge of the FET and 50% at the level of the celiac artery. In our study, the false lumen rates at each aortic level were as well or higher than those reported by Inoue and colleagues.1 Our results revealed that the fenestrated FET technique provided comparable false lumen thrombosis.
Study Limitations
This study has several limitations. The 2-center retrospective design with a relatively small sample size is a drawback of this study and it remains unclear whether the results are generalizable to other centers. Our data were combinations of results of 2 separate groups of surgeons. A longer follow-up period is required to assess the safety and efficacy of this technique. Late contrast-enhanced CT data are required to confirm whether the blood flow via the fenestrations was truly restored and endoleaks via the fenestrations are safe.
Conclusions
The fenestrated FET technique is a simple and effective procedure to treat ATAAD (Figure 3). This technique can expedite conventional TAR with FET. Further large-scale studies are warranted to confirm our results.
Figure 3.
The methods, results, and implications of this study. This study aimed at investigating the outcomes of the fenestrated frozen elephant trunk (FET) technique. LSCA, Left subclavian artery; LCCA, left common carotid artery; BCA, brachiocephalic artery.
Conflict of Interest Statement
Dr Okamura received lecture fees from Japan Lifeline. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
Date and number of IRB approval: March 13, 2023, 23020902.
Supplementary Data
Intraoperative video of the fenestrated frozen elephant trunk technique. Video available at: https://www.jtcvs.org/article/S2666-2507(24)00335-3/fulltext.
Appendix E1
References
- 1.Inoue Y., Matsuda H., Omura A., et al. Comparative study of the frozen elephant trunk and classical elephant trunk techniques to supplement total arch replacement for acute type A aortic dissection. Eur J Cardiothorac Surg. 2019;56(3):579–586. doi: 10.1093/ejcts/ezz104. [DOI] [PubMed] [Google Scholar]
- 2.Yan Y., Xu L., Zhang H., et al. Proximal aortic repair versus extensive aortic repair in the treatment of acute type A aortic dissection: a meta-analysis. Eur J Cardiothorac Surg. 2016;49(5):1392–1401. doi: 10.1093/ejcts/ezv351. [DOI] [PubMed] [Google Scholar]
- 3.Colli A., Carrozzini M., Francescato A., et al. Acute DeBakey Type I aortic dissection without intimal tear in the arch: is total arch replacement the right choice? Interact Cardiovasc Thorac Surg. 2018;26(1):84–90. doi: 10.1093/icvts/ivx229. [DOI] [PubMed] [Google Scholar]
- 4.Roselli E.E., Idrees J.J., Bakaeen F.G., et al. Evolution of simplified frozen elephant trunk repair for acute DeBakey type I dissection: midterm outcomes. Ann Thorac Surg. 2018;105(3):749–755. doi: 10.1016/j.athoracsur.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 5.Azuma S., Shimada R., Motohashi Y., Yoshii Y. Postoperative results of the in situ fenestrated open stent technique for acute aortic dissection type A. Gen Thorac Cardiovasc Surg. 2023;71(6):331–338. doi: 10.1007/s11748-022-01878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okamura H., Arakawa M., Takeuchi T., Adachi H. The fenestrated frozen elephant trunk technique for acute type A aortic dissection. J Thorac Cardiovasc Surg. 2018;156(2):e75–e77. doi: 10.1016/j.jtcvs.2018.03.098. [DOI] [PubMed] [Google Scholar]
- 7.Hashizume K., Matsuoka T., Mori M., et al. Total arch replacement with extended branched stented anastomosis frozen elephant trunk repair for type A dissection improves operative outcome. J Thorac Cardiovasc Surg Tech. 2022;17:1–9. doi: 10.1016/j.xjtc.2022.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okamura H., Kitada Y., Miyagawa A., Arakawa M., Adachi H. Clinical outcomes of a fenestrated frozen elephant trunk technique for acute type A aortic dissection. Eur J Cardiothorac Surg. 2021;59(4):765–772. doi: 10.1093/ejcts/ezaa411. [DOI] [PubMed] [Google Scholar]
- 9.Fullmer T., Wang D.C., Price M.D., et al. Incidence and treatment outcomes of vocal fold movement impairment after total arch replacement. Laryngoscope. 2019;129(3):699–703. doi: 10.1002/lary.27347. [DOI] [PubMed] [Google Scholar]
- 10.Leone A., Di Marco L., Coppola G., et al. Open distal anastomosis in the frozen elephant trunk technique: initial experiences and preliminary results of arch zone 2 versus arch zone 3. Eur J Cardiothorac Surg. 2019;56(3):564–571. doi: 10.1093/ejcts/ezz103. [DOI] [PubMed] [Google Scholar]
- 11.Guo M., Naeem A., Yang B. The challenges of novel interventions in complex aortic disease. J Thorac Cardiovasc Surg Tech. 2020;4:57–60. doi: 10.1016/j.xjtc.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intraoperative video of the fenestrated frozen elephant trunk technique. Video available at: https://www.jtcvs.org/article/S2666-2507(24)00335-3/fulltext.