Abstract
Objective:
The objective of this study was to quantify the effects of a 4-week, supervised, high-intensity interval training (HIIT) on intrahepatic triglyceride content (IHTG, percentage), cardiorespiratory fitness (CRF), and cardiometabolic markers in adolescents with obesity.
Methods:
A total of 40 adolescents (age 13–18 y, BMI 36.7 ± 5.8 kg/m2) at risk for metabolic dysfunction-associated steatotic liver disease (MASLD) based on obesity and elevated Fibroscan measured controlled attenuation parameter (CAP) scores were randomized to HIIT three times a week for 4 weeks (n = 34) or observation (control; n = 6). Liver magnetic resonance imaging proton-density fat-fraction (MRI-PDFF), CAP, oral glucose tolerance test, serum alanine aminotransferase, dual-energy x-ray absorptiometry, and CRF tests were performed before and after intervention. Within- and between-group differences were compared.
Results:
A total of 13 (38%) and 4 (66%) children had MASLD by MRI-PDFF (IHTG ≥ 5%) in the HIIT and control groups, respectively. The implemented HIIT protocol had no impact on CRF or IHTG (baseline 5.26%, Δ = −0.31 percentage points, 95% CI: −0.77 to 0.15; p = 0.179), but it decreased the 2-h glucose concentration (baseline 116 mg/dL, Δ = −11 mg/dL; 95% CI: −17.6 to −5.5; p < 0.001). When limiting the analysis to participants with MASLD (n = 17), HIIT decreased IHTG (baseline 8.81%, Δ = −1.05 percentage points, 95% CI: −2.08 to −0.01; p = 0.048). Between-group comparisons were not different.
Conclusions:
The implemented exercise protocol did not reduce IHTG, but it led to modest improvement in markers of cardiometabolic health.
INTRODUCTION
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the leading cause of chronic liver disease in children. It is strongly associated with obesity, insulin resistance (IR), and cardiovascular disease (CVD). The spectrum of MASLD ranges from simple steatosis to metabolic dysfunction-associated steatohepatitis (MASH), cirrhosis, and liver failure [1–4]. The degree of intrahepatic triglyceride (IHTG) accumulation may cause an independent role in the development of MASH [5, 6]. Simple steatosis is associated with increased mortality from cardiometabolic disease [7].
Lifestyle modifications are the first-line treatment for MASLD, but weight loss is difficult for most people to attain and maintain [8]. Reduced fitness level is associated with MASLD status and progression, and exercise alone decreases IHTG without associated weight loss [9–12]. The Physical Activity Guidelines for Americans recommend that children should do at least 60 min of moderate-to-vigorous activity daily, but most youth are far from reaching this activity goal [13]. Examining the relationship among IHTG, cardiorespiratory fitness (CRF, maximum rate of oxygen consumption [VO2 peak]), and IR through exercise intervention could help clinicians to individualize treatment strategies in patients with MASLD. Given the increasing frequency and unfavorable outcomes of youth-onset MASLD compared with adult-onset [2,14], every effort should be made at an early stage to mitigate the negative consequences of MASLD.
The primary objective of this study was to determine the effect of a 4-week, supervised, high-intensity interval training (HIIT) on IHTG by magnetic resonance imaging (MRI) in adolescents at high risk for MASLD. HIIT has been recognized as a time-efficient and effective exercise modality in improving cardiovascular health [15]. Our second objective was to examine the effects of the HIIT intervention on CRF and indirect markers of IR. In addition, we evaluated the ability of vibration-controlled transient elastrography (VCTE)-based controlled attenuation parameter (CAP) in identifying children diagnosed with MASLD. Based on the published evidence, we hypothesized that 4-week HIIT decreases IHTG and improves cardiovascular health markers of adolescents with obesity.
METHODS
Trial design
This was a single-center, two-arm, randomized controlled trial conducted between April 2021 and May 2022 at the Arkansas Children’s Research Institute (ACRI) and the Arkansas Children’s Nutrition Center (ACNC; Little Rock, Arkansas). The target allocation ratio was approximately five exercise participants to one control participant. The protocol was approved by the Institutional Review Board of the University of Arkansas for Medical Sciences. Written informed consent and assent were obtained from the legal representatives and participants aged <18 years, respectively, before participation. The study was registered at ClinicalTrials.gov (NCT04342390).
Participants
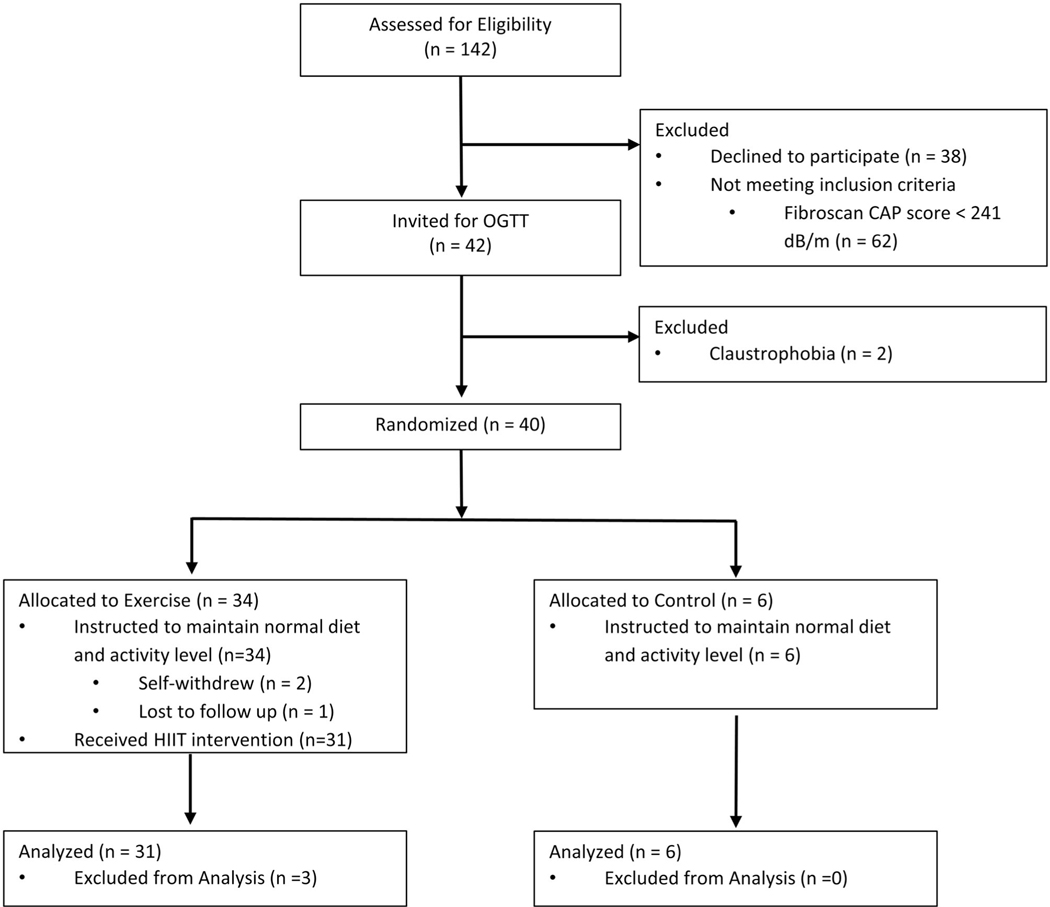
A total of 42 children aged 13 to 18 years were recruited from pediatric clinics at the Arkansas Children’s Hospital. Participants were instructed to maintain their habitual diet throughout the study. A flow diagram of the participants is shown in Figure 1 [16]. Participants with obesity (body mass index [BMI] ≥ 95th percentile for age and sex), in later stages of puberty (Tanner stage IV–V), and at high risk for MASLD, defined as VCTE-CAP ≥ 241 dB/m, were included. This CAP threshold was chosen because of its high sensitivity (98%) and specificity (80%) in MASLD diagnosis against the reference standard liver MRI [17]. Participants with diabetes and liver disease (except MASLD) or any diagnosis that would prevent them from participating in an exercise program were excluded. Those taking any medications that are known to affect hepatic lipid metabolism (i.e., metformin, statins, therapeutic doses of vitamin E) were also excluded.
FIGURE 1.
Participant flowchart (Consolidated Standards of Reporting Trials [CONSORT] diagram)
Study procedures
Screening was completed at two phases. First, all participants underwent medical history and pubertal assessment and completed “The Physical Activity Readiness Questionnaire for Everyone” to determine exercise safety [18]. Eligible participants were invited for a second screening visit, which involved a weight and height measurement to calculate BMI (percentile, z score and extended z score; https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm) and an oral glucose tolerance test (OGTT). Those without diabetes and with a CAP ≥ 241 dB/m were invited for the fitness test. Randomization took place after the fitness test. All randomized participants underwent liver fat assessment via VCTE-CAP and MRI, OGTT, fitness test, body composition analysis, and food frequency screening before and after 4 weeks of intervention. Each participant was provided with a wrist-worn fitness tracker to wear through the study period.
Outcomes
Three prespecified primary outcomes were the following: 1) IHTG content (expressed in percentage of liver volume), measured via MRI proton-density fat-fraction (PDFF); 2) CRF (VO2 peak, milliliters per kilogram of lean body mass [LBM]), measured by gas exchange during an incremental cycle ergometer test; and 3) systemic IR, defined by homeostatic model assessment of IR (HOMA-IR). Prespecified secondary outcomes were the following: 1) VCTE-CAP score; 2) alanine aminotransferase (ALT) level; 3) VCTE liver stiffness measurement (LSM) score; and 4) total body and visceral fat, assessed by dual-energy x-ray absorptiometry (DXA) scan. Exploratory outcomes were the following: 1) 2-h glucose during OGTT; and 2) other serum metabolic markers (lipids, aspartate aminotransferase [AST], adiponectin, leptin, and fibroblast growth factor [FGF]-21).
Vibration-controlled transient elastography
Fibroscan 530 Compact (Echosens, Waltham, Massachusetts) was used to determine CAP and LSM. A scan was considered valid when 10 consecutive measurements were taken with >60% overall successful scan rate and an interquartile range/median ratio < 30% per manufacturer.
Liver MRI
The primary outcome of this exercise intervention was IHTG percentage before and after the intervention or control period via MRI-PDFF (1.5 T MRI scanner, Philips Healthcare, Best, Netherlands). In brief, a multiecho, multislice, gradient-echo pulse sequence with repetition time (TR) 150 ms, flip angle of 25°, and echo times of 2.3, 4.6, and 9.2 ms with breath-hold were used to acquire in/out of phase images of the whole liver. Triple-echo method was used to control the confounding effects of intrinsic T2/T1 relaxation. Raw MRI images were downloaded to a workstation with MATLAB software (MathWorks, Natick, Massachusetts) and customized scripts for quantitative data analysis. Two raters, blinded to participants’ data, sketched a region-of-interest (ROI) for each participant, which included the whole liver as much as possible but avoided intrahepatic vessels and perihepatic fat as well at all edges. The average signal intensity in the selected ROI for each echo time was computed, and the liver fat content for the participant was calculated from these signal intensities [19]. MASLD was defined as IHTG percentage ≥ 5%. Of note, MASLD is an umbrella term referring to the full spectrum of the disease, which includes simple steatosis, MASH, fibrosis, and cirrhosis. MRI-PDFF is unable to distinguish different stages of the disease. Therefore, IHTG is the primary study outcome because IHTG ≥ 5% is a sine qua non for MASLD diagnosis, regardless of the staging or nomenclature used [4].
OGTT
A standard 2-h OGTT to measure glucose and insulin was performed after an overnight fast. Additional blood samples were collected at baseline to measure other biomarkers of interest.
Fitness test
Peak oxygen uptake (VO2 peak) was measured during an incremental cycle ergometer stress test. The participants wore a heart rate monitor (Zephyr Bluetooth Wireless Heart Rate Sensor, Medtronic, Boulder, Colorado) and an indirect calorimetry face mask to measure oxygen uptake and carbon dioxide excretion and calculate respiratory exchange ratio (carbon dioxide [CO2] expiration/oxygen [O2] uptake) throughout the test. Exertion level was assessed via the OMNI scale [20]. Peak VO2 was accepted to be achieved if the following criteria were met: a rating of perceived exertion ≥8 on the OMNI scale; and/or a peak heart rate ≥ 185 beats/min; and/or a respiratory exchange ratio ≥ 1.1.
Body composition
Body fat mass, percent body fat, and LBM were measured using DXA (Horizon-A with Advanced Body Composition™; Hologic, Bedford, Massachusetts).
Dietary assessment
Participants completed a Block Food Frequency Questionnaire online at http://nutritionquest.com/ to determine and compare average daily macronutrient intake and glycemic index.
Exercise intervention
A block randomization scheme, stratified by sex, was generated by RDL (code provided in online Supporting Information). Arm assignment was revealed after the participant completed the fitness test. Owing to the nature of the interventions, participants, study personnel (except the MRI raters), and the statistician were not blinded to treatment arm assignment.
Participants in the exercise arm received supervised HIIT three times a week for 4 weeks (total of 12 sessions) provided by a certified trainer at the ACNC. Each HIIT session lasted approximately 45 min and included a 5- to 10-min low-intensity warm-up followed by 10 1-min intervals using exercise equipment (elliptical machine, bike, or treadmill) at a work rate that elicited 80% to 90% of the maximal heart rate determined during the VO2 peak test. Participants had 2 min of recovery time (walking) between each effort. Participants performed a 5- to 10-min cool-down at a low intensity to finish the session. Heart rate and OMNI scores were monitored and recorded throughout the sessions. Participants were considered compliant with the exercise program if they attended at least nine sessions.
Although the optimum type, duration, volume, and intensity of exercise to treat MASLD is not known, studies in youth and adults show that as little as 4 weeks of moderate- to high-intensity aerobic exercise two to three times a week reduces IHTG by 17% to 21% [10, 21, 22]. Furthermore, 4 weeks of exercise training, in the absence of dietary changes, is unlikely to induce significant changes in body weight. Therefore, it would allow us to measure the direct effect of HIIT on IHTG.
Tracking of physical activity
Each participant, upon randomization to a group, received a wrist-worn activity tracker (Fitbit Inspire 2, FitBit, San Francisco, California) and were instructed to wear it throughout the study period. Step data were collected at the end of the study period.
Sample analyses
Glucose, liver enzymes (ALT, AST), lipid profile (triglyceride [TG], total cholesterol [TC], high-density lipoprotein cholesterol [HDL-c], low-density lipoprotein [LDL]) were measured in serum via colorimetric enzymatic kit using RX Daytona clinical analyzer (Randox Laboratories, Kearneysville, West Virginia). Serum insulin, adiponectin, leptin, and FGF-21 were measured via enzyme-linked immunosorbent assay (ELISA) per manufacturer’s protocols (R&D Systems, Minneapolis, Minnesota). All measurements were done at the Metabolism and Bioenergetics Core at the ACNC.
Statistical analyses
Demographic and laboratory data of the participants at baseline are summarized in Table 1. Because these measures were gathered before randomization (HIIT or control), we did not statistically compare the groups as recommended in the explanation of Consolidated Standards of Reporting Trials (CONSORT) guidelines; any statistical differences in baseline measures would be by chance [23].
TABLE 1.
Baseline characteristics of participants who completed the study in the HIIT and control groups
| Exercise group (n = 31) | Control group (n = 6) | |
|---|---|---|
| Age | 15.2 ± 1.5 | 15.4 ± 1.0 |
| Sex (male) | 14 (41%) | 2 (33%) |
| Race (White) | 15 (48%) | 6 (100%) |
| Ethnicity (Hispanic) | 11 (35%) | 5 (83%) |
| Weight (kg) | 101 ± 18.9 | 92.5 ± 17.8 |
| BMI (kg/m2) | 36.9 ± 6 | 34.9 ± 5.9 |
| BMI percentile | 99.2 [94.8, 100.0] | 97.8 [96.6, 99.8] |
| BMI z score | 2.56 ± 0.73 | 2.22 ± 0.45 |
| BMI extended z score | 3.13 ± 1.15 | 2.56 ± 0.71 |
| Body fat percent (%) | 44.7 ± 6.1 | 44.7 ± 6.6 |
| LBM (kg) | 52.1 ± 9.8 | 48.5 ± 9.6 |
| Visceral fat area (cm2) | 82.7 ± 24.6 | 90.5 ± 29.1 |
| Resting heart rate (beats/min) | 77 ± 10 | 71 ± 10 |
| Systolic blood pressure (mm Hg) | 120 ± 8 | 121 ± 7 |
| Diastolic blood pressure (mm Hg) | 67 ± 7 | 64 ± 6 |
| VO2 peak max (mL/min/kg per LBM) | 40.8 ± 7.3 | 43.2 ± 7.2 |
| CAP score (dB/m) | 279 ± 34 | 281 ± 28 |
| LSM score (kPa) | 5.25 ± 1.34 | 5.55 ± 3.37 |
| IHTG (%; all participants) | 5.26 ± 3.01 | 8.97 ± 6.61 |
| MASLD-positive by MRI-PDFF (n, %) | 11 (35%) | 4 (66%) |
Note: Values are means ± SD, count (%), or medians [min, max].
Abbreviations: CAP, controlled attenuated parameter; IHTG, intrahepatic triglyceride; LBM, lean body mass; LSM, liver stiffness measurement; MASLD, metabolic dysfunction-associated steatotic liver disease; TE, transient elastrogram; VO2, maximum rate of oxygen consumption.
Primary analyses
To address the primary aims, we originally planned a repeated-measures analysis of variance (ANOVA) with treatment (HIIT, control), time (baseline, week 4), and their interaction as fixed effects and participant as a random effect. We assumed within-participant observations were correlated and that among-participant variances constant across the two time points. We kept this analysis plan for IHTG and CAP. However, before conducting final analyses of all remaining measures, we changed this analysis plan for the following reason: the CAP measure used to screen for MASLD was imprecise, and 23 of the 40 randomized participants (57%) did not have MASLD compared with MRI. Given high false-positive screening rates for MASLD using CAP, we terminated the trial before reaching the originally proposed recruitment goal. In fact, new data published during this trial show that CAP thresholds for hepatic steatosis in children with severe obesity may be higher, which may explain this difference [24]. Consequently, we included baseline IHTG value as a participant-specific covariate in this, now, repeated-measures analysis of covariance (ANCOVA). The primary comparison of interest in both the ANOVA and ANCOVA models was the difference in means between baseline and week 4 for HIIT participants. Of secondary importance was the same comparison within control participants, along with the HIIT × time interaction. We report 95% confidence intervals (CIs) for these comparisons. Because we analyzed several measures, thus increasing the probability of false discoveries, we report the positive false discovery rate (pFDR) using the method described by Storey [25]. Finally, for several outcomes, residuals were not normal in distribution. We used a bootstrap procedure to determine whether violations of the normal assumption affected the normal-based inferences. There were no statistical differences in inferences between the original and bootstrap results for the three comparisons of interest noted earlier; therefore, we present the normal-based inferences as the primary results.
Power analysis
The target sample size of the study was 38 HIIT and 8 control participants with MRI diagnosis of MASLD who would complete all scheduled visits. With this sample size in the repeated-measures ANOVA described earlier, we would have had 0.80 power to detect a 2.4% point (pp) decrease in IHTG from baseline to week 4 in HIIT participants (the primary comparison of interest). This calculation assumed that the standard deviation (SD) of IHTG was 5.4 pp and that the first-order autocorrelation was 0.60. The obtained sample size was smaller than targeted: 34 HIIT participants, with 31 of these returning for the 4-week time point, and 6 control participants providing data at both time points. The updated power under the same assumptions earlier was 0.74.
Secondary analyses
To evaluate our secondary hypothesis that HIIT-induced changes in liver fat were negatively associated with HIIT-induced changes in fitness, we used an ANCOVA model. Changes in liver fat were expressed as the difference in IHTG (pp) between baseline and week 4, with positive values indicating an increase in IHTG over time. Likewise, changes in fitness were the difference in VO2 peak between baseline and week 4, with positive values indicating an increase in fitness. The ANCOVA modeled the change in IHTG as a linear function of change in fitness for HIIT participant and for control participants; thus, four parameters in the model. We also hypothesized that HIIT-induced changes in IHTG would be positively associated with HIIT-induced changes in HOMA-IR. We expressed changes in HOMA-IR as for IHTG and fitness, with negative values indicating worsening IR. From ANCOVA, we report the slopes and 95% CIs on the covariates.
All analyses described earlier, except for the computation of the pFDR, were performed with the MIXED procedure in SAS/STAT software, version 9.4 (SAS Institute; Cary, North Carolina). Custom code in R version 4.1.0 was used for computing pFDR. The statistical code and source data are provided in the online Supporting Information.
RESULTS
Compliance with the intervention
A total of 40 participants were randomized: 34 in the exercise group (20 female individuals), and 6 in the control group (4 female individuals). The mean age was 15.3 ± 1.4 years. Three (all female individuals) participants in the exercise group did not complete the study, with one participant dropping out (unknown reason) before attending any HIIT sessions, and two withdrew after the third and fourth HIIT sessions (lack of transportation). A total of 37 participants completed the intervention and all study procedures. Of the 31 participants who completed HIIT, 27 attended all 12, 2 attended 11 of 12, and the final 2 attended 9 of 12 sessions over 4 weeks. Thus, the overall exercise attendance rate was 97%. On average, participants exercised for 130 ± 12 min/wk at the research facility. No study-related adverse events occurred.
Liver fat assessment by VCTE-CAP and MRI
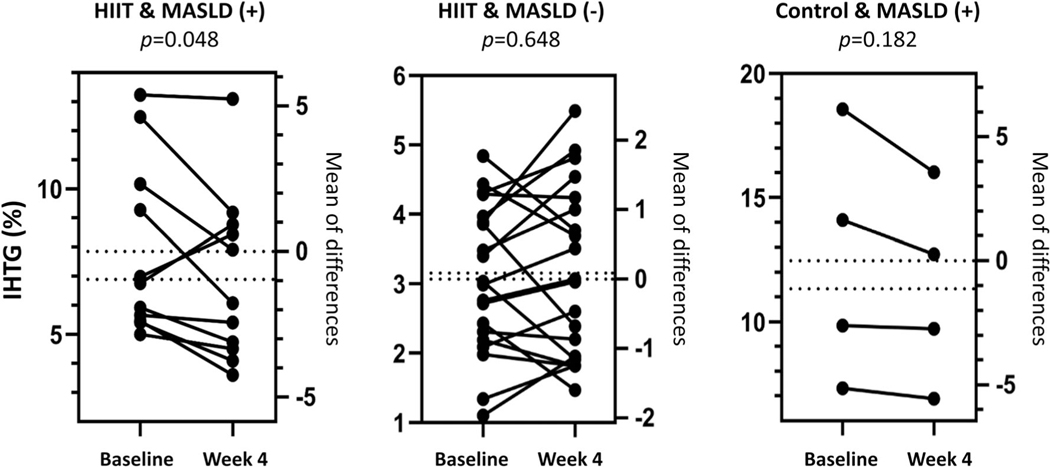
Each participant had a CAP score ≥ 241 dB/m per inclusion criteria. However, only 17 out of 40 participants (13 in the HIIT and 4 in the control group) had IHTG ≥ 5% as determined by liver MRI-PDFF. Therefore, the positive predictive value of VCTE-CAP of ≥241 dB/m in diagnosing MASLD in this cohort was only 43% compared with MRI-PDFF [26]. Whereas MASLD prevalence was 38% in the HIIT group, it was 66% in the control group at baseline. When everyone in the HIIT group, regardless of the baseline MASLD status by MRI, was considered, 4 weeks of HIIT training had little impact on decreasing IHTG from the baseline mean of 5.26% (difference [Δ] = −0.31 pp, 95% CI: −0.77 to 0.15; p = 0.179). Similarly, the control group exhibited a 0.83-pp decrease from their baseline mean of 8.97% (95% CI: −1.87 to 0.22; p = 0.117). The difference in decreases between the two groups was not significant (HIIT × time interaction p = 0.363). Subgroup analysis: Although not a planned analysis, we limited the sample to only the 17 participants who were MASLD-positive by MRI-PDFF and analyzed IHTG as described earlier. The HIIT group (n = 13) experienced a significant decrease from their baseline 8.81% (Δ = −1.05 pp, 95% CI: −2.08 to −0.01; p = 0.048). The control group (n = 4) also experienced a decrease from their baseline of 12.45% (Δ = −1.12 pp, 95% CI: −0.60 to 2.85; p = 0.182). The decreases from baseline did not differ between the HIIT and control groups (HIIT × time interaction p = 0.935; Figure 2).
FIGURE 2.
Intrahepatic triglyceride content (IHTG; percentage) levels and mean of differences before and after the intervention period in high-intensity interval training (HIIT) and control groups by baseline metabolic dysfunction-associated steatotic liver disease (MASLD) status (positive or negative)
The HIIT group had a statistically significant decrease in CAP score at the end of week 4 compared with their baseline score of 279 dB/m (Δ = −27, 95% CI: −43 to −11; p = 0.001), whereas the control group’s decrease was not significant (baseline mean 280 dB/m, Δ = −16, 95% CI: −51 to 19; p = 0.359). However, the decreases in CAP between groups were not significantly different (HIIT × time interaction, p = 0.550).
Anthropometrics, body composition, and markers of CRF
Body weight, BMI, body fat percentage, LBM, and visceral fat were similar between groups at baseline. Changes in these markers were little and not significant between baseline and week 4 in both groups. Similarly, for both groups, changes were negligible in VO2 peak, resting heart rate, and blood pressures. For all of these measures, the changes experienced by each of the two groups did not statistically differ between the groups (Table 2).
TABLE 2.
Biomarkers before and after the intervention period in all participants who started the study
| Exercise group (n = 34a) |
Control group (n = 6) |
|||||
|---|---|---|---|---|---|---|
| Outcome | Baseline | Week 4 | Baseline | Week 4 | SDb | HIIT × time p value |
| Weight (kg) | 101.18 | 101.34 | 90.85 | 91.64 | 18.17 | 0.496 |
| BMI extended z score | 3.17 | 3.14 | 2.39 | 2.42 | 1.10 | 0.397 |
| Body fat percent (%) | 45.35 | 45.19 | 44.14 | 44.05 | 6.18 | 0.921 |
| LBM (kg) | 52.02 | 52.04 | 48.39 | 48.62 | 9.35 | 0.801 |
| Visceral fat area (cm2) | 95.78 | 95.85 | 72.99 | 82.06 | 28.66 | 0.185 |
| IHTG (%) | 5.26 | 4.96 | 8.97 | 8.15 | 3.96 | 0.363 |
| CAP score (dB/m) | 279.18 | 251.57c | 280.83 | 264.67 | 35.48 | 0.554 |
| LSM score (kPa) | 5.3 | 5.56 | 5.19 | 6.41 | 1.59 | 0.186 |
| VO2 peak (mL/min/kg per LBM) | 40.36 | 40.93 | 42.88 | 44.41 | 7.23 | 0.663 |
| Fasting glucose (mg/dL) | 92 | 91.65 | 88.85 | 91.02 | 8.78 | 0.317 |
| 2-h glucose (mg/dL) | 116.13 | 104.59c | 104.17 | 99.92 | 25.45 | 0.333 |
| Fasting insulin (uIU/mL) | 37.99 | 35.85 | 24.57 | 28.86 | 22.41 | 0.387 |
| 2-h insulin (uIU/mL) | 221.9 | 217.15 | 62.55 | 77.88 | 199.47 | 0.747 |
| HOMA-IR | 8.69 | 8.28 | 5.29 | 6.4 | 5.46 | 0.433 |
| TC (mg/dL) | 149.97 | 146.26c | 152.55 | 147.72c | 27.07 | 0.642 |
| HDL-c (mg/dL) | 41.33 | 40.33c | 44.85 | 44.26 | 8.76 | 0.532 |
| LDL (mg/dL) | 95.12 | 91.68c | 94.82 | 93.01 | 21.19 | 0.402 |
| TG (mg/dL) | 104.56 | 104.61 | 111.86 | 96.71 | 47.26 | 0.199 |
| ALT (IU/L) | 20.13 | 19.6 | 29.76 | 29.48 | 17.66 | 0.822 |
| AST (IU/L) | 21.29 | 20.43 | 27.6 | 27.34 | 19.4 | 0.694 |
| Adiponectin (ng/mL) | 5.31 | 4.99 | 4.66 | 5.11 | 2.32 | 0.096 |
| Leptin (pg/mL) | 66.32 | 71.36 | 61.09 | 69.91 | 41.05 | 0.622 |
| FGF-21 (pg/mL) | 225.64 | 203.49 | 277.62 | 356.32 | 187.02 | 0.212 |
Note: All parameters were estimated within the repeated-measures ANCOVA/ANOVA model.
Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; CAP, controlled attenuation parameter; FGF, fibroblast growth factor; HDL-c, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; IHTG, intrahepatic triglyceride; LBM, lean body mass; LDL, low-density lipoprotein; LSM, liver stiffness measurement; TC, total cholesterol; TG, triglyceride; VO2, maximum rate of oxygen consumption.
Three participants did not provide week 4 values.
SD is the square root of the mean square error from the model; assumed equal for both arms at both time points.
Indicates that the week 4 mean within the group is significantly different from the baseline mean. An expanded version of this table, including data for the BMI and BMI percentiles, can be found in Supporting Information Table S1.
Assessment of the relationship between the changes in IHTG and CRF
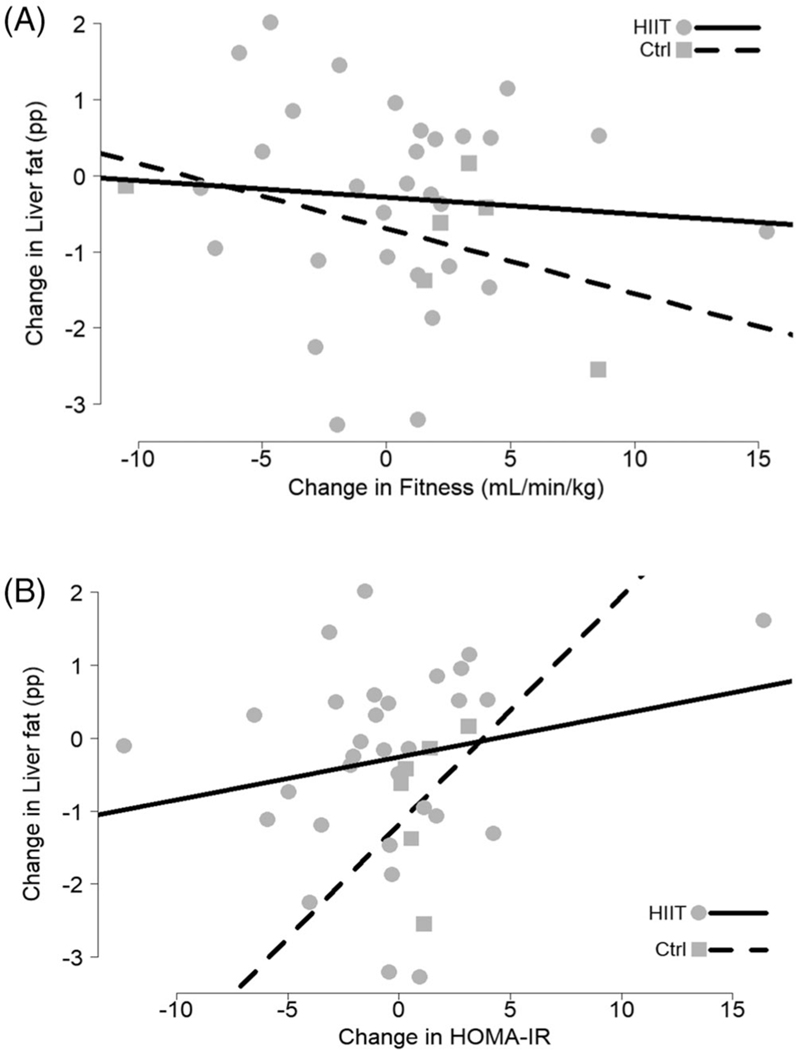
Changes in IHTG (pp) were not related to changes in CRF (VO2 peak per LBM) for either of the two groups (HIIT slope: −0.02, 95% CI: −0.13 to 0.08; control slope: −0.09, 95% CI: −0.27 to 0.10; Figure 3A). Even when assuming a common slope for the two groups, IHTG changes were still not related to changes in VO2 peak (p = 0.404).
FIGURE 3.
Changes in liver fat (expressed in percentage points, pp) plotted (A) by changes in cardiorespiratory fitness and (B) by changes in homeostatic model assessment of insulin resistance (HOMA-IR)
Assessment of IR and other cardiometabolic markers
There were no significant baseline-to-week-4 changes in serum glucose, insulin, HDL-c, LDL, triglyceride, leptin, adiponectin, and FGF-21 concentrations, as well as HOMA-IR values, within each of the HIIT and control groups (Table 2). However, at week 4, the HIIT group exhibited statistically significant decreases in 2-h glucose compared with their baseline mean of 116 mg/dL (Δ = −11.5, 95% CI: −17.6 to −5.5; p < 0.001), TC (baseline mean 150 mg/dL, Δ = −3.7, 95% CI: −5.5 to −1.9; p < 0.001), LDL (baseline mean of 95 mg/dL, Δ = −3.4, 95% CI: −4.8 to −2.0; p < 0.001), and HDL-c (baseline mean of 41 mg/dL, Δ = −1.0, 95% CI: −1.5 to −0.5; p < 0.001). However, the changes from baseline in these markers did not differ between groups (p > 0.332 for HIIT × time interaction for all 4 outcomes). The control group also exhibited a decrease in TC at week 4 from their mean of 152 mg/dL at baseline (Δ = −4.8 mg/dL, 95% CI: −9.3 to −0.3; p = 0.036; Table 2).
Assessment of the relationship between the changes in IHTG and IR
Changes in IHTG (pp) were not related to changes in HOMA-IR for either of the two groups (HIIT slope: 0.06, 95% CI: −0.04 to 0.16; control slope: 0.31, 95% CI: −0.72 to 1.34; Figure 3B). Even when assuming a common slope for the two groups, IHTG changes were still not related to changes in HOMA-IR (p = 0.216).
Assessment of macronutrient intake
There were no differences within the groups between baseline and week 4 regarding estimated intake of average macronutrients and consumption of food groups including fruit/fruit juice, saturated fat, sugar/syrup added to foods/beverages, and average daily glycemic index.
Assessment of physical activity
Although all participants were provided with a wrist-worn activity tracker, compliance was very low and inconsistent. Therefore, we were unable to collect reliable data on participants’ activity level.
FDR
Altogether, we reported on 69 comparisons in Table 2 and its extension in Table S1, of which 6 were significant at α = 0.05. Among these six significant results, the estimated pFDR was 28%.
DISCUSSION
In this randomized controlled study, we showed that 4 weeks of exercise training induced a significant decrease in CAP score but did not induce a significant change in IHTG in adolescents with obesity who had normal mean serum ALT levels at baseline. In the subgroup analysis of only those with MASLD at baseline, IHTG levels were significantly lower in the HIIT group, but the two groups’ decreases did not statistically differ. Four weeks of exercise did not induce significant changes in most secondary measures, including serum ALT, but it decreased 2-h glucose, TC, and LDL cholesterol levels. However, the mean changes in these markers were not different between the HIIT or control groups. These results demonstrate that supervised HIIT intervention for 4 weeks may not be enough to elicit measurable improvements in IHTG in adolescents with obesity when baseline MASLD status is not considered, but HIIT intervention could improve cardiometabolic health markers, including 2-h glucose and total and LDL cholesterol levels, regardless of the baseline MASLD status.
HIIT, defined as alternating cycles of short periods of intense aerobic and anaerobic exercise at near-maximum capacity, has been suggested as a time-efficient and effective exercise modality in MASLD treatment [15,27]. Many different HIIT protocols with varying workout times have been described, but available literature does not support superiority of one protocol over the others; HIIT was shown to improve CRF in healthy adolescents with obesity regardless of body composition, and the effects of prolonged high-volume versus short-term low-volume HIIT programs were comparably effective [28]. In this study, 4 weeks of HIIT training (10 1-min intervals each separated by 2-min slower efforts in between, three times per week) did not induce significant changes in CRF markers.
We did not detect significant differences in changes of IHTG as assessed by MRI-PDFF in the entire cohort. However, in subgroup analysis, we found lower IHTG levels in participants with baseline MASLD. Our findings are consistent with adult literature that has shown that short-term HIIT is effective in reducing IHTG in patients with MASLD [12,15,29,30]. Surprisingly, the participants in the control group also had a reduction in their IHTG levels at week 4. We cannot rule out type 2 error in the subgroup analysis given small sample sizes and large variability in baseline IHTG levels (i.e., groups were not matched for IHTG levels before intervention). In addition, there is the possibility that awareness of the presence of hepatic steatosis in our population altered behavior in the control group as well as the experimental group.
We detected significant reductions in 2-h glucose, TC, and LDL in the HIIT group and in TC in the control group, regardless of baseline MASLD status. Our findings are consistent with previous studies in children with obesity that have shown that HIIT is effective in reducing fasting glucose, insulin, and HOMA-IR as well as mitigating atherogenic lipid profile [11,31–33]. Adolescents with obesity who had higher IR at baseline had the most decrease in IR following a total of six sessions of HIIT over 2 weeks [34–36]. Considering the strong positive association between IR and IHTG accumulation and MASLD development, interventions aiming to decrease IR should be expected to have beneficial effects on liver fat metabolism, particularly in patients with confirmed MASLD diagnosis.
Our study has several limitations. The target population was children with obesity and MASLD; however, high false-positive detection rate of MASLD using VCTE-CAP introduced MASLD status heterogeneity into the sample. That heterogeneity may have reduced power in detecting whether 4 weeks of HIIT could reduce IHTG levels, i.e., our primary hypothesis. Also, the study was powered to detect a change in before/after levels in the HIIT group; the study was not powered to detect a difference in changes from baseline between the two groups. Longer exercise duration would have decreased IHTG more effectively, but it could also have induced weight loss, which would add another layer of complexity to the interpretation of results. Participants knew the results of their Fibroscan (i.e., MASLD-positive status) and, owing to nature of the study, were not blinded by the intervention. It is possible that participants in the control arm might have adapted lifestyle changes such as increased physical activity that went undetected through the methods employed in this study. Compliance to using wrist-worn activity tracker was very low. Finally, we estimated insulin sensitivity and lipid metabolism via surrogate markers rather than the gold-standard clamp studies.
In conclusion, short-term HIIT alone has only modest therapeutic effects on metabolism relevant to steatotic liver disease in adolescents with obesity. Also, the recommended VCTE-CAP score of ≥241 dB/m has low specificity in diagnosing MASLD in this population with significant obesity. Further studies with a larger number of participants with MASLD are needed to better evaluate the effects and mechanisms through which HIIT regulate hepatic lipid metabolism.
Supplementary Material
Study Importance.
What is already known?
Reduced cardiorespiratory fitness (CRF) is associated with the development and progression of metabolic dysfunction-associated steatotic liver disease (MASLD) in children and adults.
Improved CRF through exercise decreases liver fat in patients with MASLD, even without clinically discernable weight loss.
What does this study add?
The present study shows that supervised exercise in the form of high-intensity interval training (HIIT) has beneficial effects on cardiometabolic health markers in adolescents with obesity over a 4-week intervention.
Whereas the effect of HIIT on reducing liver fat was insignificant in participants with varying degrees of liver fat at baseline, HIIT can reduce liver fat percentage in those with MASLD.
How might the results of this study change the direction of research or the focus of clinical practice?
HIIT is a safe and time-sparing exercise mode that can be prescribed for the treatment of metabolic complications of obesity in adolescents.
Exploration of the effects of different HIIT protocols on cardiometabolic health markers in children with obesity is needed.
ACKNOWLEDGMENTS
The authors would like to thank the study participants and the staff at the ACRI and the ACNC who assisted with data collection.
FUNDING INFORMATION
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under award number P20GM109096 and was partially supported by the United States Department of Agriculture/Agricultural Research Service (USDA-ARS; USDA-ARS Project 6026-51000-012-06S). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the USDA-ARS.
Agricultural Research Service, Grant/Award Number: 6026-51000-012-06S; National Institute of General Medical Sciences, Grant/Award Number: P20GM109096
Footnotes
CLINICAL TRIAL REGISTRATION
ClinicalTrials.gov identifier NCT04342390.
CONFLICT OF INTEREST STATEMENT
The authors declared no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
Individual deidentified data used for this article (text, tables, and figures), study protocol, statistical analysis plan, and analytic code are provided in the online Supporting Information.
REFERENCES
- 1.Grander C, Grabherr F, Moschen AR, Tilg H. Non-alcoholic fatty liver disease: cause or effect of metabolic syndrome. Visc Med. 2016; 32(5):329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nobili V, Alisi A, Newton KP, Schwimmer JB. Comparison of the phenotype and approach to pediatric vs adult patients with nonalcoholic fatty liver disease. Gastroenterology. 2016;150(8):1798–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. 2008;118(3):277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. 2017;64(2):319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dongiovanni P, Stender S, Pietrelli A, et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med. 2018;283(4):356–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148–1155. [DOI] [PubMed] [Google Scholar]
- 7.Simon TG, Roelstraete B, Hartjes K, et al. Non-alcoholic fatty liver disease in children and young adults is associated with increased long-term mortality. J Hepatol. 2021;75(5):1034–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Ventura AL, Pelaez-Ballestas I, Sámano-Sámano R, Jimenez-Gutierrez C, Aguilar-Salinas C. Barriers to lose weight from the perspective of children with overweight/obesity and their parents: a sociocultural approach. J Obes. 2014;2014:575184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medrano M, Arenaza L, Migueles JH, Rodríguez-Vigil B, Ruiz JR, Labayen I. Associations of physical activity and fitness with hepatic steatosis, liver enzymes, and insulin resistance in children with overweight/obesity. Pediatr Diabetes. 2020;21(4):565–574. [DOI] [PubMed] [Google Scholar]
- 10.Medrano M, Cadenas-Sanchez C, Álvarez-Bueno C, et al. Evidence-based exercise recommendations to reduce hepatic fat content in youth- a systematic review and meta-analysis. Prog Cardiovasc Dis. 2018;61(2):222–231. [DOI] [PubMed] [Google Scholar]
- 11.Thivel D, Masurier J, Baquet G, et al. High-intensity interval training in overweight and obese children and adolescents: systematic review and meta-analysis. J Sports Med Phys Fitness. 2019;59(2):310–324. [DOI] [PubMed] [Google Scholar]
- 12.van der Windt DJ, Sud V, Zhang H, Tsung A, Huang H. The effects of physical exercise on fatty liver disease. Gene Expr. 2018;18(2):89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crespo M, Lappe S, Feldstein AE, Alkhouri N. Similarities and differences between pediatric and adult nonalcoholic fatty liver disease. Metabolism. 2016;65(8):1161–1171. [DOI] [PubMed] [Google Scholar]
- 15.Hallsworth K, Thoma C, Hollingsworth KG, et al. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci. 2015;129(12):1097–1105. [DOI] [PubMed] [Google Scholar]
- 16.Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2011;9(8):672–677. [DOI] [PubMed] [Google Scholar]
- 17.Shin J, Kim MJ, Shin HJ, et al. Quick assessment with controlled attenuation parameter for hepatic steatosis in children based on MRI-PDFF as the gold standard. BMC Pediatr. 2019;19(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warburton DE, Gledhill N, Jamnik VK, et al. Evidence-based risk assessment and recommendations for physical activity clearance: consensus document 2011. Appl Physiol Nutr Metab. 2011;36(Suppl 1):S266–S298. [DOI] [PubMed] [Google Scholar]
- 19.Tas E, Bai S, Ou X, et al. Fibroblast growth factor-21 to adiponectin ratio: a potential biomarker to monitor liver fat in children with obesity. Front Endocrinol. 2020;11:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson RJ, Goss FL, Boer NF, et al. Children’s OMNI scale of perceived exertion: mixed gender and race validation. Med Sci Sports Exerc. 2000;32(2):452–458. [DOI] [PubMed] [Google Scholar]
- 21.Johnson NA, Sachinwalla T, Walton DW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50(4):1105–1112. [DOI] [PubMed] [Google Scholar]
- 22.Keating SE, Hackett DA, Parker HM, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. 2015; 63(1):174–182. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. [DOI] [PubMed] [Google Scholar]
- 24.Runge JH, van Giessen J, Draijer LG, et al. Accuracy of controlled attenuation parameter compared with ultrasound for detecting hepatic steatosis in children with severe obesity. Eur Radiol. 2021;31(3):1588–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Storey JD. A direct approach to false discovery rates. J R Stat Soc Series B Stat Methodology. 2002;64(3):479–498. [Google Scholar]
- 26.Caussy C, Reeder SB, Sirlin CB, Loomba R. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology. 2018;68(2):763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houttu V, Bouts J, Vali Y, et al. Does aerobic exercise reduce NASH and liver fibrosis in patients with non-alcoholic fatty liver disease? A systematic literature review and meta-analysis. Front Endocrinol. 2022;13:1032164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin-Smith R, Cox A, Buchan DS, Baker JS, Grace F, Sculthorpe N. High intensity interval training (HIIT) improves cardiorespiratory fitness (CRF) in healthy, overweight and obese adolescents: a systematic review and meta-analysis of controlled studies. Int J Environ Res Public Health. 2020;17(8):2955–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keating SE, Hackett DA, George J, Johnson NA. Exercise and nonalcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57(1):157–166. [DOI] [PubMed] [Google Scholar]
- 30.Winn NC, Liu Y, Rector RS, Parks EJ, Ibdah JA, Kanaley JA. Energy-matched moderate and high intensity exercise training improves nonalcoholic fatty liver disease risk independent of changes in body mass or abdominal adiposity - a randomized trial. Metabolism. 2018;78:128–140. [DOI] [PubMed] [Google Scholar]
- 31.Cao M, Quan M, Zhuang J. Effect of high-intensity interval training versus moderate-intensity continuous training on cardiorespiratory fitness in children and adolescents: a meta-analysis. Int J Environ Res Public Health. 2019;16(9):1533–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tjonna AE, Stølen TO, Bye A, et al. Aerobic interval training reduces cardiovascular risk factors more than a multitreatment approach in overweight adolescents. Clin Sci (Lond). 2009;116(4):317–326. [DOI] [PubMed] [Google Scholar]
- 33.Cao M, Li S, Tang Y, Zou Y. A meta-analysis of high-intensity interval training on glycolipid metabolism in children with metabolic disorders. Front Pediatr. 2022;10:887852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cockcroft EJ, Bond B, Williams CA, et al. The effects of two weeks high-intensity interval training on fasting glucose, glucose tolerance and insulin resistance in adolescent boys: a pilot study. BMC Sports Sci Med Rehabil. 2019;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balagopal P, George D, Yarandi H, Funanage V, Bayne E. Reversal of obesity-related hypoadiponectinemia by lifestyle intervention: a controlled, randomized study in obese adolescents. J Clin Endocrinol Metab. 2005;90(11):6192–6197. [DOI] [PubMed] [Google Scholar]
- 36.Racil G, Ben Ounis O, Hammouda O, et al. Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur J Appl Physiol. 2013;113(10):2531–2540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual deidentified data used for this article (text, tables, and figures), study protocol, statistical analysis plan, and analytic code are provided in the online Supporting Information.