Abstract
An enzyme-linked immunosorbent assay (ELISA) for Lawsonia intracellularis was developed and compared with a whole-cell antigen-based immunofluorescence antibody test (IFAT). The antigen-containing lipopolysaccharide (LPS) was derived from Percoll gradient purified cultures of L. intracellularis by using a modification of the Westphal hot phenol procedure. The antigen was bound directly to polystyrene 96-well microtiter plates, and the assay was performed in an indirect ELISA format. Specificity and sensitivity values based on 80 known positive and 80 known negative serum samples from controlled experimental trials were 93.7% and 88.7%, respectively. Serological results from a controlled L. intracellularis challenge exposure study confirmed the high specificity and sensitivity of this assay (100% and 99.5%, respectively). Comparisons between the LPS ELISA and the IFAT in detecting anti-Lawsonia antibodies in this controlled study revealed significantly more LPS ELISA-positive pigs than IFAT-positive pigs on days 21, 28, 35, and 42 (P = 0.003, 0.030, 0.002, and 0.006, respectively). This indirect ELISA (LPS ELISA) test is an improved method of detecting antibodies in pigs soon after exposure to L. intracellularis, regardless of isolate type (vaccine or wild type) in experimental studies. The LPS ELISA may be used as a tool to support future research trials on vaccine efficacy and to further understand the immune response induced by L. intracellularis.
Proliferative enteropathy (PE) is a common enteric disease of pigs after weaning that is caused by the obligate intracellular bacterium, Lawsonia intracellularis (20, 21). The infection and disease are widespread in pig farms across America and Europe, and prevalence among groups of pigs on these affected farms can be over 30% (4, 23, 29, 31). This leads to a considerable economic impact of the disease, due to diarrhea, weight loss, and subclinical illness in growing pigs (22, 31). Since the identification of L. intracellularis as the cause of PE in 1993, a number of studies aimed at establishing the best diagnostic methods for identifying Lawsonia exposure in live animals have been conducted. These have focused on DNA detection via PCR of feces and whole-cell immunoassays (8, 12, 13, 15, 17), due to the extreme difficulty of isolation of the obligate intracellular L. intracellularis from the contaminated environment of feces (13, 17, 18). In situations where samples of ileum are available, immunohistochemistry (IHC) is considered to provide the criterion-referenced measure or “gold standard” for assessment of the actual infection status of an individual pig (9, 16, 19, 26, 28).
PCR testing of fresh feces involves considerable laboratory effort and cost to extract amplifiable bacterial DNA from each sample (9, 11, 13, 15). False positives due to pre-laboratory sample contamination during the collection of numerous samples from a group of pigs or due to contamination during the laboratory testing phase may occur. False negatives due to the regular presence of PCR inhibitors in feces may also occur (9, 10, 11). Serologic testing methods have therefore also been widely explored for detecting L. intracellularis exposure of pigs. Indirect immunofluorescence or immunoperoxidase assays have been used to examine antibody responses of pigs infected experimentally with L. intracellularis in virulent challenge exposure studies and of pigs with PE from farms (3, 4, 7, 11, 14, 29). An indirect enzyme-linked immunosorbent assay (ELISA) was developed previously for testing pig serum antibodies, with crude antigen derived directly from pig intestines affected with PE (12). However, the antigen used in that study was not fully characterized for L. intracellularis content. The development of a specific L. intracellularis antigen-based ELISA would therefore be of considerable benefit in improving the feasibility of a more universally available and standardized diagnostic assay to study the epidemiology of this economically significant disease.
We describe the development of an ELISA for detecting L. intracellularis infection based on a lipopolysaccharide antigen extract in an indirect ELISA format.
MATERIALS AND METHODS
Bacterial antigen preparation.
The lipopolysaccharide (LPS) used in this study was derived from L. intracellularis isolate 15540. This isolate was acquired from a Danish sow affected with acute hemorrhagic proliferative enteropathy (confirmed by routine histology and immunohistochemistry staining techniques) whose intestines were cocultured to obtain a pure culture of L. intracellularis by methods previously described (18, 21). Multiple 30-liter batches of L. intracellularis 15540 (ATCC PTA-4927) were propagated using fresh McCoy cell (ATCC 1696) suspensions in bioreactors (Applicon, Inc., Foster City, CA). Active cultures were allowed to reach 80 to 100% cell infectivity and then were harvested by centrifugation using an Avanti Beckman J-20I centrifuge with JA-10 rotor at 17,000 × g for 15 min at 4°C. The supernatants of each batch were discarded, and cell pellets containing both harvested extracellular L. intracellularis and McCoy cells infected with L. intracellularis were resuspended in 30 ml of sterile 0.2 M phosphate-buffered saline (PBS) at pH 7.3 and stored at −80°C.
For purifying L. intracellularis from McCoy cells, a discontinuous Percoll gradient was prepared following methods previously described, with slight modifications (12). Briefly, 225 ml of Percoll (Amersham Biosciences, Uppsala, Sweden) was mixed with 260 ml of distilled water and 15 ml of 5 M NaCl to form the stock Percoll gradient. Harvested L. intracellularis culture was passed at least 20 times through a 25-gauge needle, and 5 ml of this bacterial-McCoy cell homogenate was mixed with 25 ml of the stock Percoll gradient in polycarbonate tubes. The tubes were then centrifuged at 37,000 × g for 1 h at 4°C. The ensuing suspension contained scattered cellular debris in the upper 50% of the tube, while one distinct cellular banding pattern was visualized at a buoyant density of 1.075 g/ml. This band was carefully collected into a 5-ml polypropylene pipette and transferred to new tubes containing 20 ml of sterile PBS. These tubes were centrifuged (Avanti Beckman J-20I, JA-17 rotor) at 37,000 × g for 15 min at 4°C, and this washing process was repeated three times to remove Percoll material from each sample. After the final centrifugation step, the bacterial pellets were suspended in sterile PBS, aliquoted into 1.8-ml Cryovial tubes, and stored at −80°C. Dark field microscopy of sampled material confirmed highly concentrated tiny curved rods and the absence of intact McCoy cells.
The LPS component was extracted from this purified L. intracellularis 15540 antigen using a hot aqueous phenol method (Westphal method) with slight modifications (34). Briefly, 4.5 ml of a preheated purified L. intracellularis suspension was gently mixed in a tube containing 4.5 ml of a 90% (vol/vol) hot aqueous phenol suspension for 25 min at 65°C and then cooled overnight at 4°C. Each tube was centrifuged at 7,700 × g for 25 min at 4°C, and the LPS-containing supernatant fluid contained in the upper portion of the biphasic aqueous layer within each tube was retained and transferred into presterilized dialysis tubing. The tubing was then dialyzed repeatedly against cold reagent-grade (reverse osmosis) water for 48 h to remove the phenol. The L. intracellularis LPS extract was collected and centrifuged at 10,000 × g for 15 min at 4°C to remove any remaining particulates and stored in sterile vials at −80°C.
Antigen characterization.
The LPS extract was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 4 to 12% Bis-Tris gel in MOPS (morpholinepropanesulfonic acid) running buffer (Invitrogen) and compared to Percoll-purified whole-cell L. intracellularis and uninfected whole McCoy cells. Samples for SDS-PAGE were prepared by diluting each 1:2 into 4× lithium salt dodecyl sulfate denaturing buffer (Invitrogen) and incubated in an 85°C water bath for 10 min. Gels were periodate silver stained (30) using a slight modification of the commercially available Bio-Rad silver stain. Briefly, gels were oxidized with 0.7% periodic acid immediately after fixing and prior to the second fixation step (10% ethanol and 5% acetic acid), and an additional reduction step with 160 μM dithiothreitol was included before the silver reagent step. In other experiments, the LPS extract was subsequently transferred from unstained SDS-PAGE gels to polyvinylidene difluoride (Invitrogen) membranes for Western blot analysis. The separated molecules were electrophoretically transferred in Towbin buffer using a Novex blot module at a constant voltage of 30 V for 1 h. After transfer, the blots were cut into strips and blocked for at least 1 h in 50 ml of a blocking solution consisting of Tris-buffered saline with 0.05% (vol/vol) Tween 20 (TTBS) and 2% (wt/vol) nonfat dry milk (Bio-Rad). Blots were incubated with numerous primary antibody sources and dilutions, as follows: (i) LPS ELISA-positive control hyperimmunized pig serum (1:2500), (ii) LPS ELISA-negative control pig serum (1:2500), (iii) swine anti-Lawsonia convalescent-phase serum (1:10, 1:20, 1:100), and (iv) swine Lawsonia-negative convalescent-phase serum (1:100) to illustrate the specificity of anti-Lawsonia antibodies to the primary antigen of the extract, Lawsonia LPS. The primary antibody dilutions used for this Western analysis were similar to those used for the LPS ELISA. The primary antibodies were diluted in the blocking reagent described above and incubated with each respective membrane at room temperature for 1 h. The blots were washed three times for 2 min at room temperature in TTBS, after which the membranes were incubated in 50 ml of the secondary antibody conjugate (goat anti-swine horseradish peroxidase [HRP]; Kirkegaard and Perry Laboratories, Inc.) at a 1:1,000 dilution in blocking reagent for 1 h at room temperature. The blots were washed twice in TTBS and once in PBS prior to incubation at room temperature for 30 min in 10 ml of Opti-4CN substrate (Bio-Rad) solution. The colorimetric reaction was stopped by rinsing the blots with reagent-grade water.
Lastly, the LPS extract was analyzed for a specific concentration by a bacterial endotoxin test with a Limulus amoebocyte lysate assay (Endosafe). The concentration of LPS within the extract was expressed as endotoxin units (EU) per ml using the kinetic chromogenic method as described in the manufacturer's instructions.
Optimization of the LPS ELISA.
The ELISA was optimized following standard assay development procedures described elsewhere (6, 27). Concentrations of key reagents used in this LPS ELISA, such as the primary antigen (LPS extract), primary antibodies, and HRP-conjugated secondary antibodies, were optimized to achieve a desired and consistent working dilution using checkerboard titrations (6, 27). Other influencing factors key to assay success, such as blocking and wash buffers, substrates, incubation temperatures, and duration of incubation time periods, were also evaluated according to standard procedures to achieve optimum levels (6, 27).
LPS ELISA for anti-Lawsonia IgG antibodies.
The Lawsonia intracellularis LPS, at a concentration of 34.8 EU/ml, was used to coat Immulon 2HB plates (Dynex) at 100 μl/well at a 1:1,000 dilution in 0.05 M sodium carbonate coating buffer, pH 9.6 (2), and incubated at 20°C for 24 h. Each plate was then washed in a buffer containing 0.05% Tween 20 (vol/vol), 0.137 M NaCl, 0.005 M KCl, 0.009 M Na2HPO4, and 0.001 M KH2PO4 (pH 7.2 to 7.4) in distilled water. Antigen-coated plates were then blocked at 300 μl/well with a blocking buffer containing 5% (wt/vol) nonfat dry milk (Bio-Rad) in SeaBlock (Pierce Biotech) at 4°C for 24 h to prevent the nonspecific binding of test serum to the plates. Each plate was then washed for three cycles as described above. Each test and control serum sample was prediluted 1:40 in blocking buffer. Fifty μl per well of each diluted serum sample was incubated with antigen at 37°C for 1 h. After three washing steps, 50 μl/well of a 1:500 dilution of goat anti-swine immunoglobulin G (IgG) heavy and light chain-specific HRP conjugate (Kirkegaard and Perry Laboratories, Inc.) in blocking buffer was added and incubated at 37°C for 1 h. After three washing steps, 50 μl/well of peroxidase color substrate (3,3′,5,5′-tetramethylbenzidine) was applied to each plate and incubated at 20°C for 5 min and then stopped with a 2 M H2SO4 solution. Absorbance values at a wavelength of 450 nm (A450) were obtained for each sample well. A range of control serum samples from high- to low-positive and negative antibodies against the L. intracellularis LPS extract were generated and included for each plate. Control sera were initially diluted 1:2,560 in blocking buffer and were then serially diluted twofold a total of five times (1:2,560, 1:5,120, 1:10,240, 1:20,480 and 1:40,960) immediately prior to sample testing. Aliquots (50-μl) of each diluted positive and negative control samples were transferred and typically placed in wells A through E in columns 11 and 12 of the test plate. A test plate was considered valid if the coefficient of determination (r2 value) of these standards' A450 values was ≥0.9 in a linear regression analysis (Microsoft Excel), with empty wells reading blank.
Serum samples derived from clinical pig trials.
Serum samples were obtained from pigs in the following studies for use in validating the LPS ELISA.
Study 1.
Two 3-week-old Lawsonia-negative pigs were hyperimmunized for antibodies to the L. intracellularis LPS extract by deep intramuscular injection with 2 ml of the L. intracellularis LPS extract mixed 1:2 with a commercial Freund's incomplete adjuvant (Sigma Aldrich). Another control 3-week-old pig received a placebo injection of cell culture medium containing 5% (vol/vol) bovine serum, mixed 1:2 with this adjuvant. Booster injections of each inoculum were administered 3 and 6 weeks post-initial inoculation (p.i.), and final specimen collections were at 8 weeks p.i. Serum samples from each pig were collected on days 0, 21, 42, and 59 of the study and tested for the presence and concentration of anti-Lawsonia IgG antibodies by using an indirect immunofluorescence assay incorporating L. intracellularis whole-cell antigen (immunofluorescence antibody test [IFAT]) as described previously (15).
Study 2.
Challenge exposure studies with L. intracellularis isolates were conducted with Lawsonia-negative 6- to 9-week-old pigs as described previously (16, 33). Serum samples from the virulent L. intracellularis challenge-exposed and strict control pigs (nonvaccinated, nonchallenged pigs) in these studies were tested to investigate the cutoff limits for anti-Lawsonia LPS antibody positive/negative absorbance values. The Lawsonia IFAT was also conducted with these samples.
Study 3.
A “proof of concept” vaccine efficacy study was conducted following good clinical practice guidelines as previously reported (16). This study utilized conventional pigs that were Lawsonia-negative at the time of vaccination. On day 0 of the study, vaccinates (n = 15) were given an oral 2-ml dose of Enterisol Ileitis (Boehringer Ingelheim Vetmedica, Inc.) per the manufacturer's label instructions, while pigs in the challenge control group (n = 10) were given an equivalent dose of placebo consisting of uninfected McCoy cells in growth media. Strict control pigs were not given vaccine, placebo, or virulent challenge during the study and were kept in separate pens to ensure biosecurity among treatment groups. On day 21, vaccinated and challenge control pigs were given a 10-ml intragastric dose of virulent L. intracellularis isolate N10194 at 107.7 tissue culture infective dose 50% end point (TCID50) per ml. On day 42 of the study, pigs were humanely euthanized, necropsied, and evaluated for Lawsonia-specific gross and microscopic lesions. Average gross and microscopic lesion scores (immunohistochemistry), average daily weight gains, seroconversion rates (IFAT), L. intracellularis fecal shedding rates (PCR), and average daily clinical scores after challenge of this clinical trial were previously reported (16). Serum samples were subsequently tested with the LPS ELISA for direct comparison to the IFAT for differences in detecting anti-Lawsonia antibodies in vaccine- and challenge-exposed pigs.
Statistical analysis.
Statistical comparisons in seroconversion among assays and treatment groups utilized Fisher's exact t test and a two-sample test of proportions (5, 25). Statistical determinations of positive and negative A450 cutoffs, as well as assay sensitivities, specificities, and 95% confidence intervals and the areas under the receiver operating characteristic (ROC) curve were conducted with ROC curve analysis using Medcalc version 7.2.1.0. These additional parameters were observed to identify the assay's ability to detect Lawsonia-specific antibodies in pigs and to gauge the assay's ability to distinguish between Lawsonia-exposed and Lawsonia-naïve pigs.
RESULTS
Characterization of the L. intracellularis LPS extract as the primary ELISA antigen.
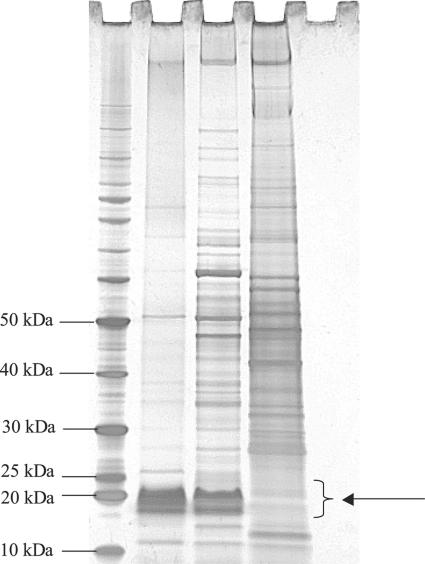
The visualization of the L. intracellularis LPS component by the SDS-PAGE-periodate silver staining of the LPS extract revealed a glycosylated molecule of approximately 18 to 25 kDa (Fig. 1). Glycosylation was confirmed by the reddish staining imparted on the species via the reaction of aldehyde groups formed by periodate oxidation of the sugars with the silver reagent. This LPS fraction was differentially stained reddish brown to specifically identify glycosylated moieties in comparison to other extracted components. The LPS was observed with the samples containing the LPS extract and whole cell L. intracellularis and not with the uninfected McCoy cell sample, suggesting that this molecule is of prokaryotic origin. The periodate silver-stained gel also revealed the presence of other, higher-molecular-weight proteins that seem to appear at lower concentrations compared to the LPS extract. These extraneous proteins perhaps are due to inadvertent carry-over of particulates from the biphasic layer during the purification step, or they could be the results of incomplete degradation of proteins during the hot aqueous phenol phase of the LPS extraction.
FIG. 1.
SDS-PAGE-periodate silver staining of L. intracellularis LPS. First lane, 10- to 220-kDa benchmark prestained protein marker (Invitrogen); second lane, L. intracellularis LPS extract; third lane, whole-cell L. intracellularis 15540; fourth lane, uninfected McCoy cells; fifth lane, blank. The arrow indicates the migration range of L. intracellularis LPS.
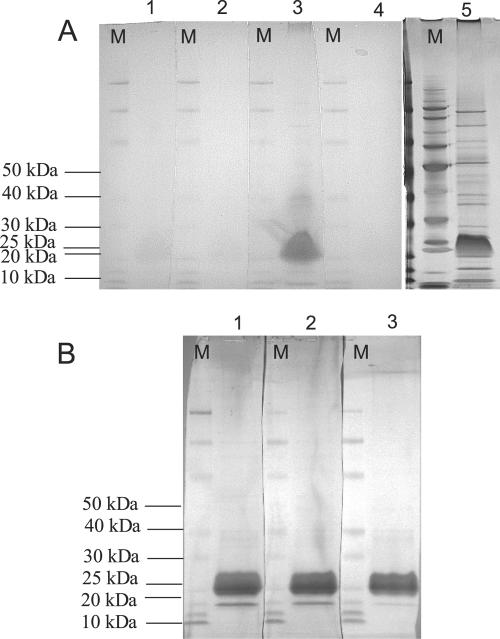
Western blots of the LPS extract were performed to determine the specificity of anti-Lawsonia antibodies to the LPS component and to assess the degree of cross-reactivity to the extraneous proteins (Fig. 2). Results of the Western blots demonstrated that the LPS ELISA negative control (1:2,500) and Lawsonia-negative pig sera (1:100) did not react to any of the extraneous proteins in the LPS extract and reacted very lightly (weak signal strength) to the LPS component itself. Positive LPS ELISA control sera (1:2,500) reacted very strongly (strong signal strength) to the LPS component and, to a lesser extent, the extraneous proteins. Slight cross-reaction of the positive control was expected, since hyperimmunized pigs would have generated antibodies to both the LPS component and the extraneous proteins. However, the Western signal for this particular interaction was very light, making up <1% of the total signal strength compared to the target LPS antigen. Additionally, Western analysis of the LPS extract to Lawsonia-positive convalescent pig serum at dilutions of 1:10, 1:20, and 1:100 revealed strong reactions to the LPS component, whereas very light reactions were noted with the higher-molecular-weight extraneous proteins at 1:10 and 1:20 dilutions only (<1% of total signal strength). An extraneous protein of lower molecular weight than the LPS component (approximately 15 kDa) did react to Lawsonia-positive convalescent pig sera but represented only ∼9% of the total signal strength of the reaction compared to the LPS component.
FIG. 2.
Western blots of L. intracellularis LPS using ELISA control or positive and negative convalescent swine sera. Each gel lane was loaded with the same amount of LPS material, and replicate lanes were cut into strips after transfer to polyvinylidene difluoride for incubation with different primary antibodies. Secondary antibody in each case was goat anti-swine-HRP (1:1,000). M, molecular weight marker. (A) Lane 1, strict control negative serum (1:100); lane 2, ELISA negative control serum (1:2,500); lane 3, ELISA positive control serum (1:2,500); lane 4, goat anti-swine-HRP conjugate only (1:1,000); lane 5, silver-stained SDS-PAGE gel. (B) Primary antibody was swine anti-Lawsonia convalescent-phase serum at different dilutions. Lane 1, 1:10; lane 2, 1:20; lane 3, 1:100.
Results from the bacterial endotoxin test for quantifying the amount of endotoxin in the purified sample of L. intracellularis LPS extract revealed an endotoxin level of 34,750 EU/ml. Furthermore, the reagent-grade water used to store the LPS extract contained <0.05 EU/ml, thus indicating that the buffer was endotoxin free and provided no interference in obtaining the true endotoxin concentration of the LPS extract.
Individual LPS ELISA plate validation via hyperimmunized pig sera.
The positive LPS ELISA control serum was generated from two 3-week-old Lawsonia-negative pigs hyperimmunized with Lawsonia LPS extract. The negative control serum was generated from one 3-week-old pig receiving adjuvant alone. Pigs that received an LPS extract inoculation were IFAT positive on days 21, 42, and 59 of the study, with mean anti-Lawsonia antibody titers of 1:200, 1:16,000, and 1:16,000, respectively. Reactivity of the positive and negative control sera against the Lawsonia LPS antigen extract (1:1,000) in the LPS ELISA plates was confirmed by performing twofold titrations in blocking buffer of each control across the plate. The positive control A450 value at a 1:40 dilution (optimal detection antibody dilution for this assay) was 1.9 and was determined to have a total anti-Lawsonia LPS endpoint titer of 1:10,000 to 1:20,000 by the LPS ELISA. The negative control A450 value at a 1:40 dilution was <0.2. Linear regression analysis of the positive control demonstrated an r2 value of 0.96. Based on the linearity of the positive control, an individual LPS ELISA assay is considered valid in detecting Lawsonia-specific antibodies when each plate demonstrates an r2 value of 0.9 or greater.
Determination of cutoff limits.
One hundred sixty pig serum samples from two L. intracellularis challenge exposure studies were evaluated to determine the anti-Lawsonia-positive and -negative antibody cutoff limits for the LPS ELISA assay. In addition, these serum samples were used to determine assay specificity, sensitivity, 95% confidence interval, and the area under the ROC curve analysis. Eighty pigs were previously identified to be positive for L. intracellularis by both IFAT and tissue immunohistochemistry analysis (16, 33). Another 80 pigs from strict control (nonvaccinated, non-challenge-exposed) and negative control (received a non-Lawsonia placebo) groups were previously identified to be negative for L. intracellularis by the same detection methods (16, 33).
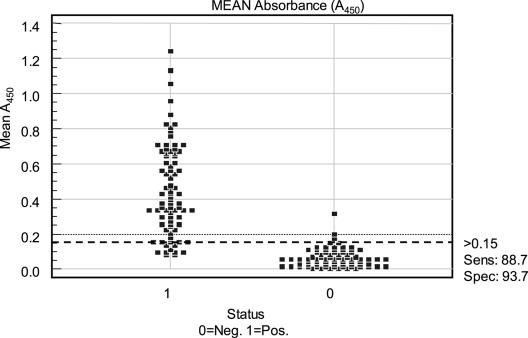
A ROC curve analysis, a graph that plots the true positive rate in function with the false-positive rate at different cutoff points, identified the critical cutoff criterion for these samples to be an A450 of ≥0.15 (Fig. 3). This value was rounded to the nearest tenth of an A450 value, to 0.2, to simplify future interpretations of LPS ELISA results. Therefore, pig serum with an A450 value of 0.2 or lower is considered Lawsonia antibody negative, whereas serum with an A450 value of greater than 0.2 is considered Lawsonia antibody positive.
FIG. 3.
Scatter plot of the mean A450 values for anti-Lawsonia antibody-positive and -negative serum samples. The y axis depicts mean A450 values, and the x axis depicts sample set identification. 0, negative (Neg.); 1, positive (Pos.) for anti-Lawsonia antibodies. The dashed black line at the A450 of 0.15 signifies the positive/negative cutoff value. Sens, sensitivity; Spec, specificity.
In addition, the ROC analysis revealed that the specificity of this assay to detect anti-Lawsonia antibodies in pigs was 93.7%, and the sensitivity was determined to be 88.7% (Fig. 3). The 95% confidence interval for this assay was 0.933 to 0.991, and the ROC under the curve analysis revealed a value of 0.972, with a standard error of 0.013.
LPS ELISA validation in a controlled, L. intracellularis challenge-exposed study.
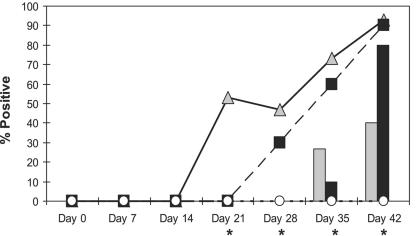
Pigs in the strict control group remained L. intracellularis IFAT and LPS ELISA negative through the study. From day 0 (vaccination) to day 14 (1 week before challenge) of the study, vaccinated and unvaccinated challenge control groups were IFAT and LPS ELISA negative for anti-L. intracellularis antibodies. On day 21 (challenge inoculation) of the study, all groups were IFAT negative (0%), whereas 53% of the vaccinated pigs exhibited LPS ELISA positive results. One week after challenge (day 28), again only the LPS ELISA was able to detect specific seroconversion to anti-Lawsonia antibodies in both the vaccinated and challenge control groups (47% and 30%, respectively). On days 35 and 42 of the study, the number of pigs in both the vaccinated and challenge control groups gradually increased in seroconversion due to virulent L. intracellularis exposure. Serological results from days 35 and 42 in the vaccinated pigs were 26.7% and 40% IFAT positive and 73% and 93% LPS ELISA positive, respectively. The challenge control group was 10% and 80% IFAT positive and 60% and 90% LPS ELISA positive on days 35 and 42, respectively.
The seroconversion rates based on the LPS ELISA identified significant (P < 0.05) increases in the percentage of anti-Lawsonia antibody positives in the vaccinate group compared to challenge control and strict control pigs at day 21 and compared to strict control pigs only on days 28, 35 and 42 of the study. Nonvaccinated challenge controls were significantly higher in the percentage of LPS ELISA positives than the strict controls on days 35 (P = 0.001) and 42 (P < 0.0001) of the study. No significant differences between vaccinates and challenge controls on days 35 and 42 were noted. Significant (P = 0.02) increases in the percentage of IFAT positives to L. intracellularis challenge exposure were evident for vaccinated pigs compared to strict control pigs on day 35 of the study. Vaccinates and challenge controls were significantly (P = 0.003) more IFAT positive than the strict control group on day 42 of the study. No significant differences in the percentage of IFAT positives were evident between vaccinates and challenge controls on days 35 and 42. Group comparisons in seroconversion rates between assays and treatment groups are summarized in Fig. 4.
FIG. 4.
IFAT versus indirect LPS ELISA; rates of seroconversion to L. intracellularis among treatment groups. *, significant differences in seroconversion among groups and assay types (chi-square/Fisher's exact test and two-sample test of proportions, P < 0.05). The bar graph represents the mean percentage of IFAT positives for treatment groups throughout the study. Vaccinates, grey bars; challenge controls, black bars; strict controls, white bars (not visible, 0%). The line graph represents the mean percentage of LPS ELISA positives for treatment groups throughout the study. Vaccinates, solid line with grey triangles; challenge controls, dashed line with black squares; strict controls, dashed line with white circles; x axis, each day of the study that serum samples were tested for anti-Lawsonia IgG; y axis, mean percentage of anti-Lawsonia antibody positive pigs.
Comparisons between the LPS ELISA and the IFAT in detecting anti-Lawsonia antibodies in this study revealed significantly more LPS ELISA-positive pigs than IFAT-positive pigs on days 21, 28, 35, and 42 (P = 0.003, 0.030, 0.002, and 0.006, respectively).
The ROC analysis of the results from this study revealed the specificity of the LPS ELISA to be 100% and the sensitivity 99.5%. The 95% confidence interval was 0.976 to 0.999, and the ROC under the curve analysis revealed a value of 0.995, with a standard error of 0.007.
DISCUSSION
We have developed a novel LPS-based indirect ELISA that specifically detects anti-Lawsonia IgG in the sera from Lawsonia-negative pigs exposed to a modified live vaccine and/or virulent challenge. This immunoassay could improve the early detection of L. intracellularis exposure in pigs by providing a user-friendly and time-efficient research tool for use in controlled clinical evaluations of pigs. The incorporation of this Lawsonia-specific and -sensitive antibody-antigen reaction into direct or sandwich ELISA approaches may allow other novel immunoassays to be developed for research purposes. Modifications to the conjugate in this LPS ELISA would also allow for detection of other anti-Lawsonia-specific IgA or IgM in pigs or antibodies in other host species. Modifications to the method for preparation of the test sample may also allow testing of tissue, feces, or sow milk samples for the test antibody.
The use of this type of LPS ELISA format has been successfully deployed in serologic assays for other enteric and intracellular bacteria, such as Salmonella dublin and Brucella spp. (1, 2, 32). The diagnostic performance of this assay is not expected to be affected by alterations in the antigen's lipid A structure or contaminating outer membrane proteins that may occur in different antigen preparations (1, 2, 24). The standard carbonate buffer coating conditions used in our study are considered suitable for the LPS ELISA format for smooth LPS antigen (2). There was also a good correlation of the LPS ELISA results in a range of field studies for Salmonella dublin infection on farms (32), and these results were superior to previous Salmonella immunoassay formats (32). The LPS ELISA described here is therefore likely to provide a robust, widely applicable diagnostic screening tool with broad benefits for disease interpretation among groups of pigs.
Current serology-based assays for L. intracellularis infection and disease are the IFAT (15) and the immunoperoxidase monolayer assay (7, 8), both incorporating whole-cell antigen on carrier wells in plates or slides. They demonstrate good sensitivity (91% and 89%, respectively) and specificity (100% for both assays) in detecting anti-Lawsonia antibodies antemortem in pig serum. However, both assays are probably not sensitive enough to detect anti-Lawsonia antibodies in lower concentrations in pig serum during the initial exposure and onset of infection. In addition, these assays rely on highly skilled technicians to accurately conduct the tests and interpret the results. Results are obtained subjectively by spending many hours looking into a microscope, analyzing wells illuminated by UV or natural light, and searching for L. intracellularis- or L. intracellularis-infected cells stained fluorescent green or enzymatic red, respectively, which represents a “positive” sample. A previous attempt to develop an ELISA for the detection of anti-Lawsonia antibodies in pigs failed to produce a sensitive and specific assay, due to its variable and nonspecific quality of antigen (derived directly from pig intestines) and limitations in sampling size related to known disease parameters for test pigs (12).
In the LPS ELISA described here, we used an antigen source consisting of phenol-purified LPS derived from pure cultures of L. intracellularis, with a specific cutoff value (A450, 0.200) that has been determined for identifying anti-Lawsonia antibody-positive and -negative pig sera. The ability of the LPS ELISA to accurately distinguish Lawsonia-exposed pigs has been established by observing the results of the ROC under the curve value and 95% confidence intervals in the controlled challenge exposure study. The strong ROC under the curve value of 0.995 and the 95% confidence intervals of 0.976 to 0.999 identify the LPS ELISA as a highly accurate serological test for detecting anti-Lawsonia antibodies in pigs exposed to L. intracellularis in these experimental trials. Serological results from a controlled study of L. intracellularis-challenge-exposed pigs reveal the superiority of the LPS ELISA in detecting early antibody onset following vaccine and virulent challenge exposure compared to the IFAT. Significantly (P < 0.05) more vaccinated pigs (53%) were LPS ELISA positive prior to receiving a virulent challenge (day 21) than nonvaccinated challenge controls (0%). The LPS ELISA was the only test sensitive enough to detect seroconversion in pigs specifically due to vaccination at 3 weeks p.i. (day 21). The LPS ELISA significantly (P < 0.05) detected more anti-Lawsonia positives than the IFAT in pigs regardless of treatment groups on the day of challenge (day 21) and 1, 2, and 3 weeks after virulent challenge. The implications for detecting earlier onset of L. intracellularis exposure in pigs using the LPS ELISA will allow a more precise and rapid implementation of L. intracellularis control strategies, such as vaccination to control and reduce PE.
Percentages of mean LPS ELISA positives tended to be higher for vaccinated pigs (47%, 73%, and 93%, respectively) than for the challenge controls (30%, 60%, and 90%, respectively) each day after challenge exposure, whereas the IFAT results revealed the first evidence of seroconversion in vaccinated pigs at 2 weeks postchallenge (26.7%) and showed a gradual increase to no more than 40% IFAT-positive pigs on the last day of the study. The challenge controls demonstrated sharp increases in the percentages of IFAT positives, with 10% and 80% on days 35 and 42 of the study, respectively. The LPS ELISA seems more sensitive in that it identified more anti-Lawsonia positive pigs in the vaccinates than in the nonvaccinates after virulent challenge; this trend differs from those seen in previous studies using the IFAT (16).
Specificity and sensitivity values based on 80 known positive and 80 known negative test samples were 93.7% and 88.7%, respectively. Serum tested from the challenge exposure study confirmed this result by demonstrating similar specificity (100%) and sensitivity (99.5%) in detecting anti-Lawsonia antibodies in pigs. Western blot analysis of the LPS extract supports this high specificity in detecting anti-Lawsonia antibodies with the LPS ELISA, even though the LPS extract contained extraneous protein carry-over. It appears that at the dilutions used, the reagents of the ELISA are quite specific for what is believed to be a component of Lawsonia LPS. The low level of carry-over protein within the LPS extract contains no confounding effects in the assay's ability to specifically detect Lawsonia-specific antibodies in pigs. Furthermore, quantification of total protein in the undiluted LPS extract by a BCA protein assay (Pierce Laboratories, Inc.) revealed concentration levels too low (0.11 mg/ml) for establishing adequate antigen-antibody complexes.
Despite the positive results noted in this investigation, there are some factors which limit the application of the described LPS ELISA beyond the research laboratory. Other antigen preparations of differing purities were examined and found not to have sufficient sensitivities and specificities for use in Lawsonia-specific research trials (data not shown). The requirement of antigen derived from Percoll gradients (low in or free from eukaryotic cellular debris) and phenol extraction methods make Lawsonia LPS antigen availability practical for only limited numbers of assays and probably cost prohibitive for uses beyond research trials. Secondly, limited evaluation of this assay under field conditions suggests that there may be differences in animal backgrounds and LPS reactivity, especially in adult pigs. If this assay were to be applied as a routine diagnostic assay, it is possible that different cutoff values would be required and sensitivity and specificity would be lower.
The technical advantages outlined above make the LPS-based indirect ELISA test an improved serological method for detection of L. intracellularis exposure in pigs compared to the IFAT, because it successfully and accurately identified early-onset exposure to this organism regardless of isolate type (vaccine or wild type) and provided a rapid and user-friendly tool for identifying specific seroconversion to L. intracellularis in experimental studies.
Acknowledgments
These projects were financially supported by Boehringer Ingelheim Vetmedica, Inc., Ames, Iowa.
We thank Sarah Gannon and Philip Utley for their technical assistance in laboratory and clinical experiments and Lyle Kesl and Ryan Saltzman, Veterinary Resources, Inc., for their involvement in the GCP challenge exposure studies that provided the samples necessary to validate the LPS ELISA.
REFERENCES
- 1.Alonso-Urmeneta, B., C. Marín, V. Aragón, J. M. Blasco, R. Díaz, and I. Moriyón. 1998. Evaluation of lipopolysaccharides and polysaccharides of different epitopic structures in the indirect enzyme-linked immunosorbent assay for diagnosis of brucellosis in small ruminants and cattle. Clin. Diagn. Lab. Immunol. 5:749-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bantroch, S., T. Buhler, and J. S. Lam. 1994. Appropriate coating methods and other conditions for enzyme-linked immunosorbent assay of smooth, rough, and neutral lipopolysaccharides of Pseudomonas aeruginosa. Clin. Diagn. Lab. Immunol. 1:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bronsvoort, M., B. Norby, D. P. Bane, and I. A. Gardner. 2001. Management factors associated with seropositivity to Lawsonia intracellularis in US swine herds. J. Swine Health Prod. 9:285-290. [Google Scholar]
- 4.Chouet, S., C. Prieto, L. Mieli, M. F. Veenhuizen, and S. McOrist. 2003. Patterns of exposure to Lawsonia intracellularis infection on European pig farms. Vet. Rec. 152:14-17. [DOI] [PubMed] [Google Scholar]
- 5.Cochran, W. G., and G. M. Cox. 1992. Notes on the statistical analysis of the results, p. 42-92. In Experimental designs, 2nd ed. John Wiley and Sons, Inc., New York, N.Y.
- 6.Crowther, J. R. 2001. The ELISA guidebook. Methods in molecular biology, vol. 149. Humana Press, Inc., Totowa, N.J. [DOI] [PubMed]
- 7.Guedes, R. M. C., C. J. Gebhart, G. Armbruster, and B. D. Roggow. 2002. Serologic follow-up of a repopulated swine herd after an outbreak of proliferative hemorrhagic enteropathy. Can. J. Vet. Res. 66:258-263. [PMC free article] [PubMed] [Google Scholar]
- 8.Guedes, R. M. C., C. J. Gebhart, N. L. Winkelman, and R. A. Mackie-Nuss. 2002. A comparative study of an indirect fluorescent antibody test and an immunoperoxidase monolayer assay for the diagnosis of porcine proliferative enteropathy. J. Vet. Diagn. Investig. 14:420-423. [DOI] [PubMed] [Google Scholar]
- 9.Guedes, R. M. C., C. J. Gebhart, N. L. Winkelman, R. A. Mackie-Nuss, T. A. Marsteller, and J. Deen. 2002. Comparison of different methods for diagnosis of porcine proliferative enteropathy. Can. J. Vet. Res. 66:99-107. [PMC free article] [PubMed] [Google Scholar]
- 10.Guedes, R. M. C., and C. J. Gebhart. 2003. Onset and duration of fecal shedding, cell-mediated and humoral immune responses in pigs after challenge with a pathogenic isolate or attenuated vaccine strain of Lawsonia intracellularis. Vet. Microbiol. 91:135-145. [DOI] [PubMed] [Google Scholar]
- 11.Hammer, J. M. 2004. The temporal relationship of fecal shedding of Lawsonia intracellularis and seroconversion in field cases. J. Swine Health Prod. 12:29-33. [Google Scholar]
- 12.Holyoake, P. K., R. S. Cutler, I. W. Caple, and R. P. Monckton. 1994. Enzyme-linked immunosorbent assay for measuring ileal symbiont intracellularis-specific immunoglobulin G response in sera in pigs. J. Clin. Microbiol. 31:1980-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones, G. F., G. E. Ward, M. P. Murtaugh, G. Lin, and C. J. Gebhart. 1993. Enhanced detection of intracellular organism of swine proliferative enteritis, ileal symbiont intracellularis, in feces by polymerase chain reaction. J. Clin. Microbiol. 31:2611-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Just, S., C. O. Thoen, B. J. Thacker, and J. U. Thompson. 2001. Monitoring of Lawsonia intracellularis by indirect serum immunofluorescence assay in a commercial swine production system. J. Swine Health Prod. 9:57-61. [Google Scholar]
- 15.Knittel, J. P., D. M. Jordan, K. J. Schwartz, B. H. Janke, M. B. Roof, S. McOrist, and D. L. Harris. 1998. Evaluation of antemortem polymerase chain reaction and serologic methods for detection of Lawsonia intracellularis-infected pigs. Am. J. Vet. Res. 59:722-726. [PubMed] [Google Scholar]
- 16.Kroll, J., M. B. Roof, and S. McOrist. 2004. Evaluation of protective immunity in pigs following oral administration of an avirulent live vaccine of Lawsonia intracellularis. Am. J. Vet. Res. 65:559-565. [DOI] [PubMed] [Google Scholar]
- 17.Lawson, G. H. K., S. McOrist, A. C. Rowland, L. Roberts, and E. McCartney. 1988. Serological diagnosis of the porcine proliferative enteropathies: implications for aetiology and epidemiology. Vet. Rec. 122:554-557. [DOI] [PubMed] [Google Scholar]
- 18.Lawson, G. H. K., S. McOrist, J. Sabri, and R. A. Mackie. 1993. Intracellular bacteria of porcine proliferative enteropathy: cultivation and maintenance in vitro. J. Clin. Microbiol. 31:1136-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McOrist, S., R. Boid, G. H. K. Lawson, and I. McConnell. 1987. Monoclonal antibodies to intracellular Campylobacter-like organisms of the porcine proliferative enteropathies. Vet. Rec. 121:421-422. [DOI] [PubMed] [Google Scholar]
- 20.McOrist, S., S. Jasni, R. A. Mackie, N. MacIntyre, N. Neef, and G. H. K. Lawson. 1993. Reproduction of porcine proliferative enteropathy with pure cultures of ileal symbiont intracellularis. Infect. Immun. 61:4286-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McOrist, S., C. J. Gebhart, R. Boid, and S. M. Barns. 1995. Characterization of Lawsonia intracellularis gen. nov., sp. nov., the obligately intracellular bacterium of porcine proliferative enteropathy. Int. J. Syst. Bacteriol. 45:820-825. [DOI] [PubMed] [Google Scholar]
- 22.McOrist, S., S. H. Smith, and L. E. Green. 1997. Estimate of direct financial losses due to porcine proliferative enteropathy. Vet. Rec. 140:579-581. [DOI] [PubMed] [Google Scholar]
- 23.McOrist, S., D. E. Barcellos, and R. J. Wilson. 2003. Global patterns of porcine proliferative enteropathy. Pig J. 51:26-35. [Google Scholar]
- 24.Moller, K., T. K. Jensen, S. E. Jorsal, T. D. Leser, and B. Carstensen. 1998. Detection of Lawsonia intracellularis, Serpulina hyodysenteriae, weakly beta-haemolytic intestinal spirochaetes, Salmonella enterica, and haemolytic Esherichia coli from swine herds with and without diarrhoea among growing pigs. Vet. Microbiol. 62:59-72. [DOI] [PubMed] [Google Scholar]
- 25.Ramsey, F. L., and D. W. Shafer. 1997. Comparisons among several samples, more tools for tables of counts, p. 108-134, 538-558. In C. Mazow and S. Gray (ed.), The statistical sleuth: a course in methods of data analysis. Wadsworth Publishing Co., Belmont, Calif.
- 26.Rowland, A. C., and G. H. K. Lawson. 1974. Intestinal adenomatosis in the pig: immunofluorescent and electron microscopic studies. Res. Vet. Sci. 17:323-330. [PubMed] [Google Scholar]
- 27.Ryan, R., and I. Kwasnik. 1985. Specific immunoglobulin detection, p. 877-881. In E. H. Lennette, A. Balows, W. J. Hausler, and H. J. Shadomy (ed.), Manual of clinical laboratory microbiology, 4th ed. American Society of Microbiology, Washington, D.C.
- 28.Schwartz, K., J. Knittel, D. Walter, M. Roof, and M. Anderson. 1999. Effect of oral tiamulin on the development of porcine proliferative enteropathy in a pure-culture challenge model. J. Swine Health Prod. 7:5-11. [Google Scholar]
- 29.Stege, H., T. K. Jensen, K. Moller, P. Baekbo, and S. E. Jorsal. 2000. Prevalence of intestinal pathogens in Danish finishing pig herds. Prev. Vet. Med. 46:279-292. [DOI] [PubMed] [Google Scholar]
- 30.Tsai, C.-M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 31.Veenhuizen, M. F., T. E. Elam, and N. Soenksen. 2002. Porcine proliferative enteropathy: diagnosis and impact. Comp. Contin. Educ. Pract. Vet. 24:S10-S15. [Google Scholar]
- 32.Veling, J., F. G. van Zuderveld, A. M. van Zuderveld-van Bemmel, H. W. Barkema, and Y. H. Schukken. 2000. Evaluation of three newly developed enzyme-linked immunosorbent assays and two agglutination tests for detecting Salmonella enterica subsp. enterica serovar Dublin infections in dairy cattle. J. Clin. Microbiol. 38:4402-4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter, D., J. Knittel, K. Schwartz, J. Kroll, and M. Roof. 2001. Treatment and control of porcine proliferative enteropathy using different tiamulin delivery methods. J. Swine Health Prod. 9:109-115. [Google Scholar]
- 34.Westphal, O., and Luderitz, O. 1954. Chemische Erforschung von Lipopolysacchariden gramnegativer Bacterien. Angew. Chem. 66:407-417. [Google Scholar]