Abstract
Focal articular cartilage defects are an important factor that leads to dysfunction of the knee joint. Several different surgical approaches have been tried, most of them showing poor results in the long term. The use of orthobiologics in the context of focal chondral lesion has emerged as a potential tool in the treatment of this condition. In this article, we present a surgical technique for the treatment of focal chondral lesions using a collagen membrane associated with microfragmented adipose tissue graft.
Technique Video
Focal articular cartilage defects are an important factor that leads to dysfunction of the knee joint, pain, and reduction in quality of life.1,2 These lesions have limited regenerative potential and may lead to premature osteoarthritis.3 Several different surgical approaches have been tried to address this type of injury, including microfractures,4 autologous chondrocyte implantation,5 autologous matrix-induced chondrogenesis,6 and hyaluronan-based scaffolds.7
Recently, the regenerative potential of mesenchymal stem cells (MSCs) has been studied in focal articular cartilage defects.8 Several different orthobiologics can supply MSCs for this purpose, such as bone marrow aspirate concentrate9 and platelet-rich plasma.10,11 One easy and readily available form of obtaining these cells without invasive procedures is the use of adipose tissue.12
The objective of this article is to describe the surgical technique of the microfragmented adipose tissue (MFAT)13 associated with a collagen membrane as an alternative option for the treatment of focal knee cartilage defect.
Surgical Technique
The patient is placed in a supine position on the surgical table, the limb is prepared, and sterile drapes are placed in the usual manner (Video 1).
Collection of Adipose Tissue
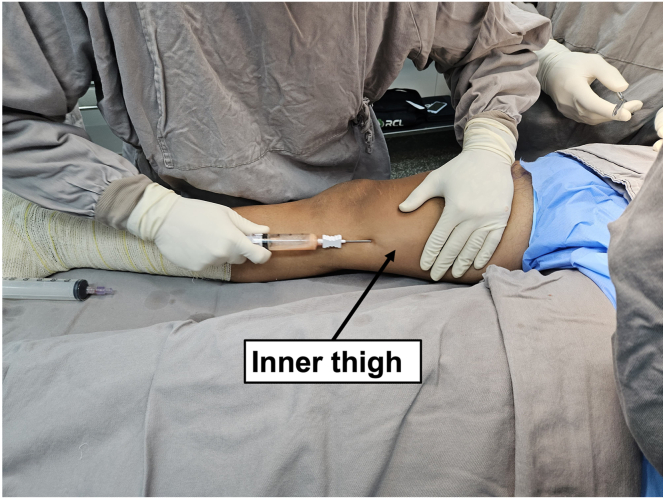
Adipose tissue can be collected in different parts of the body. In this technique, we decided to collect in the inner face of the thigh. A stab incision is performed on the inner face of the thigh to create a portal on the skin, from where the adipose tissue is harvested. A smaller-diameter 19-gauge cannula is used to infiltrate approximately 50 mL of Klein’s solution (20 mL of 2% lidocaine, 20 mL of 0.5% bupivacaine, 1 mL of 1 mg/mL adrenaline, and 250 mL of 0.9% saline) into the subcutaneous tissue. This infiltration should be performed in a fan-shaped manner from the portal, filling the entire subcutaneous donor area with this intumescent solution, aiming to achieve local anesthesia and vasoconstriction, as well as facilitate the detachment of adipose tissue. Five minutes after the subcutaneous infiltration, a larger-diameter cannula (13 gauge) is connected to a 20-mL syringe. Once the cannula is introduced into the subcutaneous tissue, the plunger of the syringe should be fully pulled to create negative pressure in the syringe. While keeping the nondominant hand flat on the skin to prevent the cannula from becoming too superficial or too deep, the other hand performs back-and-forth movements, taking care to avoid the cannula to exit the skin portal and lose negative pressure. The collection of approximately 20 mL of adipose tissue is sufficient for the procedure (Fig 1).
Fig 1.
Medial view of the right limb. Inner thigh fat harvesting using a 20-mL VacLok syringe (Merit Medical Systems).
Preparation of MFAT
The MFAT is obtained with minimal manipulation, without the addition of enzymes or any additives. This closed system uses gentle mechanical forces, washing and filtering tissue to eliminate proinflammatory agents, such as oil from ruptured adipocytes and red blood cells. The first step is a volumetric reduction of the adipose cluster in the initial lipoaspirate. Up to 100 mL of harvested subcutaneous tissue can be prepared at one time. The device is carefully prefilled with saline to avoid the presence of air throughout all the steps, producing a completely closed system. During a subsequent shaking step, stainless steel marbles inside the device emulsify oil residues, which are removed together with contaminating blood components and cellular debris by the gravity counterflow of the saline solution, while the washed reduced fat clusters float to the top of the device.
When the solution inside the device appears clear and the lipoaspirate is yellow, the device is turned upside-down and the fat tissue passes through a narrower-size reduction filter. The second adipose cluster reduction is obtained by passing the floating adipose clusters through this second-size reduction filter by pushing additional fluid from the lower opening of the device using a 10-mL syringe.
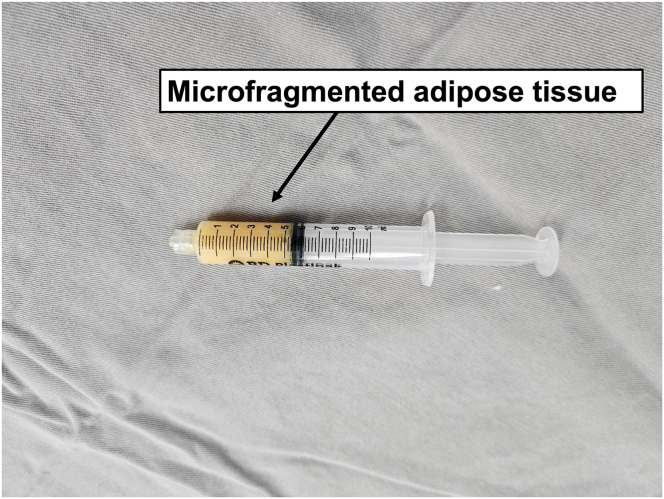
The procedure usually takes 10 to 15 minutes, and the MFAT obtained is ready to use. The tissue can easily flow through a small-caliber needle, up to 20 gauge14 (Fig 2).
Fig 2.
A syringe containing 5 mL microfragmented adipose tissue obtained after Lipogems processing (Lipogems International SpA).
Surgical Access
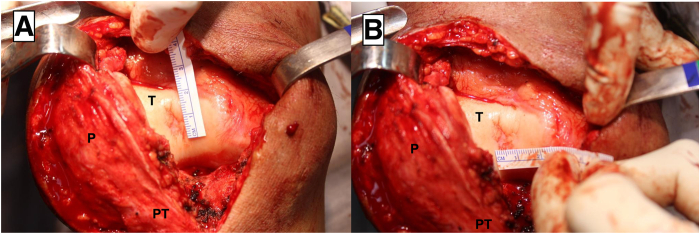
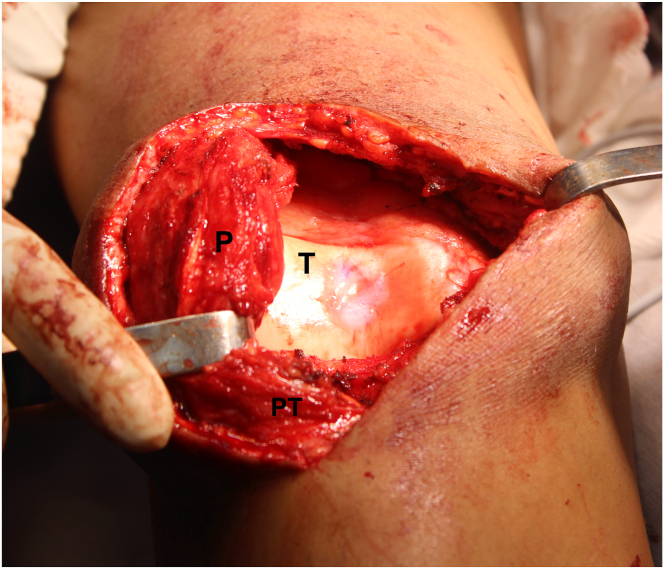
The surgical access must be performed to properly expose the chondral defect to be treated. In the case presented, a median incision of approximately 10 cm is made to the affected knee, a medial parapatellar arthrotomy is performed, and the patella is then retracted laterally, exposing the chondral lesion on the center of the trochlea (Fig 3).
Fig 3.
Front view of the right knee. Measurement of the chondral defect on the center of the trochlea on the vertical (A) and horizontal axis (B). (P, patella; PT, patellar tendon; T: trochlea.)
Debridement and Preparation of the Chondral Defect Bed
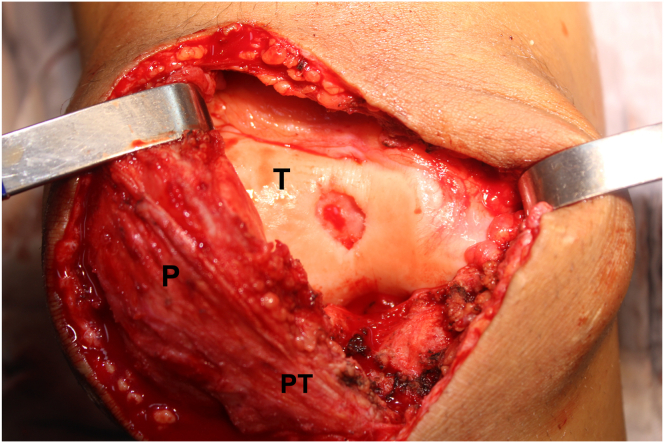
The contour of the lesion is outlined, and a No. 15 scalped blade is used to regularize the borders of the lesion and ensure there is no unstable cartilage at the periphery. After delineating the defect, debridement of its bed is performed using a scoop curette, keeping the borders of the lesion smooth and at a 90° angle to the subchondral bone surface. Care should be taken to avoid violating the subchondral bone in this process, thus reducing the risk of intralesional osteophyte formation (Fig 4).
Fig 4.
Front view of the right knee. Aspect of the chondral defect on the center of the trochlea after removing residual cartilage fragments from the lesion bed, down to the subchondral bone, and regularizing the edges of the lesion. (P, patella; PT, patellar tendon; T: trochlea.)
Preparation and Fixation of the Collagen Membrane
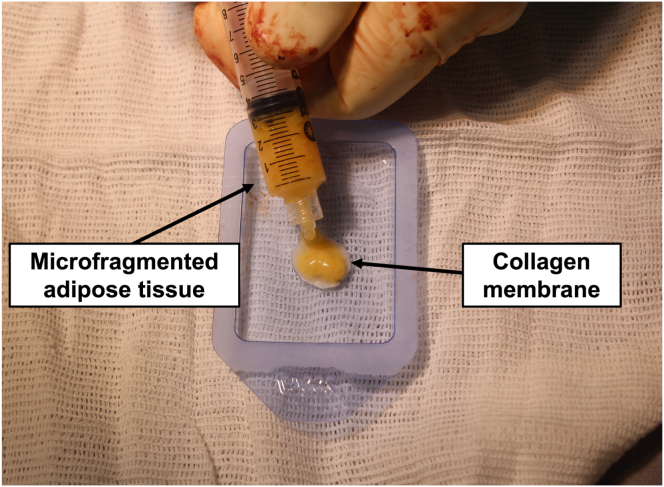
The shape of the chondral lesion is copied to a sterile aluminum template. Subsequently, the template is used to guide the cut of the collagen membrane Chondro-Gide (Geistlich Pharma AG) to exactly the shape of the lesion. Then, approximately 1 mL MFAT is deposited onto the collagen membrane rough surface (Fig 5). Allow for about 5 minutes for the collagen membrane to fully expand, and then the membrane is placed in the lesion bed with its rough surface in contact with the subchondral bone and its smooth surface facing the joint surface. The membrane is secured to the surrounding cartilage with 8 cardinal sutures using a No. 5-0 Vicryl thread (Ethicon), and then the space beneath the membrane is filled with MFAT until the membrane lies flat on the articular surface. Finally, fibrin glue is applied around the lesion, and complete drying is awaited. A final test of knee flexion-extension with the patella over the trochlea is performed to check for the stability of the membrane (Fig 6).
Fig 5.
On the auxiliary table, the prepared microfragmented adipose tissue is deposited on the rough surface of the collagen membrane.
Fig 6.
Front view of the right knee. The patella is retracted laterally, allowing proper exposure of the trochlea. In the center of the trochlea can be visualized the final aspect of the collagen membrane associated with the microfragmented adipose tissue. (P, patella; PT, patellar tendon; T: trochlea.)
Discussion
Symptomatic chondral lesions are difficult to treat due to the structural complexity of hyaline cartilage and its avascular nature, which impairs tissue repair processes. Over the past few decades, a variety of treatment options have been proposed, including scaffold techniques using porcine-derived collagen matrix combined with subchondral bone microfractures to stimulate bone marrow, inducing bleeding and the migration of mesenchymal progenitor cells to the lesion site.6 However, despite the favorable short-term outcomes, studies have shown cartilage deterioration 2 years after the microfracture procedure, with bone overgrowth and intralesional osteophyte formation in 60% of cases.15
To avoid subchondral bone violation and, consequently, intralesional osteophyte formation, other sources of progenitor cells have been investigated. MSCs, which can be obtained from various adult tissues (umbilical cord blood, periosteum, muscle, bone marrow, and adipose tissue), have demonstrated significant chondrogenic potential with promising clinical results.16, 17, 18 Due to their widespread availability, ease of acquisition, and minimally invasive nature, the use of adipose tissue as a source of MSCs has become increasingly popular.12 Therefore, this article presents an alternative surgical technique for the treatment of focal chondral lesions, especially in the trochlea or patella, which are difficult to treat, and the use of an adjuvant method may be advantageous.
As possible complications, extensive bruising and localized pain are common but typically have no significant long-term repercussions. During the adipose tissue graft collection process, care should be taken to keep the hand flat over the area to be harvested to prevent cannula superficializing and, consequently, aesthetic deformity at the site. Another crucial consideration is to accurately measure the dimensions of the lesion and ensure that the membrane is not too small, failing to adequately cover the entire lesion, or too large and redundant at the lesion’s edges, which could impair its fixation and patellar tracking. The pearls and pitfalls of this technique are described in Table 1, and the advantages and disadvantages are described in Table 2.
Table 1.
Pearls and Pitfalls of the Combined Porcine-Derived Collagen Membrane and Microfragmented Adipose Tissue Graft Procedure
| Pearls | Pitfalls |
|---|---|
| Keep the nondominant hand flat on the skin to prevent the cannula from becoming too superficial or too deep; the other hand performs back-and-forth movements. | While harvesting, avoid getting too close to the portal. This prevents the cannula from completely exiting the skin and losing negative pressure. |
| Pinch the skin to evaluate the amount of subcutaneous tissue harvested. | In the fan-shaped harvesting, collect more adipose tissue farther from your portal than closer. This avoids extra harvesting near the portal, avoiding cosmetic defects. |
Table 2.
Advantages and Disadvantages of the Combined Porcine-Derived Collagen Membrane and Microfragmented Adipose Tissue Graft Procedure
| Advantages | Disadvantages |
|---|---|
| Adipose tissue is easily available Several harvesting sites are possible: inner thigh, abdomen, flanks, axilla, thigh, and others |
Learning curve on adipose harvesting |
| Adipose mesenchymal cells are less likely to senescence in the older population compared to other mesenchymal stem cells | Elevated costs |
| Easy to use associated with collagen membrane | Increase of approximately 15 minutes in the surgical time of treatment with collagen membrane |
The rehabilitation protocol consists of a period of 6 weeks of partial weightbearing with crutches in full extension with a knee immobilizer (isometric exercises are allowed in this period). After 6 weeks, the knee immobilizer is discontinued, and partial weightbearing is maintained for up to 8 weeks. Passive range of motion in the continuous passive motion device is allowed from the second week after surgery, with progressive active range of motion being permitted from 6 weeks onward. Return to full sports activity is allowed 1 year after the procedure.
The use of collagen membrane combined with MFAT grafting is simple and reproducible, being a promising technique for the treatment of focal chondral defects of the knee. In case of failure, the technique does not preclude the possibility of performing osteochondral transplantation (autologous or allogeneic).
Disclosures
All authors (D.P.L., H.F., B.B.V., A.G.M.d.S., M.K.D., R.G.G., L.E.P.T.) declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Data
A stab incision is performed on the medial aspect of the right thigh. After infiltration with Klein’s solution, liposuction is performed with back-and-forward movements in a fan shape. Keep the nondominant hand flat on the skin. The harvested fat is introduced through a filter filled with saline solution. Gently shaken for about 30 seconds, waiting for adipose tissue to decant, the system is washed out to remove impurities. This procedure is carried out for approximately 3 cycles, as instructed by the device manual. Finally, microfragmented fat is obtained and extracted. Reserve carefully the microfragmented fat. The liposuction fat is transferred to the device with saline solution and shaken for about 30 seconds, waiting for it to decant and remove impurities. This procedure is carried out for approximately 3 cycles. Finally, the fat is captured as instructed by the device manual. Reserve carefully the microfragmented fat and perform an anterior midline skin incision and medial parapatellar approach to the knee. The following lesion is identified at the groove of the trochlea. With a 15-mm blade, outline the contours of the chondral lesion. With the aid of a hollow curette, the chondral lesion is debrided, taking care not to harm the subchondral bone. This is the final aspect of the chondral lesion after debridement. Using a metallic paper as a template, the dimensions of the chondral lesion are assessed, double checking after cutting the template is performed. The template is used to cut the collagen membrane to the correct dimensions of the lesion. Placing the collagen membrane between the template and a sheet of paper helps with stability for proper cutting of the membrane. This is the final appearance of the collagen membrane with microfragmented fat. Around 1 mm of microfragmented fat is deposited on the rough surface of the membrane, waiting for 5 minutes until it is completely expanded. The membrane is placed, with the rough side facing the lesion bed and the smooth side facing the articular surface. Eight cardinal stitches with Vicryl 6.0 (Ethicon) thread are used. The lesion bed is filled with microfragmented fat. Fibrin glue is used to completely seal the membrane. Wait for the glue to dry. Membrane stability is tested with flexion-extension movements of the knee with the patella over the trochlea. This is the final aspect of the lesion after the procedure.
References
- 1.Makris E.A., Gomoll A.H., Malizos K.N., Hu J.C., Athanasiou K.A. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeuken R.M., van Hugten P.P.W., Roth A.K., et al. A systematic review of focal cartilage defect treatments in middle-aged versus younger patients. Orthop J Sports Med. 2021;9 doi: 10.1177/23259671211031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magnussen R.A., Dunn W.R., Carey J.L., Spindler K.P. Treatment of focal articular cartilage defects in the knee: A systematic review. Clin Orthop Relat Res. 2008;466:952–962. doi: 10.1007/s11999-007-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraeutler M.J., Aliberti G.M., Scillia A.J., McCarty E.C., Mulcahey M.K. Microfracture versus drilling of articular cartilage defects: A systematic review of the basic science evidence. Orthop J Sports Med. 2020;8 doi: 10.1177/2325967120945313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niemeyer P., Albrecht D., Andereya S., et al. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: A guideline by the working group “Clinical Tissue Regeneration” of the German Society of Orthopaedics and Trauma (DGOU) Knee. 2016;23:426–435. doi: 10.1016/j.knee.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Gille J., Reiss E., Freitag M., Schagemann J., Steinwachs M., Piontek T. Autologous matrix-induced chondrogenesis for treatment of focal cartilage defects in the knee: A follow-up study. Orthop J Sports Med. 2021;9 doi: 10.1177/2325967120981872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcacci M., Berruto M., Brocchetta D., et al. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res. 2005;435:96–105. doi: 10.1097/01.blo.0000165737.87628.5b. [DOI] [PubMed] [Google Scholar]
- 8.Southworth T.M., Naveen N.B., Nwachukwu B.U., Cole B.J., Frank R.M. Orthobiologics for focal articular cartilage defects. Clin Sports Med. 2019;38:109–122. doi: 10.1016/j.csm.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Cotter E.J., Wang K.C., Yanke A.B., Chubinskaya S. Bone marrow aspirate concentrate for cartilage defects of the knee: From bench to bedside evidence. Cartilage. 2018;9:161–170. doi: 10.1177/1947603517741169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang Y., Li J., Wang Y., et al. Platelet rich plasma in the repair of articular cartilage injury: A narrative review. Cartilage. 2022;13 doi: 10.1177/19476035221118419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berrigan WA, Bailowitz Z, Park A, Reddy A, Liu R, Lansdown D. A greater platelet dose may yield better clinical outcomes for PRP in the treatment of knee osteoarthritis: A systematic review [published online March 19, 2024]. Arthroscopy. doi:10.1016/j.arthro.2024.03.018. [DOI] [PubMed]
- 12.Bosetti M., Borrone A., Follenzi A., Messaggio F., Tremolada C., Cannas M. Human lipoaspirate as autologous injectable active scaffold for one-step repair of cartilage defects. Cell Transplant. 2016;25:1043–1056. doi: 10.3727/096368915X689514. [DOI] [PubMed] [Google Scholar]
- 13.Hohmann E, Keough N, Frank RM, Rodeo S. Micro-fragmented adipose tissue demonstrates comparable clinical efficacy to other orthobiologics injections in treating symptomatic knee osteoarthritis. A systematic review of level I-IV clinical studies [published online March 11, 2024]. Arthroscopy. doi:10.1016/j.arthro.2024.03.002. [DOI] [PubMed]
- 14.Tremolada C., Ricordi C., Caplan A.I., Ventura C. Mesenchymal stem cells in Lipogems, a reverse story: From clinical practice to basic science. Methods Mol Biol. 2016;1416:109–122. doi: 10.1007/978-1-4939-3584-0_6. [DOI] [PubMed] [Google Scholar]
- 15.Krych A.J., Saris D.B.F., Stuart M.J., Hacken B. Cartilage injury in the knee: Assessment and treatment options. J Am Acad Orthop Surg. 2020;28:914–922. doi: 10.5435/JAAOS-D-20-00266. [DOI] [PubMed] [Google Scholar]
- 16.Freyria A.M., Mallein-Gerin F. Chondrocytes or adult stem cells for cartilage repair: The indisputable role of growth factors. Injury. 2012;43:259–265. doi: 10.1016/j.injury.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi Y., Sekiya I., Yagishita K., Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 18.Xue J.X., Gong Y.Y., Zhou G.D., Liu W., Cao Y., Zhang W.J. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells induced by acellular cartilage sheets. Biomaterials. 2012;33:5832–5840. doi: 10.1016/j.biomaterials.2012.04.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A stab incision is performed on the medial aspect of the right thigh. After infiltration with Klein’s solution, liposuction is performed with back-and-forward movements in a fan shape. Keep the nondominant hand flat on the skin. The harvested fat is introduced through a filter filled with saline solution. Gently shaken for about 30 seconds, waiting for adipose tissue to decant, the system is washed out to remove impurities. This procedure is carried out for approximately 3 cycles, as instructed by the device manual. Finally, microfragmented fat is obtained and extracted. Reserve carefully the microfragmented fat. The liposuction fat is transferred to the device with saline solution and shaken for about 30 seconds, waiting for it to decant and remove impurities. This procedure is carried out for approximately 3 cycles. Finally, the fat is captured as instructed by the device manual. Reserve carefully the microfragmented fat and perform an anterior midline skin incision and medial parapatellar approach to the knee. The following lesion is identified at the groove of the trochlea. With a 15-mm blade, outline the contours of the chondral lesion. With the aid of a hollow curette, the chondral lesion is debrided, taking care not to harm the subchondral bone. This is the final aspect of the chondral lesion after debridement. Using a metallic paper as a template, the dimensions of the chondral lesion are assessed, double checking after cutting the template is performed. The template is used to cut the collagen membrane to the correct dimensions of the lesion. Placing the collagen membrane between the template and a sheet of paper helps with stability for proper cutting of the membrane. This is the final appearance of the collagen membrane with microfragmented fat. Around 1 mm of microfragmented fat is deposited on the rough surface of the membrane, waiting for 5 minutes until it is completely expanded. The membrane is placed, with the rough side facing the lesion bed and the smooth side facing the articular surface. Eight cardinal stitches with Vicryl 6.0 (Ethicon) thread are used. The lesion bed is filled with microfragmented fat. Fibrin glue is used to completely seal the membrane. Wait for the glue to dry. Membrane stability is tested with flexion-extension movements of the knee with the patella over the trochlea. This is the final aspect of the lesion after the procedure.