Abstract
The Cryptococcus neoformans Ras1 protein serves as a central regulator for several signaling pathways. Ras1 controls the induction of the mating pheromone response cascade as well as a distinct signaling pathway that allows this pathogenic fungus to grow at human physiological temperature. To characterize elements of the Ras1-dependent high-temperature growth pathway, we performed a multicopy suppressor screen, identifying genes whose overexpression allows the ras1 mutant to grow at 37°C. Using this genetic technique, we identified a C. neoformans gene encoding a Rac homolog that suppresses multiple ras1 mutant phenotypes. Deletion of the RAC1 gene does not affect high-temperature growth. However, a rac1 mutant strain demonstrates a profound defect in haploid filamentation as well as attenuated mating. In a yeast two-hybrid assay, Rac1 physically interacts with the PAK kinase Ste20, which similarly regulates hyphal formation in this fungus. Similar to Rac1, overexpression of the STE20α gene also restores high-temperature growth to the ras1 mutant. These results support a model in which the small G protein Rac1 acts downstream of Ras proteins and coordinately with Ste20 to control high-temperature growth and cellular differentiation in this human fungal pathogen.
Ras proteins are highly conserved, small guanine-nucleotide binding proteins that control the activation of diverse signaling pathways. Among microorganisms, Ras proteins regulate fundamental and varied cellular processes such as morphological transitions, mating, cyclic AMP (cAMP) metabolism, and microbial pathogenesis (1, 12, 25, 40). In mammalian systems, Ras mutations are associated with a large percentage of malignancies (4), confirming the central roles that Ras proteins play in cellular differentiation.
Cryptococcus neoformans is an important opportunistic fungal pathogen in humans, most frequently causing disease in the lungs and central nervous systems of immunocompromised patients (29). This organism typically grows as a haploid yeast, but it can undergo hyphal differentiation in response to environmental stresses or when confronted with an appropriate mating partner (22, 46). Such differentiation events likely allow survival in harsh environmental conditions. Also, the spores produced during hyphal phases of growth may be the most important infectious propagule of this fungus (39).
Ras proteins are required for C. neoformans hyphal development. Activation of Ras signaling in C. neoformans results in an accelerated hyphal response. Additionally, strains with null mutations of the RAS1 gene are sterile, failing to undergo a hyphal transition when coincubated with a mating partner (1). The mating defects of the ras1 mutant are multifactorial. First, ras1 mutant strains fail to appropriately activate the components of the pheromone response pathway when incubated with a mating partner (44). Additionally, the ras1 mutants demonstrate a primary defect in filamentation, unable to form hyphae even when the pheromone response pathway is genetically activated (44). The morphological defects associated with a ras1 mutation are also evident in this strain's inability to undergo haploid fruiting, a form of hyphal differentiation distinct from mating that occurs in response to starvation and desiccation (1, 44).
The C. neoformans Ras1 signaling pathway also controls the ability of this fungus to grow at 37°C. Strains with ras1 mutations are unable to grow at mammalian body temperatures and are accordingly avirulent in animal models of cryptococcosis (1, 43, 44). Additionally, the ras1 mutant strain demonstrates alterations in actin polarization at these higher temperatures; when incubated at 37°C, this strain arrests as large, unbudded cells with depolarized actin (43).
Similar temperature-dependent alterations of growth and actin polarization have also been observed in Saccharomyces cerevisiae ras2 mutants (15). At 35°C, these mutant yeast strains demonstrate temperature-dependent morphological changes as well as altered cellular localization of the polarity-directing proteins Myo2p and Cdc42p. Although Ras proteins are major activators of the cAMP pathway in yeast, activating cAMP/PKA signaling did not complement the ras2 mutant defects in actin polarization and high-temperature growth. These data suggest a cAMP-independent pathway by which Ras proteins control fungal cytoskeletal integrity and growth at elevated temperatures (15).
In both S. cerevisiae and C. neoformans, the cAMP-independent targets of Ras proteins that control these cellular processes are poorly defined. The recent availability of a completed and annotated genome for C. neoformans, as well as the increased molecular pliability of this pathogenic microorganism, allowed us to pursue multicopy suppressor analysis to identify downstream effectors of Ras1 involved in fungal growth at 37°C and cellular differentiation.
MATERIALS AND METHODS
Strains and media.
The strains used in this study are listed in Table 1 and are derived from the C. neoformans var. neoformans (serotype D) congenic mating pair JEC20 and JEC21. The MATa rac1 mutant (CBN1) was isolated by spore micromanipulation from a cross between MVC47 and JEC20. Standard yeast media was prepared as previously described (38). 5-Fluoroorotic acid (5-FOA) was added to media (1 g/liter) as indicated. V8 medium was used for all mating experiments (23). Filament agar was prepared as described previously (46). When indicated, 3-amino-1,2,4-triazole (3AT) was added to media at a concentration of 5 mM.
TABLE 1.
Strain list
| Strain | Genotype | Reference |
|---|---|---|
| JEC20 | MATa, congenic with JEC21 | 24 |
| JEC21 | MATα, congenic with JEC20 | 24 |
| JEC30 | MATa lys1 | 32 |
| JEC43 | MATα ura5 | 45 |
| JEC53 | MATa ura5 lys1 | 32 |
| MWC1 | MATα ade2 ura5 ras1::ADE2 | 44 |
| MWC8 | MATα ade2 ras1::ADE2 | 44 |
| MVC47 | MATα ura5 rac1::URA5 | This study |
| MVC48 | MATα ura5 rac1::URA5 RAC1-nat1 | This study |
| MVC39 | MATα rac1::URA5, 5-FOA resistant | This study |
| CBN1 | MATa rac1::URA5 | This study |
| CBN2 | MATα ade2 ura5 ras1::ADE2 + pCN3 (pGPD-RAC1 URA5) | This study |
| CBN3 | MATα ade2 ura5 ras1::ADE2 + pRCD83 (pGPD-URA5) | This study |
| CBN4 | MATa rac1::URA5 lys1, 5-FOA resistant | This study |
| CBN5 | MATa rac1::URA5 lys1 | This study |
| CBN6 | MATα ade2 ura5 ras1::ADE2 + pCBN19 (pGPD-STE20αURA5) | This study |
| CBN7 | MATαade2 ura5 ste20α::ADE2 rac1::URA5 | This study |
| CBN8 | MATa ade2 ura5 ste20a::ADE2 rac1::URA5 | This study |
| CSB15 | MATα ura5 ste20α::URA5 | 42 |
| CSB5 | MATa ade2 ste20a::ADE2 | 42 |
Multicopy suppressor library.
The multicopy suppressor library was made as previously described (6, 11). Briefly, genomic DNA from the serotype D C. neoformans strain C21F2 (11) was partially digested with Sau3A, and genomic fragments ranging from 6 to 12 kb were cloned into the BamHI-digested pPM8 vector. pPM8 is maintained as an episome in multiple copies in each cell (30), providing functional overexpression of genes cloned into this vector. The size-selected genomic library was linearized by I-SceI digestion and transformed by electroporation into the ras1 ura5 mutant strain MWC1 (44). Transformants were initially selected on synthetic medium lacking uracil to maintain the episomal plasmid. Total DNA was recovered from transformants that were able to grow at 37°C, and the insert in the associated episome was PCR amplified using primers 5050 and 5051 that recognize pPM8 vector sequences flanking the BamHI restriction site (6).
Overexpression of RAC1.
The RAC1 gene was amplified by PCR using primers AAO489 (5′-CTGAGGATCCATGGCTACTACTAGAAACATC-3′, BamHI sequence underlined) and AAO572 (5′-GCAGGATCCGCTTGATGGCCTGGCTAATTCC-3′) that added BamHI sites onto the ends of the sequence. The gene was subsequently cloned into the BamHI-digested pRCD83 vector, which contains the GPD promoter and the URA5 selectable marker (43). This created plasmid pCN3 in which the RAC1 gene is under transcriptional control of a highly active and constitutive promoter. Plasmid pCN3 was biolistically transformed into the ras1 ura5 mutant strain MWC1 as previously described (8, 41), and transformants were selected on synthetic medium lacking uracil. One transformant, CBN2 (ras1+pGPD-RAC1), was chosen for further analysis. Stable genomic integration of plasmid pCN3 was documented by incubating the transformants for multiple generations in nonselective yeast-peptone-dextrose (YPD) medium, followed by incubation on selective medium lacking uracil. The ras1 mutant was similarly transformed with the empty pRCD83 plasmid as a control strain (CBN3).
Disruption of the RAC1 gene.
A rac1::URA5 mutant allele was constructed by PCR overlap extension as previously described (7), replacing the entire RAC1 coding region with the URA5 selectable marker. This allele was transformed into the MATα ura5 strain JEC43 by biolistic transformation (8). Transformants were selected on synthetic medium lacking uracil. Mutation of the RAC1 locus was confirmed by PCR and Southern hybridization (see Results).
Fusion and recombination assays.
Fusion assays were performed as described previously (1) with the following modifications. Mixtures of wild-type (JEC43 × JEC30) and rac1 (MVC39 × CBN5) mutant cells were cocultured on V8 mating medium. Equal numbers of cells were used for each cross. Each mating partner contained a complementing auxotrophic mutation (lys1 and ura5) to allow selection of prototrophic fusion products. After 48 h, the mating mixes were excised, resuspended in 1 ml of H2O, and plated in serial dilutions onto minimal medium. Colonies were counted after 3 days of incubation.
Recombinant spore production was assessed in a quantitative mating assay as described previously (1) with the following modifications. Bilateral crosses consisting of the wild-type (JEC21 × JEC53) and rac1 (MVC47 × CBN5) mutant strains containing equal numbers of cells were cocultured on V8 medium. After 7 days the mating patches were excised, resuspended in 1 ml of H2O, and plated in serial dilutions onto synthetic medium lacking lysine and containing 5-FOA. Strains were genetically marked so that only recombinant colonies grew on the selective medium. Colonies were counted after 3 days of incubation.
Microscopy.
Bright-field, differential interference microscopy (DIC), and fluorescent images were captured with a Zeiss Axioskop 2 Plus fluorescent microscope equipped with an AxioCam MRM digital camera. Additional bright-field images were captured with a Nikon Eclipse E400 microscope equipped with a Nikon Coolpix 4500 digital camera.
For filament staining, slide mounts were prepared as previously described (33). Briefly, wild-type and rac1 crosses were prepared on V8 agar-coated slides and incubated for 3 days prior to processing. To visualize septa and DNA, the slide mounts were fixed in 70% ethanol, washed, and incubated in a 10-ml preparation of calcofluor (fluorescent brightener 28 F-3397; Sigma) at 40 μg/ml and a 5,000× dilution of a 5 mM solution of Sytox Green (Molecular Probes) in phosphate-buffered saline for 20 min. Agar sections containing filaments were excised from each slide and mounted on a fresh microscope slide with 5 μl of antifade (1 mg/ml p-phenylenediamine in 50% glycerol). To visualize actin and DNA, similar slide mounts were fixed in 10% formaldehyde and incubated in a 10-ml preparation of rhodamine-conjugated phalloidin (P-195; Sigma) at 10 μg/ml. Agar sections were mounted on fresh microscope slides with 5 μl antifade mix containing 8 μg/ml DAPI (4′,6′-diamidino-2-phenylindole) (D-21490; Molecular Probes).
Yeast two-hybrid experiments.
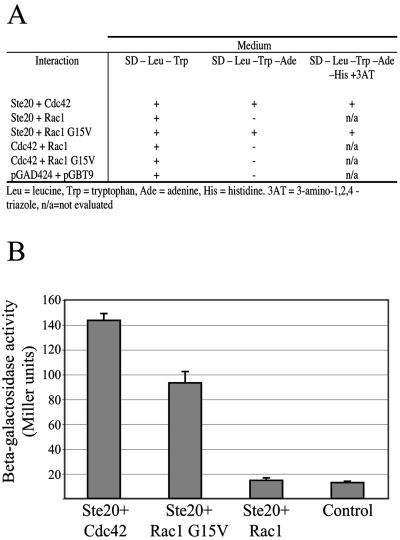
This yeast two-hybrid technique was used to explore physical interactions between C. neoformans proteins. C. neoformans cDNA sequences were PCR amplified and cloned into the yeast two-hybrid vectors pGAD424 and pGBT9 (Clontech). These plasmids were cotransformed into the S. cerevisiae strain PJ69-4A, and transformants were selected on synthetic medium lacking leucine and tryptophan to confirm the presence of both plasmids. Physical interaction between proteins encoded in these plasmids was tested by assessing restoration of adenine prototrophy, restoration of histidine prototrophy, and increased β-galactosidase activity. Yeast transformations and determination of β-galactosidase activity were performed as previously described (5).
Molecular methods.
Genomic DNA was isolated from lyophilized C. neoformans cells (36). For genomic Southern hybridizations, 20 μg of genomic DNA was digested with PvuI; electrophoresis, DNA transfer, hybridization, and autoradiography were performed as previously described (37). The cDNA sequence of the RAC1 gene was determined using the Marathon cDNA amplification kit (Clontech). All PCRs were performed using a Techne Genius thermocycler with 50 ng of template DNA, 100 ng of each oligonucleotide primer, and standard reagents from a TaKaRa kit (Takara Shuzo Co.). The PCR conditions were 95°C for 5 min followed by 35 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 1 min for each kb of DNA amplified in the reaction.
Nucleotide sequence accession number.
The RAC1 gene sequence has been submitted to the NCBI database under accession number AY780547.
RESULTS
A Rac homolog suppresses the ras1 mutant high-temperature growth defect.
To identify downstream effectors of the Ras1 signaling pathway, we pursued a multicopy suppressor strategy to define those genes whose overexpression restored growth at 37°C to the ras1 mutant strain. Ten thousand transformants in the MWC1 strain background were initially analyzed, representing approximately 4 to 5× genome equivalents, given an estimated genome size of 20 Mb (28).
In this initial screen, four transformants were isolated with a restored ability to grow at 37°C. When the strains were cured of the URA5-containing plasmid on a medium containing 5-FOA, they were no longer able to grow at this elevated temperature. Three of the plasmids contained overlapping C. neoformans genomic fragments, with 7,729 nucleotides in common. This genomic sequence contained two putative genes: a theoretical gene of unknown function and a gene encoding a Rac protein homolog. Rac proteins are highly conserved, Ras-like G proteins initially identified as substrates of C-botulinum toxin (9). In mammalian systems, Rac proteins are involved in maintaining the assembly and organization of the actin cytoskeleton (13, 34).
The C. neoformans RAC1 gene contains eight introns within the coding sequence and one intron upstream of the start codon, encoding a predicted protein of 198 amino acids. Similar to many of the proteins in the Ras superfamily, the Rac1 peptide sequence has a C-terminal CAAX motif (Cys-Leu-Val-Met), suggesting that this protein is prenylated and thereby associated with the plasma membrane (10).
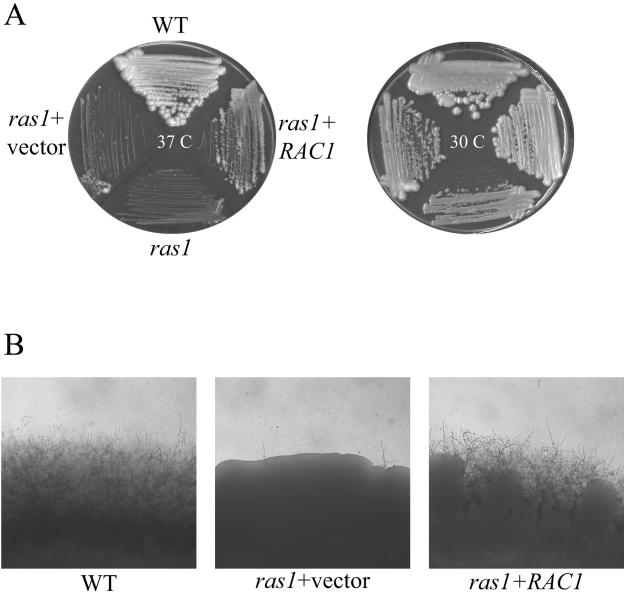
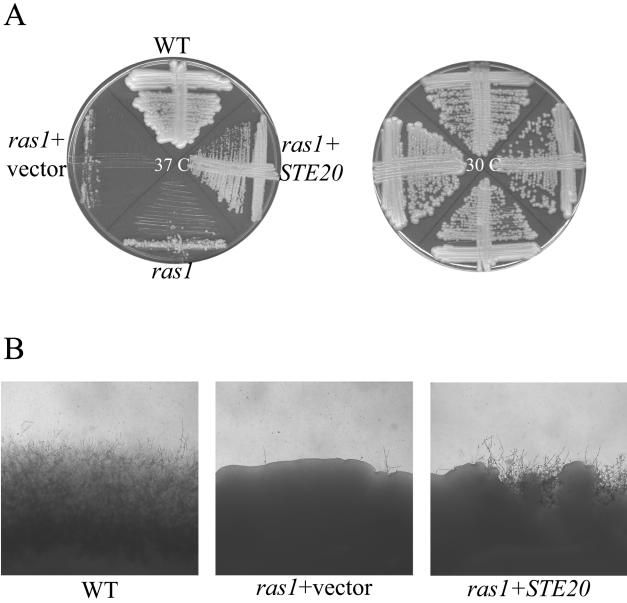
In order to confirm that overexpression of the RAC1 gene was responsible for the suppression of ras1 mutant phenotypes, C. neoformans RAC1 was subcloned under control of the constitutive GPD promoter and retransformed into the ras1 mutant strain. The ras1+pGPD-RAC1 strain (CBN2) grew well at 25°C and 30°C and displayed no altered colony or cellular morphology on YPD medium. However, in contrast to the ras1 mutant strain, CBN2 was able to grow at 37°C (Fig. 1). Therefore, in accordance with the suppressor screen, specific overexpression of the RAC1 gene suppresses the ras1 high-temperature growth defect.
FIG. 1.
RAC1 overexpression complements the ras1 mutant high-temperature growth and mating defects. (A) The wild-type (WT) strain (JEC21), ras1 mutant (MWC8), ras1 strain containing the empty pRCD83 plasmid (ras1+vector), and ras1 mutant overexpressing the RAC1 gene (ras1+RAC1) were incubated on YPD medium at 30°C or 37°C for 48 h. (B) The wild-type strain (JEC21), ras1 strain containing the empty pRCD83 plasmid (ras1+vector), and ras1 mutant overexpressing the RAC1 gene (ras1+RAC1) were coincubated with the MATa strain JEC20 in mating reactions on V8 medium. After 7 days of incubation, the edges of the mating patches were assessed for mating filamentation and photographed (40×).
RAC1 overexpression complements ras1 mutant hyphal defects.
When confronted with a mating partner, C. neoformans cells initiate a mating process involving cell fusion and hyphal differentiation in response to pheromone and nutrient signals. In the absence of a mating partner, this fungus can undergo a similar yeast-to-hyphal transition in response to extreme nutrient deprivation and desiccation, a process known as haploid fruiting, which is characterized by the formation of hyphae with unfused clamp connections, basidia, and mitotic spores (46). The ras1 mutant strain displays striking defects in both of these types of hyphal differentiation (1, 44).
Overexpression of the RAC1 gene suppresses the ras1 mutant mating defect. Microscopic inspection of JEC20 (MATa wild type) × CBN2 (MATα ras1+pGPD-RAC1) crosses revealed that mating filamentation was restored to wild-type levels (Fig. 1). These hyphae contained all of the characteristic structures of wild-type C. neoformans mating reactions, including fused clamp connections, basidia, and basidiospores. Thus, in addition to restoring growth at 37°C, RAC1 overexpression suppresses the mating defect of the ras1 mutant. These data support a model in which Rac1 functions downstream of Ras1 in the C. neoformans mating process.
Deletion of the RAC1 gene results in altered hyphal differentiation.
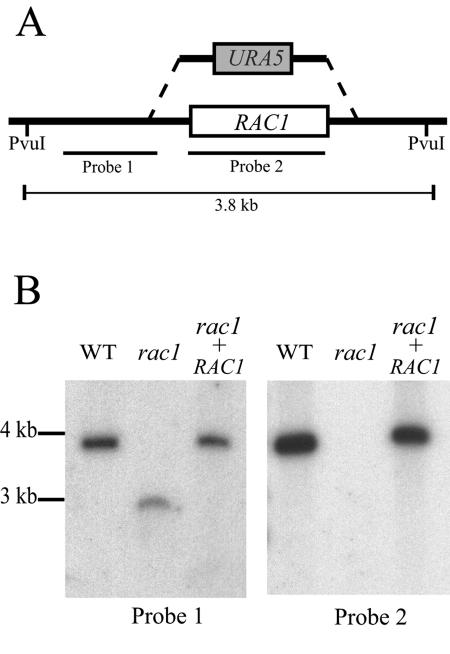
To further investigate the biological roles of the Rac1 protein, we generated a strain with a rac1 null allele (Fig. 2). By using biolistic transformation, we replaced the native RAC1 locus with a rac1::URA5 mutant allele. Of 40 transformants, 24 were rac1 mutants. Each of these demonstrated identical phenotypes, and one of these strains (MVC47) was selected as a representative rac1 mutant for subsequent experiments.
FIG. 2.
Southern hybridization confirms RAC1 gene deletion. (A) A rac1::URA5 mutant allele was made by replacing the entire RAC1 coding sequence with the URA5 selectable marker. (B) Genomic DNA was obtained from the wild-type (WT) (JEC43), rac1 mutant (MVC47), and rac1+RAC1 reconstituted strain (MVC48), digested with PvuI, and analyzed by Southern hybridization using two probes as indicated in panel A.
In contrast to the ras1 mutant strain, the rac1 mutant demonstrated no temperature-sensitive growth defects at 30°C, 37°C, or 39°C. Also, the rac1 mutant strain's cellular morphology was similar to that of the wild-type strain at all temperatures tested. The rac1 mutant was able to grow well on minimal media, indicating no auxotrophies associated with the gene mutation.
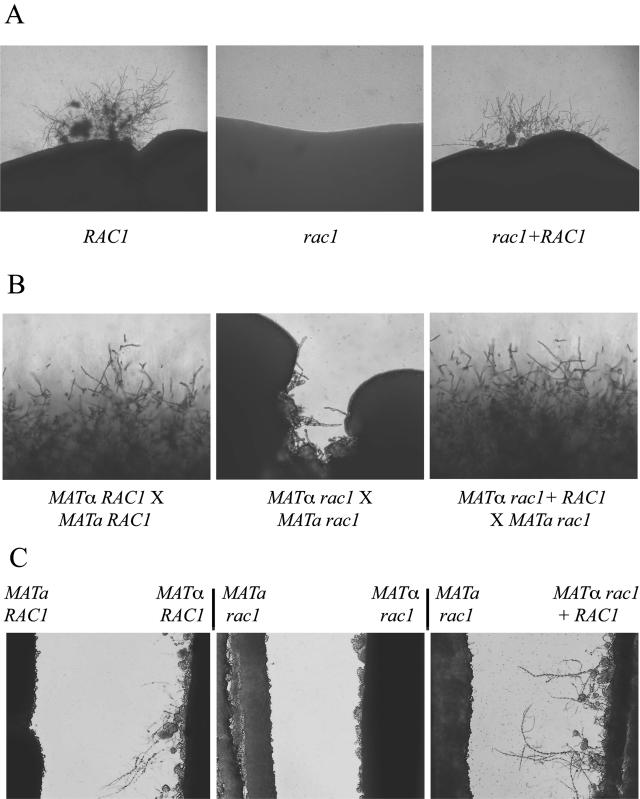
However, mutation of the rac1 gene was associated with a dramatic defect in hyphal differentiation. When incubated on the nutrient-poor filament agar, C. neoformans strains typically undergo a form of hyphal differentiation known as haploid fruiting (46). After 3 weeks of incubation on filament agar, no haploid fruiting was observed among the rac1 mutants. When incubated under identical conditions, wild-type strains displayed a consistent and reproducible hyphal transition (Fig. 3A). To ensure that the hyphal defect was due to the rac1 mutation, a wild-type copy of the RAC1 gene was ectopically introduced into the rac1 mutant to create a rac1+RAC1 reconstituted strain (MVC48). This strain underwent haploid fruiting in a manner indistinguishable from the wild type (Fig. 3A).
FIG. 3.
Rac1 is required for hyphal differentiation. (A) Haploid fruiting. The RAC1 wild-type, rac1 mutant, and rac1+RAC1 reconstituted strains were incubated for 3 weeks on filament agar. The edges of the culture patches were photographed to assess for filamentation due to haploid fruiting (40×). (B) Mating. The effect of a rac1 mutation on the mating process was assessed by coculturing MATα and MATa strains on V8 mating medium. Each strain possessed either a wild-type (RAC1) or a mutant (rac1) allele. The edges of the mating patches were assessed at 7 days for mating filamentation (100×). (C) Pheromone-induced hyphal formation. MATa and MATα strains were streaked on filament agar in close proximity to one another without physically touching. The cellular response to diffusible pheromone was assessed in this confrontation assay at 48 h (100×).
In addition to reduced haploid fruiting, mutation of the RAC1 gene also resulted in defective mating. Mating reactions in which MATα or MATa rac1 mutants were crossed with a wild-type strain demonstrated absolutely normal hyphal mating structures and basidiospores. However, bilateral mutant crosses, in which both partners were rac1 mutants, displayed markedly reduced mating filamentation (Fig. 3B). Mating was restored to wild-type levels in these crosses by reintroducing a wild-type RAC1 allele into either one of the rac1 mutant mating partners (Fig. 3B). These observations indicate that Rac1 is involved in the C. neoformans mating process and that the requirement for Rac1 activity is dose dependent; one copy of the RAC1 gene is sufficient for mating. These data contrast with the mating defects observed in the ras1 mutant strains in which a ras1 mutation in either of the parental strains results in profound reductions in mating (44).
The rac1 mutation affects mating at several steps by altering hyphal development. (i) Pheromone production and response.
To determine the precise cellular mechanisms by which Rac1 influences mating, we divided the C. neoformans mating process into distinct steps. The first step involves the production of mating pheromone in response to nutrient deprivation and to a mating partner. This process was assessed using a confrontation assay, the microscopic analysis of the initial cellular response of MATa and MATα cells to strains of the opposite mating type (44). In response to an appropriate mating partner, MATα cells produce hyphal structures known as conjugation tubes. Unlike MATα cells, MATa cells produce few hyphae in confrontation assays, but they undergo a distinct morphological change in which they become larger and more refractile (44).
In contrast to wild-type MATα cells, MATα rac1 mutant cells did not produce conjugation tubes in response to either MATa wild-type or rac1 strains (Fig. 3C and data not shown). However, MATα rac1 cells were able to induce cellular changes in MATa cells in confrontation, suggesting that this rac1 mutant produces α-pheromone. In contrast, the MATa rac1 mutant strain responded normally to either MATα wild-type or MATα rac1 mutant cells (Fig. 3C). These observations indicate that Rac1 does not regulate the production of pheromone in either MATα or MATa cells; however, Rac1 is required for the initial hyphal response of MATα cells to the MATa pheromone.
(ii) Fusion.
After the initial pheromone response, MATα and MATa cells fuse to form a dikaryon. We hypothesized that the MATα rac1 mutant defect in the morphological response to pheromone would influence its ability to find and fuse with its mating partner. To determine if rac1 mutants have a defect in the cell fusion process, rac1 auxotrophic strains were generated to allow for the selection of prototrophic isolates formed by cell fusion. Crosses were incubated on mating medium for 48 h prior to plating onto selective medium. In this assay, the wild-type and the rac1 crosses produced equal numbers of prototrophic isolates, indicating that Rac1 is not required for cell fusion. However, the overall colony size of these diploid rac1 isolates was reduced compared to those resulting from wild-type crosses. Therefore, Rac1 is not required for the vegetative growth of haploid C. neoformans yeast cells, but it may be required for the growth of the transient diploid phase. The normal cell fusion observed among rac1 mutants contrasts with ras1 mutant strains, which display a marked defect in cell fusion, even in unilateral crosses (1).
(iii) Dikaryotic hyphal development.
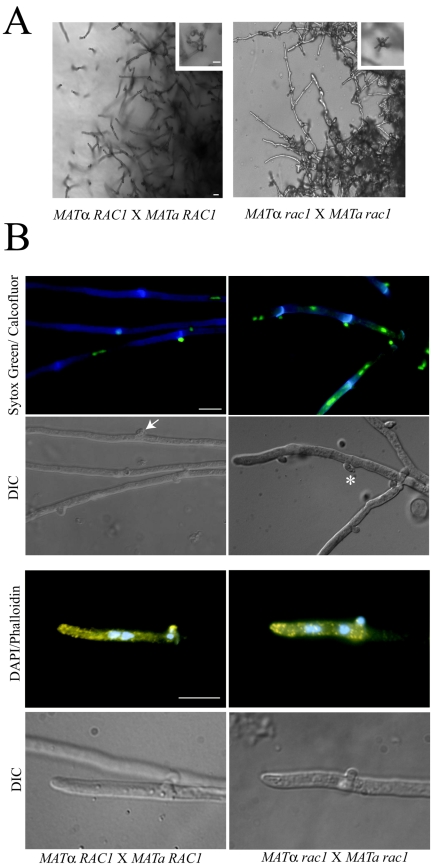
After mating cell fusion, the dikaryotic cells undergo dramatic hyphal differentiation. Wild-type C. neoformans mating filaments consist of a series of hyphal cells separated by septa and containing fused clamp cells. These hyphal structures are markedly reduced in bilateral rac1 mutant crosses. Additionally, the few filaments that are produced in these crosses exhibit morphological defects. Initial microscopic observation of the filaments resulting from a bilateral rac1 cross demonstrated that the hyphal cells are shorter and thicker than those resulting from a wild-type cross (Fig. 4A). The filaments from the bilateral rac1 cross also contained more protrusions than wild-type filaments (Fig. 4A).
FIG.4.
Altered morphology of the rac1 mutant mating hyphae. (A) Wild-type and bilateral rac1 crosses were incubated on V8 mating medium for 7 days. Areas at the edge of the mating reaction containing filaments were photographed at 200× magnification with DIC optics to visualize filaments, basidia (inset), and basidiospores (inset). Bar, 10 μm. (B) Bilateral wild-type and rac1 crosses were prepared on V8 agar-coated glass slide mounts. After incubation, filaments were fixed and costained with calcofluor and Sytox Green to visualize septa, clamp cell junctions, and DNA (first panel). Filaments and clamp cells were visualized using DIC optics (second panel). Wild-type clamp cells are fused (arrow) while rac1 clamp cells are unfused (*). Wild-type and bilateral rac1 filaments were also costained with rhodamine-conjugated phalloidin and DAPI to visualize actin and DNA (third panel). The fourth panel depicts the corresponding DIC images. Magnification, 1,000×. Bar, 10 μm.
To further assess the morphological defect of rac1 filaments, wild-type and rac1 filament samples were costained with Sytox Green and calcofluor to visualize DNA and septa, respectively. C. neoformans mating filaments are dikaryotic (two nuclei per hyphal cell). Additionally, the associated clamp cell functions in mitosis to ensure that each resulting hyphal cell receives two nuclei: one MATα nucleus and one MATa nucleus. Sytox Green staining demonstrated that, for the most part, rac1 mating filament cells contained two nuclei (Fig. 4B, first panel). Calcofluor staining and DIC microscopy revealed that the clamp cells were abnormal in shape and did not appropriately fuse with the subapical filament cell (Fig. 4B, first and second panels).
In wild-type mating filaments, polarized (new growth) occurs primarily at the tip of the leading cell and clamp cell. However, new growth can also occur from subapical clamp cells to generate secondary filaments or branches (21). Polarized growth is characterized by the accumulation of cortical actin patches. In C. neoformans, cortical actin patches localize throughout the filament but concentrate at the growing tip of the filament and at the tips of clamp cells (33). To determine if actin polarization is abnormal in the rac1 filaments, wild-type and rac1 mating hyphae were visualized with rhodamine-conjugated phalloidin. In both wild-type and rac1 filaments, actin patches concentrated in areas of polarized growth (Fig. 4B, third panel). Thus, the defects in clamp cell morphogenesis observed in rac1 mating filaments are not due to improper actin localization.
(iv) Basidium formation, meiosis, and sporulation.
The tips of maturing mating hyphae differentiate into flask-shaped basidia, the site of meiosis and spore formation. Microscopic examination revealed that the abnormal rac1 mating filaments produced wild-type-appearing basidia and basidiospores, suggesting that the predominant defect in the rac1 mating process is in hyphal differentiation (Fig. 4A, insets).
To confirm that the basidiospores produced by rac1 bilateral crosses were viable, we measured the production of recombinant meiotic progeny in rac1 bilateral crosses (1). Wild-type and rac1 bilateral crosses were incubated for 7 days on mating medium, excised, and plated onto selective medium. Each strain was genetically marked to allow for the selection of recombinant progeny. We were able to recover recombinant progeny from both the wild-type and from the bilateral rac1 crosses. Therefore, although the overall production of rac1 filaments was reduced and the filaments exhibited morphological abnormalities, the Rac1 protein is not required for a complete sexual cycle in C. neoformans. These results contrast greatly to crosses involving ras1 mutants, which result in profound decreases in mating competence and recombinant spore production (44). Ras1 is required both for mating pheromone production and for hyphal differentiation. In contrast, the Rac1 protein plays its major role in the C. neoformans mating process by influencing hyphal formation but not in regulating pheromone activity.
Rac1 interacts with the Ste20 PAK kinase in the yeast two-hybrid system.
PAK kinases are signaling molecules that often function downstream of Ras proteins and Rho-like GTPases. In C. neoformans, three PAK kinases have been identified and play distinct roles in mating and cellular differentiation (33). The genes encoding two of these proteins, Ste20α and Ste20a, are present in the mating type loci of MATα and MATa strains, respectively. The Ste20 proteins play an important role in haploid fruiting and mating. Additionally, these PAK kinases are required for high-temperature growth and for full virulence of serotype A strains of C. neoformans (42). These proteins also contain an N-terminal consensus motif for the binding of Rac and Cdc42 proteins. This CRIB (Cdc42/Rac-interacting and binding) domain exerts an autoinhibitory effect on kinase activity, which is released by the binding of the Rac or Cdc42 proteins (3).
To determine whether Rac1 binds Ste20α in C. neoformans, we cloned the cDNAs corresponding to these and other related signaling molecules into the yeast two-hybrid vectors pGAD424 and pGBT9 (Clontech). Yeast strains cotransformed with C. neoformans CDC42 and STE20α demonstrated physical interaction between these two signaling molecules by restoration of adenine and histidine prototrophy and by induction of β-galactosidase activity, as previously described (42). C. neoformans Rac1 and Ste20α demonstrated no evidence of physical interaction when the wild-type alleles were tested. However, the introduction of a dominant activating mutation into the RAC1 gene resulted in physical interaction between the Rac1-G15V mutant protein and Ste20α, as assessed by this technique (Fig. 5). This result is consistent with a similar observation in the basidiomycete fungus Ustilago maydis, in which a dominant active Rac protein demonstrated evidence of interaction with PAK kinases in a two-hybrid assay (26). The finding that Ste20α interacts with a dominant active form of Rac1 and not the native protein suggests two things. First, the Ste20α PAK kinase acts directly downstream of Rac1 in a signal transduction pathway. Second, these proteins likely only interact when Rac1 is activated by upstream signals.
FIG. 5.
Physical interaction of Rac1 and Ste20α assessed using the yeast two-hybrid system. (A) The cDNA sequences of the C. neoformans STE20α, RAC1, CDC42, and RAC1-G15V alleles were cloned into the two-hybrid vectors pGBT9 and pGAD424 and cotransformed in pair-wise combinations into the yeast strain PJ69-4A. One isolate transformed with the empty vectors was also tested as a control. Transformants were selected on synthetic medium lacking tryptophan and leucine (SD−Leu−Trp). These strains were assessed for growth on synthetic medium lacking tryptophan, leucine, and adenine (SD−Leu−Trp−Ade) and on synthetic medium lacking tryptophan, leucine, adenine, and histidine and containing 5 mM 3AT (SD−Leu−Trp−Ade−His+3AT). β-Galactosidase activity of four of these transformants was determined and expressed in Miller units (B). Data represent the means of triplicate samples; error bars indicate standard deviations.
Genetic interactions between RAC1 and STE20.
Similar to Rac1, the Ste20 PAK kinase is required for proper filament morphogenesis during C. neoformans mating. Ste20 is required to maintain polarity during hyphal development, and dikaryotic filaments lacking Ste20 become highly branched due to a tip-splitting defect (33).
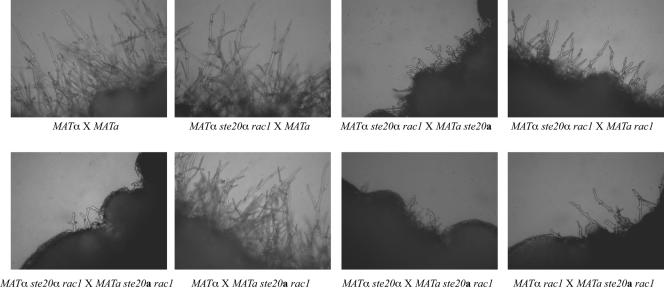
To further analyze the genetic relationship between STE20 and RAC1, we examined the morphology of filaments produced from crosses of strains with mutations in one or both of these genes. Similar to unilateral rac1 crosses, ste20 mutants crossed with wild-type strains produce mating hyphae with wild-type morphology. However, due to a defect in maintaining tip polarity, the filaments produced in bilateral ste20 crosses are short and highly branched (33). The mating hyphae in crosses between two rac1 mutant strains are also shorter, thicker, and more highly branched than those of wild-type strains. However, they do not exhibit the tip-splitting defect of the ste20 mutant hyphae. Instead, the additional branches and protrusions of the rac1 hyphae appear to be the result of deformed clamp cells (Fig. 4).
A single copy of STE20 and RAC1 among either of the crossed strains is sufficient to support wild-type mating. Mating reactions involving MATα ste20α × MATa rac1, MATα ste20α rac1 × MATa, and MATa ste20a rac1 × MATα produced completely wild-type mating filaments (Fig. 6 and data not shown). Moreover, the filaments produced in a MATα ste20α rac1 × MATa rac1 cross exhibit a rac1 phenotype, with reduced numbers of hyphae that were shorter and thicker than those of the wild type. Therefore, a single copy of the STE20 gene in one of the mating partners was able to provide adequate Ste20 protein function for its role in mating hyphal development. Similarly, the filaments produced by a MATα ste20α rac1 × MATa ste20a cross exhibit a ste20 phenotype, with short and highly branched hyphae (Fig. 6). When both mating partners were rac1 ste20 double mutants, the resulting hyphae were indistinguishable from those produced in a bilateral ste20 mutant cross (Fig. 6). Therefore, the ste20 mutation is epistatic to rac1 in mating filament morphogenesis.
FIG. 6.
Combined effect of ste20α and rac1 mutations on mating. MATa and MATα strains with the indicated genotypes were coincubated on V8 mating medium for 7 days. The edges of the mating patches were assessed for mating filamentation and photographed (67×).
Overexpression of the STE20α gene suppresses the ras1, but not the rac1, mutant phenotypes.
STE20 overexpression did not suppress the mutant mating defect of the rac1 mutant. Plasmid pCBN19, which contains the STE20α gene under control of the constitutive GPD promoter and the URA5 selectable marker (33), was transformed into a MATα rac1 ura5 mutant strain. When several independent transformants were crossed with a MATa rac1 strain, the resulting mating hyphal morphology was identical to that from bilateral rac1 mutant crosses. Similarly, introduction of the pGPD-RAC1 overexpression transgene (pCN3) into a ste20 mutant strain failed to restore normal mating morphology in a ste20 bilateral cross.
In contrast, overexpression of the STE20α gene suppressed multiple ras1 mutant phenotypes. Plasmid pCBN19 (33) was transformed with the ras1 ura5 mutant strain MWC1. Unlike ras1 strains transformed with empty vector, the ras1 strains overexpressing STE20α grew well at 37°C (Fig. 7). Similarly, STE20α overexpression suppressed the ras1 mutant mating defect (Fig. 7).
FIG. 7.
STE20α overexpression complements the ras1 mutant high-temperature growth and mating defects. (A) The wild-type (WT) strain (JEC21), ras1 mutant (MWC8), ras1 strain containing the empty pRCD83 plasmid (ras1+vector), and ras1 mutant overexpressing the STE20α gene (ras1+ STE20α) were incubated on YPD medium at 30°C or 37°C for 48 h. (B) The wild-type strain (JEC21), ras1 strain containing the empty pRCD83 plasmid (ras1+vector), and ras1 mutant overexpressing the STE20α gene (ras1+STE20α) were coincubated with the MATa strain JEC20 in mating reactions on V8 medium. After 7 days of incubation, the edges of the mating patches were assessed for mating filamentation and photographed (40×).
DISCUSSION
In mammalian cells, the Rho family of G proteins controls the organization of the actin cytoskeleton (14). For example, Rho, Rac, and Cdc42 proteins play complementary roles in adhesion to extracellular surfaces and in cellular migration. Rac proteins, in particular, regulate the polymerization of actin at the cell periphery, which is important for mammalian cell protrusion and migration (14).
Proteins that control mammalian cell morphology, motility, and malignant transformation by regulating cytoskeletal architecture may serve analogous roles in microorganisms. Major reorganization of actin polymerization must occur for microbial cells to undergo morphological transitions. Additionally, altered actin cytoskeleton function can result in dysfunctional growth and cell division among microorganisms. In fungi, the transition from the yeast to hyphal state requires actin reorientation and represents an important way in which these cells adapt to new environments. For example, S. cerevisiae changes its morphology from a yeast to a pseudohyphal form in response to extreme nutrient deprivation, perhaps as a way to forage for a better growth environment (12). Pathogenic fungi such as Candida albicans must be able to switch between yeast and hyphal forms to survive in the various tissues of the infected host. Mutant C. albicans strains that are unable to alter their cellular morphology in this way are not virulent in animal models of candidiasis (27). Therefore, those proteins that control cytoskeletal organization likely play major roles in microbial adaptation to new environments and in pathogenicity.
We have previously demonstrated that Ras proteins are required for actin polarization and normal cell division in the pathogenic yeast C. neoformans, especially at elevated temperatures (1, 43). In these studies, we have identified the gene encoding a Rac protein in a genetic screen for downstream effectors of C. neoformans Ras1. Overexpression of the RAC1 gene restores mating competence and 37°C growth to a ras1 mutant strain. Since Rac proteins play similar roles in mammalian cells, they are plausible downstream effectors of Ras to control fungal cytoskeletal architecture.
Several lines of evidence, however, suggest that C. neoformans Rac1 works with other, similar proteins to coordinate normal cytoskeletal function. For example, even though RAC1 overexpression suppresses the high-temperature growth defect in a ras1 mutant background, rac1 mutant strains grow well at elevated temperatures. Additionally, the yeast forms of the rac1 mutant cells display no obvious defects in actin polarization. Lastly, even though the hyphal transition in C. neoformans rac1 mutant strains is greatly reduced, those hyphal cells that are observed also have normal actin staining patterns. These results suggest that other proteins are able to play complementary or redundant roles with Rac1 as a downstream target of Ras.
Two genes encoding homologs of the Cdc42 GTPase have been identified in C. neoformans. These proteins are highly similar to Rac1. Interestingly, both C. neoformans Rac1 and one of the Cdc42 homologs interact with Ste20α in the yeast two-hybrid system; therefore, these two G proteins may perform similar functions to regulate Ste20α function. If so, residual Cdc42 activity in a rac1 mutant may explain why this strain does not itself have a defect in growth at 37°C. Our preliminary studies suggest that the CDC42 homolog genes may be essential in C. neoformans. Therefore, alternative strategies other than gene disruption may be required to further elucidate functional interactions among this family of G proteins. However, these related proteins are likely to play interacting, and potentially redundant, roles in cytoskeletal organization.
Many fungal species that grow primarily as yeasts, such as S. cerevisiae, do not possess RAC genes, suggesting that Rac proteins play a primary role in fungi to control hyphal development. In contrast, the dimorphic fungus Yarrowia lipolytica has a RAC gene, YlRAC1, that is required for hyphal growth (16). Additionally, as this fungus transitions from the yeast to hyphal form, YlRAC1 transcript levels increase. Interestingly, strains with mutations in this gene are able to mate, and they demonstrate normal actin localization and polarization (16).
A gene encoding a Rac homolog has also been identified and disrupted in the dimorphic human fungal pathogen Penicillium marneffei (2). Deletion of the cflB (rac) gene results in severe disruption of proper actin polarization such that growth is altered in both the conidiophore and hyphal states (2). Together these results suggest that Rac proteins have been specifically adapted for dramatic morphological changes, such as those required for mammalian cell motility or for the yeast-hyphal transition in fungi. However, the precise mechanisms by which Rac proteins regulate cytoskeletal organization and actin polarization may differ between species.
Ras and Rac function coordinately in mammalian cells.
Several lines of investigation demonstrate that Ras and Rac proteins act together to coordinate cellular functions. For example, the Ras-mediated oncogenic transformation of cultured mammalian cells requires the activation of Rac and Rho proteins. Dominant negative mutations in either Rac1 or RhoA reduce the ability of oncogenic Ras mutations to induce the transformation of cultured NIH 3T3 fibroblasts. Moreover, dominant activating mutations in Rac1 and RhoA enhance the transforming ability of Ras (20).
Many investigators have suggested potential mechanisms by which Ras and Rac proteins might mediate oncogenic transformation. Ras and Rac increase the production of reactive oxygen intermediates such as superoxide. These molecular species can in turn damage DNA and induce mutations leading to altered cell physiology and perhaps immortalization (17, 18). Rac proteins also regulate the progression of mammalian cells from G2 to M phases of the cell cycle (31), and altered Ras/Rac signaling could therefore disrupt normal cell cycle regulation. Activating Rac mutations can suppress Ras-induced apoptosis in both primary and immortalized mammalian cells in culture (19), further suggesting ways in which Rac proteins contribute to Ras effects on cell growth, morphology, and malignant transformation.
The interaction of Ras proteins and Rho/Rac proteins was also recently described in mammalian cells as a means of controlling cell migration. When R-Ras signaling was activated in T47D breast epithelial cells, Rho activity increased, and Rac activity decreased along the cell periphery. These studies suggest that R-Ras spatially activates Rho and inactivates Rac to control two elements of cell migration—membrane protrusion and adherence (47).
In the fission yeast Schizosaccharomyces pombe, Ras is upstream of two distinct signal transduction pathways. One of these pathways includes the Byr2 MEK kinase and mediates the pheromone signal. The other pathway includes Scd1, a presumed guanine-nucleotide exchange factor for the Cdc42 GTPase (35). The Scd1/Cdc42 pathway is involved in cell polarity, spindle formation, and chromosome segregation (35). Therefore, there appears to be a conserved signaling paradigm in which Ras proteins regulate the activity of Rac and similar Rho-like GTPases to control cell morphology, both in mammalian systems and in microorganisms.
Interaction of Ras, Rac, and PAK kinases.
In addition to Rac and Cdc42 proteins, Ras also controls microbial morphogenesis by regulating PAK kinases. In the basidiomycete U. maydis, a fungal pathogen of corn, Ras proteins are required for filamentation, mating, and pathogenesis (25). Also, the U. maydis PAK kinase Cla4 was recently demonstrated to control fungal cell budding. A cla4 mutant strain was viable but unable to bud properly, growing as constitutively hyphal cells with frequent septa formation and branching and with delocalized deposition of chitin in the cell wall. Although these mutant strains could still fuse with wild-type cells in a mating reaction, the cla4 mutant strain was avirulent in plant models of pathogenesis. These observations suggest that this PAK kinase regulates cell polarity during budding and hyphal growth (26). These authors demonstrated a functional interaction between PAK kinases and activated forms of Rac in a yeast two-hybrid assay (26).
Our studies similarly suggest that Rac proteins and PAK kinases functionally interact to coordinate cellular functions in C. neoformans. The PAK kinase CRIB domain is conserved in the C. neoformans Ste20α protein (33), and both Cdc42 and Rac1 physically interact with Ste20α in the yeast two-hybrid system. Also, overexpression of either RAC1 or STE20α complements the ras1 mutant defects in mating and high-temperature growth. Therefore, both proteins likely function genetically downstream of Ras1.
Even though the Rac1 and Ste20 proteins may physically interact, our results are not consistent with these proteins merely acting in a simple, linear signaling pathway to control cytoskeletal organization in C. neoformans. The phenotypes of mating hyphae resulting from bilateral rac1 crosses and bilateral ste20 crosses are quite distinct. Additionally, the ste20-like hyphae resulting from a bilateral cross of rac1 ste20 double mutants suggests that the Ste20 PAK kinase plays a more substantial role in mating hyphal morphology than Rac1. The apparent complexity of these molecular and phenotypic interactions further suggests that the Rho-like GTPases (Rac, Cdc42) and PAK kinases (Pak1, Ste20a, Ste20α) play distinct but coordinated roles to regulate cell morphology.
One of the main ways in which Rac proteins may act is by recruiting other proteins to cell membranes. Therefore, simply overexpressing downstream targets of Rac may not result in the proper localization of these proteins required for activation of downstream effectors. This may explain our observation that overexpression of Ste20α fails to restore filamentous differentiation to a rac1 mutant. We are currently defining the cellular localization patterns of the Rac and PAK kinase proteins to explore this possibility.
In conclusion, these studies further define the complex ways in which microbial cells respond and adapt to their surroundings. Fungi such as C. neoformans must be able to precisely regulate their morphology, whether in a specific host tissue or in the external environment. Many of these morphological events are driven by cytoskeleton changes controlled by Ras, Rho-like G proteins, and PAK kinases. In addition to elucidating mechanisms of microbial morphogenesis, a better understanding of the molecular interaction of these conserved signaling proteins will also demonstrate the central roles that they play in mammalian cellular adherence, motility, and malignant transformation.
Acknowledgments
We thank Joseph Heitman for the use of a Zeiss Axioskop 2 Plus fluorescent microscope and AxioCam MRM digital camera.
This work was supported by PHS grant 1R01-AI050128. Andrew Alspaugh is a Burroughs Wellcome Fund New Investigator in Molecular Pathogenic Mycology. Connie Nichols is supported by an Interdisciplinary Training Grant in AIDS (5T32AI07392). We also acknowledge the C. neoformans Genome Project, Stanford Genome Technology Center, funded by the NIAID/NIH under cooperative agreement AI47087; and the TIGR Cryptococcus neoformans Genome Project, supported by PHS grant U01 AI48594-01 (28).
REFERENCES
- 1.Alspaugh, J. A., L. M. Cavallo, J. R. Perfect, and J. Heitman. 2000. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol. Microbiol. 36:352-365. [DOI] [PubMed] [Google Scholar]
- 2.Boyce, K. J., M. J. Hynes, and A. Andrianopoulos. 2003. Control of morphogenesis and actin localization by the Penicillium marneffei RAC homolog. J. Cell Sci. 116:1249-1260. [DOI] [PubMed] [Google Scholar]
- 3.Burbelo, P. D., D. Drechsel, and A. Hall. 1995. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J. Biol. Chem. 270:29071-29074. [DOI] [PubMed] [Google Scholar]
- 4.Campbell, S. L., R. Khosravi-Far, K. L. Rossman, G. J. Clark, and C. J. Der. 1998. Increasing complexity of Ras signaling. Oncogene 17:1395-1413. [DOI] [PubMed] [Google Scholar]
- 5.Cardenas, M. E., C. Hemenway, R. S. Muir, R. Ye, D. Fiorentino, and J. Heitman. 1994. Immunophilins interact with calcineurin in the absence of exogenous immunosuppressive ligands. EMBO J. 13:5944-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz, M. C., D. S. Fox, and J. Heitman. 2001. Calcineurin is required for hyphal elongation during mating and haploid fruiting in Cryptococcus neoformans. EMBO J. 20:1020-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson, R. C., J. R. Blankenship, P. R. Kraus, M. de Jesus Berrios, C. M. Hull, C. D'Souza, P. Wang, and J. Heitman. 2002. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148:2607-2615. [DOI] [PubMed] [Google Scholar]
- 8.Davidson, R. C., M. C. Cruz, R. A. Sia, B. Allen, J. A. Alspaugh, and J. Heitman. 2000. Gene disruption by biolistic transformation in serotype D strains of Cryptococcus neoformans. Fungal Genet. Biol. 29:38-48. [DOI] [PubMed] [Google Scholar]
- 9.Didsbury, J., R. F. Weber, G. M. Bokoch, T. Evans, and R. Snyderman. 1989. rac, a novel ras-related family of proteins that are botulinum toxin substrates. J. Biol. Chem. 264:16378-16382. [PubMed] [Google Scholar]
- 10.Didsbury, J. R., R. J. Uhing, and R. Snyderman. 1990. Isoprenylation of the low molecular mass GTP-binding proteins rac1 and rac2: possible role in membrane localization. Biochem. Biophys. Res. Commun. 171:804-812. [DOI] [PubMed] [Google Scholar]
- 11.Fox, D. S., G. M. Cox, and J. Heitman. 2003. Phospholipid-binding protein Cts1 controls septation and functions coordinately with calcineurin in Cryptococcus neoformans. Eukaryot. Cell 2:1025-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gimeno, C. J., P. O. Ljungdahl, C. A. Styles, and G. R. Fink. 1992. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68:1077-1090. [DOI] [PubMed] [Google Scholar]
- 13.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 14.Hall, A., and C. D. Nobes. 2000. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos. Trans. R. Soc. Lond. B 355:965-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho, J., and A. Bretscher. 2001. Ras regulates the polarity of the yeast actin cytoskeleton through the stress response pathway. Mol. Biol. Cell 12:1541-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurtado, C. A., J. M. Beckerich, C. Gaillardin, and R. A. Rachubinski. 2000. A Rac homolog is required for induction of hyphal growth in the dimorphic yeast Yarrowia lipolytica. J. Bacteriol. 182:2376-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irani, K., Y. Xia, J. L. Zweier, S. J. Sollott, C. J. Der, E. R. Fearon, M. Sundaresan, T. Finkel, and P. J. Goldschmidt-Clermont. 1997. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 275:1649-1652. [DOI] [PubMed] [Google Scholar]
- 18.Joneson, T., and D. Bar-Sagi. 1998. A Rac1 effector site controlling mitogenesis through superoxide production. J. Biol. Chem. 273:17991-17994. [DOI] [PubMed] [Google Scholar]
- 19.Joneson, T., and D. Bar-Sagi. 1999. Suppression of Ras-induced apoptosis by the Rac GTPase. Mol. Cell. Biol. 19:5892-5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khosravi-Far, R., P. A. Solski, G. J. Clark, M. S. Kinch, and C. J. Der. 1995. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol. Cell. Biol. 15:6443-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon-Chung, K. J. 1976. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia 68:821-833. [PubMed] [Google Scholar]
- 22.Kwon-Chung, K. J. 1975. A new genus, Filobasidiella, the perfect state of Cryptococcus neoformans. Mycologia 67:1197-1200. [PubMed] [Google Scholar]
- 23.Kwon-Chung, K. J., and J. E. Bennett. 1992. Cryptococcosis, p. 397-446. In K. J. Kwon-Chung and J. E. Bennett (ed.), Medical mycology. Lea & Febiger, Malvern, Pa.
- 24.Kwon-Chung, K. J., J. C. Edman, and B. L. Wickes. 1992. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect. Immun. 60:602-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, N., and J. W. Kronstad. 2002. ras2 controls morphogenesis, pheromone response, and pathogenicity in the fungal pathogen Ustilago maydis. Eukaryot. Cell 1:954-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leveleki, L., M. Mahlert, B. Sandrock, and M. Bolker. 2004. The PAK family kinase Cla4 is required for budding and morphogenesis in Ustilago maydis. Mol. Microbiol. 54:396-406. [DOI] [PubMed] [Google Scholar]
- 27.Lo, H. J., J. R. Kohler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 28.Loftus, B. J., E. Fung, P. Roncaglia, D. Rowley, P. Amedeo, D. Bruno, J. Vamathevan, M. Miranda, I. J. Anderson, J. A. Fraser, J. E. Allen, I. E. Bosdet, M. R. Brent, R. Chiu, T. L. Doering, M. J. Donlin, C. A. D'Souza, D. S. Fox, V. Grinberg, J. Fu, M. Fukushima, B. J. Haas, J. C. Huang, G. Janbon, S. J. Jones, H. L. Koo, M. I. Krzywinski, J. K. Kwon-Chung, K. B. Lengeler, R. Maiti, M. A. Marra, R. E. Marra, C. A. Mathewson, T. G. Mitchell, M. Pertea, F. R. Riggs, S. L. Salzberg, J. E. Schein, A. Shvartsbeyn, H. Shin, M. Shumway, C. A. Specht, B. B. Suh, A. Tenney, T. R. Utterback, B. L. Wickes, J. R. Wortman, N. H. Wye, J. W. Kronstad, J. K. Lodge, J. Heitman, R. W. Davis, C. M. Fraser, and R. W. Hyman. 2005. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 307:1321-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell, T. G., and J. R. Perfect. 1995. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 8:515-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mondon, P., Y. C. Chang, A. Varma, and K. J. Kwon-Chung. 2000. A novel episomal shuttle vector for transformation of Cryptococcus neoformans with the ccdB gene as a positive selection marker in bacteria. FEMS Microbiol. Lett. 187:41-45. [DOI] [PubMed] [Google Scholar]
- 31.Moore, K. A., R. Sethi, A. M. Doanes, T. M. Johnson, J. B. Pracyk, M. Kirby, K. Irani, P. J. Goldschmidt-Clermont, and T. Finkel. 1997. Rac1 is required for cell proliferation and G2/M progression. Biochem. J. 326:17-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore, T. D., and J. C. Edman. 1993. The alpha-mating type locus of Cryptococcus neoformans contains a peptide pheromone gene. Mol. Cell. Biol. 13:1962-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nichols, C. B., J. A. Fraser, and J. Heitman. 2004. PAK kinases Ste20 and Pak1 govern cell polarity at different stages of mating in Cryptococcus neoformans. Mol. Biol. Cell 15:4476-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson, M. F., A. Ashworth, and A. Hall. 1995. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science 269:1270-1272. [DOI] [PubMed] [Google Scholar]
- 35.Papadaki, P., V. Pizon, B. Onken, and E. C. Chang. 2002. Two Ras pathways in fission yeast are differentially regulated by two ras guanine nucleotide exchange factors. Mol. Cell. Biol. 22:4598-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pitkin, J. W., D. G. Panaccione, and J. D. Walton. 1996. A putative cyclic peptide efflux pump encoded by the TOXA gene of the plant-pathogenic fungus Cochliobolus carbonum. Microbiology 142:1557-1565. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.Sherman, F. 1991. Getting started with yeast, p. 3-21. In C. Guthrie and G. R. Fink (ed.), Methods in enzymology, vol. 194. Academic Press, Inc., San Diego, Calif. [DOI] [PubMed] [Google Scholar]
- 39.Sukroongreung, S., K. Kitiniyom, C. Nilakul, and S. Tantimavanich. 1998. Pathogenicity of basidiospores of Filobasidiella neoformans var. neoformans. Med. Mycol. 36:419-424. [PubMed] [Google Scholar]
- 40.Toda, T., I. Uno, T. Ishikawa, S. Powers, T. Kataoka, D. Broek, S. Cameron, J. Broach, K. Matsumoto, and M. Wigler. 1985. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell 40:27-36. [DOI] [PubMed] [Google Scholar]
- 41.Toffaletti, D. L., T. H. Rude, S. A. Johnston, D. T. Durack, and J. R. Perfect. 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 175:1405-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, P., C. B. Nichols, K. B. Lengeler, M. E. Cardenas, G. M. Cox, J. R. Perfect, and J. Heitman. 2002. Mating-type-specific and nonspecific PAK kinases play shared and divergent roles in Cryptococcus neoformans. Eukaryot. Cell 1:257-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waugh, M. S., C. B. Nichols, C. M. DeCesare, G. M. Cox, J. Heitman, and J. A. Alspaugh. 2002. Ras1 and Ras2 contribute shared and unique roles in physiology and virulence of Cryptococcus neoformans. Microbiology 148:191-201. [DOI] [PubMed] [Google Scholar]
- 44.Waugh, M. S., M. A. Vallim, J. Heitman, and J. A. Alspaugh. 2003. Ras1 controls pheromone expression and response during mating in Cryptococcus neoformans. Fungal Genet. Biol. 38:110-121. [DOI] [PubMed] [Google Scholar]
- 45.Wickes, B. L., U. Edman, and J. C. Edman. 1997. The Cryptococcus neoformans STE12alpha gene: a putative Saccharomyces cerevisiae STE12 homologue that is mating type specific. Mol. Microbiol. 26:951-960. [DOI] [PubMed] [Google Scholar]
- 46.Wickes, B. L., M. E. Mayorga, U. Edman, and J. C. Edman. 1996. Dimorphism and haploid fruiting in Cryptococcus neoformans: association with the α-mating type. Proc. Natl. Acad. Sci. USA 93:7327-7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wozniak, M. A., L. Kwong, D. Chodniewicz, R. L. Klemke, and P. J. Keely. 2005. R-Ras controls membrane protrusion and cell migration through the spatial regulation of Rac and Rho. Mol. Biol. Cell 16:84-96. [DOI] [PMC free article] [PubMed] [Google Scholar]