Abstract
Much remains to be understood about quorum-sensing factors that allow cells to sense their local density. Dictyostelium discoideum is a simple eukaryote that grows as single-celled amoebae and switches to multicellular development when food becomes limited. As the growing cells reach a high density, they begin expressing discoidin genes. The cells secrete an unknown factor, and at high cell densities the concomitant high levels of the factor induce discoidin expression. We report here the enrichment of discoidin-inducing complex (DIC), an ∼400-kDa protein complex that induces discoidin expression during growth and development. Two proteins in the DIC preparation, DicA1 and DicB, were identified by sequencing proteolytic digests. DicA1 and DicB were expressed in Escherichia coli and tested for their ability to induce discoidin during growth and development. Recombinant DicB was unable to induce discoidin expression, while recombinant DicA1 was able to induce discoidin expression. This suggests that DicA1 is an active component of DIC and indicates that posttranslational modification is dispensable for activity. DicA1 mRNA is expressed in vegetative and developing cells. The mature secreted form of DicA1 has a molecular mass of 80 kDa and has a 24-amino-acid cysteine-rich repeat that is similar to repeats in Dictyostelium proteins, such as the extracellular matrix protein ecmB/PstA, the prespore cell-inducing factor PSI, and the cyclic AMP phosphodiesterase inhibitor PDI. Together, the data suggest that DicA1 is a component of a secreted quorum-sensing signal regulating discoidin gene expression during Dictyostelium growth and development.
There appear to exist factors secreted by cells in higher eukaryotes that function as quorum sensors (these are also known as cell density sensors), so that as the number or density of cells of a specific type increases in a tissue or the body, the concentration of the factor increases, allowing the cells to sense their number or density (23). However, in most cases the factors are unknown. For instance, a major problem in treating cancer is the phenomenon of tumor dormancy: often, when a patient has a primary tumor and metastases, surgical removal of the primary tumor appears to stimulate cell proliferation in the metastatic foci. This postsurgery proliferation appears to be due to the tumor cells secreting an unknown quorum factor that inhibits their proliferation, so that when a major source of the quorum factor (the primary tumor) is removed, the resulting reduction in the levels of the factor allows the metastases to proliferate faster (15, 28).
The simple eukaryote Dictyostelium discoideum is an excellent system in which to study quorum sensing. When an adequate bacterial food source is present, Dictyostelium cells live as unicellular amoebae and divide by fission. However, when food is scarce, Dictyostelium cells enter a developmental cycle that begins with the aggregation of cells into groups of up to 105 cells. These groups then behave as multicellular organisms, migrating towards light, humidity, and optimal temperature. Ultimately, each aggregate forms a fruiting body consisting of a stalk supporting a mass of spores. During aggregation and the subsequent multicellular stages, Dictyostelium cells communicate using extracellular signaling molecules that regulate development (20, 27, 52). The best known of these is extracellular cyclic AMP (cAMP), which coordinates chemotactic aggregation by an oscillatory relay mechanism (32). Other molecules that participate in signaling include CMF, an 80-kDa glycoprotein that allows cells to sense the local density of starving cells (25, 31, 35, 56), counting factor (CF), a 450-kDa protein complex made up of several proteins, including countin, CF45-1, and CF50, which mediates cell density sensing during aggregation and regulates the final size of the aggregates by regulating cell motility and adhesion (6, 48, 50), and countin2, a secreted protein with sequence similarity to countin, which also regulates group size (39). A chlorinated hydrocarbon called DIF regulates stalk cell differentiation (7, 37, 51, 55), a 106-kDa glycoprotein called PSI factor is implicated in prespore differentiation (38, 40), and the prespore-to-spore transition is mediated by SDF2, a small peptide (2, 3). Secreted molecules, such as adenosine and ammonia, also exert control over gene expression and the prestalk/prespore ratio (16, 27).
While most efforts to characterize signaling molecules in Dictyostelium have focused on the multicellular stages and the cell type differentiation processes, it is clear that cells also communicate during vegetative growth and the transition from growth to development. During growth, at least two secreted polypeptide factors, CMF450 and PSF, affect growth and gene expression (14, 30, 54). CMF450 is a 450-kDa complex that causes feeding cells to stop cell division and begin development (30). To monitor population density, the protein PSF is continuously secreted during growth and accumulates in the medium in proportion to cell density (13). PSF induces early developmental gene expression in a dose-dependent manner (45, 46). This action is antagonized by food bacteria, which probably allows cells to fine tune the decision of when to enter multicellular development (12, 14).
One of the genes regulated by PSF is discoidin I (46). In cells growing at low density, discoidin mRNA and protein levels are very low, and the levels increase during late growth phase and early development (13, 14, 46). Discoidin is an intracellular N-acetylgalactosamine binding protein of ∼30 kDa and is essential for proper organization of the cytoskeleton and motility (1).
In this report we describe an ∼400-kDa protein complex secreted by growing and starving cells that induces discoidin expression. This complex, which is different from CF (6) and from CMF450 (30), was enriched, and partial amino acid sequences were used to identify the putative components. We find that for one of the putative components (DicA1), the recombinant version of the protein induces discoidin expression. Given the assumption that the extracellular concentration of discoidin-inducing complex (DIC) increases with increasing cell density, the induction of discoidin by recombinant DicA1 identifies it as a Dictyostelium quorum-sensing factor component.
MATERIALS AND METHODS
Cell culture.
Axenic Dictyostelium discoideum strains Ax2 and its derivatives were used in all experiments. Strain AX2p358 contains a plasmid that carries the retrotransposon DRE (Dictyostelium repetitive element) (34), a fusion gene containing the discoidin Iγ promoter driving expression of luciferase, and the V18-Tn5 selection cassette (53). This cassette allows selection with G418 during growth on bacteria. PDE-minus and PDE-overexpressing strains (29, 49) were a kind gift from Richard Kessin, Columbia University. For growth in shaking culture with bacteria, Enterobacter aerogenes was grown on SM plates, harvested, and resuspended to an optical density at 600 nm of 8 in KK2 (50 mM potassium phosphate, pH 6.2) or PBM (20 mM KH2PO4, 0.01 mM CaCl2, 1 mM MgCl2, pH 6.1 with KOH). Ax2 cells were then grown in the bacterial suspension in shaking culture (150 rpm, 5-cm-diameter orbit) at room temperature. To induce development, cells from cultures growing on bacteria at densities below 1 × 106 cells/ml were washed free of bacteria with PBM and spread at 5 × 106 cells/cm2 on nitrocellulose filters (Schleicher and Schuell) supported by filter paper (Whatman 3M) soaked in PBM.
Preparation of CM.
Conditioned media (CM) were prepared as previously described (14, 25). To make vegetative CM, cells were inoculated at 5 × 104 cells/ml and allowed to grow in shaking culture with bacteria for approximately 30 h to a density of 5 × 106 cells/ml. The medium conditioned by growing cells was recovered by centrifugation at 1,500 × g for 3 min, and the bacteria were then removed by two centrifugations at 5,000 × g for 10 min each. To make developmental CM, vegetative cells from the first centrifugation were washed free of bacteria and resuspended at 1 × 107 cells/ml in KK2 and incubated for either 6 h or 16 h. The medium (CM) was recovered after two successive centrifugations at 1,500 × g for 3 min each. Both preparations were stored at −80°C. CM were size fractionated and concentrated by ultrafiltration using Centricon 80 and Ultrafree 15 spin filters (Millipore) (pore size of 100 kDa unless otherwise indicated) following the manufacturer's directions.
Discoidin induction assay with the luciferase reporter.
AX2p358 cells were grown in a suspension of bacteria with 50 μg/ml G418 and harvested between 0.5 × 106 and 1.5 × 106 cells/ml. Cells were washed free of bacteria by repeated centrifugation and resuspended to 2 × 105 cells/ml in PBM. A 0.1-ml sample of the suspended cells was placed in each well of a 24-well plate (Becton Dickinson, Franklin Lake, NY) and immediately supplemented with 0.4 ml of either PBM, crude conditioned medium, or a 1:40 dilution of a fraction from the purification. After incubation for 6 h, cells were lysed by adding 250 μl of 3× lysis buffer (Berthold, Pforzheim, Germany) after which luciferase activity was measured as previously described (44).
Discoidin induction assay using immunofluorescence.
For measurement of discoidin induction by immunofluorescence, wild-type cells were inoculated at 2 × 103 cells/ml in HL5 and grown for 2 days to roughly 6 × 103 cells/ml. The cells were collected by centrifugation, resuspended in PBM, collected by centrifugation, and resuspended to 2 × 105 cells/ml in PBM. A 200-μl sample of cells was placed in each well of an eight-well chambered slide (Nalge, Naperville, IL), and 10 μl of buffer or recombinant protein was added. After 4 h, cells were fixed in a mixture of 3 ml of 37% formaldehyde and 100 ml of methanol at −15°C for 5 min and then washed for 25 min in Tris-buffered saline (TBS) (10 mM Tris, 100 mM NaCl, pH to 7.5 with HCl) (13). Cells were stained with 1:200 rabbit antidiscoidin antibodies (13) in TBS for 30 min at 37°C, washed in three changes of TBS for 5 min each, stained with 1:300 Alexa 488 goat anti-rabbit (Molecular Probes) in TBS for 30 min at 37°C, and washed as described above. The slide was then dipped into distilled water, mounted, and viewed as previously described (24).
Protein purification.
Unless stated otherwise, medium conditioned by starving Ax2 cells was used. Five hundred milliliters of CM was concentrated to 1 to 1.5 ml by ultrafiltration with 100-kDa-cutoff Centricon Plus-80 centrifugal filter devices (Millipore, Bedford, MA). This and all subsequent steps were done at 4°C. The concentrate was applied to a Biogel A 1.5-m (Bio-Rad, Hercules, CA) gel filtration column (1.5 by 50 cm) equilibrated in PBM. Fractions (1.4 ml) were collected, and 20 μl of protease inhibitor stock (1 tablet of complete protease inhibitor [Roche, Indianapolis, IN] dissolved in 1 ml of PBM) was added to each fraction. The column was calibrated using molecular mass markers ranging from 44 to 650 kDa (Bio-Rad). Ten-microliter portions of the fractions were tested as described above for their ability to induce discoidin expression using the luciferase reporter assay. Active fractions were pooled and concentrated to 0.5 ml using Microcon ultrafiltration units (Millipore) before application to a 50-ml Econo-Pac High Q (Bio-Rad) ion-exchange column. The ion-exchange column was run with an Econo system (Bio-Rad) at 1 ml/min, collecting 1-ml fractions. After the column was washed with 5 ml of PBM, proteins were eluted with a 35-ml gradient of 0.01 to 0.5 M NaCl in PBM. Active fractions eluted at ∼300 mM. Protease inhibitors were added to the active fractions (typically 5 to 6 ml), which were then concentrated with 30-kDa-cutoff Microcon ultrafiltration units (Millipore) to 20 μl, and separated on a native 7% polyacrylamide gel as described previously (6). The gel was cut in 10 slices, each slice was crushed in 600 μl PBM, and the proteins were eluted overnight at 4°C with gentle rotation. The crushed acrylamide pieces were removed by centrifugation at 17,000 × g for 10 min in an Eppendorf centrifuge. Soluble acrylamide was removed by three washes of the protein fraction with 0.5 ml PBM on a 30-kDa-cutoff Microcon (Millipore) ultrafiltration unit before testing on cells. The active fractions were concentrated to 50 μl, and 10 μl was loaded on a sodium dodecyl sulfate (SDS)-polyacrylamide gel.
For an analytical run on a hydrophobic interaction column (Econo-Pac HIC cartridge; Bio-Rad), active fractions from a High Q column were concentrated on Microcon ultrafiltration units (10-kDa cutoff; Amicon) and equilibrated to 0.8 M potassium phosphate in PBM, pH 6.1. The column was run at 1 ml/min, collecting 1-ml fractions. After the column was washed with 5 ml of 0.8 M potassium phosphate, pH 6.1, proteins were eluted with a 35-ml gradient of 0.8 M potassium phosphate, pH 6.1, to distilled water. All fractions were concentrated on Amicon spin columns (10-kDa cutoff) and washed five times with PBM to remove salt before testing on cells.
Protein sequencing.
For N-terminal amino acid sequence analysis, proteins of three preparations were pooled and separated on a preparative 10% SDS-polyacrylamide gel and then transferred onto a polyvinylidene difluoride membrane by the method of Eckerskorn and Lottspeich (17). The blotted protein bands were stained with Coomassie blue for 1 min. After destaining, the bands were excised and sequenced on a Procise 492 pulsed liquid phase sequencer (Applied Biosystems, Foster City, CA).
All bands except PDE were N-terminally blocked. For analyzing internal peptides, in situ digestion of the proteins was performed by the method of Patterson (42) using endoproteinase LysC. Proteolytic fragments were separated by high-performance liquid chromatography (Hewlett Packard 1100) on a Purosphere RP18 reversed-phase column (Merck, Darmstadt) (1 mm by 150 mm). Solvent A was 0.1% trifluoroacetic acid, and solvent B was 0.1% trifluoroacetic acid in acetonitrile. The gradient was 0 to 60% solvent B over a period of 90 min at a flow rate of 30 μl/min. The peptides were sequenced (18) on the Procise 492 sequencer according to the manufacturer's instructions.
Production of recombinant DicA1 and DicB in Escherichia coli.
Dictyostelium vegetative and 3-h developmental RNA was extracted using a QIAGEN RNeasy Mini kit (Valencia, CA) following the manufacturer's protocol. cDNA was obtained using a Smart RACE cDNA amplification kit (Clontech, Palo Alto, CA). PCRs were performed with the Advantage 2 PCR kit (Clontech, Palo Alto, CA) using cDNA as a template and primers CCCCGAGCTCGCTCGAGCAAAAGAAATTCCTTGTCAATATGTATG and CCGGGGATCCTTATTCAGCTGGGGCAGAGTATAATGG for SLC522 (encoding the DicA1 protein) and prim-ers CTTGGAGCTCGCTCGAGCAGTTTGGATTGGTGGTAGTGGTTGand CCCCGGATCCTTATAATTTTCTTTCAACATCGATATCATCAG for SLE544 (encoding the DicB protein) to generate PCR products corresponding to nucleotides 136 to 2298 of the dicA1 cDNA and 194 to 1953 of the dicB cDNA. The primers created XhoI and SacI sites at the 5′ ends and BamHI sites at the 3′ ends of the PCR products. After purification with a Geneclean III kit (Qbiogene, Carlsbad, CA), the two PCR products were ligated into PCR2.1 (Invitrogen, Carlsbad, CA) and sequenced. The plasmid containing the dicA1 cDNA was digested with XhoI and EcoRV and ligated into the pThioHisA plasmid (Invitrogen), which was digested with EcoRI, blunted using a fill-in reaction with Klenow fragment, and then digested with XhoI. The plasmid containing the dicB cDNA was digested with XhoI and EcoRI and ligated into the same sites of the pThioHisB expression vector. The expression vectors were transformed into E. coli TOP 10 (Novagen, Madison, WI), and the recombinant proteins were expressed following the manufacturer's direction. Recombinant DicA was purified using the Probond purification system (Invitrogen) following the manufacturer's purification protocol for denaturing conditions. The protein was dialyzed against a 50× volume of PBM and 0.1 mM dithiothreitol at 4°C for 9 h with three changes of buffer, dialyzed against PBM at 4°C for 24 h with three changes of buffer, and then stored at 4°C. The recombinant DicB protein was purified using the same system following the manufacturer's purification protocol for native conditions, except that the bacteria were lysed in B-PER bacterial protein extraction reagent (Pierce, Rockford, IL). After the solution was clarified by centrifugation, the cell lysate was mixed with 10× binding buffer and distilled water to a final concentration of 1× binding solution. Purified DicB was dialyzed against PBM at 4°C for 9 to 12 h with three changes of buffer and stored at 4°C.
Northern blots.
Vegetative Ax2 cells growing in a shaking suspension of bacteria were harvested at various cell densities and cells developing on filter pads were harvested at 3-h intervals. RNA was prepared by the method of Chomczynski and Sacchi (11) with guanidinium chloride. Ten micrograms of RNA from each sample was separated on gels containing 1% agarose in 10 mM sodium phosphate (43) and blotted onto hybridization membranes (GeneScreen). The ∼2-kb inserts of the dicA1 and dicB cDNAs were cut with EcoRI and HindIII and labeled with [32P]dCTP using a Prime-it II kit (Stratagene, La Jolla, CA) following the manufacturer's directions. Hybridization was performed by the method of Brock et al. (5).
Nucleotide sequence accession numbers.
The cDNAs of clones SLC522 (dicA1) and SLE544 (dicB) were sequenced and deposited in the Tsukuba and EBI databases under accession numbers AJ548837 (dicA1) and AJ548836 (dicB).
RESULTS
CM induces expression of a luciferase reporter regulated by the discoidin Iγ promoter.
Medium conditioned by growing and starving cells induces discoidin expression in growing cells (8, 13, 45). In order to characterize and purify the active components in CM, we developed a rapid assay based on reporter gene induction during development. Cells carrying a discoidin promoter-luciferase reporter cassette were harvested at 1 × 106 cells/ml from a culture growing in a suspension of bacteria and cultured at a density of 2 × 104 cells/cm2 in 24-well plates with buffer or with CM from developing cells. Luciferase activity in the cells was then measured after 6 h. Induction by CM was determined as the ratio of luciferase activity with and without CM. CM from developing cells was able to induce luciferase expression during starvation 36-fold ± 17-fold (mean ± standard error of the mean [SEM]; n = 4). When cells were inoculated at a density of 1 × 105 cells/ml in bacteria suspended in either buffer or CM, grown overnight, and harvested during growth, the CM-treated cells had 9.8-fold ± 2.8-fold-higher luciferase activity than control cells (mean ± SEM; n = 4). Together, the data indicate that CM from developing cells can induce expression of the discoidin-luciferase fusion gene in growing and developing cells.
CMF is not the active component of CM.
CMF is a protein which is necessary for proper aggregation and may regulate discoidin expression during early development. It is synthesized during growth, stored in vesicles, and secreted only when cells starve (57). A crude preparation of CMF induced discoidin expression (25), and cmf− cells (4) express less discoidin protein than their DH1 parental line during growth for unknown reasons (data not shown). Furthermore, after removal of food bacteria, cmf− cells fail to increase discoidin protein levels during starvation (data not shown). We therefore assumed that CMF might be the signal responsible for discoidin induction in medium conditioned by starving cells. This is unlikely because the induction of discoidin expression by CM from cmf− cells was indistinguishable from the induction caused by CM from DH1 parental cells. Second, during the first purification step, CM from cmf− cells showed the same activity peak as wild-type cells (see below for purification from wild-type cells), and third, recombinant CMF was unable to increase expression of the AX2p328 luciferase reporter, in both the growth assay and the developmental assay. Together, these data suggest that some secreted factor other than CMF is needed for discoidin induction.
Purification of the discoidin-inducing component of CM.
To identify the compound(s) in CM responsible for discoidin expression, we first estimated the size of the compound(s) and their sensitivity to heat treatment. When CM from developing cells was fractionated by ultrafiltration using cutoffs of 5 kDa, 10 kDa, 30 kDa, and 100 kDa, about two-thirds of the activity was found in the retentate and one-third in the flowthrough. This ratio remained constant regardless of the ultrafiltration unit exclusion size, suggesting the presence of a low-molecular-mass compound smaller than 5 kDa and a high-molecular-mass compound larger than 100 kDa. The low-molecular-mass compound was found to be resistant to incubation at 80°C for 20 min, while the high-molecular-mass compound was inactivated by the heat treatment. Therefore, there appear to be at least two different fractions with discoidin-inducing activity in crude CM.
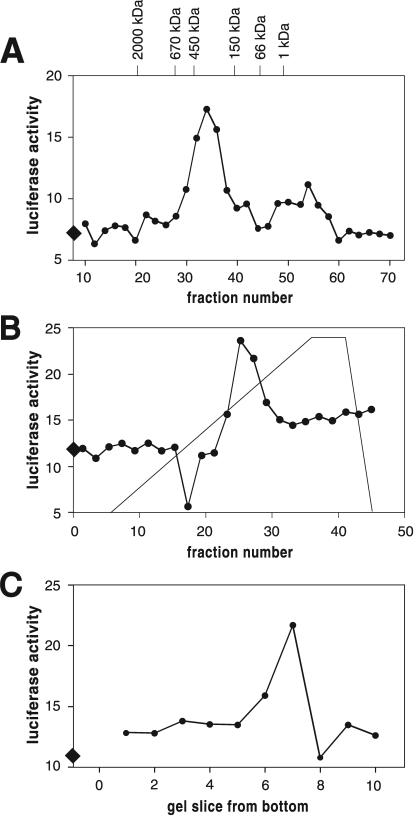
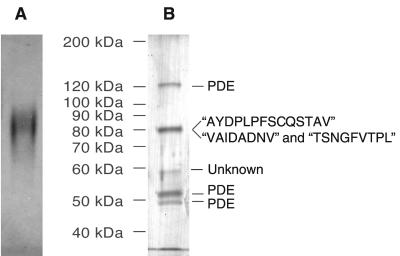
To characterize the high-molecular-mass fraction, we used conventional chromatographic methods to purify the factor from medium conditioned by starving cells. Typically 300 to 500 ml of CM was concentrated to 1 ml using ultrafiltration units with a cutoff of 100 kDa. The retentate was fractionated on a gel filtration column equilibrated with PBM and the fractions tested in the developmental assay described above. Active fractions eluted at approximately 400 kDa (Fig. 1A). A second peak of activity eluted after vitamin B as a marker (1,350 Da), i.e., outside the resolution range of the column (possibly because the factor interacted with the column material). These late-eluting fractions were not followed further. Active fractions from the 400-kDa range were concentrated and chromatographed on an HighQ ion-exchange column where they eluted at approximately 300 mM NaCl (Fig. 1B). When the active fractions from the ion-exchange column were further separated on a native polyacrylamide gel and eluted from the gel, typically one or two adjacent slices were capable of inducing discoidin expression (Fig. 1C). When proteins eluted from the active slices were rerun on a native gel and stained with silver, one diffuse band was found (Fig. 2A). Denaturing SDS-polyacrylamide gel electrophoresis of the proteins from the active slice revealed proteins in the preparation at approximately 120, 80, 58, 53, and 49 kDa (Fig. 2B).
FIG. 1.
Profiles of discoidin-inducing activity in different purification steps. (A) Discoidin-inducing activity in fractions from a gel filtration column. (B) Activity of fractions from the anion-exchange column. (C) Activity of eluates of slices of a native polyacrylamide gel. Discoidin expression was determined with luciferase as a reporter using arbitrary units. Active fractions increase discoidin expression over buffer control. Buffer controls (diamonds on the y axis) are indicated for each column.
FIG. 2.
Protein composition of the active fraction on polyacrylamide gels. (A) The enriched activity migrates as one complex on native polyacrylamide gels. (B) N-terminal sequences of the 49-, 53-, and 120-kDa proteins were identical to that of PDE. The 80-kDa fraction was N-terminally blocked. Peptide sequences were obtained after digestion with LysC, and the obtained sequences are indicated in quotation marks. Proteins in panels A and B were detected by silver staining.
In an attempt to separate these proteins, an analytical hydrophobic interaction exchange column was run. Active fractions from an ion-exchange column were loaded in 0.8 M potassium phosphate and eluted with a gradient of decreasing salt concentration. Active fractions could be recovered and eluted at about 0.6 M potassium phosphate. After this purification step, essentially the same banding pattern was observed on the SDS-polyacrylamide gel as on the native gel (data not shown). Thus, the purified protein complex is not dissociated by the high-salt conditions applied during chromatographic steps. We were thus unable to further purify the discoidin-inducing activity using a hydrophobic interaction column.
Proteins from three preparations were pooled and run on a denaturing gel, and the N-terminal sequences were determined. The N-terminal sequences of the 120-, 53-, and 49-kDa proteins matched the N-terminal sequence of the extracellular phosphodiesterase of Dictyostelium. The size heterogeneity of PDE is surprising, since it is transcribed from a single gene and the differentially spliced transcripts code for identical protein products (19). Nevertheless, previously described PDE purification protocols resulted in similar observations with molecular masses of ∼150, 55, and 50 kDa (41).
There were insufficient amounts of the 58-kDa band for sequencing, and the N-termini of the 80-kDa proteins were blocked. Preparations of the 80-kDa band were thus sequenced after digestion with LysC. From the 80-kDa band, three peptide sequences were obtained (Fig. 2B). Comparison of the peptide sequences of the 80-kDa band to the Tsukuba cDNA library revealed that they belong to two different proteins, which we named DicA1 and DicB. The two proteins correspond to cDNA clones SLC522 and SLE544 from the Tsukuba slug-specific cDNA library (36) (http://dictycdb.biol.tsukuba.ac.jp/cDNAproject.html). The two proteins are unrelated, as they have only 26% identity over a short 100-amino-acid overlap and share no domains. However, they both contain a signal sequence, which is cleaved between amino acids 19 and 20.
Role of extracellular phosphodiesterase PDE.
cAMP inhibits discoidin expression in the low cell density assay at concentrations as low as 10−8 M (data not shown). It was thus conceivable that PDE promotes discoidin expression solely by lowering cAMP levels. To investigate this possibility, we tested whether PDE-minus cells still secrete discoidin-inducing activity. In this series of experiments CM conditioned by starving PDE-minus cells induced discoidin expression of AX2p358 3.9-fold ± 0.6-fold (mean ± SEM; n = 4), while the CM from wild-type AX3 parental cells produced a 4.9-fold ± 0.9-fold (mean ± SEM; n = 4) induction. Thus, there was no significant difference between the amount of discoidin-inducing activity secreted by the PDE-minus and wild-type cells. To further test this hypothesis, we fractionated CM from the PDE-minus cells on a gel filtration column as described above. Activity was detected in the range of 400 kDa (data not shown). This suggested that PDE is dispensable for discoidin-inducing activity in CM and that PDE may be a contaminant in the DIC preparation.
Recombinant DicA1 has discoidin-inducing activity.
To determine whether either of the putative 80-kDa components of DIC have DIC activity, the two proteins were expressed in bacteria. The expression of these proteins proved to be very difficult. After trying a variety of different expression vectors and host cells, we were able to get a low level of expression using pThioHisB in E. coli BL21(DE3) cells. This resulted in the production of proteins consisting of a modified E. coli thioredoxin fused to DicA1 or DicB.
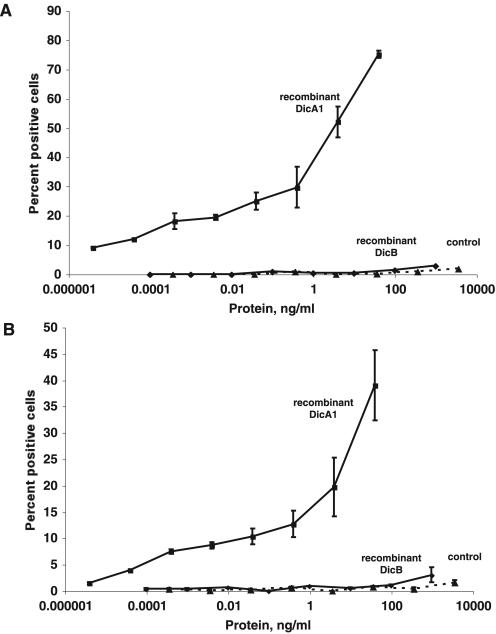
When added to wild-type cells, the fusion protein containing DicA1 caused a large percentage of cells to express discoidin I protein (Fig. 3A). At similar and higher concentrations, neither the fusion protein containing DicB or the modified thioredoxin alone induced discoidin expression. Because PSF induces discoidin expression in cells lacking the Gβ subunit (8), a similar assay was done with Gβ− cells. As shown in Fig. 3B, these cells also responded to DicA1 but not DicB or the modified thioredoxin alone.
FIG. 3.
Recombinant DicA1 induces the expression of discoidin in cells grown and starved at low cell density. (A) Wild-type cells were grown at a low cell density to prevent induction of discoidin and then starved at a low cell density in the indicated concentration of either crude recombinant thioredoxin-DicA1, crude recombinant thioredoxin-DicB, or a crude preparation of the thioredoxin protein (control). After 3 h, cells were fixed and stained for discoidin I by immunofluorescence and the number of positive cells and the total number of cells were counted in three randomly chosen microscope fields containing a total of approximately 150 to 200 cells. (B) A similar induction was done with Gβ− cells (33). For both panels A and B, results are the means ± SEMs from three (A) or four (B) independent experiments. The absence of error bars indicates the error was smaller than the plot symbol. When cells were grown to 1 × 106 cells/ml to induce discoidin expression by endogenous PSF, 79% ± 11% of Ax4 cells and 43% ± 14% of Gβ− cells expressed discoidin.
DicA1 has some similarity to other secreted proteins.
The protein sequences obtained were compared to the Tsukuba cDNA databases from slug stage and vegetative cells (36). The cDNA clones SLC522 (dicA1) and SLE544 (dicB) coding for two 80-kDa proteins were identified in the slug-specific library. cDNA sequencing of the clones was completed and the sequences deposited in the Tsukuba and EBI databases. The cDNA sequences were compared to the genomic sequence by assembling single runs from the Dictyostelium genome sequencing project (http://dictybase.org/).
The dicA1 cDNA codes for a protein of 708 amino acids with a calculated molecular mass of 76 kDa. The amino terminus of the predicted DicA1 protein is MKYLFIAIILILYCSFTKAD, which is a consensus signal sequence. A search of the National Center for Biotechnology Information database indicates that DicA1 has an ∼30% identity with three putative Dictyostelium proteins (AAO52378, AAB07590, and AAO52466) of unknown function. All four proteins have similar sizes and domain structures with highly conserved blocks in the N-terminal third of the proteins. This region corresponds to the PA14 domain (PF07691), which is a β-barrel structure that has been identified as a novel carbohydrate-binding module (47). Other proteins containing this motif include glycosidases, glycosyltransferases, proteases, amidases, adhesins, and bacterial toxins as well as the mammalian protein fibrocystin. DicA1 and the three similar proteins have cysteine-rich repeats of 24 amino acids. This repeat is identified in the InterPro database(http://www.ebi.ac.uk/InterProScan/) as the S_mold_repeat(PF00526), a conserved repeat found in several Dictyostelium proteins and in proteins from other species. It is similar to repeats found in other Dictyostelium extracellular proteins, including ecmB/PstA, a prestalk cell extracellular matrix protein (9); PDI, an inhibitor of the extracellular phosphodiesterase PDE (21); and PSI, an extracellular signaling molecule that induces prespore gene expression (38). Cysteine repeats are also found in proteins of other organisms, many of which are involved in protein-protein interactions in the extracellular matrix and in extracellular signaling. Among the most similar proteins are Notch, various Notch ligands, fibrillin, fibrosurfin, tenascin, and latent transforming growth factor β binding protein 1.
DicA1 is expressed in and secreted by vegetative and developing cells.
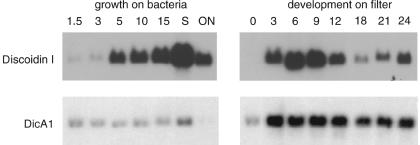
Northern blots showed that whereas discoidin I mRNA levels increase as cell density increases, dicA1 mRNA is expressed constitutively during vegetative growth (Fig. 4). When growing cells were washed free of bacteria and allowed to develop on filters, there was an initial increase of dicA1 expression in the first 3 hours of development, after which expression levels were unchanged. These results suggested that the DicA1 protein might be present during vegetative growth as well as during development. Since CMF is expressed in vegetative cells but is secreted only after starvation, we tested for the presence of the protein complex containing DicA1 in culture supernatants from vegetative cells. This crude CM from vegetative cells induced discoidin expression as did CM from developing cells. Ultrafiltration of CM from vegetative cells using a 30-kDa cutoff filter unit indicated the presence of a heat-stable low-molecular-mass compound and a heat-labile high-molecular-mass compound, both of which induced discoidin expression. The elution profiles of the high-molecular-mass compound from the gel filtration and anion-exchange columns were identical to the material purified from CM from developing cells, and the protein bands detected after elution from a native gel were indistinguishable (data not shown). Together, the data suggest that DicA1 is expressed by vegetative cells and that DIC activity is secreted by vegetative cells.
FIG. 4.
Expression pattern of the DicA1 mRNA. RNAs from cells growing in shaking culture on bacteria at various cell densities (left panel; numbers are densities [106 cells/ml]; S, early stationary phase; ON, stationary overnight) and cells developing for various times on filter pads (right panel, numbers are developmental time in hours) were hybridized to a probe for DicA1 and a discoidin probe as a control. Equal loading was verified by ethidium bromide staining.
DISCUSSION
We found that cells secrete a factor smaller than 5 kDa and an approximately 400-kDa compound, both of which induce discoidin expression. The small factor is heat stable and may well be a previously described heat-stable low-molecular-mass factor present in CM that induces accumulation of N-acetylglucosaminidase during early development (26). It is interesting that after concentration of CM by ultrafiltration with 100-kDa-cutoff filters, sieving gel fractionation shows two peaks of activity (Fig. 1A). This suggests that either the high-molecular-mass activity breaks down to a low-molecular-mass activity, as we previously observed for the secreted factor CMF (57), or that there are two different high-molecular-mass factors, one of which binds to the column resin and thus elutes anomalously. The second compound that induces discoidin expression, DIC, was analyzed and from it we identified DicA1, a cysteine-rich secreted protein with discoidin-inducing activity. This indicates that DicA1 is a functional component of DIC. Whether native DicA1 elutes at 400 kDa or whether DIC is a multimer of DicA1 or a complex of DicA1 and other proteins remains to be determined.
The cysteine residues in DicA1 are arranged in repeat units similar to those in other Dictyostelium proteins that may be involved in protein-protein interactions, such as the extracellular matrix protein ecmB, PSI, and PDI. The best characterized of these proteins is PDI, which acts by binding stoichiometrically to cyclic nucleotide phosphodiesterase, changing the Km of the enzyme for cAMP from 10 μM to 2 mM, thereby inhibiting the enzyme activity (10, 22). These repeats may allow DicA1 to interact with itself, DicB, or other proteins to form the 400-kDa DIC complex.
DicA1 also contains a PA14 domain. Most of the experimentally characterized PA14-containing proteins appear to be involved in carbohydrate binding and/or metabolism (47). This domain is named for the 14-kDa fragment of the anthrax protective antigen (PA). It is interesting that anthrax toxin is made up of three ∼80-kDa polypeptides: PA; the edema factor (EF); and the lethal factor (LF). For the anthrax toxin to be delivered into a host cell, the PA first binds to the cell and forms a point of entry for the remaining factors, LF and EF. The PA14 domain of DciA1 may thus help it to bind to Dictyostelium or bacterial cells.
The DIC preparation contained PDE. However, PDE-minus strains still secrete the discoidin-inducing activity, and gel filtration showed that the apparent size of the active complex enriched from PDE-minus strains is identical to that of the active complex from wild-type cells. Together, this indicates that PDE is not part of the active complex but that it copurified due to similar physical and biochemical properties.
DicB has some similarity to several putative cell membrane anchor proteins. Recombinant DicB has no ability to induce discoidin expression, so it is unclear whether DicB is a contaminant in the preparation or is a component of the DIC complex.
Dictyostelium cells secrete several large extracellular factors. CF is a protein complex with a size (450 kDa) similar to that of DIC, which is secreted during early development and which plays a role in determining group size. CF's biochemical properties during purification are different from those of DIC, and its subunit composition is different (6). In addition, we found that countin− cells still produce discoidin-inducing activity with an unchanged molecular mass on gel filtration columns (data not shown). Iijima et al. described a 450-kDa protein complex, CMF450, with subunits of 49, 79, and 94 kDa (30), which is secreted at the transition from growth to development and favors entry into development. Proteins with a molecular mass of 45 or 94 kDa were not obvious in our preparation, and the peptide sequence determined for the 45-kDa subunit, EQNEDKDDDFSGTH, is not found in DicA1 or DicB (no sequence data were obtained for the 79-kDa subunit of Iijima and colleagues). In addition, since this protein complex does not induce discoidin gene expression, we conclude that the two complexes are different. PSI, which induces prespore cell differentiation and which shows significant sequence similarity to DicA1, is probably a homodimer under native conditions, giving a native size of 180 kDa (38). DIC is thus different from known Dictyostelium signaling complexes.
An interesting possibility is that DicA1 is a component of PSF. PSF is a protein secreted during growth and early development that accumulates in the medium and induces discoidin expression in a dose-dependent manner (8, 12, 45, 46). PSF has not yet been purified to homogeneity; however, it has been enriched from medium conditioned by growing cells, and we observe DicA1 in medium conditioned by growing as well as starving cells. In addition, the dicA1 mRNA is expressed in growing cells, supporting the idea that like PSF, DicA1 is produced and secreted by growing cells. The similarities between PSF and DicA1 are thus their ability to induce discoidin expression, secretion during growth and early development, and the fact that transduction of the signal does not require Gβ. In addition, PSF has been described as a protein of about 70 kDa on gel filtration columns (8). Whether this size difference is due to different purification conditions, which for PSF included reducing agents and a low concentration of nonionic detergent to minimize nonspecific protein-protein interactions remains unclear. Irrespective of whether DicA1 is PSF, it is part of a factor secreted by a population of cells that induces gene expression (in this case, discoidin) in the cells that secrete it. DicA1 is thus a component of a quorum sensor or a cell density sensor and is a new example of this intriguing class of signals.
Acknowledgments
We thank Harry MacWilliams and Horst Feldmann for advice, the Tsukuba cDNA sequencing consortium and the international genome sequencing consortium for generating sequences used in this work, and Jacob Franke and Rich Kessin for the PDE knockout strains. We thank Margaret Clarke for advice and assistance with the manuscript and Margaret Clarke and Lucinda Maddera for assaying DicA1 for PSF activity.
This work was supported by the Deutsche Forschungsgemeinschaft: Graduiertenkolleg “Developmental biology” to A.K. and SFB190 “Factors and mechanisms of gene regulation” to B.W. R.H.G. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Alexander, S., L. M. Sydow, D. Wessels, and D. R. Soll. 1992. Discoidin proteins of Dictyostelium are necessary for normal cytoskeletal organization and cellular morphology during aggregation. Differentiation 51:149-161. [DOI] [PubMed] [Google Scholar]
- 2.Anjard, C., M. van Bemmelen, M. Veron, and C. D. Reymond. 1997. A new spore differentiation factor (SDF) secreted by Dictyostelium cells is phosphorylated by the cAMP dependent protein kinase. Differentiation 62:43-49. [DOI] [PubMed] [Google Scholar]
- 3.Anjard, C., C. Zeng, W. F. Loomis, and W. Nellen. 1998. Signal transduction pathways leading to spore differentiation in Dictyostelium discoideum. Dev. Biol. 193:146-155. [DOI] [PubMed] [Google Scholar]
- 4.Brazill, D. T., D. F. Lindsey, J. D. Bishop, and R. H. Gomer. 1998. Cell density sensing mediated by a G protein-coupled receptor activating phospholipase C. J. Biol. Chem. 273:8161-8168. [DOI] [PubMed] [Google Scholar]
- 5.Brock, D. A., F. Buczynski, T. P. Spann, S. A. Wood, J. Cardelli, and R. H. Gomer. 1996. A Dictyostelium mutant with defective aggregate size determination. Development 122:2569-2578. [DOI] [PubMed] [Google Scholar]
- 6.Brock, D. A., and R. H. Gomer. 1999. A cell-counting factor regulating structure size in Dictyostelium. Genes Dev. 13:1960-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brookman, J. J., K. A. Jermyn, and R. R. Kay. 1987. Nature and distribution of the morphogen DIF in the Dictyostelium slug. Development 100:119-124. [DOI] [PubMed] [Google Scholar]
- 8.Burdine, V., and M. Clarke. 1995. Genetic and physiologic modulation of the prestarvation response in Dictyostelium discoideum. Mol. Biol. Cell 6:311-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceccarelli, A., S. J. McRobbie, K. A. Jermyn, K. Duffy, A. Early, and J. G. Williams. 1987. Structural and functional characterization of a Dictyostelium gene encoding a DIF inducible, prestalk-enriched mRNA sequence. Nucleic Acids Res. 15:7463-7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chassy, B. M. 1972. Cyclic nucleotide phosphodiesterase in Dictyostelium discoideum: interconversions of two enzyme forms. Science 175:1016-1018. [DOI] [PubMed] [Google Scholar]
- 11.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 12.Clarke, M., and R. H. Gomer. 1995. PSF and CMF, autocrine factors that regulate gene expression during growth and early development of Dictyostelium. Experientia 51:1124-1134. [DOI] [PubMed] [Google Scholar]
- 13.Clarke, M., S. C. Kayman, and K. Riley. 1987. Density-dependent induction of discoidin-I synthesis in exponentially growing cells of Dictyostelium discoideum. Differentiation 34:79-87. [DOI] [PubMed] [Google Scholar]
- 14.Clarke, M., J. Yang, and S. Kayman. 1988. Analysis of the prestarvation response in growing cells of Dictyostelium discoideum. Dev. Genet. 9:315-326. [DOI] [PubMed] [Google Scholar]
- 15.Demicheli, R. 2001. Tumour dormancy: findings and hypotheses from clinical research on breast cancer. Semin. Cancer Biol. 11:297-306. [DOI] [PubMed] [Google Scholar]
- 16.Dormann, D., B. Vasiev, and C. Weijer. 2000. The control of chemotactic cell movement during Dictyostelium morphogenesis. Philos. Trans. R. Soc. London B 355:983-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckerskorn, C., and F. Lottspeich. 1989. Chromatographia 28:92-94. [Google Scholar]
- 18.Edman, P., and G. Begg. 1967. A protein sequenator. Eur. J. Biochem. 1:80-91. [DOI] [PubMed] [Google Scholar]
- 19.Faure, M., J. Franke, A. L. Hall, G. J. Podgorski, and R. H. Kessin. 1990. The cyclic nucleotide phosphodiesterase gene of Dictyostelium discoideum contains three promoters specific for growth, aggregation, and late development. Mol. Cell. Biol. 10:1921-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Firtel, R. A. 1996. Interacting signaling pathways controlling multicellular development in Dictyostelium. Curr. Opin. Genet. Dev. 6:545-554. [DOI] [PubMed] [Google Scholar]
- 21.Franke, J., M. Faure, L. Wu, A. L. Hall, G. J. Podgorski, and R. H. Kessin. 1991. Cyclic nucleotide phosphodiesterase of Dictyostelium discoideum and its glycoprotein inhibitor: structure and expression of their genes. Dev. Genet. 12:104-112. [DOI] [PubMed] [Google Scholar]
- 22.Franke, J., and R. H. Kessin. 1981. The cyclic nucleotide phosphodiesterase inhibitory protein of Dictyostelium discoideum. Purification and characterization. J. Biol. Chem. 256:7628-7637. [PubMed] [Google Scholar]
- 23.Gomer, R. H. 2001. Not being the wrong size. Nat. Rev. Mol. Cell Biol. 2:48-54. [DOI] [PubMed] [Google Scholar]
- 24.Gomer, R. H. 1987. A strategy to study development and pattern formation: use of antibodies against products of cloned genes. Methods Cell Biol. 28:471-487. [DOI] [PubMed] [Google Scholar]
- 25.Gomer, R. H., I. S. Yuen, and R. A. Firtel. 1991. A secreted 80x103 Mr protein mediates sensing of cell density and the onset of development in Dictyostelium. Development 112:269-278. [DOI] [PubMed] [Google Scholar]
- 26.Grabel, L., and W. F. Loomis. 1978. Effector controlling accumulation of N-acteylglucosaminidase during development of Dictyostelium discoideum. Dev. Biol. 64:203-209. [DOI] [PubMed] [Google Scholar]
- 27.Gross, J. D. 1994. Developmental decisions in Dictyostelium discoideum. Microbiol. Rev. 58:330-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guba, M., G. Cernaianu, G. Koehl, E. K. Geissler, K.-W. Jauch, M. Anthuber, W. Falk, and M. Steinbauer. 2001. A primary tumor promotes dormancy of solitary tumor cells before inhibiting angiogenesis. Cancer Res. 61:5575-5579. [PubMed] [Google Scholar]
- 29.Hall, A. L., J. Franke, M. Faure, and R. H. Kessin. 1993. The role of the cyclic nucleotide phosphodiesterase of Dictyostelium discoideum during growth, aggregation, and morphogenesis: overexpression and localization studies with the separate promoters of the pde. Dev. Biol. 157:73-84. [DOI] [PubMed] [Google Scholar]
- 30.Iijima, N., T. Takagi, and Y. Maeda. 1995. A proteinous factor mediating intercellular communication during the transition of Dictyostelium cells from growth to differentiation. Zool. Sci. 12:61-69. [DOI] [PubMed] [Google Scholar]
- 31.Klein, C., and M. Darmon. 1976. A differentiation stimulating factor induces cell sensitivity to 3,5′-cyclic AMP pulses in Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 73:1250-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konijn, T. M., J. G. C. van de Meene, Y. Y. Chang, D. S. Barkley, and J. T. Bonner. 1969. Identification of adenosine-3′,5′-monophosphate as the bacterial attractant for myxoamoebae of Dictyostelium discoideum. J. Bacteriol. 99:510-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lilly, P., L. J. Wu, D. L. Welker, and P. N. Devreotes. 1993. A G-protein beta-subunit is essential for Dictyostelium development. Genes Dev. 7:986-995. [DOI] [PubMed] [Google Scholar]
- 34.Marschalek, R., J. Hofmann, G. Schumann, R. Gosseringer, and T. Dingermann. 1992. Structure of DRE, a retrotransposable element which integrates with position specificity upstream of Dictyostelium discoideum tRNA genes. Mol. Cell. Biol. 12:229-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehdy, M. C., and R. A. Firtel. 1985. A secreted factor and cyclic AMP jointly regulate cell-type-specific gene expression in Dictyostelium discoideum. Mol. Cell. Biol. 5:705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morio, T., H. Urushihara, T. Saito, Y. Ugawa, H. Mizuno, M. Yoshida, R. Yoshino, B. Mitra, M. Pi, T. Sato, K. Takemoto, H. Yasukawa, J. Williams, M. Maeda, I. Takeuchi, H. Ochiai, and Y. Tanaka. 1998. The Dictyostelium developmental cDNA project: generation and analysis of expressed sequence tags from the first-finger stage of development. DNA Res. 5:335-340. [DOI] [PubMed] [Google Scholar]
- 37.Morris, H. R., G. W. Taylor, M. S. Masento, K. A. Jermyn, and R. R. Kay. 1987. Chemical structure of the morphogen differentiation inducing factor from Dictyostelium discoideum. Nature 328:811-814. [DOI] [PubMed] [Google Scholar]
- 38.Nakagawa, M., A. Oohata, H. Tojo, and S. Fujii. 1999. A prespore-cell-inducing factor in Dictyostelium discoideum: its purification and characterization. Biochem. J. 343:265-271. [PMC free article] [PubMed] [Google Scholar]
- 39.Okuwa, T., T. Katayama, A. Takano, K. Kodaira, and H. Yasukawa. 2001. Two cell-counting factors regulate the aggregate size of the cellular slime mold Dictyostelium discoideum. Dev. Growth Differ. 43:735-744. [DOI] [PubMed] [Google Scholar]
- 40.Oohata, A. A., M. Nakagawa, M. Tasaka, and S. Fujii. 1997. A novel prespore-cell-inducing factor in Dictyostelium discoideum induces cell division of prespore cells. Development 124:2781-2787. [DOI] [PubMed] [Google Scholar]
- 41.Orlow, S. J., I. Shapiro, J. Franke, and R. H. Kessin. 1981. The extracellular cyclic nucleotide phosphodiesterase of Dictyostelium discoideum. Purification and characterization. J. Biol. Chem. 256:7620-7627. [PubMed] [Google Scholar]
- 42.Patterson, S. 1994. From electrophoretically separated protein to identification: strategies for sequence and mass analysis. Anal. Biochem. 221:1-15. [DOI] [PubMed] [Google Scholar]
- 43.Pelle, R., and N. Murphy. 1993. Northern hybridization: rapid and simple electrophoretic conditions. Nucleic Acids Res. 21:2783-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Primpke, G., V. Iassonidou, W. Nellen, and B. Wetterauer. 2000. Role of cAMP-dependent protein kinase during growth and early development of Dictyostelium discoideum. Dev. Biol. 221:101-111. [DOI] [PubMed] [Google Scholar]
- 45.Rathi, A., and M. Clarke. 1992. Expression of early developmental genes in Dictyostelium discoideum is initiated during exponential growth by an autocrine-dependent mechanism. Mech. Dev. 36:173-182. [DOI] [PubMed] [Google Scholar]
- 46.Rathi, A., S. C. Kayman, and M. Clarke. 1991. Induction of gene expression in Dictyostelium by prestarvation factor, a factor secreted by growing cells. Dev. Genet. 12:82-87. [DOI] [PubMed] [Google Scholar]
- 47.Rigden, D. J., L. V. Mello, and M. Y. Galperin. 2004. The PA14 domain, a conserved all-β domain in bacterial toxins, enzymes, adhesins and signaling molecules. Trends Biochem. Sci. 29:335-339. [DOI] [PubMed] [Google Scholar]
- 48.Roisin-Bouffay, C., W. Jang, D. R. Caprette, and R. H. Gomer. 2000. A precise group size in Dictyostelium is generated by a cell-counting factor modulating cell-cell adhesion. Mol. Cell 6:953-959. [PubMed] [Google Scholar]
- 49.Sucgang, R., C. J. Weijer, F. Siegert, J. Franke, and R. H. Kessin. 1997. Null mutations of the Dictyostelium cyclic nucleotide phosphodiesterase gene block chemotactic cell movement in developing aggregates. Dev. Biol. 192:181-192. [DOI] [PubMed] [Google Scholar]
- 50.Tang, L., R. Ammann, T. Gao, and R. H. Gomer. 2001. A cell number-counting factor regulates group size in Dictyostelium by differentially modulating cAMP-induced cAMP and cGMP pulse sizes. J. Biol. Chem. 276:27663-27669. [DOI] [PubMed] [Google Scholar]
- 51.Thompson, C., and R. Kay. 2000. The role of DIF-1 signaling in Dictyostelium development. Mol. Cell 6:1509-1514. [DOI] [PubMed] [Google Scholar]
- 52.Weeks, G. 2000. Signalling molecules involved in cellular differentiation during Dictyostelium morphogenesis. Curr. Opin. Microbiol. 3:625-630. [DOI] [PubMed] [Google Scholar]
- 53.Wetterauer, B., P. Morandini, I. Hribar, I. Murgia-Morandini, U. Hamker, C. Singleton, and H. K. MacWilliams. 1996. Wild-type strains of Dictyostelium discoideum can be transformed using a novel selection cassette driven by the promoter of the ribosomal V18 gene. Plasmid 36:169-181. [DOI] [PubMed] [Google Scholar]
- 54.Whitbread, J. A., M. Sims, and E. R. Katz. 1991. Evidence for the presence of a growth factor in Dictyostelium discoideum. Dev. Genet. 12:78-81. [DOI] [PubMed] [Google Scholar]
- 55.Williams, J. G., A. Ceccarelli, S. McRobbie, H. Mahbubani, R. R. Kay, A. Farly, M. Berks, and K. A. Jermyn. 1987. Direct induction of Dictyostelium prestalk gene expression by DIF provides evidence that DIF is a morphogen. Cell 49:185-192. [DOI] [PubMed] [Google Scholar]
- 56.Yuen, I. S., R. Jain, J. D. Bishop, D. F. Lindsey, W. J. Deery, P. J. M. Van Haastert, and R. H. Gomer. 1995. A density-sensing factor regulates signal transduction in Dictyostelium. J. Cell Biol. 129:1251-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuen, I. S., C. Taphouse, K. A. Halfant, and R. H. Gomer. 1991. Regulation and processing of a secreted protein that mediates sensing of cell density in Dictyostelium. Development 113:1375-1385. [DOI] [PubMed] [Google Scholar]