Abstract
We demonstrate here that SAP155 encodes a negative modulator of K+ efflux in the yeast Saccharomyces cerevisiae. Overexpression of SAP155 decreases efflux, whereas deletion increases efflux. In contrast, a homolog of SAP155, called SAP185, encodes a positive modulator of K+ efflux: overexpression of SAP185 increases efflux, whereas deletion decreases efflux. Two other homologs, SAP4 and SAP190, are without effect on K+ homeostasis. Both SAP155 and SAP185 require the presence of SIT4 for function, which encodes a PP2A-like phosphatase important for the G1-S transition through the cell cycle. Overexpression of either the outwardly rectifying K+ channel, Tok1p, or the putative plasma membrane K+/H+ antiporter, Kha1p, increases efflux in both wild-type and sit4Δ strains. However, overexpression of the Na+-K+/H+ antiporter, Nha1p, is without effect in a sit4Δ strain, suggesting that Sit4p signals to Nha1p. In summary, the combined activities of Sap155p and Sap185p appear to control the function of Nha1p in K+ homeostasis via Sit4p.
K+, implicated in translation and thus replication (22, 25-27), is the most abundant cation found in cells. K+ has been implicated in numerous other important cellular processes. For example, depletion of K+ causes inhibition of endocytosis (13, 19-21), presumably because clathrin cages cannot be disassembled by Hsc70, an enzyme requiring K+ for function (35, 48). K+ is crucial for establishing membrane potential, for import and export of molecules into and out of cells, for pH control, and for osmolarity control in plants and fungi (11, 42). Finally, recent work has shown that ion homeostasis is linked to cell cycle regulation (7, 9, 10, 31, 50) and is mediated in part by a G1-S regulatory protein, the phosphatase, Sit4p (31). Despite the importance of K+ in this broad spectrum of cell functions, little is understood about the molecular mechanisms controlling intracellular K+ levels.
Cytosolic K+ homeostasis is controlled by the competing activities of influx, efflux, and storage. In the yeast, Saccharomyces cerevisiae, Trk1p mediates high-affinity uptake (12). Trk2p, which is 55% identical to Trk1, mediates medium-affinity uptake (17, 18, 38). Low-affinity uptake is mediated by a variety of other transporters, including NSC1, an activity described electrochemically, but for which no protein has yet been identified (5, 6, 39).
Efflux is thought to be mediated by one of three different transporters. The K+ channel, Tok1p, shows similarities to outward rectifying channels of higher eukaryotes (4, 23, 24, 51), although evidence suggests that this protein can also mediate influx under certain conditions (4). Nha1p, a Na+-K+/H+ antiporter, localizes to the plasma membrane and is involved in regulation of both intracellular ion concentration and regulation of the cell cycle (2, 3, 47). Another antiporter, Kha1p, which exchanges K+ for H+, was initially thought to localize to the plasma membrane (37), but recent evidence suggests that it is instead localized to internal membranes, perhaps the Golgi apparatus (30). The vacuole is responsible for K+ storage (36), carried out by proteins whose identities are currently unknown.
The regulation of K+ influx and efflux activities across the plasma membrane so as to maintain the cytosolic level of K+ is not well understood. Recently, we found that loss of the guanine-nucleotide-binding protein gene, ARL1, causes a defect in K+ influx (33), suggesting a novel means for regulation of this process. Loss of ARL1 is accompanied by sensitivity to a number of different toxic cations, including the translation inhibitor, hygromycin B (33, 34). A high-copy suppressor screen for genes that permit growth of arl1Δ strains in the presence of hygromycin B identified SAP155 (33).
SAP155 and three homologs—SAP4, SAP185, and SAP190—encode proteins that interact with Sit4p (28). Sit4p, a PP2A-like phosphatase, was first identified as a regulator of G1-S transition (46) but also plays a role in K+ homeostasis, since overexpression of SIT4 causes increased K+ efflux from cells (31). The Sap proteins may be substrates for Sit4p's phosphatase activity since they are hyperphosphorylated in a sit4Δ strain (28). Recent work has demonstrated that the Sap proteins, in conjunction with Sit4, function to control Tor-mediated transcription and translation (40). Interestingly, only SAP155 overexpression protects cells from the deleterious effects of the cell cycle inhibitor, zymocin, from Kluyveromyces lactis (16). Similarly, only SAP155 suppresses the hygromycin B-sensitive phenotype of the arl1Δ mutant (33).
Based on this information, we hypothesized that Sap155p inhibits Sit4p's ability to promote K+ efflux. Through a variety of experiments, we establish the validity of this hypothesis and further demonstrate that a homolog of Sap155p, Sap185p, promotes K+ efflux. Finally, we identify Nha1p as a downstream target of Sit4p. Thus, the combined activities of Sap155p and Sap185p control Sit4p's ability to regulate K+ efflux through Nha1p.
MATERIALS AND METHODS
Yeast strains.
For strains used in the present study, see Table 1. All haploid strains were derived from BY4742 from the Deletion Collection (Open Biosystems, Huntsville, AL) (49), and all mutations were confirmed by PCR analysis with an oligonucleotide specific to the gene used to replace the open reading frame (KanMX or HIS3) and one specific to the gene deletion of interest in the reverse direction from the 3′ downstream region. Strains were routinely grown in either rich medium (YPAD) or minimal medium (SD) with appropriate supplements to cover auxotrophies (1, 43). In one experiment, dextrose was replaced with galactose for SGal medium. Genotypes of the diploid strains from the deletion collection were also confirmed by PCR analysis. Antibiotics (Geneticin [0.2 mg/ml] and hygromycin B [up to 0.1 mg/ml]) were added to YPAD medium after autoclaving.
TABLE 1.
Strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| Haploids | ||
| AM150 (BY4742) | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | 49 |
| AM151 | AM150 arl1Δ::HIS3 | This study |
| SP101 | AM150 sap155Δ::KanMX | 49 |
| DH101 | AM150 arl1Δ::HIS3 sap155Δ::KanMX | This study |
| CM101 | AM150 sit4Δ::KanMX | 49 |
| Diploids | ||
| BY4743 | MATa/α his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 +/lys2Δ0 +/met15Δ0 ura3Δ0/ura3Δ0 | 49 |
| Sap4 | BY4743 sap4Δ::KanMX/ sap4Δ::KanMX | 49 |
| Sap155 | BY4743 sap155Δ::KanMX/sap155Δ::KanMX | 49 |
| Sap185 | BY4743 sap185Δ::KanMX/sap185Δ::KanMX | 49 |
| Sap190 | BY4743 sap190Δ::KanMX/sap190Δ::KanMX | 49 |
| Sit4 | BY4743 sit4Δ::KanMX/sit4Δ::KanMX | 49 |
Plasmids.
For plasmids used in the present study, see Table 2. Plasmids were propagated in Escherichia coli (DH10B or DH5α) grown on LB medium containing ampicillin (100 mg/liter), which was added after autoclaving. Plasmids were isolated from E. coli by using commercially available kits from either Promega (Madison, WI) or Qiagen (Valencia, CA). Transformation of yeast was done by using the method of Ito et al. (15), but the 42°C incubation was extended from 15 min to a maximum of 2 h.
TABLE 2.
Plasmids used in this studya
| Plasmid | Description | Source or reference |
|---|---|---|
| YEp352 | Parental vector | 14 |
| pARY1-3 | ARL1 in YEp352 | 41 |
| YEp24 | Parental vector | 8 |
| CB2643 | SAP155 in YEp24 | 28 |
| CB2606 | SAP4 in YEp24 | 28 |
| CB2819 | SAP185 in YEp24 | 28 |
| CB2925 | SAP190 in YEp24 | 28 |
| pSIT4 | SIT4 in high-copy vector | Kim Arndt |
| pYGW1 | pGAL1/10::TOK1 in Geneweaver vector | Clifford Slayman |
| pNHA1 | NHA1 in YEp352 | Joaquin Ariño |
| pKHA1 | KHA1 in YEp352 | Joaquin Ariño |
All vectors used in this study contain URA3 as the yeast selectable marker.
86Rb+ studies.
86Rb+ influx was performed as previously described (33) by the method of Mulet et al. (32). Efflux was measured by using the following protocol. Cells were grown overnight in SD medium (with appropriate supplements to cover auxotrophies) to an optical density at 600 nm of 0.8, washed twice with distilled H2O, resuspended in K+ starvation medium (50 mM succinic acid [pH 5.5], 2% glucose, 1% casein digest), and incubated for 4 h at 30°C. Cells were then washed three times with distilled H2O and resuspended in reaction buffer (100 mM succinic acid [pH 5.5], 8% glucose) to a concentration of 10 optical density units (OD)/ml. 86RbCl2 (2.2 μCi/ml, 0.2 mM RbCl2 [final concentration]) was added to each 10 OD of cells. One 100-μl aliquot (corresponding to 1 OD of cells) was taken immediately (zero time) and a second was taken after incubation at room temperature for 60 min (the loading/influx period). Cells were then collected by centrifugation, resuspended in room temperature reaction buffer containing 0.2 mM cold RbCl2, and then incubated for up to 3 h. Aliquots were removed over time and then added to 10 ml of ice-cold 20 mM MgCl2. Cells were collected on nitrocellulose and washed extensively with ice-cold 20 mM MgCl2, and the filters counted by scintillation spectroscopy.
Atomic absorption spectroscopy.
Total internal K+ concentrations were determined by atomic absorption spectroscopy using a method similar to that described previously (36). Briefly, cells were grown in YPAD medium overnight and then grown for 12 h in SD medium with the required growth supplements. Cells (15 OD units) were then incubated for 8 h in 5 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid); pH 6.5] containing 100 mM KCl and 30 mM glucose. Cells were then washed three times in 5 mM PIPES with 30 mM glucose. Cell pellets were dissolved in 500 μl of 6 M HNO3 and incubated at room temperature for 4 h. Samples were then diluted to 5 ml total with distilled H2O. K+ content was determined by using a Buck 200A spectrophotometer with a K+ lamp tuned to 763.5 nm compared to standards.
RESULTS AND DISCUSSION
SAP155 and SAP185 modulate hygromycin B sensitivity.
We previously demonstrated that an arl1Δ mutant strain was sensitive to hygromycin B and other toxic cations and that SAP155, but not SAP4, SAP185, or SAP190, suppressed the hygromycin B-sensitive phenotype (33). We initially assumed that SAP155 was downstream of ARL1 and expected that an arl1Δ sap155Δ double mutant would have the same phenotype as an (ARL1) sap155Δ mutant. However, the double mutant was actually more sensitive to hygromycin B than either single mutant (Fig. 1), suggesting that SAP155 functions in a separate pathway, one that does not contain ARL1. As observed in our previous studies with the arl1Δ mutant, increased K+ in the medium suppressed the hygromycin B-sensitive phenotype of the sap155Δ and arl1Δ sap155Δ mutants (data not shown).
FIG. 1.
An arl1Δ sap155Δ mutant is more sensitive to hygromycin B than either single mutant. Strains AM150 (ARL1 SAP155), AM151 (arl1Δ SAP155), SP101 (ARL1 sap155Δ), and DH101 (arl1Δ sap155Δ) were grown overnight in YPAD medium and then pelleted by centrifugation, resuspended to an optical density at 600 nm of 5 OD/ml, and subjected to 10-fold serial dilutions in water. Cells were spotted onto solid YPAD medium with or without 0.05 mg of hygromycin B/ml by using a replicator tool. Plates were photographed after overnight incubation at 30°C.
To examine the effects of ARL1 and SAP155 on steady-state levels of intracellular K+, cells were prepared for atomic absorption spectroscopy. Wild-type (ARL1 SAP155) cells contained 83.4 ± 3.0 μmol/OD, arl1Δ SAP155 cells contained 39.5 ± 1.2 μmol/OD (47.1% of wild type), ARL1 sap155Δ cells contained 63.1 ± 2.4 μmol/OD (75.7% of wild type), and the double mutant (arl1Δ sap155Δ) cells contained 34.7 ± 3.0 μmol/OD (41.6% of wild type). Thus, loss of either gene lowers the steady-state levels of K+ in cells, whereas loss of both genes lowers the steady state more. This result, like the hygromycin B sensitivity phenotypes of these strains, is consistent with the hypothesis that ARL1 and SAP155 act in separate pathways.
The presence of SAP155 on a high-copy vector conferred resistance to hygromcyin B in wild-type cells. Surprisingly, SAP185 conferred sensitivity. The presence of either SAP4 or SAP190 on a high-copy vector had no effect compared to empty vector (Fig. 2, top panel). Similar results were observed in the arl1Δ strain, although such strains were more sensitive to hygromycin B overall (not shown). Further, loss of SAP155 conferred hygromycin B sensitivity, whereas loss of SAP185 conferred resistance. Loss of either SAP4 or SAP190 was without effect (Fig. 2, bottom panel).
FIG. 2.
SAP155 and SAP185 modulate responses of cells to hygromycin B. (Top) Overexpression of the SAP genes. Wild-type cells (strain BY4743) were transformed with either YEp24 (empty vector), CB2643 (SAP155 in YEp24), CB2606 (SAP4 in YEp24), CB2819 (SAP185 in YEp24), or CB2925 (SAP190 in YEp24) and selected for growth on SD medium lacking uracil. Transformants were then grown overnight in selective medium, diluted to an optical density at 600 nm of 5 OD/ml, serially diluted as described above, and spotted onto solid YPAD with or without 0.075 mg of hygromcyin B/ml. Plates were photographed after overnight incubation at 30°C. (Bottom) Deletion of the SAP genes. Homozygous diploid strains lacking both copies of each SAP gene were grown overnight in YPAD and then treated as in the top panel.
SAP155 and SAP185 modulate K+efflux.
Since intracellular K+ levels are controlled by both influx and efflux, we hypothesized that SAP155 encodes a negative modulator of K+ efflux, reasoning that preventing the loss of K+ from cells should protect against taking up too much hygromycin B. Indirect support for this hypothesis came from the genetic experiment shown in Fig. 1, since the loss of SAP155 in an arl1Δ mutant exacerbated the arl1Δ phenotype, suggesting that SAP155 was not involved in K+ influx.
To test the efflux hypothesis directly, we loaded cells with 86Rb+, a congener of K+ that has been used extensively for studies of K+ flux in yeast (29, 31-33, 50) and then monitored the loss of radioactivity over time. As predicted, overexpression of SAP155 inhibited efflux, whereas overexpression of SAP185 increased efflux (Fig. 3, top panel). In contrast, a sap155Δ strain exhibited increased efflux and a sap185Δ strain exhibited decreased efflux relative to wild type (Fig. 3, bottom panel). The presence or absence of SAP4 or SAP190 was without effect (not shown). These results are completely consistent with the hygromycin B sensitivity data shown in Fig. 2.
FIG. 3.
SAP155 and SAP185 modulate K+ efflux. Strains as listed in both panels were grown and prepared for efflux as described in Materials and Methods. After a 60-min influx period, cells were washed and resuspended in buffer containing cold RbCl. Aliquots were removed over time, cells were collected by filtration, and filters were counted by scintillation spectroscopy. The data shown are average of triplicate determinations (± the standard deviation). Each of these experiments was repeated twice with similar results. (Top) Overexpression of the SAP genes. 86Rb+ efflux from wild-type cells (strain BY4743) transformed with either YEp24 (empty vector), CB2643 (SAP155 in YEp24), or CB2819 (SAP185 in YEp24) was determined. (Bottom) Deletion of the SAP genes. 86Rb+ efflux from wild-type, sap155Δ, and sap185Δ strains was determined.
We examined the steady-state levels of K+ in strains with either SAP155 or SAP185 deleted or overexpressing one of these two SAP genes. Wild-type cells contained 86.1 ± 4.0 μmol/OD, the sap155Δ strain contained 40.4 ± 2.05 μmol/OD (46.9% of wild type), and the sap185Δ strain contained 129.9 ± 2.0 μmol/OD (151% of wild type). Wild-type cells with empty vector contained 87.2 ± 8.0 μmol/OD, with the SAP155 vector contained 130.0 ± 5.4 μmol/OD (149% of wild type with empty vector), and with the SAP185 vector contained 43.8 ± 4.3 μmol/OD (50% of wild type with empty vector). Thus, the results from steady-state analysis of intracellular K+ levels are consistent with the 86Rb+ efflux and hygromycin B sensitivity data.
Further, overexpression of SAP155 in a sap185Δ mutant tipped the balance more toward retention, whereas overexpression of SAP185 in a sap155Δ mutant tipped the balance more toward efflux (Table 3). In summary, these results suggest that the combined activities of Sap155p and Sap185p modulate K+ efflux. In contrast, the loss of ARL1 had no effect on efflux (data not shown).
TABLE 3.
Sap155p inhibits efflux and Sap185 promotes effluxa
| Expt | Strain | Plasmid | Growth on hygromycin B | 86Rb+ efflux (% change/min) ± SD |
|---|---|---|---|---|
| A | WT | + EV | ++ | −0.390 ± 0.038 |
| + SAP155 | ++++ | −0.271 ± 0.063 | ||
| + SAP185 | ± | −0.525 ± 0.022 | ||
| + SIT4 | ++ | −0.484 ± 0.061 | ||
| B | WT | + EV | ++ | −0.387 ± 0.042 |
| + SAP4 | ++ | −0.372 ± 0.021 | ||
| + SAP190 | ++ | −0.401 ± 0.032 | ||
| C | WT | + EV | ++++ | −0.375 ± 0.019 |
| sap155Δ | + EV | ++ | −0.507 ± 0.037 | |
| + SAP155 | ++++ | −0.243 ± 0.067 | ||
| + SAP185 | − | −0.630 ± 0.026 | ||
| + SIT4 | ± | −0.605 ± 0.020 | ||
| D | WT | + EV | ++++ | −0.324 ± 0.037 |
| sap185Δ | + EV | ++ | −0.417 ± 0.007 | |
| + SAP155 | +++ | −0.233 ± 0.054 | ||
| + SAP185 | − | −0.658 ± 0.010 | ||
| +SIT4 | − | −0.533 ± 0.002 | ||
| E | WT | + EV | +++ | n.d. |
| sit4Δ | + EV | + | −0.489 ± 0.060 | |
| + SAP155 | + | −0.505 ± 0.039 | ||
| + SAP185 | + | −0.513 ± 0.036 | ||
| + SIT4 | +++ | −0.650 ± 0.008 |
Each division (A to E) shows a single experiment. Each experiment was repeated at least twice. 86Rb+ efflux experiments were performed as outlined in Materials and Methods. Data from experiment A are shown graphically in Fig. 3 (top). Hygromycin B experiments were performed with different concentrations of hygromycin B to account for the differences in hygromycin B sensitivity observed in the different deletion strains. Therefore, although the 86Rb+ efflux experiments can in general be directly compared between experiments, the hygromcyin B experiments can only be compared to other data within that division. In experiments A and B strains were compared on medium containing 0.075 mg of hygromycin B/ml (as shown in Fig. 2), in experiments C and E strains were compared on medium containing 0.05 mg of hygromycin B/ml, and in experiment D strains were compared on medium containing 0.125 mg of hygromycin B/ml. EV, empty vector; WT, wild type.
Previous work by Arndt's laboratory (28) suggested that SAP185 and SAP190 were functional homologs of one another, since these two proteins are 42% identical overall, the highest level of homology between any two of the Sap proteins; nevertheless, our study clearly distinguishes between the functions of these two: SAP185 encodes a regulator of K+ efflux, whereas SAP190 does not. This result may suggest that instead of two pairs of functional homologs (SAP185 and SAP190 compared to SAP4 and SAP155), all four Sap proteins have independent, unique functions.
Sap155p and Sap185p require Sit4p for function.
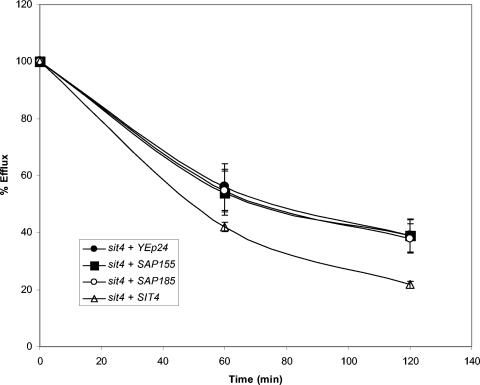
The Sap proteins bind to Sit4p and Sit4p requires the Saps for function, since a strain missing all four SAP genes has the same phenotype as a sit4Δ strain (28). The Sap proteins are hyperphosphorylated in a sit4Δ strain, thus potential substrates for the phosphatase activity of Sit4p (28). Further, overexpression of SIT4 causes increased K+ efflux and expression of SIT4 is induced by K+ (31). We therefore investigated the role of SIT4 with respect to the two SAP genes of interest. A sit4Δ strain was transformed with empty vector or vector containing SAP155, SAP185, or SIT4, and then transformants were loaded with 86Rb+. Neither overexpression of SAP155 or SAP185 affects K+ efflux in a sit4Δ strain, demonstrating that both SAP155 and SAP185 require the presence of SIT4 for the modulation of K+ efflux (Fig. 4 and Table 3).
FIG. 4.
SIT4 is required for the function of the SAP genes. 86Rb+ efflux from the sit4Δ strain transformed with YEp24 (empty vector), CB2643 (SAP155 in YEp24), CB2819 (SAP185 in YEp24), and pSIT4 was determined as in Fig. 3 above. This experiment was repeated twice with similar results.
The ability of Nha1p to promote K+ efflux is modulated by Sit4p.
Three proteins have been suggested to play roles in efflux of K+ from cells. These are Tok1p, an inward rectifying K+ channel (4, 23, 24, 51); Nha1p, a Na+-K+/H+ antiporter (2, 3, 47), and Kha1p, a K+/H+ antiporter (37). However, recent evidence suggests this last protein is not localized to the plasma membrane and may therefore not play roles in efflux from the cytosol across the plasma membrane (30).
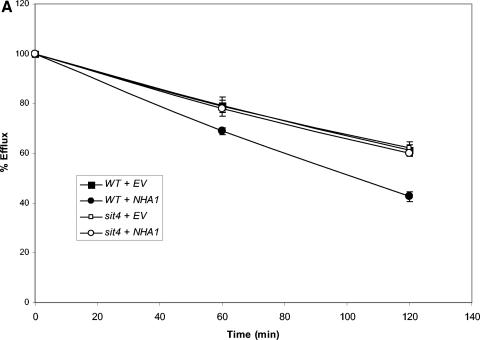
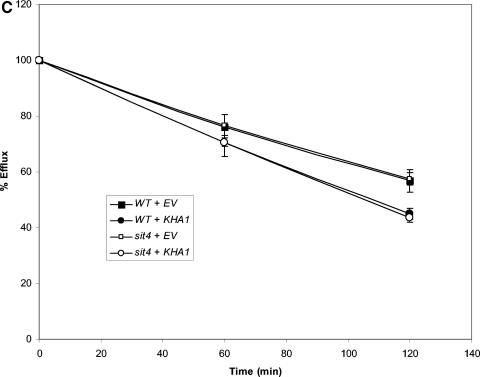
To distinguish among these three possibilities, we examined the effect of overexpression of each of the three in wild-type and sit4Δ strains, reasoning that the absence of SIT4 would render overexpression of one or more of these proteins without effect. Overexpression of any one of the three increased efflux in wild-type cells (Fig. 5). Overexpression of TOK1 (Fig. 5B) or KHA1 (Fig. 5C) in the sit4Δ strain also led to increased efflux. In contrast, overexpression of NHA1 (Fig. 5A) was without effect in the sit4Δ strain, supporting the hypothesis that Sit4p signals to Nha1p to direct K+ efflux but not to Kha1p or Tok1p.
FIG. 5.
Overexpression of NHA1, but not TOK1 or KHA1, is without effect in a sit4Δ strain. 86Rb+ efflux was measured in cells transformed with either empty vector (EV) or vector containing NHA1, TOK1, or KHA1 to determine which gene required the presence of SIT4 for efflux activity. These experiments were repeated once with similar results. (A) Overexpression of NHA1. Wild-type and sit4Δ strains transformed with empty vector (EV) or vector containing NHA1 (pNHA1). Transformants were grown in SD medium lacking uracil and then loaded with 86Rb+ as described in Materials and Methods. Efflux was measured as in Fig. 3. (B) Overexpression of TOK1. Wild-type and sit4Δ strains were transformed with empty vector or vector containing TOK1 under the control of the GAL1/10 promoter (pYGW1). The experiment was performed as described in panel A, except transformants were grown in SGal medium lacking uracil to induce expression of TOK1. Efflux was then measured as in Fig. 3. (C) Overexpression of KHA1. Wild-type and sit4Δ strains transformed with empty vector (EV) or vector containing KHA1 (pKHA1). Transformants were grown in SD medium lacking uracil and then loaded with 86Rb+ as described in Materials and Methods. Efflux was measured as described for Fig. 3.
Our results suggest that the competing functions of Sap155p and Sap185p act as a rheostat to control Sit4p-mediated K+ efflux from cells. This is the first description of the involvement ofany of the Sap proteins in control of intracellular K+ levels. Of the four Sap proteins, these two are the least homologous to one another, showing only 14% identity overall. The most homologous region among the four proteins is the central region which may represent the Sit4p-binding domain, whereas the N- and C-terminal regions are more divergent. Previous results demonstrated that binding of Sap155p and Sap190p to Sit4p was mutually exclusive (28). Although the same experiment was not performed with Sap155p and Sap185p in that study, mutually exclusive binding is a plausible mechanism for the results observed here. Sap155p and Sap185p may therefore control Sit4p's phosphatase activity, further suggesting that Nha1p is regulated by phosphorylation. If Nha1p is indeed phosphorylated, not only is there regulation at the level of the kinase and phosphatase, but from the present study the activity of the phosphatase, Sit4p, may be regulated by Sap155p and Sap185p. Future experiments will be geared toward identifying the specific regions of Sap155p and Sap185p that inhibit and promote K+ efflux, respectively.
Further, our results demonstrate that Sit4p signals to the Na+-K+/H+ antiporter, Nha1p, a finding consistent with results from Ariño's laboratory, which have shown that overexpression of NHA1 suppresses the synthetic lethal phenotype caused by loss of both SIT4 and HAL3. In addition, the C-terminal domain of Nha1p contains regions that are important for regulation of the cell cycle (44, 45). Future experiments will be aimed at determining whether Nha1p is in fact phosphorylated and whether it is directly dephosphorylated by Sit4p.
Acknowledgments
We thank the members of the Rosenwald laboratory for comments about this study. Judith F. Rubinson (Chemistry Department, Georgetown University) assisted with the atomic absorption spectroscopy studies. Finally, we thank Joaquin Ariño (Universitat Autòma de Barcelona) for the KHA1 and NHA1 plasmids, Kim Arndt (Wyeth-Ayherst) for the SAP plasmids, and Clifford Slayman (Yale University) for the TOK1 plasmid.
C.M.A.M. and D.H.H. were supported by Zukowski Summer Research Awards. C.M.A.M. is supported by a grant to Joseph Neale from the Howard Hughes Medical Institute and a scholarship from the Clare Booth Luce Foundation. Work in A.G.R.'s laboratory is supported by a CAREER grant (MCB-9875762) from the National Science Foundation.
REFERENCES
- 1.Adams, A., D. E. Gottschling, C. A. Kaiser, and T. Stearns. 1997. Methods in yeast genetics: a Cold Spring Harbor Laboratory course manual. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 2.Banuelos, M. A., M. C. Ruiz, A. Jimenez, J.-L. Souciet, S. Potier, and J. Ramos. 2002. Role of the Nha1 antiporter in regulating K+ influx in Saccharomyces cerevisiae. Yeast 19:9-15. [DOI] [PubMed] [Google Scholar]
- 3.Banuelos, M. A., H. Sychrova, C. Bleykasten-Grosshans, J. L. Souciet, and S. Potier. 1998. The Nha1 antiporter of Saccharomyces cerevisiae mediates sodium and potassium efflux. Microbiology 144:2749-2778. [DOI] [PubMed] [Google Scholar]
- 4.Bertl, A., J. Ramos, J. Ludwig, H. Lichtenberg-Frate, J. Reid, H. Bihler, F. Calero, P. Martinez, and P. O. Ljungdahl. 2003. Characterization of potassium transport in wild-type and isogenic yeast strains carrying all combinations of trk1, trk2, and tok1 null mutations. Mol. Microbiol. 47:767-780. [DOI] [PubMed] [Google Scholar]
- 5.Bihler, H., C. L. Slayman, and A. Bertl. 2002. Low-affinity potassium uptake by Saccharomyces cerevisiae is mediated by NSC1, a calcium-blocked nonspecific cation channel. Biochim. Biophys. Acta 1558:109-118. [DOI] [PubMed] [Google Scholar]
- 6.Bihler, H., C. L. Slayman, and A. Bertl. 1998. NSC1: a novel high-current inward rectifier for cations in the plasma membrane of Saccharomyces cerevisiae. FEBS Lett. 432:59-64. [DOI] [PubMed] [Google Scholar]
- 7.Binley, K. M., P. A. Radcliffe, J. Trevethick, K. A. Duffy, and P. E. Sudbery. 1999. The yeast PRS3 gene is required for cell integrity, cell cycle arrest upon nutrient deprivation, ion homeostasis and the proper organization of the actin cytoskeleton. Yeast 15:1459-1469. [DOI] [PubMed] [Google Scholar]
- 8.Broach, J. R. 1983. Construction of high copy yeast vectors using 2-micron circle sequences. Methods Enzymol. 101:307-325. [DOI] [PubMed] [Google Scholar]
- 9.Clotet, J., E. Gar, M. Aldea, and J. Arino. 1999. The yeast Ser/Thr phosphatases Sit4 and Ppz1 play opposite roles in regulation of the cell cycle. Mol. Cell. Biol. 19:2408-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrando, A., S. J. Kron, G. Rios, G. R. Fink, and R. Serrano. 1995. Regulation of cation transport in Saccharomyces cerevisiae by the salt tolerance gene HAL3. Mol. Cell. Biol. 15:5470-5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaber, R. F. 1992. Molecular genetics of yeast ion transport. Int. Rev. Cytol. 137A:299-353. [DOI] [PubMed] [Google Scholar]
- 12.Gaber, R. F., C. A. Styles, and G. R. Fink. 1988. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol. Cell. Biol. 8:2848-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heuser, J. E., and R. G. Anderson. 1989. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 108:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill, J. E., A. M. Myers, T. J. Koerner, and A. Tzagoloff. 1986. Yeast/Escherichia coli shuttle vectors with multiple unique restriction sites. Yeast 2:163-167. [DOI] [PubMed] [Google Scholar]
- 15.Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jablonowski, D., A. R. Butler, L. Fichtner, D. Gardiner, R. Schaffrath, and M. J. R. Stark. 2001. Sit4p protein phosphatase is required for sensitivity of Saccharomyces cerevisiae to Kluyveromyces lactis zymocin. Genetics 159:1479-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko, C. H., A. M. Buckley, and R. F. Gaver. 1990. TRK2 is required for low affinity K+ transport in Saccharomyces cerevisiae. Genetics 125:305-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko, C. H., and R. F. Gaber. 1991. TRK1 and TRK2 encode structurally related K+ transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:4266-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larkin, J. M., M. S. Brown, J. L. Goldstein, and R. G. Anderson. 1983. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell 33:273-285. [DOI] [PubMed] [Google Scholar]
- 20.Larkin, J. M., W. C. Donzell, and R. G. Anderson. 1985. Modulation of intracellular potassium and ATP: effects on coated pit function in fibroblasts and hepatocytes. J. Cell Physiol. 124:372-378. [DOI] [PubMed] [Google Scholar]
- 21.Larkin, J. M., W. C. Donzell, and R. G. Anderson. 1986. Potassium-dependent assembly of coated pits: new coated pits form as planar clathrin lattices. J. Cell Biol. 103:2619-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ledbetter, M. L. S., and M. Lubin. 1977. Control of protein synthesis in human fibroblasts by intracellular potassium. Exp. Cell Res. 105:223-236. [DOI] [PubMed] [Google Scholar]
- 23.Loukin, S., B. Vaillant, X.-L. Zhou, E. P. Spalding, C. Kung, and Y. Saimi. 1997. Random mutagenesis reveals a region important for gating of the yeast K+ channel Ykc1. EMBO J. 16:4817-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loukin, S. H., J. Lin, U. Athar, C. Palmer, and Y. Saimi. 2002. The carboxy tail forms a discrete functional domain that blocks closure of the yeast K+ channel. Proc. Natl. Acad. Sci. USA 99:1926-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubin, M. 1964. Intracellular potassium and control of protein synthesis. Fed. Proc. 23:994-1001. [PubMed] [Google Scholar]
- 26.Lubin, M. 1967. Intracellular potassium and macromolecular synthesis in mammalian cells. Nature 213:451-453. [DOI] [PubMed] [Google Scholar]
- 27.Lubin, M., and H. L. Ennis. 1964. On the role of intracellular potassium in protein synthesis. Biochim. Biophys. Acta 80:614-631. [DOI] [PubMed] [Google Scholar]
- 28.Luke, M. M., F. Della Seta, C. J. Di Como, H. Sugimoto, R. Kobayashi, and K. T. Arndt. 1996. The SAPs, a new family of proteins, associate and function positively with the SIT4 phosphatase. Mol. Cell. Biol. 16:2744-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madrid, R., M. J. Gomez, J. Ramos, and A. Rodriguez-Navarro. 1998. Ectopic potassium uptake in trk1 trk2 mutants of Saccharomyces cerevisiae correlates with a highly hyperpolarized membrane potential. J. Biol. Chem. 273:14838-14844. [DOI] [PubMed] [Google Scholar]
- 30.Maresova, L., and H. Sychrova. 2005. Physiological characterization of Saccharomyces cerevisiae kha1 deletion mutants. Mol. Microbiol. 55:588-600. [DOI] [PubMed] [Google Scholar]
- 31.Masuda, C. A., J. Ramirez, A. Pena, and M. Montero-Lomeli. 2000. Regulation of monovalent ion homeostasis and pH by the Ser-Thr protein phosphatase SIT4 in Saccharomyces cerevisiae. J. Biol. Chem. 275:30957-30961. [DOI] [PubMed] [Google Scholar]
- 32.Mulet, J. M., M. P. Leube, S. J. Kron, G. Rios, G. R. Fink, and R. Serrano. 1999. A novel mechanism of ion homeostasis and salt tolerance in yeast: the Hal4 and Hal5 protein kinases modulate the Trk1-Trk2 potassium transporter. Mol. Cell. Biol. 19:3328-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munson, A. M., D. H. Haydon, S. L. Love, G. L. Fell, V. R. Palanivel, and A. G. Rosenwald. 2004. Yeast ARL1 encodes a regulator of K+ influx. J. Cell Sci. 117:2309-2320. [DOI] [PubMed] [Google Scholar]
- 34.Munson, A. M., S. L. Love, J. Shu, V. R. Palanivel, and A. G. Rosenwald. 2004. Loss of both ARL1 and ATC1/LIC4 in Saccharomyces cerevisiae causes lithium sensitivity. Biochem. Biophys. Res. Commun. 315:617-623. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien, M. C., and D. B. McKay. 1995. How potassium affects the activity of the molecular chaperone Hsc70. I. Potassium is required for optimal ATPase activity. J. Biol. Chem. 270:2247-2250. [DOI] [PubMed] [Google Scholar]
- 36.Perkins, J., and G. M. Gadd. 1993. Accumulation and intracellular compartmentation of lithium ions in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 107:255-260. [DOI] [PubMed] [Google Scholar]
- 37.Ramirez, J., O. Ramirez, C. Saldana, R. Coria, and A. Pena. 1998. A Saccharomyces cerevisiae mutant lacking a K+/H+ exchanger. J. Bacteriol. 180:5860-5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramos, J., R. Aligo, R. Haro, and A. Rodriguez-Navarro. 1994. TRK2 is not a low-affinity potassium transport in Saccharomyces cerevisiae. J. Bacteriol. 176:249-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts, S. K., M. Fischer, G. K. Dixon, and D. Sanders. 1999. Divalent cation block of inward currents and low-affinity K+ uptake in Saccharomyces cerevisiae. J. Bacteriol. 181:291-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rohde, J. R., S. Campbell, S. A. Zurita-Martinez, N. S. Cutler, M. Ashe, and M. E. Cardenas. 2004. TOR controls transcriptional and translational programs via Sap-Sit4 protein phosphatase signaling effectors. Mol. Cell. Biol. 24:8332-8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenwald, A. G., M. A. Rhodes, H. Van Valkenburgh, V. Palanivel, G. Chapman, A. Boman, C.-J. Zhang, and R. A. Kahn. 2002. ARL1 and membrane traffic in Saccharomyces cerevisiae. Yeast 19:1039-1056. [DOI] [PubMed] [Google Scholar]
- 42.Serrano, R., M. C. Kielland-Brandt, and G. R. Fink. 1986. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature 319:689-693. [DOI] [PubMed] [Google Scholar]
- 43.Sherman, F., G. Fink, and C. Lawrence. 1974. Methods in yeast genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 44.Simon, E., A. Barcelo, and J. Arino. 2003. Mutagenesis analysis of the yeast Nha1 Na+/H+ antiporter carboxy-terminal tail reveals residues required for function in cell cycle. FEBS Lett. 545:239-245. [DOI] [PubMed] [Google Scholar]
- 45.Simon, E., J. Clotet, F. Calero, J. Ramos, and J. Arino. 2001. A screening for high copy suppressors of the sit4 hal3 synthetically lethal phenotype reveals a role for the yeast Nha1 antiporter in cell cycle regulation. J. Biol. Chem. 276:29740-29747. [DOI] [PubMed] [Google Scholar]
- 46.Sutton, A., D. Immanuel, and K. T. Arndt. 1991. The SIT4 protein phosphatase functions in late G1 for progression into S phase. Mol. Cell. Biol. 11:2133-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sychrova, H., J. Ramirez, and A. Pena. 1999. Involvement of Nha1 antiporter in regulation of intracellular pH in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 171:167-172. [DOI] [PubMed] [Google Scholar]
- 48.Wilbanks, S. M., and D. B. McKay. 1995. How potassium affects the activity of the molecular chaperone Hsc70. II. Potassium binds specifically in the ATPase active site. J. Biol. Chem. 270:2251-2257. [DOI] [PubMed] [Google Scholar]
- 49.Winzeler, E. A., D. D. Shoemaker, A. Astromoff, H. Liang, K. Anderson, B. Andre, R. Bangham, R. Benito, J. D. Boeke, H. Bussey, A. M. Chu, C. Connelly, K. Davis, F. Dietrich, S. W. Dow, M. El Bakkoury, F. Foury, S. H. Friend, E. Gentalen, G. Giaever, J. H. Hegemann, T. Jones, M. Laub, H. Liao, N. Liebundguth, D. J. Lockhart, A. Lucau-Danila, M. Lussier, N. M'Rabet, P. Menard, M. Mittmann, C. Pai, C. Rebischung, J. L. Revuelta, L. Riles, C. J. Roberts, P. Ross-MacDonald, B. Scherens, M. Snyder, S. Sookhai-Mahadeo, R. K. Storms, S. Véronneau, M. Voet, G. Volckaert, T. R. Ward, R. Wysocki, G. S. Yen, K. Yu, K. Zimmermann, P. Philippsen, M. Johnston, and R. W. Davis. 1999. Functional characterization of the Saccharomyces cerevisiae genome by gene deletion and parallel analysis. Science 285:901-906. [DOI] [PubMed] [Google Scholar]
- 50.Yenush, L., J. M. Mulet, J. Arino, and R. Serrano. 2002. The Ppz protein phosphatases are key regulators of K+ and pH homeostasis: implications for salt tolerance, cell wall integrity and cell cycle progression. EMBO J. 21:920-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou, X.-L., B. Vaillant, S. H. Loukin, C. Kung, and Y. Saimi. 1995. YKC1 encodes the depolarization-activated K+ channel in the plasma membrane of yeast. FEBS Lett. 373:170-176. [DOI] [PubMed] [Google Scholar]