Abstract
The current study developed a method for quantifying four drugs—Sulfamethoxazole, Trimethoprim, Isoniazid, and Pyridoxine—in rabbit plasma. The method uses gradient liquid chromatography based on analytical quality by design. To achieve separation, a Eclip Plus C18 (250 mm × 5 mm, 4.6 µm) column with L1 packing was used, and analytes were detected at 254 nm at ambient temperature. The optimized mobile phase consisted of 50 mM potassium dihydrogen phosphate buffer (pH 6.5) and Methanol. The concentration of Methanol was 3% (0–5 min), 15% (5–15 min), 55% (15–27 min), and 3% Methanol until the end of the 30-min runtime, and the flow rate was set at 0.95 mL/min. Control Noise Experimentation was used to screen studies, revealing that flow rate, pH, and Methanol concentration significantly affected the analytical attributes. The study identified critical attributes (resolution and asymmetric factor) and developed a quality target method profile. A central composition design was used to optimize the essential parameters. The method developed for the drugs showed peaks at retention times of 6.990 min for Isoniazid, 7.880 min for Pyridoxine, 15.530 min for Sulfamethoxazole, and 26.890 min for Trimethoprim, respectively. The method was validated with linearity in the range of 10–640 ng ml−1, with R2 of 0.9993, 0.9987, 0.9993, and 0.9992 for Sulfamethoxazole, Trimethoprim, Isoniazid, and Pyridoxine, respectively.
Keywords: Bioanalytical quality by design, Gradient elution, Sulfamethoxazole, Trimethoprim, Isoniazid, Pyridoxine
Subject terms: Analytical chemistry, Bioanalytical chemistry
Introduction
The HIV virus, which causes AIDS, is one of the world’s most dangerous public health problems. Efforts are being made to stop the spread of HIV and guarantee treatment for all those living with the virus globally1. Recent statistics from UNAIDS indicate that there are more than 39 million HIV-positive people worldwide. Despite significant efforts, there is currently no cure for HIV/AIDS, and some individuals are still unaware of prevention, care, and treatment2.
HIV targets CD4 + T cells and decreases their count, making individuals more susceptible to opportunistic infections3. Patients with low CD4 + levels or AIDS-defining illness are at higher risk of lethal infections caused by various pathogens, including Herpes simplex viruses, Cryptococcus neoformans, Pneumocystis jirovecii, and others4. High HIV prevalence has been linked to a wide range of opportunistic infections globally5,6. In India, TB is the most common opportunistic illness among HIV-positive individuals. Oral candidiasis, herpes zoster, cryptococcal meningitis, cerebral toxoplasmosis, and cytomegalovirus retinitis are some opportunistic infections that have been documented7,8.
Patients with HIV/AIDS are treated with a combination of sulfamethoxazole (Sul), trimethoprim (Trim), isoniazid (INH), and pyridoxine (B6) to prevent opportunistic infections9–11. This treatment is highly effective in preventing tuberculosis, isosporiasis, pneumonia, and toxoplasmosis and has reduced mortality and hospitalizations among HIV/AIDS patients. It is an essential tool in the management of HIV/AIDS and plays a critical role in improving the quality of life for those suffering from this disease12,13.
Analyzing four drugs-Sul/Trim/INH/B6—requires accounting for their varying polarities to develop a successful assessment method. The Ultra Flow Liquid Chromatography (UFLC)-UV method with gradient elution is necessary to ensure the retention of individual analytes with optimal peak shape. The technique plays a critical role in retaining variables and the peak shape of the analytes14. The composition of the mobile phase impacts the solute properties of the analytes, making it challenging to manage all variables through trial and error. Therefore, a structured process becomes crucial to attaining the desired outcomes. Applying the bioanalytical Quality by Design (BQbD) approach assists in identifying, understanding, and controlling Critical Method Parameters (CMP) that influence the quality of outcomes15,16. By integrating the gradient principle and BQbD in bioanalytical development through UFLC, a robust method capable of eliminating the need for revalidation and enhancing separation can be achieved, further improving the effectiveness of the bioanalytical method and making it a state-of-the-art process17.
Using gradient elution in chromatography enhances the separation of four different polarities (Sul/Trim/INH/B6) with better peak resolution. Additionally, applying Quality by Design (QbD) analysis ensures the quality and safety of the bio analytical method.
Result
Bio analytical method Risk assessment by QbD
A Control-Noise-Experimentation (CNX) approach was utilized for SUL/TRIM/INH/B6 to identify the significant variables that impact the method’s attributes18–20. This approach used a Cause-and-Effect (C&E) Risk Assessment matrix (Table 1).
Table 1.
Control-noise-experimentation (CNX) approach.
| Critical method parameter | Critical method attributes | Initial risk assessment scores | C,N,X | Experimental strategy | ||
|---|---|---|---|---|---|---|
| Retention time | Asymmetric factor | Resolution | ||||
| Isocratic Binary Parameter | 2 | 2 | 2 | 40 | C | Calibrated |
| Flow Rate | 10 | 10 | 10 | 300 | X | DOE |
| Stationary Phase | 5 | 5 | 5 | 100 | C | New Column |
| Particle size | 2 | 2 | 2 | 40 | C | Optimum |
| Dimension | 2 | 2 | 2 | 40 | C | Standard |
| Column Temp | 5 | 5 | 5 | 100 | N | Ambient |
| Buffer pH | 10 | 10 | 10 | 300 | X | DOE |
| % organic Modifier | 10 | 10 | 10 | 300 | X | DOE |
| Solvent Grade | 5 | 5 | 5 | 100 | N | UFLC grade |
| Injection Vol | 2 | 2 | 2 | 40 | C | 20µL |
| Flow Cell temp | 5 | 5 | 5 | 100 | C | 400 C |
| PDA | 5 | 5 | 5 | 100 | N | Standard |
C-Control, N-Noise & X-Experiment Score Low Risk-2, Medium Risk-5 & High Risk- 10. Total Score = (Risk level of First CMA × 10) + (Risk level of Second CMA × 10).
Design of experiment
The Central Composite design (CCD) was used to enhance the quality and separation using Design Expert 13, as shown in Table 221. All experiments were conducted randomly to minimize the impact of uncontrolled variables, as per the standard practice. This design facilitated optimizing and assessing main, interactions, and quadratic effects22–24. The impact of the factors on the dependent variables was examined using ANOVA. Tables 3 and 4 depicts the ANOVA coefficients with p-values for the SUL/TRIM/INH/B6 responses (Resolution and Asymmetric factor) and interprets the relationship between factors and responses25,26.
Table 2.
Experimental design: central composite design: 3 factors with 2 responses for SUL/TRIM/INH/B6.
| Run | Factor :A | Factor :B | Factor :C | R1: Response1: Resolution (Rs) | R2: Response2 : Asymmetric factor (As) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R1(SUL) | R1(TRIM) | R1(INH) | R1(B6) | R2(SUL) | R2(TRIM) | R2(INH) | R2(B6) | ||||
| 1 | 0.95 | 6.5 | 13.0454 | 15.001 | 28.782 | 6.322 | 1.954 | 1.068 | 0.954 | 0.962 | 1.052 |
| 2 | 0.95 | 6.5 | 8 | 13.111 | 26.254 | 7.254 | 1.641 | 0.992 | 0.837 | 0.730 | 1.007 |
| 3 | 1.2 | 7.5 | 5 | 13.011 | 39.329 | 6.800 | 1.574 | 0.965 | 1.134 | 0.990 | 1.307 |
| 4 | 0.95 | 6.5 | 8 | 13.145 | 26.254 | 7.254 | 1.641 | 0.992 | 0.835 | 0.730 | 1.270 |
| 5 | 0.95 | 6.5 | 8 | 13.185 | 26.254 | 6.904 | 1.522 | 0.992 | 0.839 | 0.730 | 1.270 |
| 6 | 0.95 | 6.5 | 8 | 13.122 | 26.254 | 7.254 | 1.614 | 0.992 | 0.838 | 0.731 | 1.270 |
| 7 | 0.95 | 6.5 | 2.95462 | 12.233 | 29.053 | 8.52 | 1.769 | 0.882 | 1.086 | 1.111 | 1.097 |
| 8 | 1.37045 | 6.5 | 8 | 16.156 | 35.551 | 6.176 | 1.193 | 1.184 | 0.822 | 0.711 | 1.118 |
| 9 | 0.95 | 6.5 | 8 | 13.155 | 26.254 | 7.254 | 1.854 | 0.992 | 0.833 | 0.732 | 1.270 |
| 10 | 0.7 | 7.5 | 5 | 12.235 | 32.060 | 8.350 | 1.733 | 0.993 | 0.988 | 0.882 | 1.076 |
| 11 | 0.7 | 5.5 | 5 | 15.089 | 17.045 | 7.556 | 1.764 | 0.989 | 0.921 | 0.824 | 1.202 |
| 12 | 0.95 | 6.5 | 8 | 13.185 | 26.254 | 7.254 | 1.726 | 0.992 | 0.837 | 0.737 | 1.270 |
| 13 | 0.529552 | 6.5 | 8 | 13.322 | 25.296 | 7.198 | 1.936 | 1.075 | 0.988 | 0.884 | 1.065 |
| 14 | 0.7 | 7.5 | 11 | 15.025 | 27.083 | 7.556 | 1.550 | 1.302 | 0.938 | 0.831 | 0.892 |
| 15 | 0.95 | 8.18179 | 8 | 10.025 | 24.43 | 6.36 | 1.625 | 2.079 | 0.896 | 0.794 | 1.020 |
| 16 | 1.2 | 5.5 | 11 | 15.798 | 28.581 | 6.045 | 1.081 | 0.98 | 0.901 | 0.801 | 1.173 |
| 17 | 0.95 | 4.81821 | 8 | 11.232 | 19.421 | 6.34 | 1.745 | 1.973 | 1.019 | 0.964 | 0.780 |
| 18 | 1.2 | 5.5 | 5 | 12.252 | 23.176 | 6.547 | 1.543 | 0.993 | 0.964 | 0.867 | 1.173 |
| 19 | 0.7 | 5.5 | 11 | 14.755 | 17.845 | 6.629 | 1.149 | 0.865 | 0.971 | 0.857 | 1.108 |
| 20 | 1.2 | 7.5 | 11 | 16.555 | 33.022 | 6.557 | 0.928 | 1.114 | 0.926 | 0.82 | 1.190 |
Factor A, Flow Rate of the mobile Phase; Factor B, Mobile phase pH; C, Organic Modifier; R1, Response1, Resolution (Rs), R2, Response2, Asymmetric factor (As); R1(SUL), Resolution of Sulphonamide; R1(TRIM), Resolution of Trimethoprim; R1(INH), Resolution of Isoniazid;R1(B6), Resolution of pyridoxine; R2(SUL), Asymmetric factor of Sulphonamide; R2(TRIM), Asymmetric factor of Trimethoprim; R2(INH), Asymmetric factor of Isoniazid; R2(B6), Asymmetric factor of pyridoxine.
Table 3.
ANOVA coefficients with p-values for response 1(Resolution).
| R1 (SUL) | p-Value | R1 (TRIM) | p-Value | R1 (INH) | p-Value | R1 (B6) | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Intercept | + 13.05 | + 26.25 | + 7.18 | + 1.20 | ||||
| A | + 0.4180 | 0.1744 | + 3.4700 | 0.0005 | −0.4029 | 0.0022 | −0.0171 | 0.0619 |
| B | −0.1970 | 0.5064 | + 3.9052 | 0.0002 | + 0.1557 | 0.1465 | −0.1988 | < 0.0001 |
| C | + 1.1001 | 0.0033 | −0.4053 | 0.5649 | −0.4020 | 0.0023 | −0.2256 | < 0.0001 |
| AB | + 0.5596 | 0.1650 | −0.4574 | 0.6183 | −0.0770 | 0.5644 | + 0.0641 | 0.0006 |
| AC | + 0.5814 | 0.1507 | + 0.4094 | 0.6552 | + 0.0770 | 0.5644 | + 0.0411 | 0.0069 |
| BC | + 0.3881 | 0.3233 | −2.1900 | 0.0338 | + 0.0657 | 0.6218 | + 0.0554 | 0.0014 |
| A2 | + 0.8136 | 0.0152 | + 1.5022 | 0.0470 | −0.0930 | 0.3566 | + 0.0152 | 0.0710 |
| B2 | −0.6139 | 0.0519 | −1.5014 | 0.0467 | −0.3359 | 0.0058 | −0.0869 | 0.0005 |
| C2 | + 0.4424 | 0.1430 | + 0.9687 | 0.1745 | + 0.0176 | 0.8583 | + 0.0571 | < 0.0001 |
Factor A: Flow Rate of the mobile Phase; Factor B: Mobile phase pH; C: Organic Modifier; R1: Response1: Resolution (Rs), R2: Response2: Asymmetric factor (As); R1(SUL): Resolution of Sulphanilamide; R1(TRIM): Resolution of Trimethoprim; R1(INH): Resolution of Isoniazid;R1(B6): Resolution of pyridoxine.
Table 4.
ANOVA coefficients with p-values for Response 2 (Asymmetric Factor).
| R2 (SUL) | p-Value | R2 (TRIM) | p-Value | R2 (INH) | p-Value | R2 (B6) | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Intercept | + 1.00 | + 0.8300 | + 0.7318 | + 1.27 | ||||
| A | + 0.0063 | 0.9164 | −0.0120 | 0.4057 | −0.0143 | 0.4312 | −0.0072 | 0.8534 |
| B | + 0.0531 | 0.3867 | + 0.0014 | 0.9212 | −0.0070 | 0.6964 | + 0.1951 | 0.0077 |
| C | + 0.0464 | 0.4474 | −0.0359 | 0.0266 | −0.0367 | 0.0625 | + 0.2832 | 0.0001 |
| AB | −0.0419 | 0.5969 | + 0.0197 | 0.2994 | + 0.0138 | 0.5607 | + 0.0176 | 0.7485 |
| AC | −0.0061 | 0.9379 | −0.0335 | 0.0931 | −0.0262 | 0.2773 | −0.0739 | 0.2049 |
| BC | + 0.0744 | 0.3549 | −0.0300 | 0.1274 | −0.0238 | 0.3231 | + 0.1931 | 0.0081 |
| A2 | −0.0305 | 0.6047 | + 0.0251 | 0.0917 | + 0.0111 | 0.5295 | −0.0631 | 0.1117 |
| B2 | + 0.2864 | 0.0005 | + 0.0451 | 0.0074 | + 0.0394 | 0.0433 | + 0.0415 | 0.5678 |
| C2 | −0.0852 | 0.1669 | + 0.0672 | 0.0005 | + 0.0965 | 0.0002 | + 0.1607 | 0.0024 |
Factor A, Flow Rate of the mobile Phase; Factor B, Mobile phase pH; C, Organic Modifier; R1, Response1, Resolution (Rs), R2, Response2, Asymmetric factor (As); R2(SUL), Asymmetric factor of Sulphanilamide; R2(TRIM), Asymmetric factor of Trimethoprim; R2(INH), Asymmetric factor of Isoniazid; R2(B6), Asymmetric factor of pyridoxine.
Method validation27
Specificity
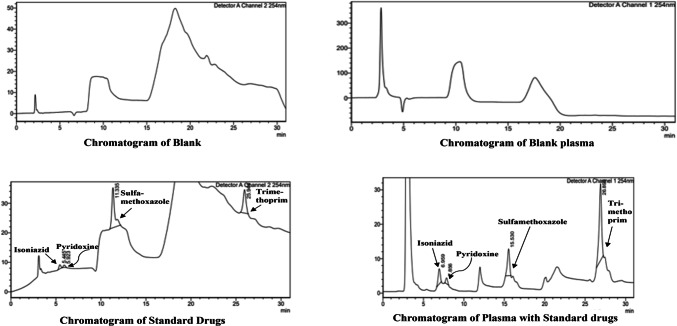
The technique employed successfully distinguished the peaks of plasma and interference from the peak of SUL/TRIM/INH/B6 gradually. This verified the specificity of the method, enabling the selective identification of four drugs in rabbit plasma28. Figure 1 is the representative chromatograms of specificity studies. As per the FDA bioanalytical guidelines, the acceptance criteria encompass blank and zero calibrators, which must be devoid of interference at the retention times of the analyte(s)29.
Fig. 1.
Specificity study of SUL/TRIM/INH/B6.
Accuracy and precision
The developed method’s accuracy and intra- and inter-day precision results are shown in Tables 5 and 6. The LLOQ, LQC, MQC, and HQC values meet the acceptable limit (RSD ≤ 15%) according to the international guideline, indicating that the established method is reliable and precise for quantifying each drug in rabbit plasma30,31.
Table 5.
Accuracy data of SUL/Trim/INH/B6 (n = 6).
| QC Levels | Nominal conc | SUL (ng mL−1) | TRIM (ng mL−1) | INH (ng mL−1) | B6 (ng mL−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean conc ± SD | %CV | %RE | Mean conc ± SD | %CV | %RE | Mean conc ± SD | %CV | %RE | Mean conc ± SD | %CV | %RE | ||
| LLOQ | 10 | 8.590 ± 0.259 | 3.020 | −14.1 | 8.535 ± 0.422 | 4.952 | −14.65 | 8.516 ± 0.5128 | 6.021 | −14.833 | 8.596 ± 0.270 | 3.147 | −14.033 |
| LQC | 30 | 27.236 ± 0.498 | 1.828 | −9.21 | 27.645 ± 1.176 | 4.257 | −7.85 | 27.583 ± 1.183 | 4.291 | −8.055 | 27.243 ± 0.491 | 1.804 | −9.188 |
| MQC | 200 | 186.990 ± 2.858 | 1.528 | −6.505 | 191.023 ± 1.785 | 0.934 | −4.486 | 184.2 ± 2.163 | 1.174 | −7.900 | 186.960 ± 2.822 | 1.509 | −6.520 |
| HQC | 600 | 578.813 ± 3.240 | 0.559 | −3.531 | 581.673 ± 2.938 | 0.505 | −3.0545 | 582.260 ± 4.028 | 0.691 | −2.956 | 575.480 ± 5.474 | 0.951 | −4.086 |
QC Level, Quality Control Level; CV, coefficient of variation; SD, Standard Deviation; LLOQ, Lower limit of quantification; LQC, Low Quality control; MQC, Mid Quality control; HQC, High Quality control; %RE, Percentage of Relative Error; CV, coefficient of variation; SD, Standard Deviation.
Table 6.
Intra and inter- day precision of SUL/Trim/INH/B6 (n = 6).
| Analyte | QC Level | Nominal concentration (ng mL−1) | Intraday precision | Interday precision | ||||
|---|---|---|---|---|---|---|---|---|
| Measured concentration (Mean ± SD) | % CV | % RE | Measured concentration (Mean ± SD) | % CV | % RE | |||
| SUL (ng mL−1) | LLOQ | 10 | 8.596 ± 0.533 | 6.200 | −14.033 | 8.616 ± 0.613 | 7.117 | −13.833 |
| QC | 30 | 26.600 ± 0.538 | 2.024 | −11.333 | 26.666 ± 0.480 | 1.800 | −11.111 | |
| MQC | 200 | 190.766 ± 2.970 | 1.557 | −4.616 | 190.200 ± 2.163 | 1.137 | −4.900 | |
| HQC | 600 | 583.800 ± 3.504 | 0.600 | −2.700 | 583.133 ± 3.115 | 0.352 | −2.811 | |
| TRIM (ng mL−1) | LLOQ | 10 | 8.557 ± 0.444 | 5.194 | −14.430 | 8.551 ± 0.434 | 5.076 | −14.490 |
| LQC | 30 | 27.637 ± 1.181 | 4.276 | −7.874 | 27.647 ± 1.189 | 4.303 | −7.842 | |
| MQC | 200 | 191.006 ± 1.813 | 0.949 | −4.497 | 191.005 ± 1.803 | 0.944 | −4.497 | |
| HQC | 600 | 588.664 ± 2.959 | 0.508 | −3.055 | 581.611 ± 3.006 | 0.5168 | −3.064 | |
| INH (ng mL−1) | LLOQ | 10 | 8.520 ± 0.563 | 6.609 | −14.800 | 8.563 ± 0.510 | 5.957 | −14.336 |
| LQC | 30 | 27.266 ± 1.549 | 5.682 | −9.111 | 27.556 ± 1.296 | 4.703 | −8.144 | |
| MQC | 200 | 184.200 ± 2.116 | 1.149 | −7.900 | 184.003 ± 1.890 | 1.027 | −7.998 | |
| HQC | 600 | 581.843 ± 3.430 | 0.589 | −3.026 | 579.790 ± 3.499 | 0.603 | −3.368 | |
| B6 (ng mL−1) | LLOQ | 10 | 8.570 ± 0.468 | 5.469 | −14.300 | 8.576 ± 0.378 | 4.414 | −14.233 |
| LQC | 30 | 27.276 ± 0.840 | 3.079 | −9.077 | 27.273 ± 0.597 | 2.190 | −9.088 | |
| MQC | 200 | 186.933 ± 3.453 | 1.847 | −6.533 | 186.893 ± 3.490 | 1.867 | −6.553 | |
| HQC | 600 | 575.446 ± 5.558 | 0.965 | −4.092 | 575.220 ± 5.558 | 0.965 | −4.070 | |
QC Level, Quality Control Level; CV, coefficient of variation; SD, Standard Deviation; LLOQ, Lower limit of quantification; LQC, Low Quality control; MQC, Mid Quality control; HQC, High Quality control; %RE, Percentage of Relative Error; CV, coefficient of variation; SD, Standard Deviation.
Linearity
This study aimed to establish a calibration curve standard for optimized chromatographic conditions using a 20 µL injection volume. The correlation between the peak area and the respective concentration created the calibration curve32. The data was analyzed using both unweighted and weighted linear regression, and the optimal weighting variables were selected based on Correlation Coefficient (R2) and percentage relative error (% ƩRE), as shown in Table 7. The regression models were statistically significant, as evidenced by the SUL/TRIM/INH/B6 F-values, which were not disclosed in the text. The p-values for SUL/TRIM/INH/B6 are below 0.05, respectively33. This indicates statistical significance at a 95% confidence level. These findings suggest that the current model is reliable for analyzing the calibration curve standard data. Acceptance Criteria include Non-zero calibrators should be ± 15% of nominal (theoretical) concentrations, except at LLOQ, where the calibrator should be ± 20% of the nominal concentrations in each validation run34,35.
Table 7.
Linearity data of SUL/TRIM/INH/B6 (n = 6).
| Drug | Weight factor(w) | weighting least square linear regression | |||
|---|---|---|---|---|---|
| X | 1/X | 1/√x | 1/x2 | ||
| SUL | R2 | 0.9993 | 0.4705 | 0.6408 | 0.2750 |
| % RE | 13.7850 | 5056.8000 | 67152.6140 | 30026.7900 | |
| TRIM | R2 | 0.9987 | 0.5164 | 0.6859 | 0.3164 |
| % RE | 18.8281 | 1606.5640 | 50.062 | 23746.0200 | |
| INH | R2 | 0.9993 | 0.4682 | 0.6374 | 0.2751 |
| % RE | 15.4101 | 751.3880 | 47.4311 | 25972.0800 | |
| B6 | R2 | 0.9992 | 0.4875 | 0.6556 | 0.2917 |
| % RE | 1.6090 | 721.7670 | 46.2760 | 28023.1100 | |
R2, Correlation Coefficient; %RE, Percentage of Relative Error; QC Level, Quality Control Level; CV, coefficient of variation; SD, Standard Deviation; LLOQ, Lower limit of quantification; LQC, Low Quality control; MQC, Mid Quality control; HQC, High Quality control %RE, Percentage of Relative Error CV, coefficient of variation SD, Standard Deviation.
Recovery
Table 8 displays the percentage recoveries for the LQC, MQ, and HQC samples. A diluent (methanol: water, 7:3) was chosen as the extraction solvent to optimise recovery36. The percentage recovery achieved for each drug was sufficient to ensure an accurate and precise determination within the defined detection range37.
Table 8.
Percentage recovery studies of SUL/TRIM/INH/B6 (n = 3).
| Analyte | QC Level | Nominal Concentration (ng mL−1) | % Recovery | |
|---|---|---|---|---|
| Mean ± SD | %CV | |||
| SUL | LLOQ | 10 | 8.526 ± 0.775 | 9.090 |
| LQC | 30 | 26.433 ± 0.508 | 1.923 | |
| MQC | 200 | 186.570 ± 2.077 | 1.113 | |
| HQC | 600 | 577.603 ± 2.143 | 0.371 | |
| TRIM | LLOQ | 10 | 8.515 ± 0.391 | 4.598 |
| LQC | 30 | 27.568 ± 1.070 | 3.882 | |
| MQC | 200 | 190.972 ± 1.841 | 0.964 | |
| HQC | 600 | 581.306 ± 2.650 | 0.455 | |
| INH | LLOQ | 10 | 8.566 ± 0.512 | 5.986 |
| LQC | 30 | 27.483 ± 1.186 | 4.318 | |
| MQC | 200 | 184.080 ± 2.131 | 1.157 | |
| HQC | 600 | 580.526 ± 2.399 | 0.413 | |
| B6 | LLOQ | 10 | 8.506 ± 0.455 | 5.354 |
| LQC | 30 | 27.196 ± 0.619 | 2.278 | |
| MQC | 200 | 186.833 ± 2.487 | 1.331 | |
| HQC | 600 | 575.050 ± 5.940 | 1.032 | |
QC Level, Quality Control Level; CV, coefficient of variation; SD, Standard Deviation; LLOQ, Lower limit of quantification; LQC, Low Quality control; MQC, Mid Quality control; HQC, High Quality control; CV, coefficient of variation SD, Standard Deviation.
Stability studies
Four investigational candidates’ stability studies were conducted under various conditions. According to the acceptance criteria, the accuracy at each level should be within ± 15% of the nominal value38. The results are presented in Table 9.
Table 9.
Stability of analytes in rabbit plasma in four QC level (n = 6).
| Stability | Measured concentration (Mean ± SD) | % CV | %RE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nominal Concentration (ng mL−1) | SUL | TRIM | INH | B6 | SUL | TRIM | INH | B6 | SUL | TRIM | INH | B6 | |
| 0 h | 10 | 8.563 ± 0.639 | 8.562 ± 0.445 | 8.620 ± 0.556 | 8.593 ± 0.272 | 7.468 | 5.197 | 6.455 | 3.168 | −14.360 | −14.370 | −13.800 | −14.060 |
| 30 | 26.566 ± 0.523 | 27.704 ± 1.124 | 27.556 ± 1.296 | 27.276 ± 0.840 | 1.938 | 4.058 | 4.703 | 3.079 | −11.440 | −7.650 | −8.140 | −9.077 | |
| 200 | 190.27 ± 2.510 | 191.02 ± 1.786 | 184.546 ± 2.120 | 186.966 ± 3.426 | 1.319 | 0.935 | 1.149 | 1.832 | −4.865 | −4.486 | −7.720 | −6.516 | |
| 600 | 583.436 ± 2.79 | 583.436 ± 2.95 | 582.293 ± 4.110 | 575.616 ± 5.546 | 0.477 | 0.508 | 0.705 | 0.980 | −2.706 | −3.055 | −2.950 | −4.063 | |
| Bench Top (24 h) Rt | 10 | 8.560 ± 0.871 | 8.559 ± 0.443 | 8.596 ± 0.505 | 8.580 ± 0.383 | 10.18 | 5.176 | 5.876 | 4.465 | −14.400 | −14.40 | −14.030 | −14.200 |
| 30 | 26.433 ± 0.515 | 27.637 ± 1.198 | 27.516 ± 1.241 | 27.240 ± 0.623 | 1.981 | 4.337 | 4.511 | 2.289 | −11.800 | −7.870 | −8.270 | −9.200 | |
| 200 | 190.04 ± 2.612 | 190.975 ± 1.830 | 184.443 ± 1.946 | 186.770 ± 2.339 | 1.374 | 0.958 | 1.055 | 1.252 | −4.980 | −4.510 | −7.770 | −6.615 | |
| 600 | 583.436 ± 3.18 | 581.56 ± 3.024 | 581.626 ± 2.089 | 574.810 ± 5.474 | 0.545 | 0.520 | 0.359 | 0.952 | −2.760 | −3.072 | −3.060 | −4.198 | |
| Freeze and Thaw (− 80 °C for 3 three cycles) | 10 | 8.530 ± 0.416 | 8.522 ± 0.394 | 8.559 ± 0.580 | 8.553 ± 0.502 | 4.885 | 4.633 | 6.785 | 5.874 | −14.700 | −14.770 | −14.400 | −14.46 |
| 30 | 26.363 ± 0.560 | 27.618 ± 1.218 | 27.446 ± 1.141 | 27.130 ± 0.588 | 2.124 | 4.411 | 4.159 | 2.170 | −12.120 | −7.940 | −8.511 | −9.566 | |
| 200 | 189.79 ± 1.978 | 190.77 ± 1.830 | 184.336 ± 1.977 | 186.373 ± 3.694 | 1.042 | 0.959 | 1.072 | 1.982 | −5.101 | −4.610 | −7.831 | −6.813 | |
| 600 | 582.43 ± 2.242 | 581.263 ± 2.67 | 581.056 ± 3.766 | 574.770 ± 5.686 | 0.385 | 0.460 | 0.648 | 0.989 | −2.927 | −3.122 | −3.157 | −4.205 | |
| Long term (−80° 45 days ) | 10 | 8.51 ± 0.434 | 8.517 ± 0.390 | 8.516 ± 0.512 | 8.503 ± 0.489 | 5.109 | 4.589 | 6.021 | 5.756 | −14.900 | −14.826 | −14.830 | −14.96 |
| 30 | 26.03 ± 0.020 | 27.564 ± 1.081 | 27.346 ± 1.360 | 27.030 ± 0.588 | 0.076 | 3.924 | 4.975 | 2.178 | −13.230 | −8.117 | −8.844 | −9.900 | |
| 200 | 188.796 ± 1.978 | 190.506 ± 1.64 | 184.110 ± 2.191 | 186.373 ± 3.786 | 1.047 | 0.861 | 1.190 | 2.031 | −5.601 | −4.747 | −7.945 | −6.813 | |
| 600 | 581.77 ± 1.955 | 580.863 ± 2.42 | 580.526 ± 2.399 | 574.043 ± 6.058 | 0.336 | 0.416 | 0.413 | 1.055 | −3.038 | −3.189 | −3.245 | −4.326 | |
QC Level, Quality Control Level; CV, coefficient of variation; SD, Standard Deviation; LLOQ, Lower limit of quantification; LQC, Low Quality control; MQC, Mid Quality control; HQC, High Quality control; %RE, Percentage of Relative Error; CV, coefficient of variation SD, Standard Deviation.
Discussion
The method aimed to determine the levels of SUL/TRIM/INH/B6 present in rabbit plasma. The reverse phase chromatographic conditions, such as the flow rate, mobile phase composition, and mobile phase pH, were optimized to separate the eluted compounds adequately in gradient mode. Several peak parameters were optimized to obtain an appropriate separation of eluted compounds, including height, capacity, theoretical plates, tailing factor, and resolution.
The ChemAxon Log-D predictor (demo version) was used to generate the Log-D curve based on which column selection and appropriate mobile phase buffer pH range were established. The flat slope of the Log-D plot in the pH range of 9–13 for SUL revealed that the retention time of SUL was constant in this range. The curved Log-D plot value for TRIM in the given pH range of 2–4 and 9–11 demonstrated that the retention values of the peak were sensitive to even a modest pH change. The Log-D plot for INH was found to be a flat slope in the pH range of 5–11, revealing that the retention time for INH was constant in this range. Similarly, the Log-D plot value for B6 was curved in the given pH range of 3–6 and 8–11, demonstrating that the retention values of the peak were sensitive to even a modest pH change.
Furthermore, the pKa values were established to be 6.16 (strongly acidic) and 1.97 (strongly basic) for SUL, 17.33 (strongly acidic) and 7.16 (strongly basic) for TRIM, 13.61 (strongly acidic), 3.35 (strongly basic), 9.4 (strongly acidic), and 5.58 (strongly basic) for INH and B6, respectively. During method development, a pH range of 4–6.5 was studied for the choice of buffer and column, considering the physiochemical properties of the APIs. After analyzing the drugs with UV, it was found that the optimal wavelength for detection is 254 nm. This wavelength resulted in a better response for all four drugs.
After exploring various mobile phase compositions to elute title ingredients, the mobile phase system containing 50 mm Potassium dihydrogen Orthophosphate pH 6.5: Methanol with 1 ml/min flow rate in gradient mode was chosen. The Eclip Plus C18 column was considered suitable for chromatographic separation at ambient temperature with an injection volume of 20 μl and runtime of 30 min for all the solutions. The pH value 6.5 of the buffer was chosen for improved selectivity and sensitivity of the APIS. The isocratic mode could not solve peaks of the four drugs with proper Resolution (Rs) and Retention time (Rt); therefore, further method development was done using gradient mode.
This study employed the protein precipitation method with methanol as the best approach for extracting four drugs from rabbit plasma. The method is relatively simple and provides quick sample clean-up in plasma.
The CNX experiment has implemented a Cause and Effect metric approach for risk assessment to identify the Critical Method Parameters (CMPs) that facilitate quality separation and elution. Among the CMPs that significantly impact the method attributes are the Flow Rate of the mobile Phase (Factor A), pH of Mobile phase (Factor B) and the percentage of organic modifier at (0.01 to 5 min) (Factor C). The Risk Assessment has determined that the Critical Method Attributes are R1, Resolution (Rs), and R2, Asymmetric factor (As). To develop an optimized and efficient process, an investigation was conducted on the interaction between these essential attributes of the method and parameters, creating a design space.
In DOE, the resolution of sulfamethoxazole was significantly influenced by model terms C and A2, with both terms having synergistic effects. Similarly, model terms A, B, BC, A2, and B2 significantly impacted Trimethoprim Resolution. The experimental design suggested that Trimethoprim Resolution could be affected by the antagonist effect of factors BC and B2, while factors A, B, and A2 had a synergistic impact. The resolution of Isoniazid was potentially affected by all three aspects, namely A, C, and B2, with an antagonistic effect. Model terms B, C, AB, AC, BC, B2, and C2 significantly impacted Pyridoxine resolution. The experimental design indicated that Pyridoxine resolution could be substantially influenced by the antagonist effect of factors B, C, and B2. In contrast, the synergistic effect of factors AB, AC, BC, and C2 was not more significant than 0.1000. The significant model term in the sulfamethoxazole Asymmetric factor was B2, which had a synergistic effect. Model terms C, B2, and C2 substantially impacted the Trimethoprim Asymmetric Factor. The experimental design suggested that Isoniazid Resolution could be affected by the antagonist effect of factor C and the synergistic effect of polynomial terms B and C. For the Isoniazid Asymmetric factor, model terms B2 and C2 had a synergistic effect. Similarly, model terms B, C, BC, and C2 significantly impacted the Asymmetric factor of pyridoxine. The experimental design indicated that the pyridoxine Asymmetric factor could be substantially influenced by the antagonist effect of all four factors. Concerning R2 and Adjusted R2 values, the present study found that sulfamethoxazole, Trimethoprim, Isoniazid, and pyridoxine had R2 and Adjusted R2 values of 0.9010, 0.9879, 0.9319, 0.9952 and 0.9220, 0.9869, 0.9807, 0.9890, respectively. For the Asymmetric factor, the Coefficient of determination R2 and Adjusted R2 values were found to be 0.9651, 0.9315, 0.9120, 0.9397 and 0.9538, 0.9799, 0.9429, 0.9622 for Sulfamethoxazole, Trimethoprim, Isoniazid and pyridoxine, respectively. An adjusted R2 value of ≥ 0.80 showed a strong correlation between the experimental data and the fitted model39. The adequate precision was 7.436 (SUL), 11.739 (Trim), 7.777 (INH), 46.510 (B6) for resolution and 7.945 (SUL), 6.934 (Trim), 7.320 (INH), 12.554 (B6) for asymmetric factor. These values indicated an adequate signal. The Coefficient of variance (C.V.), which measures the model reproducibility, was found to be 3.830 (SUL), 5.350 (TRIM), 5.290 (INH), 2.990 (B6) for resolution and 4.350 (SUL), 5.530 (Trim), 7.770 (INH), 7.130 (B6) for Asymmetric factor. A Coefficient of variance (C.V.) value of < 10% is considered reasonably reproducible40. Therefore, the model was considered significant for undergoing the separation.
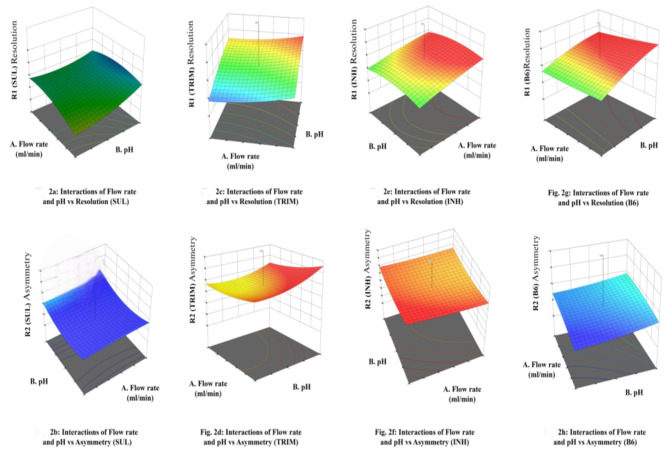
Response Surface Methodology (RSM) was employed to study the influence of individual variables on responses41. Figure 2 depicts the relationship between selected responses and variables in 3D contour plots for Sulfamethoxazole, Trimethoprim, Isoniazid, and Pyridoxine. The independent variables were analyzed for their impact on the individual responses using RSM The 3D-response surface plot of Sulfamethoxazole showed a linear increase in resolution with an increase in flow rate and a decrease in the pH of the mobile phase. The asymmetric factor is maximum when the flow rate is minimum and the mobile phase pH is lowest. The 3D-response surface plot of Trimethoprim demonstrated that Trimethoprim resolution increases with a decrease in flow rate and an increase in pH of the mobile phase. The asymmetric factor for Trimethoprim will be at its maximum if the flow rate and mobile phase PH are increased. The 3D-response surface plot of Isoniazid depicted that resolution increases with a decrease in the flow rate and pH of the mobile phase. The response plot showed that the asymmetric factor of Isoniazid increases with a reduction in flow rate and mobile phase pH. The 3D-response surface plot of Pyridoxine showed a linear increase in resolution with an increase in flow rate and pH of the mobile phase. The plot showed a linear decline in the asymmetric factor with decreased flow rate and mobile phase pH.
Fig. 2.
Interference study of SUL/Trim/INH/B6.
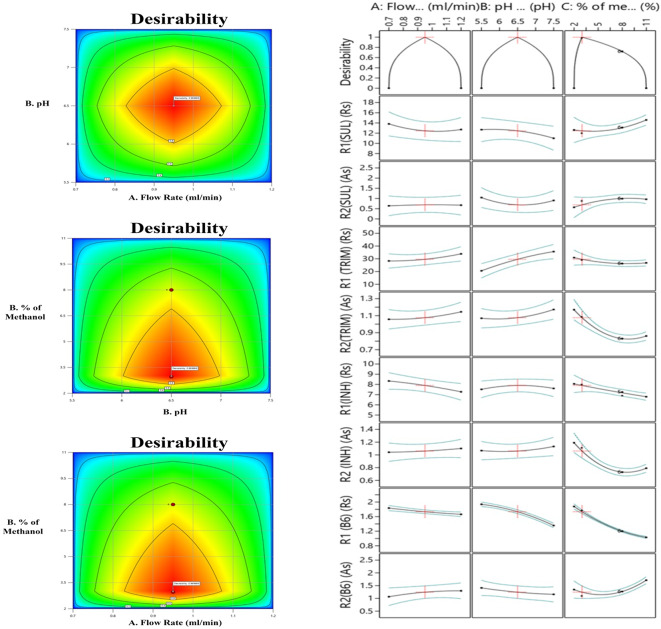
The software “Design Expert” visualizes each factor’s impact on the separation process through multi-dimensional plots. This approach entails establishing and verifying acceptable ranges for different parameters of the analytical process under defined conditions. As a result, a dependable and robust analytical process that consistently provides accurate results is obtained42. Using three factors, namely flow rate, mobile phase pH, methanol concentration up to five minutes during a 30-min run time, and three responses, namely retention time, resolution, and asymmetric factor, a two-dimensional design space (DS) has been created43, as shown in Fig. 3. The 2D contour plots in this space highlight the design space for resolution and the asymmetric factor, represented by the shaded red region. This region indicates the robustness of the method and the results that meet the predefined criteria44. The primary objective was to minimize asymmetric factors and maximize the resolution of the symmetrical peak45. Derringer’s desirability function (D) is thus an appropriate strategy where several responses are available to optimize with various targets46. The maximum desirability function’s response surface plot (D = 1) is shown in Fig. 3, demonstrating the mathematical model’s effectiveness. The coordinates result in a maximum desirability value at a mobile phase flow rate of 0.95 ml min−1, a mobile phase of pH 6.5, a concentration of 3% V/V methanol from initial to five minutes, and an exact concentration of methanol from twenty-seven to thirty minutes.
Fig. 3.
Design Space and Derringer’s desirability function of SUL/Trim/INH/B6.
The final chromatographic conditions employed for the separation of analytes. A Eclip Plus C18 column (250 mm × 5 mm × 4.6 µm, L1 packing) was utilized at a 0.95 ml/min flow rate. The optimized mobile phase consisted of a 50 mM potassium dihydrogen phosphate buffer mixture with pH 6.5 and methanol. The methanol concentration was set to 3% from the start to five minutes of runtime, 15% from five to fifteen minutes, and 55% from fifteen to twenty-seven minutes. The column was re-equilibrated with 3% methanol concentration until 30 min of runtime. The analytes were detected at 254 nm at ambient temperature for 30 min. The chromatogram showed the peaks of the drugs at retention times of 5.309 min for Isoniazid, 5.787 min for Pyridoxine, 11.317 min for Sulfamethoxazole, and 25.150 min for Trimethoprim, respectively.
Method
Material
The SUL/TRIM/INH/B6 compound (purity ≥ 99.00%) was acquired from Yarrow Chem Products at Swastik disa corporate park, opp. Shreyas Talkies, lbs Road, Ghatkopar (west), Mumbai-India. The solvents utilized in the experiment, including Acetonitrile (UFLC grade), Methanol (UFLC grade), Ortho phosphoric acid (UFLC grade), and Formic acid (UFLC grade), were obtained from s d fine chem limited at 1502, marathon Icon, Marathon Nextgen veer santaji marg, Lower Parel, Mumbai India. Potassium dihydrogen orthophosphate, NaOH (AR grade), was procured from Thermo Fisher Scientific India Pvt. Ltd. Navi Mumbai, India. Type-I water employed in the study was obtained from the Millipore Ultrapure Water Purification System (AnaMatrix, Chamrajpet, Bengaluru – 560018, Karnataka, India).
Instrument and software
The chromatographic separation process has been performed using Prominence LC-20A, Quaternary Gradient UFLC from Shimadzu, Japan. The UFLC integrated software (LC Realtime Analysis) was employed for integration and data processing purpose. The development of the chromatographic method was aided by a weighing balance (ACCULAB, Sartorius, Bangalore, India) and digital pH meters (MK VI, Systronics, Ahmedabad, Gujarat, India).
Experimental animal
The study used New Zealand White rabbits weighing between 1.5 and 2.5 kg. These animals were kept in the central animal facility at Sri Adichunchanagiri College of Pharmacy, B G Nagara, and were provided with standard laboratory conditions, that is, 22 ± 2 °C temperature and 12 h cycle of light and dark. They were also given a standard pellet diet and unrestricted water access. The Institutional Animal Ethics Committee (IAEC) at Sri Adichunchanagiri College of Pharmacy, B G Nagara, approved the experimental protocols (Approval Number: SACCP-IAEC/2020/01/28). Blood samples were obtained from the ear veins of rabbits (which require no anaesthetising) and subjected to centrifugation to extract Drug-free plasma. The animals used in this study were not euthanized and sacrificed at any point. Instead, a single blood sample was collected from each animal for the express purpose of isolating plasma. All procedures were implemented according to the CPCSEA guidelines for laboratory animal facilities, and the ARRIVE guidelines reported the study.
Statistical design
The Design of Experiments (DOE) uses statistical principles to control and estimate the association between factors and the output. It involves randomization, replication, and blocking techniques. “Central Composite Designs” (CCD) and “Box-Behnken” design (BBD) are famous for optimizing processes in experimental design under the Response Surface Methodology (RSM)47. RSM helps understand the relationship between factors and responses. The CCD is employed to estimate a quadratic model. This design is derived from a two-level factorial design, incorporating centre and axial points. It provides exceptional predictive capability within the design space’s central region, often referred to as the bullseye. When comparing BBD and CCD, it is essential to note that BBD involves fewer design points than the axial points of CCD, resulting in fewer experiments. Additionally, CCD tests are conducted under extreme conditions, making them better suited for quadratic models25. The Central Composite Design (CCD) was used to generate and test the data with the help of the trial version of Design-Expert® 13 software.
Risk assessment
The primary goal of the risk assessment (RA) is to identify the factors influencing critical quality attributes (CQA). Risk can be expressed quantitatively as a risk priority number (RPN) or qualitatively as ‘high’, ‘medium’, and ‘low’. In addition, ‘risk scores’ further describe the descriptors for ranking. The assessment of CQAs is crucial for evaluating the quality of the developed method48. The risk assessment tools utilized in quality by design (QBD) play a vital role in assessing and managing potential risks associated with pharmaceutical development and manufacturing processes. These tools assist in identifying and prioritizing risks, determining the level of risk for each identified factor, and implementing appropriate controls and mitigation strategies. The methods for determining risks include: (1) Control-noise-experimentation, or cause-effect approach, (2) Failure mode effect analysis, (3) Failure mode effect and criticality analysis, (4) Fault tree analysis, risk ranking and filtering, and ishakaba diagramatic tool. This study employed the control-noise-experimentation (CNX) tools to screen the risk factors in method development.
Stock solution preparation
A solution containing SUL/TRIM/INH/B6 was prepared by dissolving approximately 10 mg of the compound in methanol: water (7:3) to create a stock solution at 1000 µg mL−1 concentration. Working solutions were then prepared by diluting the stock solution with the same solvent system. The working and stock solutions were stored in a cold environment, maintained at a temperature range of 2–8 °C49.
Preparation quality control sample
A solution was prepared by combining a 200 µL working standard, 200 µL plasma, and 600 µL methanol as a protein precipitating agent. This solution contains four analytes (SUL/TRIM/INH/B6). The concentrations of the combination were set at 10, 20, 40, 80, 120, 160, 240, 320, and 640 ng mL−1, respectively. Quality control solutions for Lower limit of quantitation (LLOQ, 10 ng mL−1), Low Quality Control sample (LQC, 30 ng mL−1), Medium Quality control sample (MQC, 200 ng mL−1), High Quality Control sample (HQC, 600 ng mL−1) of SUL/TRIM/INH/B6 in combination were prepared using the working standard solution. All solutions were vortex-mixed for one minute and then centrifuged at 5000 rpm at 4 °C for five minutes. The clear supernatant underwent filtration through a 0.22 µm pore size and was subsequently sonicated for five minutes before the analysis.
Preparation of plasma sample
The bioanalytical samples were prepared by adding 200 µL of drug solution and 600 µL of methanol to 200 µL plasma (blank) in 1.5 ml Eppendorf tubes. The solutions were vigorously mixed for a minute and then centrifuged at 5000 rpm for five minutes at 4 °C50. Then, clear supernatant liquid went through filtration by a 0.22 µm membrane filter51,52.
Initial chromatographic condition
Chromatographic separation process of four investigational candidates was executed on a C18 Eclipse column (250 mm × 5 mm × 4.6 µm) through a mobile phase flow rate of 1.00 mL/min. The optimal composition for symmetrical peaks with a peak purity index of 0.999 in gradient elution was obtained by incorporating methanol into the mobile phase containing a mixer 50 m M Phosphate Buffer (pH 6.5). The concentration of methanol was 8% from the initial to five minutes. From six to fifteen minutes, the percentage of methanol in the mobile phase was fifteen cents, steeply increasing to thirty-five cents up to twenty-seven minutes. Finally, the mobile phase was re-equilibrated with eight per cent up to thirty minutes. Both analytes were detected at 254 nm with a 30-min runtime at ambient temperature.
Method validation
The proposed method has been evaluated for specificity, linearity, accuracy, precision, recovery and stability according to the US FDA Bioanalytical Technique Validation guideline27,53.
Conclusion
The QbD approach has been implemented successfully to develop a robust method for estimating Sulfamethoxazole, trimethoprim, Isoniazid, and Pyridoxine in rabbit plasma. The physicochemical properties of these drugs were considered while selecting input variables for the Design of the Experiment using a Central Composite Design. The concentration of Sulfamethoxazole, trimethoprim, Isoniazid, and Pyridoxine was not considered a quantitative variable in this design due to their narrow concentration range. Qualitative variables such as mobile phase pH, % organic modifier mobile phase & flow rate were considered and controlled. Each step of the Bioanalytical QbD process has been studied to determine the Design Space. Response surface plots graphically illustrated the significant effects of mobile phase pH, % organic modifier mobile phase, and flow rate on the separation. Using the QbD approach, the method’s robustness was established even before validation. The process was validated for accuracy and precision, and the results were highly satisfactory. The technique is cost-effective but also linear, precise, and accurate. The entire process of method development was rationalized by the BQbD approach through an empirical approach. Various experimental designs were applied to the screening and optimization of factor values, helping to understand the variability in responses. The selection of optimum conditions was justified, leaving no scope for uncertainty. The BQbD approach aided in upholding the method’s quality and performance by establishing a design space. It demonstrated method conditions having flexibility and high reliability. Validation further added to the reliability of the process for the intended purpose. Thus, the BQbD approach is proposed to be rational, safe, and reliable over regular method development techniques. Using the QbD approach has resulted in a highly reliable and effective method for estimating the four drugs in rabbit plasma.
Acknowledgements
The authors thank Sri Adichunchanagiri College of Pharmacy for providing research facilities to complete the study.
Abbreviations
- SUL
Sulfamethoxazole
- Trim
Trimethoprim
- INH
Isoniazid
- B6
Pyridoxine
- UFLC
Ultra flow liquid chromatography
- BQbD
Bioanalytical quality by design
- CAA
Critical analytical attributes
- QTMP
Quality target method profile
- CNX
Control noise experimentation
- CMP
Critical method parameters
- RPN
Risk priority number
- C&E
Cause-and-effect
- CCD
Central composition design
- BBD
Box-Behnken design
- RSM
Response surface methodology
- DS
Design space
- API
Active pharmaceutical ingredient
- IAEC
Institutional Animal Ethical Committee
- QC Level
Quality control level
- CV
Coefficient of variation
- SD
Standard deviation
- LLOQ
Lower limit of quantification
- LQC
Low quality control
- MQC
Mid quality control
- HQC
High quality control
- %RE
Percentage of relative error
- CV
Coefficient of variation
- Factor A
Flow rate of the mobile phase
- Factor B
Mobile phase pH
- C
Organic modifier
- R1
Response1
- Rs
Resolution
- R2
Response 2
- As
Asymmetric factor
- R1(SUL)
Resolution of sulphonamide
- R1(TRIM)
Resolution of trimethoprim
- R1(INH)
Resolution of isoniazid
- R1(B6)
Resolution of pyridoxine
- R2(SUL)
Asymmetric factor of sulphonamide
- R2(TRIM)
Asymmetric factor of trimethoprim
- R2(INH)
Asymmetric factor of isoniazid
- R2(B6)
Asymmetric factor of pyridoxine
Author contributions
Premsagar K M, Bhagyalakshmi C: Data curation, Methodology Visualization, Investigation, Akramol Ansary, Tridib Kumar Das: Data curation, Visualization, Methodology, investigation, Piyongsola: Writing- Original draft preparation, Investigation, Software, Original draft preparation, Investigation, Software, T Yunus Pasha, B Ramesh: Supervision, Writing- Reviewing and Editing, Manish Majumder, Koushik Nandan Dutta: Conceptualization, Supervision, writing, Reviewing and Editing. All authors reviewed the manuscript.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.del Rio, C. The global HIV epidemic: What the pathologist needs to know. Semin. Diagn. Pathol.34, 314–317 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ratnam, M. V. R. et al. CD4 cell counts and oral manifestations in HIV infected and AIDS patients. J. Oral Maxillofac. Pathol.22 (2018). [DOI] [PMC free article] [PubMed]
- 3.Birmeka, M. Distribution pattern and prevalence of opportunistic infections and their possible reciprocal effects among HIV patients, Burayu Health Centers. 10.3855/jidc.15105 [DOI] [PubMed]
- 4.Henderson, H. I. et al. Predicting risk of multidrug-resistant enterobacterales infections among people with HIV. Open Forum Infect. Dis.9, 487 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdoli, A., Falahi, S. & Kenarkoohi, A. COVID-19-associated opportunistic infections: A snapshot on the current reports. Clin. Exp. Med.22, 327–346 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali, M. et al. HIV and AIDS-defining opportunistic illnesses in the state of Qatar: A cohort population-based retrospective study covering 17 years (2000–2016). Ann. Med. Surg.78 (2022). [DOI] [PMC free article] [PubMed]
- 7.Madhavan, A., Sachu, A., Samuel, A. & Vasudevapanicker, J. Cryptococcal antigen prevalence in HIV patients from a tertiary care centre in South India. 14, 740–745 (2022). [DOI] [PMC free article] [PubMed]
- 8.Indira, P., Kumar, P. M., Shalini, S. & Vaman, K. Opportunistic infections among people living with HIV (PLHIV) with diabetes mellitus (DM) attending a tertiary care hospital in coastal city of South India. PLoS One10, e0136280 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duff, P. Prevention of opportunistic infections in women with HIV infection. Clin. Obstet. Gynecol.62 (2019). [DOI] [PubMed]
- 10.Harries, A. D., Lawn, S. D., Suthar, A. B. & Granich, R. Benefits of combined preventive therapy with co-trimoxazole and isoniazid in adults living with HIV: Time to consider a fixed-dose, single tablet coformulation. Lancet. Infect. Dis.15, 1492–1496 (2015). [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. WHO-PQ recommended summary of product haracteristics. 3–3 (2021).
- 12.Letang, E. et al. Minimally invasive tissue sampling: a tool to guide efforts to reduce AIDS-related mortality in resource-limited settings. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am.73, S343–S350 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coelho, L. E. et al. Predictors of opportunistic illnesses incidence in post combination antiretroviral therapy era in an urban cohort from Rio de Janeiro. Brazil. BMC Infect. Dis.16, 134 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui, Y. et al. Development of an ultra fast liquid chromatography-tandem mass spectrometry method for simultaneous determination of cefazedone and etimicin in beagle dog plasma: Application to the pharmacokinetic study of the combination of cefazedone and etimicin inje. J. Chromatogr. Anal. Technol. Biomed. Life Sci.973, 97C–103 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Chiarentin, L. et al. Drilling into ‘quality by design’ approach for analytical methods. Crit. Rev. Anal. Chem.8, 1–42. 10.1080/10408347.2023.2253321 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Sharma, G., Thakur, K., Raza, K. & Katare, O. P. Stability kinetics of fusidic acid: Development and validation of stability indicating analytical method by employing analytical quality by design approach in medicinal product(s). J. Chromatogr. B Anal. Technol. Biomed. Life Sci.1120, 113–124 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Tumpa, A., Stajić, A., Jančić-Stojanović, B. & Medenica, M. Quality by design in the development of hydrophilic interaction liquid chromatography method with gradient elution for the analysis of olanzapine. J. Pharm. Biomed. Anal.134, 18–26 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Rašević, M., Malenović, A., Protić, A. & Zečević, M. Analytical method development supported by DoE-DS approach for enantioseparation of (S, S)- and (R, R)-moxifloxacin. J. Pharm. Biomed. Anal.235, 115645 (2023). [DOI] [PubMed] [Google Scholar]
- 19.Aboelghar, S. M., Hegazy, M. A. & Wagdy, H. A. Ecofriendly bioanalytical validated RP-HPLC method for simultaneous determination of COVID-19 co-prescribed drugs employing quality by design and green chemistry. Microchem. J.200, 110292 (2024). [Google Scholar]
- 20.Prajapati, P. B., Bagul, N. & Kalyankar, G. Implementation of DoE and risk-based enhanced analytical quality by design approach to stability-indicating RP-HPLC method for stability study of bosutinib. J. AOAC Int.104, 1742–1753 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Muchakayala, S. K. et al. AQbD based green UPLC method to determine mycophenolate mofetil impurities and Identification of degradation products by QToF LCMS. Sci. Rep.12, 19138 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taheri, M., Bagheri, M., Moazeni-Pourasil, R. S. & Ghassempour, A. Response surface methodology based on central composite design accompanied by multivariate curve resolution to model gradient hydrophilic interaction liquid chromatography: Prediction of separation for five major opium alkaloids. J. Sep. Sci.40, 3602–3611 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Prajapati, P., Salunkhe, M., Pulusu, V. S. & Shah, S. Integrated approach of white analytical chemistry and analytical quality by design to multipurpose RP-HPLC method for synchronous estimation of multiple fixed-dose combinations of paracetamol. Chem. Africa7, 1353–1371 (2024). [Google Scholar]
- 24.Prajapati, P., Rana, B., Pulusu, V. S. & Mishra, A. Multipurpose RP-HPLC method for simultaneous estimation of fixed-dose combinations of anti-diabetic drugs: Integrating green, economical, and robust approaches with design of experiments and white analytical chemistry. Chem. Afr.7, 1385–1400 (2024). [Google Scholar]
- 25.Prajapati, P., Rana, B., Pulusu, V. S. & Shah, S. Method operable design region for robust RP-HPLC analysis of pioglitazone hydrochloride and teneligliptin hydrobromide hydrate: Incorporating hybrid principles of white analytical chemistry and design of experiments. Futur. J. Pharm. Sci.9, 93 (2023). [Google Scholar]
- 26.Konduru, N., Gundla, R., Dongala, T., Katari, N. K. & Mallavarapu, R. Development and validation of liquid chromatography method for determination of Ibrutinib in finished dosage forms using quality by design approach. Sep. Sci. PLUS5, 254–266 (2022). [Google Scholar]
- 27.FDA, F. & D. A. Bioanalytical Method Validation Guidance. Food Drug Adm.1043, 25 (2018).
- 28.Dalvi, A. V., Uppuluri, C. T., Bommireddy, E. P. & Ravi, P. R. Design of experiments-based RP–HPLC bioanalytical method development for estimation of Rufinamide in rat plasma and brain and its application in pharmacokinetic study. J. Chromatogr. B1102–1103, 74–82 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Yang, G., Tazoe, H. & Yamada, M. Rapid determination of 135Cs and precise 135Cs/137Cs atomic ratio in environmental samples by single-column chromatography coupled to triple-quadrupole inductively coupled plasma-mass spectrometry. Anal. Chim. Acta908, 177–184 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Hailat, M., Al-Ani, I., Hamad, M., Zakareia, Z. & Abu Dayyih, W. Development and validation of a method for quantification of favipiravir as COVID-19 management in spiked human plasma. Molecules26 (2021). [DOI] [PMC free article] [PubMed]
- 31.Abdel-Moety, E. M., Rezk, M. R., Wadie, M. & Tantawy, M. A. A combined approach of green chemistry and quality-by-design for sustainable and robust analysis of two newly introduced pharmaceutical formulations treating benign prostate hyperplasia. Microchem. J.160, 105711 (2021). [Google Scholar]
- 32.Meng, M. et al. Simultaneous quantitation of polymyxin B1, polymyxin B2 and polymyxin B1–1 in human plasma and treated human urine using solid phase extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. B1012–1013, 23–36 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Surve, D. H. & Jindal, A. B. Development and validation of reverse-phase high-performance liquid chromatographic (RP-HPLC) method for quantification of Efavirenz in Efavirenz-Enfuvirtide co-loaded polymer-lipid hybrid nanoparticles. J. Pharm. Biomed. Anal.175, 112765 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Alladio, E. et al. Effective validation of chromatographic analytical methods: The illustrative case of androgenic steroids. Talanta215, 120867 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Gu, H., Liu, G., Wang, J., Aubry, A. F. & Arnold, M. E. Selecting the correct weighting factors for linear and quadratic calibration curves with least-squares regression algorithm in bioanalytical LC-MS/MS assays and impacts of using incorrect weighting factors on curve stability, data quality, and assay perfo. Anal. Chem.86, 8959–8966 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Pant, A. et al. Quality by design-steered development and validation of analytical and bioanalytical methods for raloxifene: Application of Monte Carlo simulations and variance inflation factor. Biomed. Chromatogr.37, e5641 (2023). [DOI] [PubMed] [Google Scholar]
- 37.Abdelwahab, N. S., Ali, N. W., Zaki, M. M., Sharkawi, S. M. Z. & Abdelkawy, M. M. Simultaneous determination of thalidomide and dexamethasone in rat plasma by validated HPLC and HPTLC with pharmacokinetic study. J. Chromatogr. Sci.57, 130–138 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Method, B., An, V., Goch, W. & Giebułtowicz, J. Replicates number for drug stability testing during bioanalytical method validation—An experimental and retrospective approach (2022). [DOI] [PMC free article] [PubMed]
- 39.Patel, K. Y., Dedania, Z. R., Dedania, R. R. & Patel, U. QbD approach to HPLC method development and validation of ceftriaxone sodium 4 (2021).
- 40.Shamim, A. et al. QbD-engineered development and validation of a RP-HPLC method for simultaneous estimation of rutin and ciprofloxacin HCl in bilosomal nanoformulation. ACS Omega8, 21618–21627 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumari, M. & Gupta, S. K. Response surface methodological ( RSM ) approach for optimizing the removal of trihalomethanes (THMs) and its precursor’s by surfactant modified magnetic nanoadsorbents (sMNP)—An endeavor to diminish probable cancer risk 1–11. 10.1038/s41598-019-54902-8 (2019). [DOI] [PMC free article] [PubMed]
- 42.Hanafi, R. S. & Lämmerhofer, M. Response surface methodology for the determination of the design space of enantiomeric separations on cinchona-based zwitterionic chiral stationary phases by high performance liquid chromatography. J. Chromatogr. A1534, 55–63 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Surapuraju, P. K. R. & Juturu, R. R. Development, robustness by design expert and validation of a method for enantiomeric impurity content determination in pretomanid drug substance and pharmaceutical dosage form. J. Chromatogr. Sci.10.1093/chromsci/bmac090 (2022). [DOI] [PubMed] [Google Scholar]
- 44.Hassan, R. M., Saleh, O. A., El-Azzouny, A. A., Aboul-Enein, H. Y. & Fouad, M. A. Experimental design optimization of simultaneous enantiomeric separation of atenolol and chlorthalidone binary mixture by high-performance liquid chromatography using polysaccharide-based stationary phases. Chirality33, 397–408 (2021). [DOI] [PubMed] [Google Scholar]
- 45.da Silva, B., Valdomiro Gonzaga, L., Fett, R. & Oliveira Costa, A. C. Simplex-centroid design and Derringer’s desirability function approach for simultaneous separation of phenolic compounds from Mimosa scabrella Bentham honeydew honeys by HPLC/DAD. J. Chromatogr. A1585, 182–191 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Variables, R. SS symmetry on the application of a design of experiments along with an ANFIS and a desirability function to model response variables (2021).
- 47.Prajapati, P., Rana, B., Pulusu, V. S. & Shah, S. Simultaneous chromatographic estimation of vildagliptin and dapagliflozin using hybrid principles of white analytical chemistry and analytical quality by design. J. AOAC Int.107, 212–222 (2024). [DOI] [PubMed] [Google Scholar]
- 48.Salwa, & Kumar, L. Quality-by-design driven analytical method (AQbD) development and validation of HPLC–UV technique to quantify rivastigmine hydrogen tartrate in lipidic nanocarriers: Forced degradation, and assessment of drug content and in vitro release studies. Microchem. J.193, 108944 (2023). [Google Scholar]
- 49.Ingle, R. G., Zeng, S., Jiang, H. & Fang, W.-J. Current developments of bioanalytical sample preparation techniques in pharmaceuticals. J. Pharm. Anal.12, 517–529 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eckernäs, E. et al. Development and application of a highly sensitive LC-MS/MS method for simultaneous quantification of N,N-dimethyltryptamine and two of its metabolites in human plasma. J. Pharm. Biomed. Anal.212, 114642 (2022). [DOI] [PubMed] [Google Scholar]
- 51.Chapter 2 Fundamental strategies for bioanalytical sample preparation. in High Throughput Bioanalytical Sample Preparation (ed. Wells, D. A. B. T.-P. in P. and B. A.) vol. 5 41–74 (Elsevier, 2003).
- 52.Baldelli, S., Marrubini, G., Cattaneo, D., Clementi, E. & Cerea, M. Application of Quality by Design Approach to Bioanalysis: Development of a Method for Elvitegravir Quantification in Human Plasma. Therapeutic Drug Monitoring vol. 39 (2017). [DOI] [PubMed]
- 53.Fares, M. Y., Hegazy, M. A., El-Sayed, G. M., Abdelrahman, M. M. & Abdelwahab, N. S. Quality by design approach for green HPLC method development for simultaneous analysis of two thalassemia drugs in biological fluid with pharmacokinetic study. RSC Adv.12, 13896–13916 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.