Abstract
Objective
The various functionalities of collagen peptides have generated a large interest in utilizing the bioactive peptides as a nutritional therapy to ameliorate various physiological degenerative conditions. Collagen peptides are observed to reduce the pain and aligned difficulties with respect to osteoarthritis. Here we report the enhanced ameliorating property of novel high-functional “Wellnex” Type J collagen peptides following a double-blind randomized active and placebo-controlled 5-arm clinical trial (n = 100) by using it as a nutritional supplement in subjects with knee joint osteoarthritis in comparison with conventional bovine collagen peptides. The efficacy, safety, and tolerability were also studied.
Design
Dosages of 2.5, 5.0, and 10.0 g of high-functional Type J bovine collagen peptides, 10.0 g of conventional collagen peptides, and 10.0 g of placebo were given to the 5 groups for a period of 90 days. The Western Ontario McMaster Universities Arthritis Index (WOMAC) score, Pain Scale, Quality of Life (QoL), Physician’s Impression of change Score (PICS), serum C-terminal cross-linked telopeptide of type II collagen (CTX-II) levels and Magnetic Resonance Imaging Osteoarthritis Knee Score (MOAKS) parameters were monitored.
Results
Type J 2.5 g showed significant improvement in WOMAC, QoL, CTX, and MOAKS and observed to be equivalent to conventional collagen peptide 10-g supplementation in terms of efficacy.
Conclusion
The two significant outcomes of the study were that Type J 10.0 g, Type J 5.0 g, Type J 2.5 g and conventional collagen peptides 10.0 g supplementation were observed to be beneficial nutraceutical therapies for knee joint osteoarthritis, and Type J 2.5 g supplementation was equivalent to conventional collagen peptides 10.0-g supplementation in terms of efficacy.
Keywords: bovine collagen peptide, osteoarthritis, WOMAC, QoL, PICs, MOAKS
Introduction
Millions of people worldwide suffer from osteoarthritis (OA), a painful and complex disease. 1 Since OA can cause cumulative damage over time, increased age is the primary risk factor for the onset and progression of OA. The most common joints affected by OA are the interphalangeal, hips, knee, and intervertebral joints. The symptoms of OA include inflammation, pain, stiffness, and loss of mobility as cartilage degrades, bone shape change, and cartilage degrades. OA is characterized by focal and progressive loss of hyaline articular cartilage with concomitant changes in the bone underneath the cartilage, including formation of osteophytes and bony sclerosis, and changes in the synovium and joint capsule. 2 Even though OA is a serious health, economic, and social problem, the exact etiology of its occurrence has not been determined. According to current hypotheses, mitochondrial dysfunction and oxidative stress 3 contribute to aging, as does accumulation of advanced glycation end products (AGEs) 4 in chondrocytes. These hypotheses are based on the classic “wear and tear” theory,5,6 senescence-related secretory phenotype, 7 and low reactivity of chondrocytes to growth factors. 8 The cumulative effect of mechanical load may lead to clinical wear and tear and pathological cartilage breakdown. 5 Hence, the current research effort is focused on management of the condition with alternatives such as supplementation with high-functional collagen peptides.
Collagen peptides (CP) produced by the hydrolysis of collagen is assimilated in human peripheral blood not only as amino acids but also as dipeptides and tripeptides. These smaller peptides remain in the blood for relatively long time.9 -11 Several collagen-derived peptides are detected in blood following the ingestion of CPs, such as Pro-Hyp, Pro-Hyp-Gly, Ala-Hyp, Ala-Hyp-Gly, Ser-Hyp-Gly, Leu-Hyp, Ile-Hyp, and Phe-Hyp. 12 Cell proliferation, growth, and hyaluronic acid synthesis have been reported to be stimulated by the Pro-Hyp in cultured dermal fibroblasts and synovium cells.13 -15 In both in vitro and in vivo studies, Pro-Hyp exerted chondroprotective effects in the articular cartilage. 16 Thus, supplementation of CPs regulates chondrocyte differentiation and stimulates synthesis of proteoglycans, resulting in the initiation of repair processes in cartilage tissue. In our previous clinical study, we have demonstrated CP as an effective supplement for the improvement in overall physical problems associated with OA and thereby help to improve the quality of life. 17 High-functional Type J CPs have active dipeptides proline-hydroxyproline (PO) and hydroxyproline-glycine (OG) at a concentration greater than 3,000 ppm. In our recent animal study using this high-functional Type J product, we have confirmed that the product at a concentration of 517 mg/kg and 1,033 mg/kg is having potent anti-OA effects against monoiodoacetic acid (MIA)-induced OA in rats when taken orally for 21 consecutive days. 18
As OA is a disease characterized by the progressive destruction of articular cartilage where a major component is collagen, it has been postulated that supplementation with collagen hydrolysates may induce the synthesis of cartilage matrix. This process involves stimulating the chondrocytes, 19 following intestinal absorption and accumulation in articular cartilage through blood circulation. The objective of this clinical study is to determine the dosage of high-functional CP (Type J) for the management of OA.
Materials and Methods
Investigational Products
High-functional bovine CPs, Type J, the investigational product ( Table 1 ), is the product of a Good Manufacturing Practice (GMP)-certified company—Nitta Gelatin India Limited—sold under the global “Wellnex” brand, with enhanced levels of active dipeptides PO and OG in it. The product is tested using a liquid chromatography with tandem mass spectrometry (LC-MS/MS) for the active dipeptide content in comparison with conventional bovine CPs. Being the key regulatory factor in functionality, the 30-fold increase in the active molecule content in newly developed Type J CP shall exhibit high functional property in joint health compared to existing conventional CPs. The average molecular weight of the new high-functional CPs is <1,000 Da in comparison with the average molecular weight of <5,000 Da in the existing conventional CPs. The active dipeptide content of the new high-functional CPs is >3,000 ppm, whereas the active dipeptide content in the existing conventional CPs is <100 ppm. The placebo used in the study was Maltodextrin purchased from Nutrimed Healthcare Pvt. Ltd.
Table 1.
The Pattern of Treatment Administration, Active Treatment, Comparator and the Placebo.
| Route of Administration | Oral | |
|---|---|---|
| Test treatment | Type J 2.5 g collagen peptide | Once a day for 90 days |
| Type J 5.0 g collagen peptide | Once a day for 90 days | |
| Type J 10.0 g collagen peptide | Once a day for 90 days | |
| Active comparator | 10 g Conv. collagen peptide | Once a day for 90 days |
| Placebo comparator | 5.0 g placebo | Once a day for 90 days |
Study Design
The study was designed as a 90-day double-blind, prospective, multicenter, randomized, active and placebo-controlled, 5-arm, clinical study to evaluate the efficacy, safety, and tolerability of high-functional bovine CPs as a nutritional therapy in the management of knee joint OA in adults. The Universal Ethics Committee India, which is an ethics committee registered with the Central Drugs Standard Control Organisation (CDSCO) (reg. no. ECR/125/Indt/TN/2013/RR-20) and Office for Human Research Protections (OHRP), and United States Food and Drug Administration (USFDA; reg. no. IORG0007234) reviewed and approved the ethical, scientific, and medical aspects of the clinical study. The induction activities of the clinical study were initiated only after the approval by the ethics committee. The study was conducted in accordance with the ethical principles as laid out in the current version of the Declaration of Helsinki, The International Council for Harmonisation (ICH) Harmonized Tripartite Guideline—Guideline for Good Clinical Practice E6 (R2), and Indian Council for Medical Research (ICMR) Ethical Guidelines for Biomedical Research on Human Participants and New Drugs and Clinical Trial Rules. The trial was further registered with the Clinical Trial Registry India (CTRI) with the registration no. CTRI/2021/04/032912 dt. 19/04/2021. The study is CONSORT (Consolidated Standards of Reporting Trials) compliant. The study was conducted at the following trial sites: S.V. MultiSpeciality Hospital, Kodambakkam, Chennai; Star Bone and Joint Center, Triplicane High Road, Chennai; Life Span Bone and Joint Clinic, Alwarthirunagar, Chennai; and Noble Super Speciality Clinic, Thiruvanmiyur, Chennai. The investigator had counseled the subjects, explained the purpose, procedure, risks, and benefits of the study on the day of screening and obtained their informed consent. The screening procedure was performed as per the clinical study protocol. Subjects who fulfilled the study criteria were enrolled into the study. The randomization allocation sequence was generated using Statistical Analysis System (SAS). The double-blinded randomization was implemented using alphanumeric coded and labeled sachets. Subjects were randomized in a 1:1:1:1:1 ratio. The randomization sequence was generated by the statistician and the unblinded pharmacist. The investigator and his study team enrolled and assigned the participants to the intervention as per the randomization sequence. Participants, investigator, and the study team were blinded to the intervention. There were no changes made to the trial design and trial outcome after the commencement. Recruitment of the subjects was between July 14, 2021, and October 29, 2021. The subjects were followed up for a period of 3 months with the last subject’s last visit on February 2, 2022. The trial ended only upon completion of follow-up of all enrolled subjects and was neither stopped nor terminated interim.
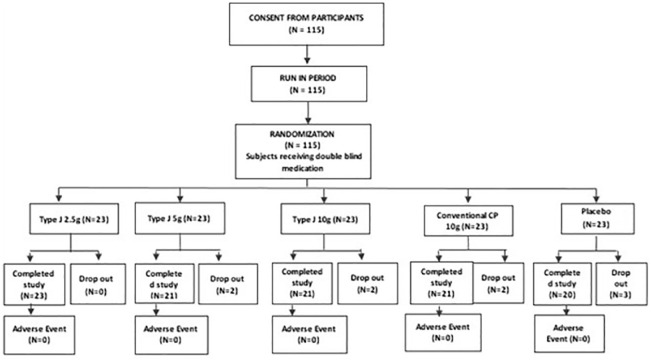
The objective of the study was to evaluate the comparative efficacy of high-functional Type J bovine CP in the management of knee joint OA ( Table 2 ). The primary objective was to evaluate the structural disease progression, to determine the optimal dose of Type J peptide and to assess the improvement in functional capacity and quality of life of the subjects. The secondary objectives were to evaluate the safety of high-functional Type J bovine CPs; the usage, dosage, and frequency of SOS (si opus sit (which in latin means - if necessary/as needed) or in general term as save our soul/rescue medication) medication during the treatment period; and to evaluate the subject compliance and satisfaction. The study design is illustrated in Figure 1 .
Table 2.
Objective, Primary Aim, Additional Objectives, and Outcome Measures of This Study.
| Study Objective |
| 1. To evaluate the comparative efficacy of Type J 2.5 g collagen peptide to conventional collagen peptide 10.0 g in the structural disease progression of knee joint osteoarthritis. 2. To evaluate the comparative efficacy of Type J 2.5 g collagen peptide to conventional collagen peptide 10.0 g in the symptomatic relief of knee joint osteoarthritis. |
| Additional Objectives |
| 1. To assess the improvement in functional capacity and quality of life of the subjects 2. To evaluate the safety of Type J collagen |
| Outcome Measures |
| 1. Reduction of WOMAC scores 2. Reduction of pain Scores 3. Clinical improvement as assessed by a physician using x-ray 4. Reduction of CTX II scores 5. Safety assessments (complete blood count, LFT, RFT) |
WOMAC = Western Ontario McMaster Universities Arthritis Index; CTX-II = C-terminal cross-linked telopeptide of type II collagen; LFT = liver function test; RFT = renal function test.
Figure 1.
Illustration of selection method of subjects for clinical study and study design. The subjects were randomized into 3 arms of Type J CP, 1 arm of conventional CP and 1 arm of placebo in 1:1:1:1:1 ratio. CP = collagen peptides.
Inclusion and Exclusion Criteria
The subjects between 30 and 65 years of age with confirmed diagnosis or known history of OA, with moderately active lifestyle (including both the aforementioned ages and both sexes) were included in the study. Subjects categorized as Grade II or III in the Kellgren Lawrence Scale, as confirmed by x-ray of target joint, were selected. Subjects who scored ≥4 on the Pain Scale (numerical rating scale 0-10) in one or both knees were screened. Subjects who were not satisfied with anti-inflammatory or anti-analgesic drugs for the past 6 months were also included for the study. Subjects with known hypersensitivity or vegetarians/vegans who object consumption of animal-origin investigational products were excluded from the study. Subjects with contraindications to magnetic resonance imaging (MRI) and subjects with known hypersensitivity to nonsteroidal anti-inflammatory drugs, aspirin, COX-2 inhibitors, and other analgesic medicine were excluded from the study. Subjects who have had hyaluronic acid injections, up to 6 months prior to the enrollment; subjects who have had intra-articular steroid, up to 3 months prior to the enrollment; and subjects with immunocompromised-state complications were excluded. Those with uncontrolled diabetes, hypertension, or congestive heart failure or with any significant medical condition were excluded. Females who are pregnant, lactating, or planning to become pregnant during the study period were also not included in the study. This clinical study was conducted as a double-blinded study. The products were packed in coded sachets that look the same, so that there were no partiality or favoritism in deciding who were given which packet.
Outcome Measures
The major parameters evaluated in this study were Western Ontario and McMaster Universities Arthritis Index (WOMAC) score, 20 Pain Scale, 21 Quality of Life (QoL), 22 Physician’s Impression of Change Scale (PICS), 23 serum C-terminal cross-linked telopeptide of type II collagen (CTX-II), 24 and Magnetic Resonance Imaging Osteoarthritis Knee Score (MOAKS) 25 parameters. Self-administered WOMAC score is the most commonly used clinical tool to measure symptoms and physical disability of patients with OA. It includes 5 questions about pain, 2 about stiffness, and 17 on degree of disability of activities of daily living. WOMAC score is widely used in the evaluation of hip and knee OA. Higher scores on the WOMAC indicate worse pain, stiffness, and functional limitations. Pain Scale score is a tool that doctors use to help assess a person’s pain. A person usually self-reports their pain using a specially designed scale, of 0 to 10 or 0 to 5. Zero means “no pain,” and 5 or 10 means “the worst possible pain.” QoL is a measure of morbidity associated with any health conditions. The specific dimensions found in most health-related QoL definitions include degrees of physical symptoms, functional limitations, emotional well-being, social functioning, role activities, life satisfaction, and health perception. PICS is a scoring system based on physician’s impression marked at baseline and at the end of the study, which is based on the x-ray report, activity limitations, symptoms, emotions, and overall quality of life. CTX-II assays measure a fragment of the C-terminal telopeptide of type II collagen. The Type II collagen degradation product CTX-II is released into the serum and urine in the case of subjects with OA, and the CTX-II concentration in body fluids reflects OA progression. MRI is advantageous in the evaluation of structural changes during the progression of knee OA. MOAKS is a semi-quantitative scoring tool that was developed from the Whole-Organ Magnetic Resonance Imaging Score (WORMS) and Boston Leeds OA Knee Score (BLOKS) scoring tools. MOAKS has been shown to have very good to excellent reliability. The demographic data of subjects in each group are described in Table 3 .
Table 3.
Demographic Data of Subjects in Each Group.
| Type J 10 g Group | Type J 5 g Group | |
|---|---|---|
| Sex (male/female) | 8/13 | 7/14 |
| Age, years | 48 ± 2.14 | 49.05 ± 2.78 |
| Height, cm | 160 ± 0.07 | 160 ± 0.06 |
| Weight, kg | 76.54 ± 6.07 | 76.10 ± 7.44 |
| Type J 2.5 g Group | Conv. CP 10 g Group | |
| Sex (male/female) | (9/11) | (11/9) |
| Age, years | 48.65 ± 2.16 | 48.70 ± 2.15 |
| Height, cm | 159 ± 0.06 | 157 ± 0.06 |
| Weight, kg | 75.00 ± 7.41 | 77.54 ± 5.34 |
| Placebo 5 g Group | ||
| Sex (male/female) | (9/9) | |
| Age, years | 49.39 ± 2.20 | |
| Height, cm | 161 ± 0.06 | |
| Weight, kg | 78.19 ± 7.09 |
CP = collagen peptides.
There is no significant difference in the intragroup demographic data. The study was conducted as a 5-arm clinical study with both active and conventional CPs and placebo comparator to evaluate the comparative effectiveness of Type J. Type J was tested in 3 doses of 2.5 g, 5.0 g, and 10.0 g so as to determine the most optimal dose for the treatment of OA. The study end points were set as the reduction of the WOMAC scale by ≥20 scores, reduction of Pain Scale score by ≥4 scores, improvement in the QoL by ≥10 scores and reduction of CTX-II by 10% from the baseline. The objective assessment was based on PICS on x-ray. The frequency and dosage of SOS medication were monitored, and improvement in MOAKS of ≥10% from baseline was noted.
Statistical Analysis
All statistical analyses are performed in accordance with the ICH E9 (The International Council for Harmonisation E9) guideline for Statistical Principles for Clinical Trials, using SAS (Version 9.4 or higher). Single Proportion Test determined the efficacy of each individual treatment arm and Two Proportion Test determined the comparative efficacy for proportion of subjects. For absolute scores, Single Mean Test determined the efficacy of each treatment arm, and the comparative efficacy was determined using Two Mean Test. All point estimates, such as mean and standard deviation, and interval estimates such as 95% confidence intervals were derived for the estimated parameters. No interim analysis was performed.
The statistical software SAS V-9.4 was used to perform both parametric and nonparametric tests. The assumption of normality was tested by the use of a nonparametric test “Kolmogorov-Smirnov Test (K-S Test).” If the normality assumption was satisfied, then a parametric test, such as Student’s t test or analysis of variance (ANOVA) test, was applied to compare efficacy parameters. Statistical tests were carried out at 5% level of significance. Descriptive measures such as mean, standard deviation, and proportions were used to model the basic characteristics of the subject/sample.
Results
As per a statistical analysis plan (SAP), it was decided that 115 subjects would be screened and enrolled into the study. Of these 115 subjects, the first 100 to complete the study would be considered for statistical analysis. This was designed to accommodate for dropouts. Without bias, the first 100 subjects to complete the study were considered for statistical analysis and reporting.
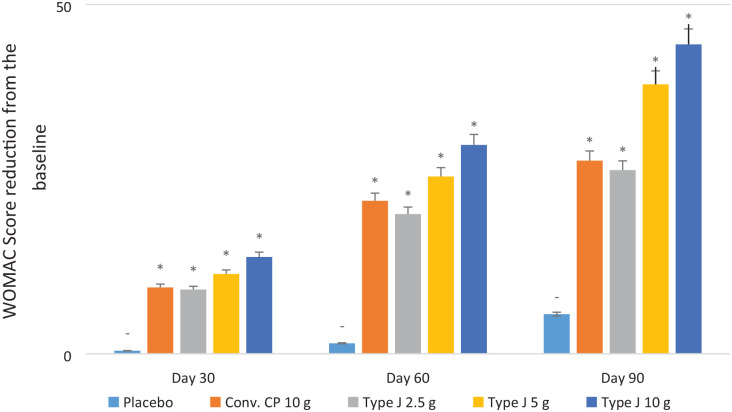
WOMAC score showed a significant reduction in all test groups compared to placebo (P < 0.05) on days 30, 60, and 90 of supplementation. The average reduction of WOMAC score at the end of the study from the baseline was 27.65, 26.30, 38.57, 44.29, and 5.67 for the conventional CPs 10.0 g, Type J 2.5 g, 5.0 g, 10.0 g, and placebo groups, respectively, which was significantly ( Table 4 ) higher than the reduction of WOMAC score (P < 0.05) ( Fig. 2 ). The endpoint of WOMAC score was the reduction by ≥20 scores from baseline, and all test groups achieved that. The proportion of the subjects that either reached or surpassed the endpoint by the end of the study in the conventional CP 10.0 g, Type J 2.5 g, 5.0 g, and 10.0 g groups were 90%, 80%, 95%, and 95%, respectively.
Table 4.
Statistical Analysis Between All Groups for All Variables (P Value) (P < 0.05 Is Significant).
| Type J 2.5 g | Type J 5 g | Type J 10 g | Conv. CP 10 g | Placebo | |
|---|---|---|---|---|---|
| WOMAC | |||||
| Type J 2.5 g | — | — | — | — | — |
| Type J 5 g | 0.0002* | — | — | — | — |
| Type J 10 g | 0.0001* | 0.0500 | — | — | — |
| Conv. CP 10 g | 0.7652 | 0.0138* | 0.0006* | — | — |
| Placebo | 0.0001* | 0.0000* | 0.0001* | 0.0001* | — |
| Pain Scale | |||||
| Type J 2.5 g | — | — | — | — | — |
| Type J 5 g | 0.0001* | — | — | — | — |
| Type J 10 g | 0.0001* | 0.4190 | — | — | — |
| Conv. CP 10 g | 0.9783 | 0.0004* | 0.0001* | — | — |
| Placebo | 0.0020* | 0.0000* | 0.0000* | 0.0010* | — |
| QoL | |||||
| Type J 2.5 g | — | — | — | — | — |
| Type J 5 g | 0.0131* | — | — | — | — |
| Type J 10 g | 0.0001* | 0.0011* | — | — | — |
| Conv. CP 10 g | 0.7111 | 0.0586 | 0.0001* | — | — |
| Placebo | 0.0000* | 0.0000* | 0.0000* | 0.0000* | — |
| PICS | |||||
| Type J 2.5 g | — | — | — | — | — |
| Type J 5 g | 0.0001* | — | — | — | — |
| Type J 10 g | 0.0001* | 0.1263 | — | — | — |
| Conv. CP 10 g | 1.0000 | 0.0001* | 0.0001* | — | — |
| Placebo | 0.0000* | 0.0000* | 0.0000* | 0.0000* | — |
| CTX-II | |||||
| Type J 2.5 g | — | — | — | — | — |
| Type J 5 g | 0.0001* | — | — | — | — |
| Type J 10 g | 0.0001* | 0.0001* | — | — | — |
| Conv. CP 10 g | 0.0001* | 0.0001* | 0.0001* | — | — |
| Placebo | 0.0001* | 0.0001* | 0.0001* | 0.0001* | — |
| MOAKS | |||||
| Type J 2.5 g | — | — | — | — | — |
| Type J 5 g | 0.0001* | — | — | — | — |
| Type J 10 g | 0.0001* | 0.0001* | — | — | — |
| Conv. CP 10 g | 0.6481 | 0.0001* | 0.0001* | — | — |
| Placebo | 0.0001* | 0.0001* | 0.0001* | 0.0001* | — |
CP = collagen peptides; WOMAC = Western Ontario McMaster Universities Arthritis Index; PICS = Physician’s Impression of change Score; CTX-II = C-terminal telopeptides of collagen type II; MOAKS = Magnetic Resonance Imaging Osteoarthritis Knee Score; QoL = Quality of Life.
Significant values.
Figure 2.
Average reduction of Western Ontario McMaster Universities Arthritis Index (WOMAC) scores from baseline (statistical significance [*] is with respect to the placebo).
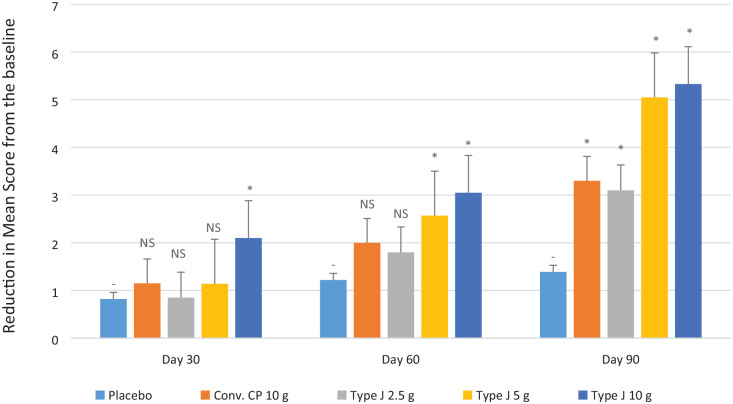
Pain Scale score showed a significant reduction in all test groups compared to placebo (P < 0.05) at the end of the study. The average reduction of Pain Scale score at the end of the study from the baseline was observed as 3.3, 3.1, 5.05, 5.33, and 1.39 in the conventional CPs 10.0 g, Type J 2.5 g, 5.0 g, 10.0 g, and placebo groups, respectively, which was significantly higher with respect to the reduction in Pain Scale score (P < 0.05) ( Fig. 3 ). The endpoint of Pain Scale score was the reduction by ≥4 scores from baseline. The proportion of the subjects that either reached or surpassed the endpoint by the end of the study in the conventional CP 10.0 g, Type J 2.5 g, 5.0 g, and 10.0 g groups was 40%, 25%, 90%, and 95%, respectively.
Figure 3.
Average reduction of Pain Scale scores from baseline. NS = nonsignificant. Statistical significance (*) is with respect to the placebo.
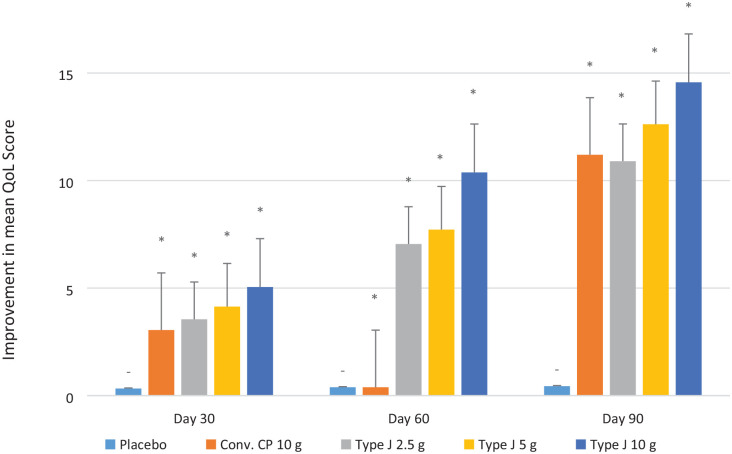
The QoL score showed a significant improvement in all test groups compared to placebo at days 30, 60, and 90 (P < 0.05). The average improvement in QoL from the baseline at the end of the study observed in conventional CPs 10.0 g, Type J 2.5 g, 5.0 g, and 10.0 g groups was 11.2, 10.9, 12.62, and 14.17, respectively, and that was significantly higher than the improvement of 0.44 in the placebo arm (P < 0.05) ( Fig. 4 ). The endpoint of QoL was the improvement by ≥10 scores from baseline, and all the test groups achieved that. The proportion of the subjects that either reached or surpassed the endpoint by the end of the study in the conventional CP 10.0 g, Type J 2.5 g, 5.0 g, and 10.0 g groups was 80%, 70%, 95%, and 95%, respectively.
Figure 4.
Average improvement of QoL scores from baseline. QoL = quality of life. Statistical significance (*) is with respect to the placebo.
PICS showed a significant reduction in all test groups compared to placebo (P < 0.05) at the end of the study. The mean PICS in conventional CPs 10.0 g, Type J 2.5 g, 5.0 g, 10.0 g, and placebo groups was 3.35, 3.35, 2.24, 2, and 4.44, respectively (P < 0.05) ( Fig. 5 ). The endpoint of PICS was 2 at visit 4, and that was achieved by Type J 10.0 g and 5.0 g groups. The proportion of the subjects that either reached or surpassed the endpoint by the end of the study in the conventional CP 10.0 g, Type J 2.5 g, 5.0 g, and 10.0 g groups was 10%, 5%, 76%, and 86%, respectively.
Figure 5.
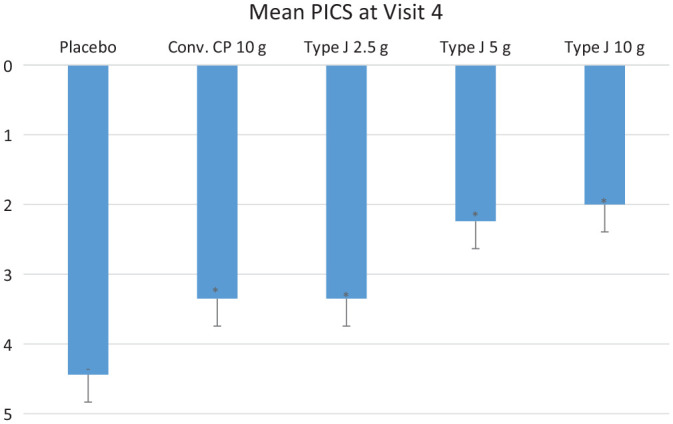
The average PICS score at the end of the study. PICS = Physician’s Impression of change Score. Statistical significance (*) is with respect to the placebo.
CTX-II levels showed a significant reduction in all test groups compared to placebo (P < 0.05) at the end of the study. The mean value of percentage reduction of CTX-II levels in conventional CPs 10.0 g, Type J 2.5 g, 5.0 g, and 10.0 g groups was 22.15%, 22.8%, 30.14%, and 34.35%, respectively, and was significantly higher than the reduction of 3.1% in placebo group (P < 0.05) ( Fig. 6 ). The endpoint of CTX-II level was set as a reduction by ≥10% from the baseline, and this was achieved by all the test groups. The proportion of the subjects that either reached or surpassed the endpoint by the end of the study in the conventional CP 10.0 g, Type J 2.5 g, 5.0 g, and 10.0 g groups was 90%, 85%, 90%, and 95%, respectively.
Figure 6.
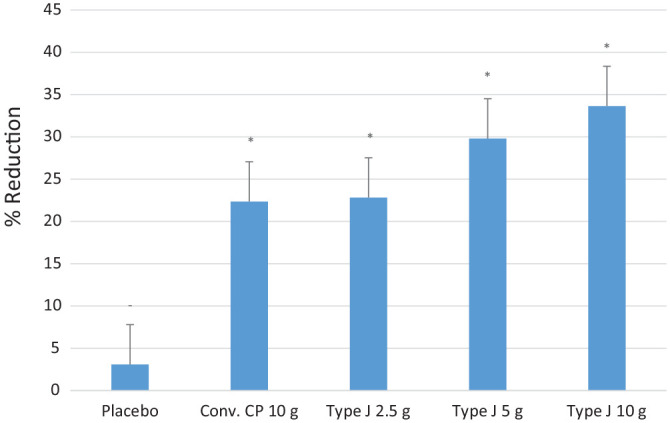
The average percentage of CTX-II reduction from baseline. CTX-II = C-terminal telopeptides of collagen type II. Statistical significance (*) is with respect to the placebo.
The MRI examination exhibited a three-dimensional visualization of cartilage, which can provide quantitative, semi-quantitative, and biochemical assessments of cartilage. A significant reduction in the MOAK score in comparison to the placebo by the end of the study was observed in all the test groups (P < 0.05) ( Fig. 7 ). The mean value of percentage reduction of MOAKS in conventional CPs 10.0 g, Type J 2.5 g, 5.0 g, and 10.0 g groups was 23.57%, 23.53%, 33.81%, and 42.86%, respectively. The mean values were significantly higher than the 7% reduction in placebo group (P < 0.05). The endpoint of CTX-II level was the reduction of >10% from the baseline, and this was achieved by all the test groups. The proportion of the subjects that either reached or surpassed the endpoint by the end of the study in the conventional CP 10.0 g, Type J 2.5 g, 5.0 g, and 10.0 g groups was 83%, 66%, 83%, and 83%, respectively.
Figure 7.
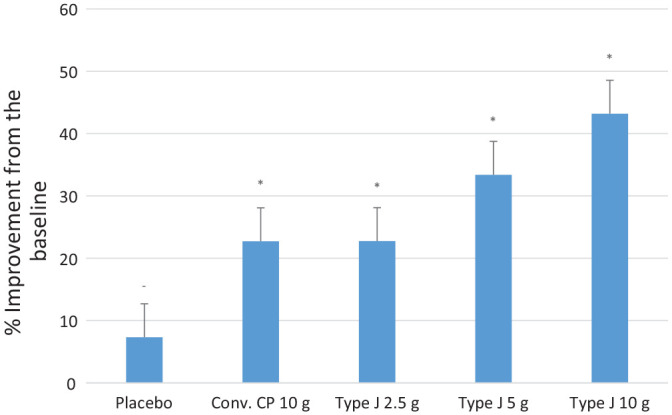
The average percentage improvement in MOAKS from baseline. MOAKS = Magnetic Resonance Imaging Osteoarthritis Knee Score. Statistical significance (*) is with respect to the placebo.
There were no subgroup or adjusted analyses performed. A total of 14 adverse events (AEs) ( Table 5 ) were reported during the study. In the Type J 2.5g group, there were 3 AEs reported (diarrhea, seasonal cold, and flu); in the Type J 5.0g group, 1 AE was reported (hypoglycemia); in the Type J 10.0g group, 5 AEs were reported (body pain, asthma exacerbation, 3 cases of seasonal cold and flu); in the conventional CP 10.0g, 3 AEs were reported (diarrhea, 2 cases of seasonal cold and flu); and in the placebo group, 2 AEs were reported (seasonal cold and flu). All the AEs were ruled as not related to the investigational product by the respective investigator.
Table 5.
Brief Summary of Adverse Effects.
| Body Pain | Diarrhea | Difficulty in Breathing (Patient Asthmatic) | Fever | Hypoglycemia | Seasonal Cold and Flu | |
|---|---|---|---|---|---|---|
| Type J 2.5 g | — | 1 a | — | — | — | 2 a |
| Type J 5 g | — | — | — | — | 1 a | — |
| Type J 10 g | 1 a | — | 1 a | — | — | 1 a |
| Conv. CP 10 g | — | 1 a | — | 2 a | — | 2 a |
| Placebo | — | — | — | — | — | 2 a |
CP = collagen peptides.
The observed adverse effects were ruled as not related to the investigational product by the respective investigator.
Discussion
The present study suggests that oral supplementation of Type J CP decreases the symptoms of OA at a low dosage level. This finding was supported by the reduction in both the total WOMAC index and Pain Scale score. By the end of the study, in comparison to the placebo, all the test groups showed a significant reduction in the WOMAC score (P < 0.05). Pain Scale and PICS score also showed a significant reduction across the test groups. In comparison with the placebo, the QoL was observed to improve across the test groups.
Severe pain being one of the most important symptoms in OA, the pain reduction indirectly indicates the mark of improvement in joint conditions in patients. Thus, the administration of CP has much relevance with respect to the reduction of pain in a patient with OA. It is considered that the accumulated CP helps to maintain structure and function of cartilage, which in turn results in joint comfort and subsequent improvements in pain. The efficacy of CP in OA has been studied in human clinical trials. In one randomized, double-blind, placebo-controlled multicenter trial, 250 subjects with knee OA were given 10.0 g of CPs daily for 6 months. At the end of the study, significant pain improvement was observed in the visual analog scales and the WOMAC subscale. 26 Other clinical trials also showed reduction in joint pain upon the daily consumption of CPs for periods ranging from 3 months to 1 year. In all these clinical trials, an improvement in the WOMAC index and other indexes of joint functionality and discomfort was observed.27 -31
Results from experimental studies have revealed that collagen is capable to reduce articular pain and a biomarker of cartilage degradation—CTX-II in the plasma and urine. 32 In the present study using high-functional “Wellnex” CPs, CTX-II levels showed a significant reduction in all test groups. The mean value of percentage reduction of CTX-II levels in the conventional CPs 10.0 g was 22.15% and that of high-functional Type J peptide 2.5 g was 22.8%. The MOAK score showed a significant reduction in all test groups. The mean value of percentage reduction of MOAKS in conventional CPs 10.0 g was 23.57% and that of high-functional Type J peptide 2.5 g was 23.53%. All the results showed a significant improvement compared to the placebo (P < 0.05). The MOAKS was introduced by a panel of experienced OA researchers and has shown very good to excellent reliability. 25 The MOAKS refined the scoring of Bone Marrow Lesions (BMLs), articular cartilage, and the elements of meniscal morphology scoring and involves scoring of 7 sub-regions such as BMLs, cartilage, osteophytes, synovitis, meniscus, ligament/tendon, and periarticular findings. 25
Throughout the study, the subjects were instructed to continue with the same routine and lifestyle as before without making any significant changes to the dietary or exercise patterns. The only addition to their everyday routine lifestyle was the investigational product; thus, an unbiased intra-comparison was performed. There are no sources of potential bias, imprecision, and multiplicity of analyses in the planned study protocol. However, we did not investigate the exercise habits of subjects, so we could not deeply consider the correlation to knee pain and subject’s background.
Supplements containing collagen are abundantly rich in amino acids such as glycine, proline, and hydroxyproline, which play a crucial role in cartilage formation. In addition, they may also exhibit anti-inflammatory and antioxidant properties and may act as signaling molecules. 33 Postprandial absorption of the processed components is enhanced after the enzymatic hydrolysis of collagen. As a result, nutraceuticals derived from hydrolyzed collagen have an increased bioavailability of its amino acids and/or peptides. 34 Bone mineral density, bone mineral content, osteoblast differentiation, and the amount of type I collagen in the bone matrix are enhanced by type I collagen synthesis. 35 It is more likely that collagen-derived peptides with a lower molecular weight will reach other parts of the body, including the joints, because they are more readily absorbed in the small intestine. 36 The low-molecular-weight peptides are resistant to hydrolysis primarily based on the amino acid composition of the molecules. In the small intestine, the peptides with the amino acids proline or hydroxyproline are not readily hydrolyzed or digested by the gastrointestinal system. Wang et al. 37 and Iwai et al. 9 have reported that collagen-derived peptides such as PO and OG, originating from the repeating motif G-X-Y, circulate in the blood up to 4 hours following oral collagen and gelatin consumption.9,37 Skov et al. 34 and León-López et al. 38 also reported that hydrolyzed collagen has greater bioavailability and solubility, resulting in better absorption from the small intestine.34,38 Nakatani et al. 16 stated that PO reduces the Alkaline phosphatases (ALP) activities and improves the Extracellular matric (ECM) in vitro. As per their findings, after oral administration, PO is absorbed in the small intestine, and the circulating PO may regulate chondrocyte differentiation and maintenance of mature chondrocytes in the permanent cartilage. The regulation mechanism of PO suggests that it may be recognized by chondrocytes, which lead to the reduction in the mRNA level for osteocalcin via regulation of the Runx1 mRNA level and thereby termination of differentiation at the mature chondrocyte stage. The postulated regulation of chondrocytes by PO may explain the mechanism of the therapeutic effect of CPs in improving joint conditions. Osawa et al. 39 studied the absorption of orally administered CPs in vascular-perfused rat intestine in situ. Results of the study imply that breakdown products of CP digestion are absorbed as small peptides. 39 In the present study, the high content of PO and OG in the high-functional product makes it an effective nutraceutical therapy for the management of OA. Based on the improvement observed on the QoL and reduction with respect to the WOMAC Scale, Pain Scale, and CTX-II levels, it can be concluded that high-functional CP is effective in providing symptomatic relief from knee joint OA. The study inclusion-exclusion criteria have been designed to include subjects within a wide age range, including subjects of all genders, socio-economic status, and demographics, in an attempt to be objective and yet with external validity, generalizability, and applicability.
Conclusion
The current study showed that oral supplementation of CPs is a potential therapeutic or supportive strategy in the management of OA. Supplementation of 2.5 g of “Wellnex” Type J CPs with high PO and OG content resulted in significant improvements in QoL and reduction in the WOMAC score, CTX-II, and MOAKS. The supplementation of 2.5 g of Type J CPs gave the same effect as that of 10.0 g of conventional CP consumption. The results clearly indicate that the new high-functional Type J CPs slow down or even inhibit the progression of OA. Safety assessments (complete blood count, liver function tests, renal functional tests) were performed at the start and end of the study for every enrolled subject, showing no significant pathological difference. Thus, the high-functional CP is proven to be a safe, clinically verified, and validated product to manage the structural disease progression and provide symptomatic relief while improving functional capacity and quality of life of people with knee joint OA.
Footnotes
Author’s Note: The work was done at Nitta Gelatin India Limited, KINFRA Industrial Parks Ltd, Kakkanad, Cochin 682042, India.
Author Contribution: S.M. and S.D. designed the study. S.D., J.T.J., S.P.S., and S.V.T. performed the experiments and acquired the data. S.V.T. and S.M. analyzed and interpreted data. S.D. drafted the first version of the manuscript. S.M., S.K., A.P.K., and L.C. reviewed the manuscript and contributed to its improvement. A.P.K. revised and finalized the manuscript, and S.D., S.M., S.K., and L.C. approved the manuscript for submission. All authors read and approved the final manuscript. Consent for publication was obtained from all individual participants included in the study.
Acknowledgment and Funding: The authors would like to thank all the participants & Nitta Gelatin India Limited, India, Nitta Gelatin Inc., Japan (NGI), Nitta Gelatin NA, USA (NGNA), and Aurous Health Care Research and Development India Private Limited, India, who supported them in carrying out this clinical study and preparing the manuscript for the journal submission. Type J, Conv. Collagen peptides & placebo (test products), the funds for carrying out the research in Type J, and the funds for conducting the clinical studies were enabled and organized by Nitta Gelatin India Limited (NGIL), KINFRA Industrial Parks Ltd, Kakkanad, Cochin, India.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: High-functional bovine collagen peptides, Type J, is the product of Nitta Gelatin India Limited and sold under the global “Wellnex” brand, with enhanced levels of active dipeptides. Nitta Gelatin Inc., 2-22, Futamata, Yao-City, Osaka, Japan, owns the “Wellnex” brand. The author S.D. is the Quality Assurance Manager, J.T.J. is the Research and Development (R&D) trainee, S.P.S. is the R&D Assistant, A.P.K. is the Head of the R&D Department (at present on chair), and S.M. is the Former Head of the R&D Department at NGIL.
Ethical Approval: The study was conducted in accordance with the ethical principles as laid out in the current version of the Declaration of Helsinki, ICH Harmonised Tripartite Guideline – Guideline for Good Clinical Practice E6 (R2) and ICMR (Indian Council for Medical Research) Ethical Guidelines for Biomedical Research on Human Participants and New Drugs and Clinical Trial Rules. The trial was registered with the Clinical Trial Registy India (CTRI) with the registration No. CTRI/2021/04/032912 dt. 19/04/2021.
ORCID iD: Abhilash Parameswaran Kailas  https://orcid.org/0000-0001-9918-5065
https://orcid.org/0000-0001-9918-5065
Data Availability: The datasets generated and/or analyzed in the current study are available from the corresponding author upon reasonable request.
References
- 1. Kloppenburg M, Berenbaum F. Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthritis Cartilage. 2020;28(3):242-8. [DOI] [PubMed] [Google Scholar]
- 2. Felson DT, La wrence RC, Dieppe PA, Hirsch R, He lmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Int Med. 2000;133:635-46. [DOI] [PubMed] [Google Scholar]
- 3. Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011;7:161-9. [DOI] [PubMed] [Google Scholar]
- 4. Huang CY, Lai KY, Hung LF, Wu WL, Liu FC, Ho LJ. Advanced glycation end products cause collagen II reduction by activating Janus kinase/signal transducer and activator of transcription 3 pathway in porcine chondrocytes. Rheumatology. 2011;50(8):1379-89. [DOI] [PubMed] [Google Scholar]
- 5. Aigner T, Rose J, Martin J, Buckwalter J. Aging theories of primary osteoarthritis: from epidemiology to molecular biology. Rejuvenation Res. 2004;7(2):134-45. [DOI] [PubMed] [Google Scholar]
- 6. Loeser RF. Aging processes and the development of osteoarthritis. Curr Opin Rheumatol. 2013;25:108-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):106-10. [DOI] [PubMed] [Google Scholar]
- 8. Messai H, Duchossoy Y, Khatib A, Panasyuk A, Mitrovic DR. Articular chondrocytes from aging rats respond poorly to insulin-like growth factor-1: an altered signaling pathway. Mech Ageing Develop. 2000;115:21-37. [DOI] [PubMed] [Google Scholar]
- 9. Iwai K, Hasegawa T, Taguchi Y, Morimatsu F, Sato K, Nakamura Y, et al. Identification of food-derived peptides in human blood after oral ingestion of gelatin hydrolysates. J Agric Food Chem. 2005;53:6531-6. [DOI] [PubMed] [Google Scholar]
- 10. Aito-Inoue M, Ohtsuki K, Nakamura Y, Park EY, Iwai K, Morimatsu F, et al. Improvement in isolation and identification of food-derived peptides in human plasma based on precolumn derivatization of peptides with phenyl isothiocyanate. J Agric Food Chem. 2006;54:5261-6. [DOI] [PubMed] [Google Scholar]
- 11. Ohara H, Matsumoto H, Ito K, Iwai K, Sato K. Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. J Agric Food Chem. 2007;55:1532-5. [DOI] [PubMed] [Google Scholar]
- 12. Ichikawa S, Morifuji M, Ohara H, Matsumoto H, Takeuchi Y, Sato K. Hydroxyproline-containing dipeptides and tripeptides quantified at high concentration in human blood after oral administration of gelatin hydrolysate. Int J Food Sci Nutr. 2010;61(1):52-60. [DOI] [PubMed] [Google Scholar]
- 13. Shigemura Y, Iwai K, Morimatsu F, Iwamoto T, Mori T, Oda C, et al. Effect of prolyl-hydroxyproline (Pro-Hyp), a food-derived collagen peptide in human blood, on growth of fibroblasts from mouse skin. J Agric Food Chem. 2007;55:1532-5. [DOI] [PubMed] [Google Scholar]
- 14. Ohara H, Ichikawa S, Matsumoto H, Akiyama M, Fujimoto N, Kobayashi T, et al. Collagen-derived dipeptide, proline–hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J Dermatol. 2010;37(4):330-8. [DOI] [PubMed] [Google Scholar]
- 15. Ohara H, Iida H, Ito K, Takeuchi Y, Nomura Y. Effects of pro-Hyp, a collagen hydrolysate-derived peptide, on hyaluronic acid synthesis using in vitro cultured synovium cells and oral ingestion of collagen hydrolysates in a guinea pig model of osteoarthritis. Biosci Biotechnol Biochem. 2010;74(10):2096-9. [DOI] [PubMed] [Google Scholar]
- 16. Nakatani S, Mano H, Sampei C, Shimizu J, Wada M. Chondroprotective effect of the bioactive peptide prolyl-hydroxyproline in mouse articular cartilage in vitro and in vivo. Osteoarthritis Cartilage. 2009;17(12):1620-7. [DOI] [PubMed] [Google Scholar]
- 17. Kumar S, Sugihara F, Suzuki K, Inoue N, Venkateswarathirukumar S. A double-blind, placebo-controlled, randomised, clinical study on the effectiveness of collagen peptide on osteoarthritis. J Sci Food Agric. 2015;95:702-7. [DOI] [PubMed] [Google Scholar]
- 18. Devasia S, Joseph JT, Stephena PS, Koizumi S, Himeno A, Gayathri V, et al. Bioactive collagen peptides ameliorate monoiodoacetic acid induced osteoarthritis in rats. J Orthop Res Ther. 2021;6:1205. [Google Scholar]
- 19. Deal CL, Moskowitz RW. Nutraceuticals as therapeutic agents in osteoarthritis. The role of glucosamine, chondroitin sulfate, and collagen hydrolysate. Rheum Dis Clin North Am. 1999;25(2):379-95. [DOI] [PubMed] [Google Scholar]
- 20. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833-40. [PubMed] [Google Scholar]
- 21. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011;63(Suppl 11):S240-152. [DOI] [PubMed] [Google Scholar]
- 22. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337-43. [DOI] [PubMed] [Google Scholar]
- 23. Guy W. ECDEU assessment manual for psychopharmacology. Revised. US Department of Health, Education, and Welfare Publication (ADM). Rockville (MD): National Institute of Mental Health; 1976. p. 76-338. [Google Scholar]
- 24. Oestergaard S, Chouinard L, Doyle N, Karsdal MA, Smith SY, Qvist P, et al. The utility of measuring C-terminal telopeptides of collagen type II (CTX-II) in serum and synovial fluid samples for estimation of articular cartilage status in experimental models of destructive joint diseases. Osteoarthritis Cartilage. 2006;14(7):670-9. [DOI] [PubMed] [Google Scholar]
- 25. Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage. 2011;19(8):990-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benito-Ruiz P, Camacho-Zambrano MM, Carrillo-Arcentales JN, Mestanza-Peralta MA, Vallejo-Flores CA, Vargas-López SV, et al. A randomized controlled trial on the efficacy and safety of a food ingredient, collagen hydrolysate, for improving joint comfort. Int J Food Sci Nutr. 2009;60(Suppl 2):99-113. [DOI] [PubMed] [Google Scholar]
- 27. McAlindon TE, Nuite M, Krishnan N, Ruthazer R, Price LL, Burstein D, et al. Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: a pilot randomized controlled trial. Osteoarthritis Cartilage. 2011;19(4):399-405. [DOI] [PubMed] [Google Scholar]
- 28. Trč T, Bohmová J. Efficacy and tolerance of enzymatic hydrolysed collagen (EHC) vs. Glucosamine sulphate (GS) in the treatment of knee osteoarthritis (KOA). Int Orthop. 2011;35(3):341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiang J, Yu S, Huang Q, Zhang X, Zhang C, Zhou J, et al. Collagen peptides improve knee osteoarthritis in elderly women—a 6-month randomized, double-blind, placebo-controlled study. Agro Food Industry Hi Tech. 2014;25(2):19-23. [Google Scholar]
- 30. Bruyère O, Zegels B, Leonori L, Rabenda V, Janssen A, Bourges C, et al. Effect of collagen hydrolysate in articular pain: a 6-month randomized, doubleblind, placebo-controlled study. Complement Ther Med. 2012;20(3):124-30. [DOI] [PubMed] [Google Scholar]
- 31. Carpenter RL, Peel JB, Carpenter MR, Lowndes J, Angelopoulos TJ. Effectiveness of a collagen hydrolysate-based nutritional supplement on the level of joint pain, range of motion and muscle function in individuals with mild osteoarthritis of the knee: a randomized clinical trial. Ann Rheum Dis. 2005;3:1544-5. [Google Scholar]
- 32. Di Cesare Mannelli L, Micheli L, Zanardelli M, Ghelardini C. Low dose native type II collagen prevents pain in a rat osteoarthritis model. BMC Musculoskel Disord. 2013;14:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paul C, Leser S, Oesser S. Significant amounts of functional collagen peptides can be incorporated in the diet while maintaining indispensable amino acid balance. Nutrients. 2019;11:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skov K, Oxfeldt M, Thøgersen R, Hansen M, Bertram HC. Enzymatic hydrolysis of a collagen hydrolysate enhances postprandial absorption rate—a randomized controlled trial. Nutrients. 2019;11:1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takeda S, Jong-Hoon P, Kawashima E, Ezawa I, Omi N. Hydrolyzed collagen intake increases bone mass of growing rats trained with running exercise. J Int Soc Sports Nutr. 2013;35:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mobasheri A, Mahmoudian A, Kalvaityte U, Uzieliene I, Larder CE, Iskandar MM, et al. A white paper on collagen hydrolyzates and ultrahydrolyzates: potential supplements to support joint health in osteoarthritis? Curr Rheumatol Rep. 2021;23(11):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang L, Wang Q, Liang Q, He Y, Wang Z, He S, et al. Determination of bioavailability and identification of collagen peptide in blood after oral ingestion of gelatin. J Sci Food Agric. 2015;95(13):2712-7. [DOI] [PubMed] [Google Scholar]
- 38. León-López A, Morales-Peñaloza A, Martínez-Juárez VM, Vargas Torres A, Zeugolis DI, Aguirre-Álvarez G. Hydrolyzed collagen sources and applications. Molecules. 2019;24:4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Osawa Y, Mizushige T, Jinno S, Sugihara F, Inoue N, Tanaka H, et al. Absorption and metabolism of orally administered collagen hydrolysates evaluated by the vascularly perfused rat intestine and liver in situ. Biomed Res. 2018;39(1):1-11. [DOI] [PubMed] [Google Scholar]