Abstract
Background
This study aims to explore the relationship between magnesium depletion score (MDS) and periodontitis in US adults using data from the National Health and Nutritional Examination Survey (NHANES) 2009–2014.
Methods
This cross-sectional study’s outcome was periodontitis, defined by the CDC/AAP using clinical periodontal parameters. The exposure of this study was MDS, which was calculated according to four parameters (diuretic use, proton pump inhibitor use, renal function and alcohol consumption). Weighted univariable and multivariable logistic regression analyses were performed to explore the association between MDS intake and periodontitis. Confounding factors included in the adjusted model were age, sex, race, income, smoking status, dietary magnesium, obesity, diabetes, hypertension, education level, recreational activity, and work activity.
Results
A total of 8,628 participants over the age 30 were included in our study. Individuals with high level of magnesium deficiency were more likely referred to poorer periodontal health in both crude model (OR = 2.01, 95% CI: 1.54–2.61, p < 0.0001) and fully adjusted model (OR = 1.35, 95% CI: 1.03–1.77, p = 0.03).
Conclusions
MDS is positively associated with moderate/severe periodontitis. Further longitudinal studies are needed to understand the impact of MDS on periodontitis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-024-05048-1.
Keywords: Periodontitis, MDS, Cross-sectional study, NHANES
Background
Periodontitis is a common chronic inflammatory disease caused by periodontal pathogens that destroy periodontal tissues, leading to tooth loss [1]. Epidemiological data suggests that nearly half of adults worldwide are affected by periodontitis, with 10% experiencing severe effects [2]. In recent years, minerals, especially magnesium, have been found to be closely associated with periodontitis [3].
Magnesium is an essential mineral that collaborates with over 300 enzymes and regulates various biochemical processes, including protein synthesis, DNA/RNA synthesis, glycolysis, and oxidative phosphorylation [4]. Magnesium deficiency is associated with chronic inflammatory stress, which predisposes to the development of chronic diseases, including periodontitis [5]. Magnesium deficiency stimulates the production of reactive oxygen species (ROS) and the subsequent release of inflammatory cytokines [6], which contribute to the occurrence and development of periodontitis. Moreover, magnesium plays a crucial role in periodontal tissue regeneration by promoting angiogenesis and bone repair [7, 8]. A significant reduction in serum magnesium levels was found in patients with periodontitis compared to normal controls, while increased magnesium intake could decrease the risk of periodontitis [9, 10]. Previous research has primarily focused on the effects of serum magnesium and dietary magnesium on periodontitis, overlooking magnesium deficiency. The Magnesium depletion score (MDS) is a comprehensive scoring tool used to assess magnesium deficiency status, considered more reliable and sensitive compared to measuring serum or urinary magnesium levels [11]. However, the relationship between MDS and periodontitis remains unclear.
In this study, we aimed to assess the relationship between MDS and periodontitis based on data from the National Health and Nutrition Examination Survey (NHANES).
Methods
Study population
The data used in this study is sourced from the NHANES 2009–2014. NHANES utilized a complex, multistage probability design to represent the non-institutionalized civilian population across all 50 states of the US. NHANES protocols were approved by the Research Ethics Review Board of the National Center for Health Statistics, U.S. Centers for Disease Control and Prevention (NCHS IRB/ERB Protocol Number: 2009–2010, Protocol #2005-06; 2011–2014, Protocol #2011-17), and data collection was carried out in accordance with the aforementioned protocol. Further details can be found at https://www.cdc.gov/nchs/nhanes/index.htm. Written informed consent was obtained from all participants. For this study, we excluded individuals who had not undergone a complete full-mouth periodontal examination (FMPE), those for whom data on assessing MDS were unavailable, had incomplete 24-hour recall data, and individuals under the age of 30 [12]. Additionally, participants whose total energy intake fell outside the range of 500–5000 kcal/day were also excluded [13].
Exposure variable
We used MDS to evaluate the overall body magnesium levels, as outlined in a previous study [11]. Four criteria were utilized in the calculation of MDS: (1) current use of diuretics (1 point), (2) current use of proton pump inhibitors (1 point), (3) renal function (1 point for eGFR between 60 and 90 mL/min/1.73m2; 2 points for eGFR < 60 mL/min/1.73m2), and (4) alcohol consumption (1 point for heavy drinkers). For the purposes of analysis, MDS was categorized into four groups: None (MDS = 0), Low (MDS = 1), Medium (MDS = 2), and High (MDS > 2). The use of diuretics and proton pump inhibitors was assessed by self-reported intake within the previous 30 days, gathered through in-home face-to-face interviews. The eGFR is calculated using the following formula: GFR = 141 * min(Scr/k, 1)^α * max(Scr/k, 1)^-1.209 * 0.993^Age * 1.018 [if female] * 1.159 [if black], where Scr represents serum creatinine, k is 0.9 for males and 0.7 for females, α is -0.411 for males and − 0.329 for females. Min and max functions denote the minimum and maximum values, respectively, of the Scr/k ratio or 1 [14]. The measurement of serum creatinine was based on the Jaffe rate method. Heavy drinkers were defined as > 1 drink/day for women and > 2 drinks/day for men, which was obtained through questionnaires. Further detailed information could be found in a previous study [11].
Outcome variable
The outcome of this study was moderate or severe periodontitis. Periodontitis was defined by employing oral examination data obtained through an assessment conducted by licensed dentists in the mobile examination center (MEC). The dental examiners received thorough training and calibration to guarantee the accuracy and quality of periodontal health data [15, 16]. This process involved ongoing monitoring and recalibration to uphold the necessary standards. The periodontal examination employed the FMPE protocol, which involved assessing attachment loss (AL) and probing pocket depth (PPD) at six sites per tooth (excluding third molars). AL refers to the distance between the cemento-enamel junction and the base of the sulcus, whereas PPD is the measurement from the free gingival margin to the base of the sulcus. The classification of periodontitis followed the CDC/AAP definitions and is presented in Table S1 [17].
Covariates
The Directed Acyclic Graph (DAG) (www.dagitty.net/dags.html) was utilized to illustrate the hypothesized associations between MDS, confounders, and periodontitis (Figure S1). Potential confounders examined based on the DAG included participants’ age (≥ 60 and < 60 years), sex (female and male), race (non-Hispanic white, Mexican American, Non-Hispanic Black, and others), obesity (non-obese: BMI < 30 and obese: BMI ≥ 30), smoking status (non-smokers and smokers), income (< US$20,000 and > US$20,000), dietary magnesium, diabetes mellitus, and hypertension. Additionally, alternative DAGs were considered, incorporating more variables such as those related to periodontitis. Education level (< high school, high school, and > high school), recreational activity (no, vigorous activity, moderate activity, and both), and work activity (no, vigorous activity, moderate activity, and both) were also included as related variables. The detailed definition of smoking status, diabetes mellitus, and hypertension was based on our previous study [18].
Statistical analysis
This study employed the statistical analysis procedures recommended by the CDC, which took into account the complexities of a multistage cluster survey design. The new six-year weights were calculated based on one-third of the two-year Mobile Examination Center (MEC) weights. Continuous variables were described as mean with standard errors while categorical variables were displayed as percentages. Baseline characteristics were assessed for differences among the MDS levels by conducting a one-way ANOVA test for continuous variables and a Chi-square test for categorical variables. The relationship between MDS and periodontitis was assessed based on weighted multivariable logistic regression models. Model I was adjusted for age, gender and race, model II was additionally adjusted for income, smoking status, dietary magnesium, obesity, diabetes, and hypertension, and model III was further adjusted for education level, recreational activity, and work activity. We also performed a sensitivity analysis based on another periodontal classification proposed by the 2017 World Workshop on Periodontal and Peri-Implant Diseases and Conditions [19]. The severity of periodontitis was categorized into four stages according to the maximum AL: 1–2 mm was designated as Stage I, 3–4 mm as Stage II, and equal to or exceeding 5 mm as Stage III/IV. Additionally, management complexity was also considered. Patients diagnosed with Stage II periodontitis were reclassified as Stage III if their maximum PPD measurement was ≥ 6 mm. Moreover, patients diagnosed with Stage III periodontitis were reclassified as Stage IV if they had fewer than 20 teeth remaining. Stage III/IV periodontitis was established as the outcome; others were designated as the reference. Our study utilized a complete case analysis approach because of the minimal missing data. The analyses were carried out based on R software (version 4.1.2), with statistical significance defined as a P-value less than 0.05.
Results
Baseline characteristics
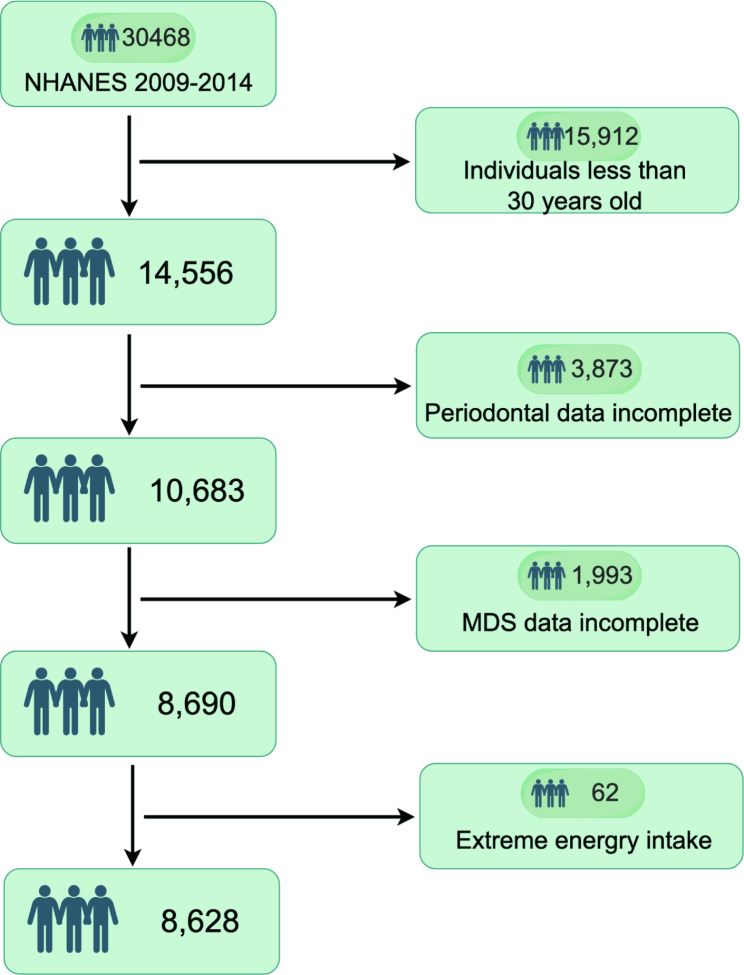
The participant screening process, as shown in Fig. 1, consisted of several phases during the NHANES 2009–2014 study. Initially, NHANES included 30,468 participants, with this study focusing on the analysis of 14,556 individuals aged 30 years and older. To ensure data integrity, participants with incomplete oral health examinations (n = 3,873), those lacking MDS data (n = 1,993), and those with extreme energy intake (n = 62) were excluded. As a result, a total of 8,628 individuals met the inclusion criteria for this study, representing approximately 120.8 million noninstitutionalized residents in the United States. Table 1 presents the baseline characteristics of the individuals, both overall and stratified by various MDS levels. On average, participants were 51.19 years old, with males accounting for 48.21% of the sample. Individuals with a high level of magnesium deficiency exhibited the lowest magnesium intake. The prevalence of moderate/severe periodontitis increased progressively with higher MDS levels, reaching 32.37%, 36.53%, 43.44%, and 48.99%, respectively.
Fig. 1.
Participant flow chart, 2009–2014 NHANES by Figdraw
Table 1.
Characteristics of the participates (NHANES 2009–2014, N = 8,628)
| Characteristics | Overall (n = 8,628) |
MDS = 0 (n = 3,607) |
MDS = 1 (n = 3,071) |
MDS = 2 (n = 1,358) |
MDS > 2 (n = 592) |
p value |
|---|---|---|---|---|---|---|
| Age | 51.19(0.26) | 43.87(0.25) | 52.46(0.33) | 59.52(0.44) | 66.44(0.67) | < 0.0001 |
| Age group (%) | < 0.0001 | |||||
| <=60 | 74.77 | 92.71 | 73.61 | 52.29 | 30.69 | |
| >60 | 25.23 | 7.29 | 26.39 | 47.71 | 69.31 | |
| Sex (%) | < 0.001 | |||||
| Female | 51.79 | 53.58 | 48.64 | 51.49 | 60.89 | |
| Male | 48.21 | 46.42 | 51.36 | 48.51 | 39.11 | |
| Race (%) | < 0.0001 | |||||
| Non-Hispanic White | 70.93 | 58.45 | 76.52 | 82.48 | 83 | |
| Mexican American | 7.67 | 12.96 | 5.32 | 3.02 | 1.79 | |
| Non-Hispanic Black | 9.83 | 11.26 | 8.73 | 8.59 | 11.03 | |
| Others | 11.57 | 17.33 | 9.43 | 5.9 | 4.18 | |
| Education a (%) | < 0.0001 | |||||
| < High school | 13.89 | 17.02 | 11.4 | 12.27 | 14.44 | |
| High school | 20.95 | 20.14 | 21.35 | 21.43 | 22.41 | |
| > High school | 65.08 | 62.84 | 67.25 | 66.3 | 63.14 | |
| Incomea (%) | 0.08 | |||||
| < US$20,000 | 11.04 | 11.47 | 10.45 | 12.52 | 13.47 | |
| > US$20,000 | 85.98 | 88.53 | 89.55 | 87.48 | 86.53 | |
| Smoking statusa (%) | 0.001 | |||||
| Non smoker | 83.93 | 82.54 | 83.51 | 85.82 | 90.4 | |
| Current smoker | 16.04 | 17.46 | 16.49 | 14.18 | 9.6 | |
| Work activitya (%) | < 0.0001 | |||||
| No | 57.9 | 59.03 | 53.19 | 62.01 | 69.41 | |
| Moderate | 22.36 | 20.08 | 24.86 | 23.28 | 18.66 | |
| Vigorous | 4.24 | 4.66 | 4.45 | 3.52 | 2.25 | |
| Both | 15.48 | 16.24 | 17.5 | 11.18 | 9.68 | |
| Recreational activity (%) | < 0.0001 | |||||
| No | 45.22 | 45.62 | 42.4 | 46.96 | 55.46 | |
| Moderate | 31.89 | 29.41 | 33.19 | 34.69 | 31.82 | |
| Vigorous | 6.72 | 7.29 | 7.93 | 4.27 | 2.19 | |
| Both | 16.17 | 17.68 | 16.47 | 14.08 | 10.53 | |
| Obesitya (%) | 0.002 | |||||
| No | 61.62 | 61.62 | 64.34 | 59.14 | 55.2 | |
| Yes | 37.98 | 38.38 | 35.66 | 40.86 | 44.8 | |
| Diabetes mellitusa (%) | < 0.0001 | |||||
| No | 85.09 | 88.2 | 87.65 | 80.64 | 71.23 | |
| Yes | 14.24 | 11.8 | 12.35 | 19.36 | 28.77 | |
| Hypertensiona (%) | < 0.0001 | |||||
| No | 59.91 | 74.27 | 62.11 | 37.5 | 16.77 | |
| Yes | 40.09 | 25.73 | 37.89 | 62.5 | 83.23 | |
| Magnesium intake (mg/day) | 315.50(2.47) | 318.04(3.52) | 322.20(3.21) | 309.00(5.18) | 276.06(6.66) | < 0.0001 |
| Periodontitis (%) | < 0.0001 | |||||
| Non/mild periodontitis | 63.16 | 67.63 | 63.47 | 56.56 | 51.01 | |
| Moderate/severe periodontitis | 36.84 | 32.37 | 36.53 | 43.44 | 48.99 | |
| Periodontitis (%) | < 0.0001 | |||||
| Non/Stage I/II | 63.53 | 67.77 | 63.72 | 57.36 | 52.44 | |
| Stage III/IV | 36.47 | 32.23 | 36.28 | 42.64 | 47.56 | |
aMissing values for total study: education (n = 5; 0.06%), income (n = 335, 3.88%), smoking status (n = 3; 0.03%), work activity (n = 2; 0.02%), obesity (n = 47; 0.54%), diabetes mellitus (n = 54; 0.63%), and hypertension (n = 1; 0.01%)
Association between MDS and periodontitis
Table 2 presents the results of the association of MDS with moderate/severe periodontitis based on weighted logistic regression analysis. As a continuous variable, MDS was found to be positively associated with moderate/severe periodontitis in all regression models (crude model: OR = 1.26, 95% CI: 1.17–1.34, p < 0.0001; Model I: OR = 1.14, 95% CI: 1.06–1.22, p < 0.001; Model II: OR = 1.10, 95% CI: 1.02–1.19, p = 0.01; and Model III: OR = 1.12, 95% CI: 1.04–1.21, p = 0.01). Compared to individuals without magnesium deficiency (MDS = 0), individuals with high level of magnesium deficiency (MDS > 2) had a higher prevalence of moderate/severe periodontitis in Crude model (OR = 2.01, 95% CI: 1.54–2.61, p < 0.0001 ), Model I (OR = 1.43, 95% CI: 1.11–1.84, p = 0.01 ), model II (OR = 1.31, 95% CI: 1.004-1.70, p = 0.047), and Model III (OR = 1.35, 95% CI: 1.03–1.77, p = 0.03). P for trend was less than 0.05 in all models.
Table 2.
Association between MDS and moderate/severe periodontitis (NHANES 2009–2014, N = 8,628)
| MDS | Crude model | Model I | Model II | Model II | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | ||||
| Continuous | 1.26 (1.17, 1.34) | < 0.0001 | 1.14 (1.06, 1.22) | < 0.001 | 1.10 (1.02, 1.19) | 0.01 | 1.12 (1.04, 1.21) | 0.01 | |||
| MDS | |||||||||||
| 0 | Ref | Ref | Ref | Ref | |||||||
| 1 | 1.20 (1.05, 1.37) | 0.01 | 1.12 (0.97, 1.29) | 0.11 | 1.12 (0.97, 1.29) | 0.13 | 1.14 (0.99, 1.32) | 0.07 | |||
| 2 | 1.60 (1.33, 1.93) | < 0.0001 | 1.31 (1.05, 1.63) | 0.02 | 1.24 (0.97, 1.57) | 0.08 | 1.28 (1.004, 1.64) | 0.047 | |||
| > 2 | 2.01 (1.54, 2.61) | < 0.0001 | 1.43 (1.11, 1.84) | 0.01 | 1.31 (1.004, 1.70) | 0.047 | 1.35 (1.03,1.77) | 0.03 | |||
| p for trend | < 0.0001 | 0.001 | 0.02 | 0.01 | |||||||
Model I: Adjusted for age, sex, and race
Model II: Model I and adjusted for income, smoking status, dietary magnesium, obesity, diabetes, and hypertension
Model III: Model I, Model II and adjusted for education level, recreational activity, and work activity
A sensitivity analysis was conducted to evaluate the correlation between MDS and Stage III/IV periodontitis. The findings also indicated a comparable pattern regarding this association (Table 3). In contrast to individuals without magnesium deficiency, those with a significant level of magnesium deficiency exhibited a greater prevalence of Stage III/IV periodontitis in the Crude model (OR = 1.91, 95% CI: 1.44–2.53, p < 0.0001), Model I (OR = 1.39, 95% CI: 1.06–1.83, p = 0.02), Model II (OR = 1.29, 95% CI: 0.98–1.69, p = 0.07), and Model III (OR = 1.34, 95% CI: 1.02–1.75, p = 0.04).
Table 3.
Association between MDS and Stage III/IV periodontitis (NHANES 2009–2014, N = 8,628)
| MDS | Crude model | Model I | Model II | Model II | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | ||||
| Continuous | 1.24 (1.15, 1.33) | < 0.0001 | 1.12 (1.05, 1.21) | 0.002 | 1.09 (1.02, 1.17) | 0.02 | 1.11 (1.03, 1.19) | 0.01 | |||
| MDS | |||||||||||
| 0 | Ref | Ref | Ref | Ref | |||||||
| 1 | 1.20 (1.03, 1.39) | 0.02 | 1.11 (0.95, 1.28) | 0.17 | 1.10 (0.95, 1.28) | 0.19 | 1.12 (0.96, 1.31) | 0.14 | |||
| 2 | 1.56 (1.31, 1.87) | < 0.0001 | 1.28 (1.05, 1.56) | 0.01 | 1.21 (0.99, 1.48) | 0.06 | 1.25 (1.03, 1.53) | 0.03 | |||
| > 2 | 1.91 (1.44, 2.53) | < 0.0001 | 1.39 (1.06, 1.83) | 0.02 | 1.29 (0.98, 1.69) | 0.07 | 1.34 (1.02, 1.75) | 0.04 | |||
| p for trend | < 0.0001 | 0.004 | 0.02 | 0.01 | |||||||
Model I: Adjusted for age, sex, and race
Model II: Model I and adjusted for income, smoking status, dietary magnesium, obesity, diabetes, and hypertension
Model III: Model I, Model II and adjusted for education level, recreational activity, and work activity
Discussion
In this cross-sectional study of 8,628 adults, we found a positive association between MDS and periodontitis in all adjusted models. However, the cross-sectional nature of this study limits our ability to establish a causal relationship between MDS and periodontitis.
In clinical practice, the most commonly used method for evaluating magnesium status is measuring serum magnesium concentrations. However, blood magnesium levels only account for 0.8% of the body’s total magnesium content [20]. Consequently, serum magnesium concentrations may not accurately reflect overall magnesium levels [21]. Another method used to assess magnesium status is measuring urinary magnesium levels; however, the fluctuation in renal magnesium reabsorption and excretion undermines its reliability as an indicator [22]. Although intravenous or oral magnesium loading tests have also been employed, they are deemed clinically inadequate, expensive, and lack standardization [23]. Recently, Fan et al. developed a novel index, referred to as MDS, for evaluating magnesium deficiency. The model based solely on MDS had an AUC of 0.60 (95% CI 0.48, 0.72), which was the highest AUC estimate among the single predictor models. In contrast, the model including only serum magnesium or urinary magnesium yielded an AUC of 0.58 (95% CI: 0.45, 0.71) or 0.58 (95% CI 0.45, 0.71), respectively [11]. Therefore, MDS emerges as a straightforward, efficient, and precise approach for evaluating overall magnesium levels in the body.
Magnesium is one of the most abundant minerals involved in multiple cell functions, and its imbalance contributes to various diseases such as diabetes and cardiovascular diseases [11, 24, 25]. A cross-sectional study of 9,476 participants indicates that higher intakes of magnesium is associated with a lower risk of periodontal disease in the UK Biobank [26]. Similar result is observed in the NHANES, with found that dietary magnesium deficiency increases the prevalence of periodontitis [10]. Regarding serum magnesium, a matched-pair study also indicate that its decreased is linked to greater probing depth, attachment loss, and fewer remaining teeth [27]. In this study, we focused on a more reliable and sensitive index (MDS), and also found that magnesium deficiency tended to increase the risk of periodontitis, which further support that magnesium plays an important role in maintaining periodontal health.
Magnesium plays a crucial role in bone regeneration, and magnesium deficiency can enhance osteoclastogenesis while reducing osteoblast activity, thus worsening bone resorption in a ligature-induced periodontitis model [28]. Mechanistically, magnesium has the potential to activate the canonical Wnt signaling pathway [29], which is vital for maintaining bone homeostasis and may serve as a promising strategy for treating periodontitis. Activation of Wnt signaling promotes osteogenic differentiation and suppresses osteoclastogenesis through the RANK/RANKL/OPG system [30]. Additionally, Wnt signaling plays a critical role in cementogenic differentiation, which is important for the regeneration of periodontal tissues [31]. Moreover, magnesium serves as an essential cofactor in the synthesis of vitamin D [32], a protective factor in periodontitis. An increase of one standard deviation in serum 25(OH)D levels is associated with a 25% decrease in the prevalence of severe periodontitis [33].
Growing evidence indicates that low magnesium levels are associated with a pro-inflammatory response. Low magnesium has been found to activate NF-κB, a common inflammatory cytokine that plays a role in RANKL-induced osteoclast formation [34, 35] and is also crucial for the development of the senescence-associated secretory phenotype (SASP) [36]. The SASP further promotes cellular senescence in nearby cells, exacerbating the inflammatory response [37, 38]. Additionally, chronic moderate magnesium deficiency in rats leads to oxidative stress [39], a characteristic feature of periodontitis. Excessive ROS can cause cytotoxicity to host cells by damaging DNA, proteins, and lipids, ultimately resulting in destruction of periodontal tissues [40]. Therefore, an imbalance in bone homeostasis, along with pro-inflammatory responses and oxidative stress, may contribute to the accelerated progression of periodontitis in individuals with MDS.
This research has several notable strengths. It is the first population-based study to uncover a positive association between MDS and periodontitis, thereby enhancing the validity and generalizability of the findings. Moreover, MDS serves as a novel indicator of magnesium status, which is considered to be more reliable and sensitive than measuring serum or urinary magnesium levels. However, it is important to acknowledge various limitations that warrant consideration. Firstly, the cross-sectional design used in this study restricts the ability to establish a causal relationship between MDS and periodontitis. To address this limitation, future multi-center prospective trials are essential to validate any potential causal link. Secondly, we were unable to assess whether MDS was a more reliable indicator of magnesium deficiency compared to serum magnesium levels due to the absence of serum magnesium data in NHANES. The ability to evaluate magnesium status using MDS compared to serum magnesium levels needs to be assessed in a larger population. Finally, we could not completely eliminate all potential residual confounders stemming from unmeasured confounding factors. Randomized controlled trials are an important design to minimize the impact of confounding factors.
Conclusion
In conclusion, magnesium deficiency is positively associated with the prevalence of periodontitis. Additional longitudinal research is required to evaluate the causal relationship between MDS and periodontitis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- MDS
Magnesium depletion score
- FMPE
Full-mouth periodontal examination
- AL
Attachment loss
- PPD
Probing pocket depth
- PI
Poverty index
- OR
Odds ratios
- CIs
Confidence intervals
- eGFR
Estimated glomerular filtration
- MEC
Mobile Examination Center
Author contributions
Study conception and design: Ruoyan Cao. Data collection, analysis, and figure preparation: Qiqi Wu and Ruoyan Cao. Manuscript writing: Qiqi Wu, Shusen Zhang, and Ruoyan Cao. All authors read and approved the final manuscript.
Funding
This study was supported by the Natural Science Foundation of Hunan Province (2021JJ40390).
Data availability
The NHANES data of our study are openly available at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Declarations
Ethics approval and consent to participate
NHANES protocol approved by NCHS Research Ethics Review Board, and obtained informed consent from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shusen Zhang, Email: zhang520425@163.com.
Ruoyan Cao, Email: caory1993@163.com.
References
- 1.Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani F, et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World workshop on the classification of Periodontal and Peri-implant diseases and conditions. J Periodontol. 2018;89(Suppl 1):S173–82. [DOI] [PubMed] [Google Scholar]
- 2.Wahlin Å, Papias A, Jansson H, Norderyd O. Secular trends over 40 years of periodontal health and disease in individuals aged 20–80 years in Jönköping, Sweden: repeated cross-sectional studies. J Clin Periodontol. 2018;45(9):1016–24. [DOI] [PubMed] [Google Scholar]
- 3.Dommisch H, Kuzmanova D, Jönsson D, Grant M, Chapple I. Effect of micronutrient malnutrition on periodontal disease and periodontal therapy. Periodontol 2000. 2018;78(1):129–53. [DOI] [PubMed] [Google Scholar]
- 4.Wang K, Wei H, Zhang W, Li Z, Ding L, Yu T, Tan L, Liu Y, Liu T, Wang H, et al. Severely low serum magnesium is associated with increased risks of positive anti-thyroglobulin antibody and hypothyroidism: a cross-sectional study. Sci Rep. 2018;8(1):9904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen FH. Magnesium, inflammation, and obesity in chronic disease. Nutr Rev. 2010;68(6):333–40. [DOI] [PubMed] [Google Scholar]
- 6.Locatelli L, Fedele G, Castiglioni S, Maier JA. Magnesium Deficiency induces lipid Accumulation in Vascular endothelial cells via oxidative stress-the potential contribution of EDF-1 and PPARγ. Int J Mol Sci 2021, 22(3). [DOI] [PMC free article] [PubMed]
- 7.Chen J, Yu L, Gao T, Dong X, Li S, Liu Y, Yang J, Xia K, Yu Y, Li Y, et al. Nanofiber-induced hierarchically-porous magnesium phosphate bone cements accelerate bone regeneration by inhibiting notch signaling. Bioact Mater. 2024;37:459–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L, Wang F, Song W, Zhang D, Lin W, Yin Q, Wang Q, Li H, Yuan Q, Zhang S. Magnesium promotes vascularization and osseointegration in diabetic states. Int J Oral Sci. 2024;16(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shetty A, Bhandary R, Thomas B, Ramesh A. A comparative evaluation of serum magnesium in diabetes Mellitus Type 2 patients with and without Periodontitis - A Clinico-biochemical study. J Clin Diagn Res. 2016;10(12):Zc59–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li XY, Wen MZ, Liu H, Shen YC, Su LX, Yang XT. Dietary magnesium intake is protective in patients with periodontitis. Front Nutr. 2022;9:976518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan L, Zhu X, Rosanoff A, Costello RB, Yu C, Ness R, Seidner DL, Murff HJ, Roumie CL, Shrubsole MJ, et al. Magnesium depletion score (MDS) predicts risk of systemic inflammation and Cardiovascular Mortality among US adults. J Nutr. 2021;151(8):2226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li A, Chen Y, Schuller AA, van der Sluis LWM, Tjakkes GE. Dietary inflammatory potential is associated with poor periodontal health: a population-based study. J Clin Periodontol. 2021;48(7):907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin D, Hur J, Cho EH, Chung HK, Shivappa N, Wirth MD, Hébert JR, Lee KW. Pre-pregnancy Body Mass Index is Associated with Dietary Inflammatory Index and C-Reactive protein concentrations during pregnancy. Nutrients 2017, 9(4). [DOI] [PMC free article] [PubMed]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dye BA, Li X, Lewis BG, Iafolla T, Beltran-Aguilar ED, Eke PI. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2009–2010. J Public Health Dent. 2014;74(3):248–56. [DOI] [PubMed] [Google Scholar]
- 16.Dye BA, Afful J, Thornton-Evans G, Iafolla T. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2011–2014. BMC Oral Health. 2019;19(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83(12):1449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miao D, Zhao L, Cao R. Association between ethylene oxide exposure and periodontitis: a cross-sectional study from NHANES 2013–2014. BMC Public Health. 2024;24(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani F, et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World workshop on the classification of Periodontal and Peri-implant diseases and conditions. J Clin Periodontol. 2018;45(Suppl 20):S162–70. [DOI] [PubMed] [Google Scholar]
- 20.Al Alawi AM, Majoni SW, Falhammar H. Magnesium and Human Health: Perspectives and Research Directions. Int J Endocrinol 2018, 2018:9041694. [DOI] [PMC free article] [PubMed]
- 21.Costello RB, Elin RJ, Rosanoff A, Wallace TC, Guerrero-Romero F, Hruby A, Lutsey PL, Nielsen FH, Rodriguez-Moran M, Song Y, et al. Perspective: the case for an evidence-based reference interval for serum magnesium: the Time has come. Adv Nutr. 2016;7(6):977–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Workinger JL, Doyle RP, Bortz J. Challenges in the diagnosis of Magnesium Status. Nutrients 2018, 10(9). [DOI] [PMC free article] [PubMed]
- 23.Pelczyńska M, Moszak M, Bogdański P. The role of Magnesium in the Pathogenesis of Metabolic disorders. Nutrients 2022, 14(9). [DOI] [PMC free article] [PubMed]
- 24.Chaudhary DP, Sharma R, Bansal DD. Implications of magnesium deficiency in type 2 diabetes: a review. Biol Trace Elem Res. 2010;134(2):119–29. [DOI] [PubMed] [Google Scholar]
- 25.Lu J, Li H, Wang S. The kidney reabsorption-related magnesium depletion score is associated with increased likelihood of abdominal aortic calcification among US adults. Nephrol Dial Transpl 2022. [DOI] [PubMed]
- 26.Watson S, Woodside JV, Winning L, Wright DM, Srinivasan M, McKenna G. Associations between self-reported periodontal disease and nutrient intakes and nutrient-based dietary patterns in the UK Biobank. J Clin Periodontol. 2022;49(5):428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meisel P, Schwahn C, Luedemann J, John U, Kroemer HK, Kocher T. Magnesium deficiency is associated with periodontal disease. J Dent Res. 2005;84(10):937–41. [DOI] [PubMed] [Google Scholar]
- 28.Belluci MM, de Molon RS, Rossa C Jr., Tetradis S, Giro G, Cerri PS, Marcantonio E Jr., Orrico SRP. Severe magnesium deficiency compromises systemic bone mineral density and aggravates inflammatory bone resorption. J Nutr Biochem. 2020;77:108301. [DOI] [PubMed] [Google Scholar]
- 29.Hung CC, Chaya A, Liu K, Verdelis K, Sfeir C. The role of magnesium ions in bone regeneration involves the canonical wnt signaling pathway. Acta Biomater. 2019;98:246–55. [DOI] [PubMed] [Google Scholar]
- 30.Bao J, Yang Y, Xia M, Sun W, Chen L. Wnt signaling: an attractive target for periodontitis treatment. Biomed Pharmacother. 2021;133:110935. [DOI] [PubMed] [Google Scholar]
- 31.Xiao Z, Han Y, Zhang Y, Zhang X. Hypoxia-regulated human periodontal ligament cells via Wnt/β-catenin signaling pathway. Med (Baltim). 2017;96(16):e6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rondanelli M, Faliva MA, Tartara A, Gasparri C, Perna S, Infantino V, Riva A, Petrangolini G, Peroni G. An update on magnesium and bone health. Biometals. 2021;34(4):715–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou F, Ma N, Su R, He X, Wang X, Zhou Y, Shi J. Serum 25-hydroxyvitamin D is negatively associated with severe periodontitis: a cross-sectional study. BMC Oral Health. 2021;21(1):479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrè S, Baldoli E, Leidi M, Maier JA. Magnesium deficiency promotes a pro-atherogenic phenotype in cultured human endothelial cells via activation of NFkB. Biochim Biophys Acta. 2010;1802(11):952–8. [DOI] [PubMed] [Google Scholar]
- 35.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salminen A, Kauppinen A, Kaarniranta K. Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell Signal. 2012;24(4):835–45. [DOI] [PubMed] [Google Scholar]
- 37.Nelson G, Wordsworth J, Wang C, Jurk D, Lawless C, Martin-Ruiz C, von Zglinicki T. A senescent cell bystander effect: senescence-induced senescence. Aging Cell. 2012;11(2):345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15(8):978–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blache D, Devaux S, Joubert O, Loreau N, Schneider M, Durand P, Prost M, Gaume V, Adrian M, Laurant P, et al. Long-term moderate magnesium-deficient diet shows relationships between blood pressure, inflammation and oxidant stress defense in aging rats. Free Radic Biol Med. 2006;41(2):277–84. [DOI] [PubMed] [Google Scholar]
- 40.Kanzaki H, Wada S, Narimiya T, Yamaguchi Y, Katsumata Y, Itohiya K, Fukaya S, Miyamoto Y, Nakamura Y. Pathways that regulate ROS scavenging enzymes, and their role in Defense Against tissue Destruction in Periodontitis. Front Physiol. 2017;8:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The NHANES data of our study are openly available at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.