Abstract
Background
The characteristics of bronchiectasis (BE) in Asia, including Japan, remain largely unknown. We aimed to provide insights into the clinical characteristics and treatment outcomes of BE, especially regarding nontuberculous mycobacteria (NTM) infection and its poorly understood impact on prognosis. We also aimed to clarify the effect of long-term macrolide antibiotic use in patients with BE, who had no history of exacerbations.
Methods
In this single-center, retrospective study, the medical records of patients who satisfied the BE criteria between January 1, 2012, and August 31, 2023, were reviewed. Severe exacerbations and mortality during the observation period were recorded. Baseline characteristics and overall survival of patients with and without NTM infection, and factors influencing the time to the first exacerbation and death were analyzed. Additionally, the effects of long-term macrolide antibiotic use in patients without a history of severe exacerbations were estimated.
Results
In a cohort of 1044 patients with BE, the rate of severe exacerbation was 22.3%, with mortality rates of 3.2% over 3 years. Notably, the high prevalence of NTM infection (n = 410, 39.3%) in this cohort was distinctive. NTM infection was not associated with either the time to first severe exacerbation (p = 0.5676, adjusted hazard ratio = 1.11) or mortality (p = 0.4139, adjusted hazard ratio = 0.78). Compared with the NTM group, the non-NTM group had a higher proportion of elevated inflammatory markers, with significant differences in C-reactive protein levels (p = 0.0301) and blood neutrophil counts (p = 0.0273). Pseudomonas aeruginosa colonization was more frequent in the non-NTM group (p = 0.0003). Among patients with non-NTM infection and without a history of exacerbation in the past 2 years, 38.2% received long-term macrolide antibiotics that did not invariably prolong the time to first severe exacerbation (p = 0.4517, IPW p = 0.3555).
Conclusions
This study highlights BE epidemiology in Japan, noting that the presence of NTM infection may not necessarily worsen the prognostic outcomes and advising caution in the casual use of macrolides for milder cases without a history of exacerbations.
Clinical trial registration
UMIN Clinical Trials Registry Number: UMIN000054726 (Registered on 21 June 2024).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-024-03337-7.
Keywords: Bronchiectasis, Macrolides, Nontuberculous mycobacteria, TB
Background
Bronchiectasis (BE) is a syndrome characterized by chronic respiratory symptoms and bronchial dilation confirmed by imaging studies, with a wide variety of causative conditions [1]. Among these, cystic fibrosis (CF) resulting from genetic abnormalities in chloride ion channels has garnered considerable attention in Western countries, leading to numerous studies. However, non-CF BE has received insufficient attention due to diagnostic difficulties, its heterogeneous nature, and limited treatment options; it was considered an orphan disease [2].
Interest in non-CF BE has increased owing to an increase in the number of patients, especially older women, and the widespread use of computed tomography (CT), which has made diagnosis easier. Large-scale registries, such as the European Multicenter Bronchiectasis Adult and Research Collaboration (EMBARC) in Europe [3] and the United States Bronchiectasis Research Registry (USBRR) in the United States [4] have been established. Consequently, guidelines have been developed, positioning non-CF BE as a major category of respiratory disease. In addition, non-CF BE is distinguished from CF, and the term BE has come to mean primarily non-CF BE.
In Asian regions, where the prevalence of CF is low [5], BE has been neglected and is often perceived as a secondary phenomenon associated with more common conditions such as chronic obstructive pulmonary disease (COPD), bronchial asthma, and nontuberculous mycobacteria (NTM) infection. Consequently, there is a lack of clear recognition of BE as a distinct clinical entity and therapeutic interventions have been insufficiently applied.
Recently, the global incidence of NTM infections has increased [6], sparking growing interest in their association with BE [7]. Japan, along with other parts of Asia, exhibits a higher incidence and prevalence of NTM infection than the rest of the world [8], due to climatic conditions (high humidity, soil environment), aging population, lifestyle factors (bathing, agriculture), and genetic factors [9], leading to increased interest in BE. However, epidemiological data on BE among Asian populations are limited, with only 147 cases reported by Kadowaki et al. in Japan [5]. Thus, sufficient epidemiological studies on BE in this region are needed. Additionally, the impact of NTM on the prognosis of BE has rarely been reported [7], highlighting the need to clarify its precise effect on disease outcomes.
Moreover, in Japan, macrolides are frequently used for non-NTM BE regardless of exacerbation history. This is due to several factors: the high prevalence of diffuse panbronchiolitis in East Asia and the success of low-dose macrolide treatment [10], numerous reports from Japan on the immunomodulatory effects of macrolides, the insurance restrictions on the use of inhaled antibiotics for non-CF BE (such as inhaled tobramycin and liposomal amikacin), and the insurance approval of clarithromycin for all neutrophilic inflammatory airway diseases based on its efficacy in COPD. However, this approach diverges from international standard treatments [11–13], which recommend long-term macrolide therapy primarily for patients with BE with a history of exacerbations, based on evidence of efficacy in this specific group. Given this discrepancy between Japanese practice and international recommendations, there is a critical need to clarify the effects of long-term macrolide use in patients with BE without a history of exacerbations.
This study aimed to elucidate the epidemiological profiles of BE in this region, with a focus on NTM. Additionally, we sought to clarify the impact of NTM on BE prognosis and evaluate the outcomes of long-term macrolide antibiotic use in patients with non-NTM BE without prior exacerbation events.
Methods
Study design and patient population
In this single-center, retrospective, observational study, we reviewed the medical records of patients diagnosed with BE between January 1, 2012, and August 31, 2023, at the National Hospital Organization Osaka Toneyama Medical Center. The inclusion criteria were based on international consensus recommendations [2]: a clinical history consistent with BE (cough, chronic sputum production, and/or recurrent respiratory infections) and chest CT demonstrating BE (bronchial dilatation) affecting one or more lobes. The exclusion criteria were BE due to known CF, traction bronchiectasis due to pulmonary fibrosis, and age < 18 years. The study was approved by the Institutional Review Board of the National Hospital Organization of Osaka Toneyama Medical Centre (approval no. TNH-R-2024002).
Baseline clinical characteristics
Baseline clinical information collected at enrolment included age, sex, body mass index (BMI), modified Medical Research Council dyspnea scale (mMRC) [14], Bronchiectasis Severity Index (BSI) score (age, BMI, forced expiratory volume in 1 s [FEV1], history of hospital admission and exacerbations, dyspnea, colonization of P. aeruginosa, colonization of other organisms, radiologic severity involving 3 or more lobes or cystic bronchiectasis) [15], FACED score (F: FEV1, A: age, C: chronic colonization by Pseudomonas aeruginosa (P. aeruginosa), E: radiological extension [number of pulmonary lobes affected], and D: dyspnea) [16], smoking history, comorbidities, blood neutrophil counts (BNCs), blood eosinophil count, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), anti-glycopeptidolipid (GPL)-core IgA antibody, pulmonary function test (PFT) results (FEV1, forced vital capacity [FVC]), disease extent, cystic bronchiectasis and cavities on CT, evidence of bacterial colonization as previously described [17, 18] (detected within 180 days before or after the date of BE diagnosis, with at least one positive sputum culture result), and exacerbation history. Laboratory ESR findings were considered elevated with values more than 15 mm/h in men and 20 mm/h in women as previously described [19]. Anti-GPL-core IgA antibodies were considered positive if the level was 0.7 U/mL [20]. One radiologist and four pulmonologists independently reviewed the CT images and reached a consensus on the findings. Idiopathic BE was diagnosed using a cause-specific algorithm as previously described [21].
Outcome measurements
The time to the first severe exacerbation was defined as the time from diagnosis to the first severe exacerbation. Overall survival (OS) was defined as the time from diagnosis to death from any cause. Censoring was performed at the point of loss to follow-up or at the end of the study on August 31, 2023. For patients monitored at external hospitals, clinical progression data, including mortality, were predominantly sourced from the regional medical liaison office given the central role of our hospital in the region.
Diagnostic criteria for NTM
Based on official clinical practice guidelines [22], the diagnostic criteria for NTM pulmonary disease required both clinical and radiological evidence. This included pulmonary or systemic symptoms accompanied by nodular or cavitary opacities on radiography or CT, which also demonstrated bronchiectasis with multiple small nodules. The diagnosis was confirmed when these criteria were met al.ong with one of the following microbiological findings: (1) positive culture results from at least two separate expectorated sputum samples (if the results were non-diagnostic, sputum acid-fast bacilli [AFB] smears and cultures were repeated); (2) positive culture results from at least one bronchial wash or lavage; or (3) transbronchial or other lung biopsy showing mycobacterial histologic features (granulomatous inflammation or AFB) and a positive culture for NTM, or biopsy evidence of mycobacterial histologic features along with at least one positive sputum or bronchial washing culture positive for NTM. Patients were classified as having NTM complications if they met these criteria either before the BE diagnosis or during the period from BE diagnosis to the end of follow-up.
Definition of severe exacerbation
BE exacerbation was defined as (1) a deterioration in three or more of the key symptoms (including cough, sputum volume and/or consistency, sputum purulence, dyspnea and/or exercise tolerance, fatigue and/or malaise, and hemoptysis) lasting at least 48 h and (2) a clinician’s assessment that a change in treatment was necessary [23]. Severe exacerbations were defined according to the British Thoracic Society guidelines as unscheduled hospitalizations or emergency department visits due to severe BE exacerbations or complications. These were recorded from patient histories and verified using administrative databases [11].
Long-term macrolide therapy exposure
To assess the impact of long-term macrolide therapy (azithromycin, clarithromycin, and erythromycin), patients were divided into two groups: those using macrolides for at least 3 months and those not receiving long-term macrolide therapy. The dosage of macrolide was not considered in this analysis. The study focused on patients without NTM infection because macrolides are key therapeutic agents for NTM, and their effects in this context are well-established.
Statistical analysis
Continuous data are presented as medians and interquartile ranges (IQRs), while categorical data are presented as frequencies and percentages. For continuous variables, the Wilcoxon rank-sum test was used to compare two groups. For categorical variables, differences between groups were assessed using Fisher’s exact test. Regarding outcomes, we conducted several Cox proportional hazards regression analyses: (1) analyses to assess the impact of NTM on severe exacerbation and mortality in the entire cohort, and (2) analyses of non-NTM and NTM groups to identify risk factors for severe exacerbation in each group. Variables were selected based on previous literature [13, 24–27] and their clinical significance, with an effort to exclude correlated variables wherever feasible. For the first analysis, the variables included NTM, sex, age, BMI, Smoking history, mMRC, use of inhaled corticosteroids (ICS), CRP, BNCs, CT findings of disease extension over three or more lobes, presence of cystic bronchiectasis, cavitary lesions, and colonization by P. aeruginosa. In the second analysis, NTM was excluded from the variables. Also, Kaplan–Meier survival analysis was conducted to compare the time to death between the non-NTM and NTM groups. Finally, to assess the effects of macrolide exposure on the time to first severe exacerbation after diagnosis, non-NTM patients without severe exacerbations for 2 years before diagnosis were divided into treatment and non-treatment groups according to the definition of long-term macrolide antibiotic exposure described prior. The time to the first exacerbation was estimated using the Kaplan-Meier method to compare the groups. To address immortal time bias, time zero was defined as 90 days after diagnosis. We also estimated the average treatment effect (ATE) of the treatment utilizing inverse probability weighting (IPW) by propensity scores calculated using all predefined covariates; ATE weight was applied to each patient to create a pseudo-population. Regarding covariates, we pre-configured sex, age, BMI, mMRC, smoking history, cystic bronchiectasis and cavities on CT, CRP, BNCs, and P. aeruginosa colonization as time-fixed covariates by referring to previous studies [13, 24–29]. To assess the balance between the groups before and after adjustment, we used the standardized mean difference (SMD). An SMD variation exceeding 25% was considered indicative of a significant imbalance.
All statistical analyses were performed using JMP Pro 17 (SAS Institute, Cary, NC, USA) and R software version 4.3.2 (https://www.r-project.org/). Statistical significance was defined as p < 0.05; all p-values reported in this study were assessed against this threshold.
Result
Study population and baseline characteristics
In total, 1044 patients were diagnosed with BE and followed up until their last visit, death, or the end of the observation period. The median follow-up time (IQR) from diagnosis was 27.8 (5.6–56.7) months. The baseline characteristics were shown in Table 1. Most patients were women (n = 807, 77.3%), with a median age of 72.0 (IQR 63.0–77.0) years and a median BMI of 19.3 (IQR 17.5–21.4) kg/m2. The sputum culture tests detecting for general bacteria, including those for P. aeruginosa, were performed a median of 1.0 times per year (IQR 1.0–3.0). During the 2 years before diagnosis, 15.5% (162/1044) of patients had experienced severe exacerbation episodes.
Table 1.
Comparative baseline characteristics between patients with and without NTM
| Characteristics | Overall (n = 1044) |
Non-NTM (n = 634) |
NTM (n = 410) |
p value§ |
|---|---|---|---|---|
| Sex, female | 807 (77.3) ‡ | 467 (73.7) | 340 (82.9) | 0.0005 |
| Age (years) |
72.0 [63.0–77.0] |
72.0 [63.0–77.0] |
72.0 [63.0–78.0] |
0.2782 |
| BMI (kg/m2) |
19.3 [17.5–21.4] |
19.6 [17.8–21.9] |
19.1 [17.1–20.7] |
0.0001 |
| mMRC > 1 | 158 (15.1) | 85 (13.4) | 73 (17.8) | 0.0631 |
| Bronchiectasis Severity Index ( n = 363), No (%) | ||||
| Mild | 32/363 (8.8) | 17/193 (8.8) | 15/170 (8.8) | 0.3106 |
| Moderate | 99/363 (27.3) | 59/193 (30.6) | 40/170 (23.5) | |
| Severe | 232/363 (63.9) | 117/193 (60.6) | 115/170 (67.7) | |
| FACED score (n = 363), No (%) | ||||
| Mild | 180/363 (49.6) | 105/193 (54.4) | 75/170 (44.1) | 0.0157 |
| Moderate | 147/363 (40.5) | 65/193 (33.7) | 82/170 (48.2) | |
| Severe | 36/363 (9.9) | 23/193 (11.9) | 13/170 (7.7) | |
| Smoking status, No (%) | ||||
| Never | 789 (75.6) | 458 (72.2) | 331 (80.7) | 0.0019 |
| Former, Current | 255 (24.4) | 176 (27.8) | 79 (19.3) | |
| Comorbidities, No (%) | ||||
| Post infection1 | 215 (20.6) | 121 (19.1) | 94 (22.9) | 0.1372 |
| Airway abnormality and aspiration syndrome2 | 82 (7.9) | 30 (4.7) | 52 (12.7) | < 0.0001 |
| COPD | 59 (5.7) | 45 (7.1) | 14 (3.4) | 0.0130 |
| Bronchial asthma | 74 (7.1) | 51 (8.0) | 23 (5.6) | 0.1405 |
| ABPA | 4 (0.4) | 3 (0.5) | 1 (0.2) | 1.0000 |
| Chronic sinusitis | 97 (9.3) | 67 (10.4) | 31 (7.6) | 0.2428 |
| Rheumatoid arthritis | 54 (5.2) | 39 (6.2) | 15 (3.7) | 0.0861 |
| Others3 | 3 (0.3) | - | - | - |
| Laboratory findings | ||||
| Blood neutrophils, /µL (n = 980) |
3705 [2820–5118] |
3760 [2905–5235] |
3600 [2690 − 490] |
0.0273 |
| Blood eosinophils, /µL (n = 980) |
130 [80–210] |
130 [80–210] |
120 [70–200] |
0.2797 |
| CRP, mg/L (n = 979) |
0.2 [0.1–0.8] |
0.2 [0.1–0.9] |
0.1 [0.1–0.7] |
0.0301 |
| Elevated ESR, (n = 704) | 457/704 (64.9) | 248/364 (68.1) | 209/340 (61.5) | 0.0693 |
| Positive anti-GPL-core IgA antibody, (n = 891) | 438/891 (49.2) | 141/495 (28.5) | 297/396 (75.0) | < 0.0001 |
| Lung function test results (n = 364) | ||||
| FEV1 |
1.6 [1.2–2.1] |
1.6 [1.1–2.1] |
1.7 [1.3–2.1] |
0.2741 |
| FVC |
2.2 [1.7–2.7] |
2.2 [1.6–2.7] |
2.2 [1.8–2.7] |
0.6118 |
| FEV1(%) |
80.7 [63.3–98.9] |
76.2 [60.5–96.6] |
87.1 [65.6-103.8] |
0.0043 |
| FEV1/FVC |
76.2 [68.9–84.7] |
75.3 [67.7–84.5] |
77.0 [70.4–85.2] |
0.2945 |
| Disease extension on CT, No (%) | ||||
| 3 or more lobes involved | 659 (63.1) | 349 (55.1) | 310 (75.6) | < 0.0001 |
| Cystic bronchiectasis | 156 (14.9) | 83 (13.1) | 73 (17.8) | 0.0409 |
| Cavities | 236 (22.6) | 101 (15.9) | 135 (32.9) | < 0.0001 |
| Bacterial colonization, No (%) | ||||
| P. aeruginosa | 119 (11.4) | 90 (14.2) | 29 (7.1) | 0.0003 |
| Staphylococcus species | 33 (3.2) | 19 (3.0) | 14 (3.4) | 0.7199 |
| H. influenzae | 22 (2.1) | 15 (2.4) | 7 (1.7) | 0.5169 |
| S. pneumoniae | 14 (1.3) | 12 (1.9) | 2 (0.5) | 0.0582 |
| K. pneumoniae | 9 (0.9) | 5 (0.8) | 4 (1.0) | 0.7439 |
| Aspergillus species | 18 (1.7) | 11 (1.7) | 7 (1.7) | 1.0000 |
1After bacterial or viral pneumonia (n = 115) or tuberculosis (n = 100)
2Airway obstruction by foreign body and vocal cord disease or dysfunction; esophageal disease; or dysmotility (prior radiation treatment, neurologic disease, esophageal motility disorder, or gastroesophageal reflux disease)
3 Primary ciliary dyskinesia (n = 1), Sjögren’s syndrome (n = 1), Scleroderma (n = 1)
§Differences among continuous variables were assessed using the Wilcoxon rank-sum test; differences among categorical variables were assessed using the Fisher’s exact test, as appropriate
Abbreviations: NTM, nontuberculous mycobacteria; SMD, standardized mean difference; BMI, body mass index; mMRC, modified Medical Research Council dyspnea scale; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GPL, glycopeptidolipid; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; CT, computed tomography; ABPA, Allergic bronchopulmonary aspergillosis; P. aeruginosa, Pseudomonas aeruginosa; H. influenzae, Haemophilus influenzae; .S. pneumoniae, Streptococcus pneumoniae, K. pneumoniae, Klebsiella pneumoniae
‡Data are presented as n (%) or median (interquartile range)
The cohort comprised 634 (60.7%) patients without NTM, 444 of which were idiopathic BE, and 410 (39.3%) patients with NTM (Table 1). In the NTM group, 263 patients (64.1%) were diagnosed with NTM at the time of inclusion. In the non-NTM group, mycobacterial culture tests were performed 7.0 times (IQR 2.0–17.0), with an average frequency of 3.1 times per year, although 28.5% of the patients tested positive for anti-GPL-core IgA antibodies.
FACED scores and disease extension on CT were significantly higher and BMI was significantly lower in NTM group (Table 1). On the other hand, patients with higher inflammatory profiles, including BNCs (p = 0.0273) and CRP (p = 0.0301), and airway colonization by P. aeruginosa (p = 0.0003) were more common in the non-NTM group (Table 1). Interestingly, in the non-NTM group, patients with a history of exacerbations in the past two years had significantly higher levels of BNCs, elevated ESR, and CRP compared to those without a history of exacerbations (all p < 0.0001) (Table S1).
Effect of NTM on severe exacerbation and death in BE
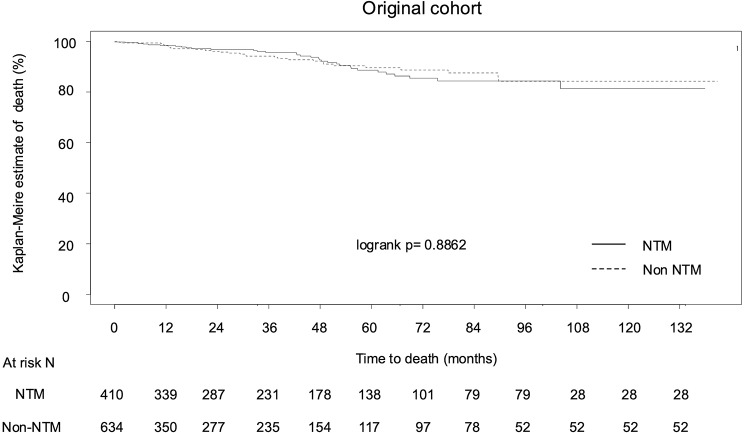
As a whole, the rates of severe exacerbation 1 and 3 years after diagnosis were 14.1% (n = 147) and 22.3% (n = 233), respectively. The mortality rates at 1 and 3 years were 1.2% (n = 13) and 3.2% (n = 33), respectively. We investigated the factors associated with the time to first severe exacerbation within 1 year post BE diagnosis using Cox proportional hazards regression (Table 2). NTM was not significantly associated with the time to first exacerbation in BE patients in both the univariate (HR = 1.27, 95% CI, 0.92–1.76) and the multivariate (adjusted HR = 1.11, 95% CI, 0.78–1.57) analyses. We further investigated the factors associated with the exacerbation with and without NTM, separately, and found P. aeruginosa was a significant exacerbation factor in the non-NTM group (HR = 2.25, 95% CI = 1.33–3.83), but it was not a significant factor in the NTM group (HR = 1.23, 95% CI = 0.54–2.83) (Table S2). Next, we investigated whether NTM was associated with OS or not. In Cox proportional hazards regression, NTM was not significantly associated with OS in BE patients in both the univariate (HR 1.06, 95% CI, 0.65–1.74) and multivariate (adjusted HR 0.78, 95% CI, 0.43–1.41) analyses (Table 3). We also compared the time to death between the non-NTM and NTM groups using Kaplan-Meier survival analysis. There was no significant difference in OS between patients with and without NTM (log-rank p = 0.8862) (Fig. 1).
Table 2.
A prognostic index model for predicting severe exacerbation
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | Adjusted HR | 95% CI | p value | |
| Sex, male | 1.38 | 0.96–1.98 | 0.0844 | 1.35 | 0.86–2.10 | 0.1895 |
| NTM | 1.27 | 0.92–1.76 | 0.1428 | 1.11 | 0.78–1.57 | 0.5676 |
| Age | 1.02 | 1.01–1.04 | 0.0028 | 1.00 | 0.99–1.02 | 0.6983 |
| BMI | 0.89 | 0.83–0.94 | < 0.0001 | 0.94 | 0.88-1.00 | 0.0392 |
| Smoking history | 1.31 | 0.92–1.86 | 0.1357 | 1.37 | 0.89–2.12 | 0.1496 |
|
mMRC mMRC = 1 mMRC = 2 mMRC = 3 mMRC = 4 |
1.43 - - - - |
0.97–2.13 - - - - |
0.0729 - - - - |
- 1.11 1.39 2.27 3.36 |
- 0.74–1.66 0.82–2.36 1.22–4.23 1.19–9.47 |
- 0.6249 0.2155 0.0100 0.0218 |
| Use of ICS | 1.94 | 1.25-3.00 | 0.0030 | 1.71 | 1.07–2.71 | 0.0236 |
| CRP | 1.08 | 1.05–1.12 | < 0.0001 | 1.03 | 0.98–1.09 | 0.1797 |
| Blood neutrophils | 1.00 | 1.00–1.00 | < 0.0001 | 1.00 | 1.00–1.00 | 0.8123 |
|
Disease extension 3 or more lobes |
3.94 | 2.43–6.38 | < 0.0001 | 2.13 | 1.26–3.60 | 0.0047 |
| Cystic bronchiectasis | 2.81 | 1.98–3.98 | < 0.0001 | 1.51 | 1.00-2.27 | 0.0482 |
| Cavity | 2.51 | 1.81–3.48 | < 0.0001 | 1.35 | 0.92–1.97 | 0.1209 |
|
Colonization of P. aeruginosa |
2.53 | 1.74–3.68 | < 0.0001 | 1.53 | 1.01–2.27 | 0.0442 |
The time to the first severe exacerbation within 1 year post-diagnosis were analyzed using univariate and multivariate Cox proportional hazards regression. NTM, sex, age, BMI, smoking history, mMRC, use of inhaled corticosteroids, CRP, blood neutrophil counts, CT findings of disease extension over three or more lobes, presence of cystic bronchiectasis, cavitary lesions, and colonization by Pseudomonas aeruginosa were included as covariates to adjust for confounding factors. Abbreviations: HR, hazards ratio; CI, confidence interval; NTM, nontuberculous mycobacteria; BMI, body mass index; mMRC, modified Medical Research Council dyspnea scale; ICS, inhaled corticosteroid; CRP, C-reactive protein; P. aeruginosa, Pseudomonas aeruginosa
Table 3.
A prognostic index model for predicting death
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | Adjusted HR | 95% CI | p value | |
| Sex, male | 1.10 | 0.62–1.96 | 0.7470 | 0.92 | 0.40–2.08 | 0.8383 |
| NTM | 1.06 | 0.65–1.74 | 0.8052 | 0.78 | 0.43–1.41 | 0.4139 |
| Age | 1.10 | 1.07–1.14 | < 0.0001 | 1.11 | 1.07–1.16 | < 0.0001 |
| BMI | 0.69 | 0.62–0.76 | < 0.0001 | 0.76 | 0.67–0.86 | < 0.0001 |
| Smoking history | 1.49 | 0.89–2.49 | 0.1328 | 3.71 | 1.83–7.52 | 0.0003 |
|
mMRC mMRC = 1 mMRC = 2 mMRC = 3 mMRC = 4 |
1.32 - - - - |
0.65–2.66 - - - - |
0.4463 - - - - |
- 1.11 1.31 3.19 5.24 |
- 0.52–2.39 0.56–3.10 1.31–7.76 1.44–18.99 |
- 0.7818 0.5321 0.0104 0.0118 |
| Use of ICS | 0.90 | 0.41–1.97 | 0.7850 | 1.12 | 0.47–2.66 | 0.7936 |
| CRP | 1.12 | 1.08–1.16 | < 0.0001 | 1.07 | 1.01–1.14 | 0.0162 |
| Blood neutrophils | 1.00 | 1.00–1.00 | < 0.0001 | 1.00 | 1.00–1.00 | 0.3811 |
|
Disease extension 3 or more lobes |
19.0 | 4.63–77.49 | < 0.0001 | 4.28 | 0.99–18.58 | 0.0521 |
| Cystic bronchiectasis | 8.56 | 5.19–14.10 | < 0.0001 | 3.22 | 1.77–6.18 | 0.0002 |
| Cavity | 7.71 | 4.54–13.10 | < 0.0001 | 1.45 | 1.66–6.24 | 0.0005 |
|
Colonization of P. aeruginosa |
2.38 | 1.37–4.15 | 0.0022 | 1.5 | 0.76–2.75 | 0.2587 |
The time to death were analyzed using univariate and multivariate Cox proportional hazards regression. NTM, sex, age, BMI, smoking history, mMRC, use of inhaled corticosteroids, CRP, blood neutrophil counts, CT findings of disease extension over three or more lobes, presence of cystic bronchiectasis, cavitary lesions, and colonization by Pseudomonas aeruginosa were included as covariates to adjust for confounding factors. Abbreviations: HR, hazards ratio; CI, confidence interval; NTM, nontuberculous mycobacteria; BMI, body mass index; mMRC, modified Medical Research Council dyspnea scale; ICS, inhaled corticosteroid; CRP, C-reactive protein; P. aeruginosa, Pseudomonas aeruginosa
Fig. 1.
Kaplan–Meier analysis was performed to compare the overall survival of patients with bronchiectasis was compared between NTM (n = 410) and non-NTM (n = 634) groups. Abbreviations: NTM, nontuberculous mycobacteria
Effect of long-term macrolide therapy
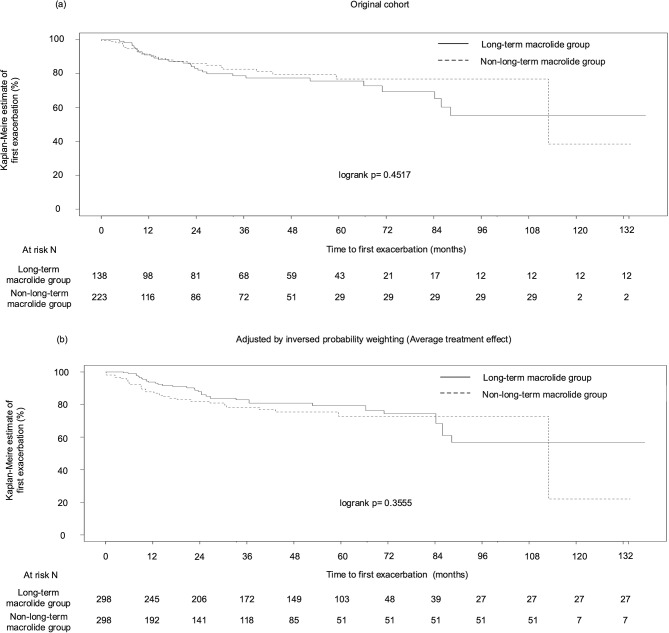
Among 634 patients in the non-NTM group, 556 patients had no history of exacerbation for two years before diagnosis of BE. We excluded 195 patients, who had an observation period of 90 or less than 90 days, to eliminate immortal time bias, and remaining 361 patients (138 who received long-term macrolide [38.2%], and 223 who did not [61.8%]) were analyzed to compare the time to first exacerbation, because the benefit of long-term macrolide therapy in BE patients who have no history of exacerbation has not been proven. The time to the first exacerbation did not differ between the groups (log-rank p = 0.4517; Fig. 2a). Similar results were observed after all covariates were adjusted for balance using the IPW (ATE-weighted) (Table S3). (log-rank p = 0.3555; Fig. 2b).
Fig. 2.
The time to the first exacerbation of patients with non-NTM BE who had no history of severe exacerbation for two years before diagnosis of BE between 138 who received long-term macrolide and 223 who did not, before (a) and after (b) the adjustment for multiple covariates (sex, age, BMI, mMRC, smoking, cystic bronchiectasis and cavities on CT, CRP, neutrophil count, and P. aeruginosa colonization) by using IPW. Abbreviations: NTM, nontuberculous mycobacteria; IPW, inverse probability weighting; BMI, body mass index; mMRC, modified Medical Research Council dyspnea scale; CT, computed tomography; CRP, C-reactive protein; P. aeruginosa, Pseudomonas aeruginosa
Discussion
In this large-scale retrospective cohort study of BE patients, the comparison between the NTM and non-NTM groups showed no significant difference in the prognosis, despite the fact that the lesion area on the chest CT image was significantly larger in the NTM group. Furthermore, we found that NTM did not significantly shorten the time to first severe exacerbation in BE patients. Recent USBRR data also showed a similar prognosis between NTM and non-NTM groups, suggesting that our findings may be applicable to different regions and races [7]. For this reason, guideline-based multidrug therapy should of course be considered; in fact, 44.9% of patients in the NTM group received. For the other reasons, in our cohort, patients in NTM group are less likely to exhibit colonization by P. aeruginosa, a poor prognostic factor in BE [30]. Also, we found P. aeruginosa was a significant exacerbation factor in the non-NTM group, but not in the NTM group. NTM colonization might provide a protective effect against other bacteria, including P. aeruginosa. Thirdly, interestingly, the NTM group showed lower levels of neutrophilic inflammation compared to the non-NTM group. The impact of neutrophils on BE has been well established [31], and re-confirmed by a recent clinical trial of brensocatib, a neutrophil serine protease inhibitor [32]. The differing role of neutrophilic inflammation in the pathogenesis of BE between NTM and non-NTM groups should be investigated in future studies.
This study also found that long-term macrolide therapy in patients with BE without a history of severe exacerbations did not significantly prolong the time to severe exacerbation. The efficacy of long-term macrolide therapy has been confirmed in patients with BE with at least one history of exacerbations [13, 33], and international guidelines only recommend macrolides for this patient group [11, 12]. Macrolides act on BE by a variety of mechanisms, including suppression of neutrophilic inflammation [33]. However, our study suggests that inflammatory markers are low in patients without a history of exacerbations and may have a limited effect. Considering the risk of side effects, drug interactions, and the potential for induction of macrolide resistance, caution should be exercised in indiscriminate use in patients without a history of exacerbations. Studies that comprehensively assess efficacy are needed to establish the optimal use of macrolides in patients with BE without a history of exacerbations.
The association of NTM with BE has recently become a globally recognized issue, as these diseases are increasing worldwide and are closely related [6, 7]. Our study revealed that the prevalence of NTM among patients with BE is extremely high, at 39.3%. This is considerably higher than the 18% reported in a previous single-center study in Japan [5]. This discrepancy may be attributed to regional differences and the fact that our facility is a referral center specialized in pulmonary disease, which may have led to a different patient population. Although the prevalence of NTM in BE is estimated at 18.0% in Europe [34], the recent USBRR reported a surprisingly high rate of 58.8% [7], which is thought to be due to the inclusion of data primarily from specialized centers. The prevalence of NTM in BE likely varies depending on factors such as regional differences, diagnostic methods, and the characteristics of the study facilities. Therefore, a thoughtful assessment considering these factors is essential.
Additionally, we demonstrated that 28.5% of the non-NTM group tested positive for anti-GPL-core IgA antibodies despite a sufficient number of sputum tests. These patients cannot be diagnosed with NTM-PD based on current diagnostic criteria, but they may be in the early stages of NTM-PD and should be closely monitored, as the serum anti-GPL-core IgA antibody test is highly specific for Mycobacterium avium complex and Mycobacterium abscessus species infection [20, 35].
In evaluating the severity of BE, the BSI tended to classify cases as more severe compared to the FACED. This might be because the BSI (and not FACED) assesses parameters including BMI, hospitalizations and exacerbations before study enrollment, chronic colonization by microorganism other than P. aeruginosa, and development of cystic bronchiectasis [3]. Our patients have a lower BMI (median 19.3 kg/m2, IQR 17.5–21.4) and many of these had respiratory colonization by microorganisms, including Staphylococcus species, Haemophilus influenzae, Streptococcus pneumoniae, Klebsiella pneumoniae, and Aspergillus species. Such characteristics of our cohort might have contributed to this trend.
This study had some limitations. First, this study was conducted in a single referral center in Japan, applicability to a broader population should be examined in future studies Although substantial cohorts exist globally, the evidence is particularly sparse in Japan, underscoring the necessity for large-scale real-world cohort study. Second, because of the retrospective nature of the study, we could not exclude potential confounding factors. However, we performed rigorous statistical correction to reduce potential biases. Third, this study lacks an assessment of symptom improvement and quality of life. Studies that comprehensively assess efficacy are needed to establish the optimal use of macrolides in patients with BE without a history of exacerbations. Fourth, the timing and selection of macrolide antibiotics were left to the discretion of individual physicians, which may have introduced potential bias in evaluating the effects of long-term use. Finally, the PFT data were insufficient, preventing us from evaluating PFT as a prognostic factor. Fifth, regarding follow-up, the timing of follow-up termination may have introduced measurement bias affecting the interpretation of results. However, we made efforts to minimize loss to follow-up by utilizing regional medical liaison offices and inter-hospital communication.
Conclusion
Our study represents an inaugural large-scale epidemiological cohort study of patients with BE in a real-world setting in Japan. This study suggests that the presence of NTM infection does not necessarily worsen the prognostic outcomes such as severe exacerbations and mortality. Furthermore, long-term macrolide therapy may not prolong the time to exacerbation without a history of exacerbations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the pulmonary radiologists at Osaka Toneyama Medical Center for their helpful discussions regarding chest computed tomography assessments. We also thank E. Akiba for assistance with data collection and helpful discussions.
Abbreviations
- NTM
nontuberculous mycobacteria
- IPW
inverse probability weighting
- EMBARC
European Multicenter Bronchiectasis Adult and Research Collaboration
- USBRR
United States Bronchiectasis Research Registry
- COPD
chronic obstructive pulmonary disease
- BMI
body mass index
- mMRC
modified Medical Research Council dyspnea scale
- P. aeruginosa
Pseudomonas aeruginosa
- BSI
Bronchiectasis Severity Index
- BNCs
blood neutrophil counts
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- GPL
glycopeptidolipid
- PFT
pulmonary function test
- FVC
forced vital capacity
- OS
overall survival
- AFB
acid-fast bacilli
- IQRs
interquartile ranges
- RA
rheumatoid arthritis
- SMD
standardized mean difference
- HR
hazard ratio
- CI
confidence interval
Author contributions
KH, YA, and KF designed the project. KH, YA, KF, and NT analyzed the clinical data. KH, KF, T Niitsu, and SM conducted the CT image analysis. KH, KF, T Niitsu, and SM conducted clinical data extraction. SM, T Nii, TM, and HK assisted with clinical data extraction. SK assisted with data analysis. KH, YA, KF, and T Niitsu performed the statistical analysis. KM and HK supervised the project. KF and HK are responsible for the overall content as guarantors. All authors read and approved the final manuscript.
Funding
This work was supported in part by AMED (grant numbers JP20fk0108129, JP21fk0108129h0702, JP21lm02007, 23fk0108673h0701), JSPS KAKENHI (Grant numbers JP21K16118, JP21K08194, JP24K11378), Takeda Science Foundation, Uehara Memorial Foundation, MSD Life Science Foundation, Japanese Respiratory Society Boehringer Ingelheim Research Grant Program, Foundation of Kinoshita Memorial Enterprise, Senri Life Science Foundation, the Japan Intractable Diseases (Nanbyo) Research Foundation (Grant Number 2020B02), Osaka Medical Research Foundation for Intractable Diseases, and Inamori Foundation. The funders had no role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The institutional research ethics board of the National Hospital Organization of Osaka Toneyama Medical Centre approved this study and waived the requirement for informed consent because of the retrospective nature of the analysis (approval no. TNH-R-2024002). The opt-out recruitment method was applied to provide an opportunity to decline participation for all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kazuki Hashimoto, Yuko Abe and Kiyoharu Fukushima contributed equally to this work.
References
- 1.O’Donnell AE. Bronchiectasis - A clinical review. N Engl J Med. 2022;387:533 – 45. 10.1056/NEJMra2202819, Pubmed:35947710. [DOI] [PubMed]
- 2.Kinney WM. Bronchiectasis; a neglected disease. Dis Chest. 1947;13:33–47. 10.1378/chest.13.1.33. Pubmed:20279925. [DOI] [PubMed] [Google Scholar]
- 3.Chalmers JD, Polverino E, Crichton ML, Ringshausen FC, De Soyza A, Vendrell M et al. Bronchiectasis in Europe: data on disease characteristics from the European Bronchiectasis registry (EMBARC). Lancet Respir Med. 2023;11:637 – 49. 10.1016/S2213-2600(23)00093-0, Pubmed:37105206. [DOI] [PubMed]
- 4.Aksamit TR, O’Donnell AE, Barker A, Olivier KN, Winthrop KL, Daniels MLA, et al. Adult patients with bronchiectasis: a first look at the US bronchiectasis research registry. Chest. 2017;151:982–92. 10.1016/j.chest.2016.10.055. Pubmed:27889361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadowaki T, Yano S, Wakabayashi K, Kobayashi K, Ishikawa S, Kimura M, Ikeda T. An analysis of etiology, causal pathogens, imaging patterns, and treatment of Japanese patients with bronchiectasis. Respir Investig. 2015;53:37–44. 10.1016/j.resinv.2014.09.004, Pubmed:25542602. [DOI] [PubMed]
- 6.Shteinberg M, Stein N, Adir Y, Ken-Dror S, Shitrit D, Bendayan D, et al. Prevalence, risk factors and prognosis of nontuberculous mycobacterial infection among people with bronchiectasis: a population survey. Eur Respir J. 2018;51. 10.1183/13993003.02469-2017. Pubmed:29545278. [DOI] [PubMed]
- 7.Aksamit TR, Locantore N, Addrizzo-Harris D, Ali J, Barker A, Basavaraj A, et al. Five-year outcomes among U.S. bronchiectasis and NTM research registry patients. Am J Respir Crit Care Med. 2024;210:108–18. 10.1164/rccm.202307-1165OC. [DOI] [PubMed] [Google Scholar]
- 8.Morimoto K, Iwai K, Uchimura K, Okumura M, Yoshiyama T, Yoshimori K et al. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann Am Thorac Soc. 2014;11:1–8. 10.1513/AnnalsATS.201303-067OC, Pubmed:24102151. [DOI] [PubMed]
- 9.Harada K, Hagiya H, Funahashi T, Koyama T, Kano MR, Otsuka F. Trends in the nontuberculous mycobacterial disease mortality rate in Japan: a nationwide observational study, 1997–2016. Clin Infect Dis. 2021;73:e321–6. 10.1093/cid/ciaa810. Pubmed:32556251. [DOI] [PubMed] [Google Scholar]
- 10.Azuma A, Kudoh S. Diffuse panbronchiolitis in East Asia. Respirology. 2006;11:249 – 61. 10.1111/j.1440-1843.2006.00845.x, Pubmed:16635082. [DOI] [PubMed]
- 11.Hill AT, Sullivan AL, Chalmers JD, De Soyza A, Elborn SJ, Floto AR et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax. 2019;74:1–69. 10.1136/thoraxjnl-2018-212463, Pubmed:30545985. [DOI] [PubMed]
- 12.Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, Loebinger MR, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50. 10.1183/13993003.00629-2017. Pubmed:28889110. [DOI] [PubMed]
- 13.Chalmers JD, Boersma W, Lonergan M, Jayaram L, Crichton ML, Karalus N et al. Long-term macrolide antibiotics for the treatment of bronchiectasis in adults: an individual participant data meta-analysis. Lancet Respir Med. 2019;7:845 – 54. 10.1016/S2213-2600(19)30191-2, Pubmed:31405828. [DOI] [PubMed]
- 14.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. 10.1378/chest.93.3.580, Pubmed: 3342669. [DOI] [PubMed]
- 15.Chalmers JD, Goeminne P, Aliberti S, McDonnell MJ, Lonni S, Davidson J et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189:576–585. 10.1164/rccm.201309-1575OC, Pubmed: 3977711. [DOI] [PMC free article] [PubMed]
- 16.Martínez-García M, de Gracia J, Vendrell Relat M, Girón RM, Máiz Carro L, de la Rosa Carrillo D et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J. 2014;43:1357-67. 10.1183/09031936.00026313, Pubmed: 24232697. [DOI] [PubMed]
- 17.Kelly MG, Murphy S, Elborn JS. Bronchiectasis in secondary care: a comprehensive profile of a neglected disease. Eur J Intern Med. 2003;14:488 – 92. 10.1016/j.ejim.2003.10.002, Pubmed:14962701. [DOI] [PubMed]
- 18.Tsang KW, Chan K, Ho P, Zheng L, Ooi GC, Ho JC, Lam W. Sputum elastase in steady-state bronchiectasis. Chest. 2000;117:420–6. 10.1378/chest.117.2.420. Pubmed:10669685. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Song MJ, Kwon BS, Kim YW, Lim SY, Lee YJ, et al. Usefulness of the BACES score in nontuberculous mycobacterial pulmonary disease for various clinical outcomes. Sci Rep. 2023;13:7495. 10.1038/s41598-023-33782-z. Pubmed:37160912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitada S, Kobayashi K, Ichiyama S, Takakura S, Sakatani M, Suzuki K et al. Serodiagnosis of Mycobacterium avium-complex pulmonary disease using an enzyme immunoassay kit. Am J Respir Crit Care Med. 2008;177:793-7. 10.1164/rccm.200705-771OC, Pubmed: 18079497. [DOI] [PubMed]
- 21.Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020;56(1):2000535. 10.1183/13993003.00535-2020. Pubmed: 32636299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Araújo D, Shteinberg M, Aliberti S, Goeminne PC, Hill AT, Fardon T, et al. Standardised classification of the aetiology of bronchiectasis using an objective algorithm. Eur Respir J. 2017;50. 10.1183/13993003.01289-2017. Pubmed:29242262. [DOI] [PubMed]
- 23.Hill AT, Haworth CS, Aliberti S, Barker A, Blasi F, Boersma W, et al. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J. 2017;49. 10.1183/13993003.00051-2017. Pubmed:28596426. [DOI] [PubMed]
- 24.Choi H, Ryu S, Keir HR, Giam YH, Dicker AJ, Perea L et al. Inflammatory molecular endotypes in bronchiectasis: A European multicenter cohort study. Am J Respir Crit Care Med. 2023;208:1166-76. 10.1164/rccm.202303-0499OC, Pubmed:37769155. [DOI] [PubMed]
- 25.Keir HR, Shoemark A, Dicker AJ, Perea L, Pollock J, Giam YH et al. Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: an international, observational, multicohort study. Lancet Respir Med. 2021;9:873 – 84. 10.1016/S2213-2600(20)30504-X, Pubmed:33609487. [DOI] [PubMed]
- 26.Chalmers JD, Moffitt KL, Suarez-Cuartin G, Sibila O, Finch S, Furrie E et al. Neutrophil elastase activity is associated with exacerbations and lung function decline in bronchiectasis. Am J Respir Crit Care Med. 2017;195:1384-93. 10.1164/rccm.201605-1027OC, Pubmed:27911604. [DOI] [PMC free article] [PubMed]
- 27.Kwok WC, Teo KC, Lau KK, Ho JC-M. High-sensitivity C-reactive protein level in stable-state bronchiectasis predicts exacerbation risk. BMC Pulm Med. 2024;24:80. 10.1186/s12890-024-02888-z, Pubmed:38350918. [DOI] [PMC free article] [PubMed]
- 28.Kwok WC, Ho JCM, Tam TCC, Ip MSM, Lam DCL. Risk factors for Pseudomonas aeruginosa colonization in non-cystic fibrosis bronchiectasis and clinical implications. Respir Res. 2021;22:132. 10.1186/s12931-021-01729-5, Pubmed:33910573. [DOI] [PMC free article] [PubMed]
- 29.Narayana JK, Aliberti S, Mac Aogáin M, Jaggi TK, Ali NABM, Ivan FX et al. Microbial dysregulation of the gut-lung axis in bronchiectasis. Am J Respir Crit Care Med. 2023;207:908 – 20. 10.1164/rccm.202205-0893OC, Pubmed:36288294. [DOI] [PMC free article] [PubMed]
- 30.Choate R, Aksamit TR, Mannino D, Addrizzo-Harris D, Barker A, Basavaraj A, et al. Pseudomonas aeruginosa associated with severity of non-cystic fibrosis bronchiectasis measured by the modified bronchiectasis severity score (BSI) and the FACED: the US bronchiectasis and NTM Research Registry (BRR) study. Respir Med. 2021;177:106285. 10.1016/j.rmed.2020.106285. [DOI] [PubMed] [Google Scholar]
- 31.Chalmers JD, Hill AT. Mechanisms of immune dysfunction and bacterial persistence in non-cystic fibrosis bronchiectasis. Mol Immunol. 2013;55:27–34. 10.1016/j.molimm.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Chalmers JD, Haworth CS, Metersky ML, Loebinger MR, Blasi F, Sibila O et al. Phase 2 trial of the DPP-1 inhibitor brensocatib in bronchiectasis. N Engl J Med. 2020;383:2127-37. 10.1056/NEJMoa2021713, Pubmed:32897034. [DOI] [PubMed]
- 33.Figueiredo C, Ibiapina C. The role of macrolides in noncystic fibrosis bronchiectasis. Pulm Med. 2011;2011:751982. 10.1155/2011/751982, Pubmed:22292118. [DOI] [PMC free article] [PubMed]
- 34.Wagner D, van Ingen J, van der Laan R, Obradovic M et al. Non-tuberculous mycobacterial lung disease in patients with bronchiectasis: perceived risk, severity and guideline adherence in a European physician survey. BMJ Open Respir Res. 2020;7:4438. 10.1136/bmjresp-2019-000498, Pubmed:32332023. [DOI] [PMC free article] [PubMed]
- 35.Shibata Y, Horita N, Yamamoto M, Tsukahara T, Nagakura H, Tashiro K et al. Diagnostic test accuracy of anti-glycopeptidolipid-core IgA antibodies for Mycobacterium avium complex pulmonary disease: systematic review and meta-analysis. Sci Rep. 2016;6:29325. 10.1038/srep29325, Pubmed:27373718. [DOI] [PMC free article] [PubMed]
- 36.Costa JC, Machado JN, Ferreira C, Gama J, Rodrigues C. The Bronchiectasis Severity Index and FACED score for assessment of the severity of bronchiectasis. Pulmonology. 2018;29306672. 10.1016/j.rppnen.2017.08.009. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.