Abstract
Background
Hyperuricemia (HUA) and gout have been demonstrated as independent risk factors for cardiovascular disease. The relationship between the recently updated Life’s Essentials 8 (LE8), which measures ideal cardiovascular health (CVH), and HUA and gout remains unclear. The aim of this study was to explore the relationship between CVH and the prevalence of HUA and gout among a nationally representative sample of US adults.
Methods
This study utilized cross-sectional analysis of data from the National Health and Nutrition Examination Survey (NHANES) for the years 2007 to 2018. The CVH scores and their corresponding components were defined according to the guidelines established by the American Heart Association. The association between the LE8 score and both HUA and gout was assessed using weighted multivariable logistic and restricted cubic spline (RCS) models.
Results
Among the 21,155 participants aged 20 years and older, the prevalence of HUA was 17.20% (95% CI, 16.05–18.36%), and the prevalence of gout was 3.58% (95% CI, 3.13–4.02%). After adjusting for potential confounders, compared to participants exhibiting low CVH, the multivariable adjusted odds ratio (OR) for HUA was 0.65 (95% CI, 0.56–0.75) in those with moderate CVH, and 0.25 (95% CI, 0.20–0.31) in those with high CVH. Additionally, compared to participants with low CVH, the multivariable adjusted OR for gout was 0.66 (95% CI, 0.53–0.81) in those with moderate CVH and 0.32 (95% CI, 0.20–0.50) in those with high CVH. The LE8 score exhibited a significant nonlinear negative association with HUA and linear negative correlation with gout. In subgroup analyses focusing on HUA, significant interactions were observed between LE8 score and age, sex, and CKD (P for interaction < 0.05). For gout, only a significant interaction between LE8 score and sex was observed (P for interaction < 0.05).
Conclusions
Among adults, there was a significant negative correlation between LE8 score and the prevalence of HUA and gout. Maintaining an ideal CVH may be beneficial in reducing the burden of HUA and gout.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41043-024-00665-6.
Keywords: Life’s essential 8, Cardiovascular health, Hyperuricemia, Gout, NHANES
Introduction
Hyperuricemia (HUA) is a disorder characterized by either excessive production or impaired excretion of uric acid, leading to abnormally high levels of uric acid above a specific threshold [1]. As uric acid levels continue to rise, they can lead to supersaturation and the deposition of urates in peripheral joints and surrounding tissues, resulting in inflammatory arthritis, commonly known as gout [2]. With the rapid development of the economy and changes in people’s lifestyles and dietary structure, the prevalence of HUA and gout has been steadily increasing and displaying a trend toward younger populations [3]. Recent studies have reported that the prevalence of HUA ranges from 2.6 to 36%, and the prevalence of gout ranges from 0.03–15.3% [4, 5]. Therefore, it is crucial to comprehend the potential risk factors contributing to the elevated prevalence of HUA and gout within the general population.
Unhealthy lifestyles significantly contribute to the increased prevalence of HUA and gout, primarily due to poor diets [6], sleep deprivation [7], exposure to nicotine [8], and lack of physical activity [9]. HUA and gout serve as independent risk factors for cardiovascular disease (CVD) and are also strongly linked to other CVD risk factors, such as hypertension [10], disorders of glucose and lipid metabolism [10, 11], and obesity [12]. Collectively, these factors detrimentally impact the cardiovascular health (CVH) of the population.
In 2010, the American Heart Association (AHA) proposed Life’s Simple 7 (LS7) to advocate for ideal CVH in the public [13]. However, the original LS7 score exhibited limitations in its practical applicability. As a result, to improve the sensitivity of scores to interindividual differences and to capture individual and population changes over time, the AHA revised the scoring algorithm and redefined existing metrics. Furthermore, sleep health was integrated into the CVH metrics, and the impact of psychological health and social determinants of health on desired CVH was underscored [14]. And the revised quantitative score for CVH was designated as “Life’s Essentials 8” (LE8) [14]. Given the strong correlation between HUA, gout, and CVD, maintaining ideal CVH may serve as a preventive and management strategy to alleviate the burden of HUA and gout. Nevertheless, to date, no study has concurrently assessed the relationship between the LE8 score and both HUA and gout. This cross-sectional study, utilizing data from the National Health and Nutrition Examination Survey (NHANES), was designed to examine the association between LE8 score and HUA and gout in US adults.
Materials and methods
Study population
NHANES is a continuous national cross-sectional survey conducted by the Centers for Disease Control and Prevention’s National Center for Health Statistics (NCHS) to assess the health and nutritional status of adults and children in the United States. The NHANES study protocol was approved by the Research Ethics Review Board of the NCHS, and all participants signed a written informed consent form prior to participation. This cross-sectional analysis obtained data from the NHANES study from 2007 to 2018. All data in this study are public and available on NHANES (https://www.cdc.gov/nchs/nhanes/index.htm). Additionally, the study adhered to the standards of the Strengthening the Reporting of Observational Studies in Epidemiology report [15].
Inclusion and exclusion criteria
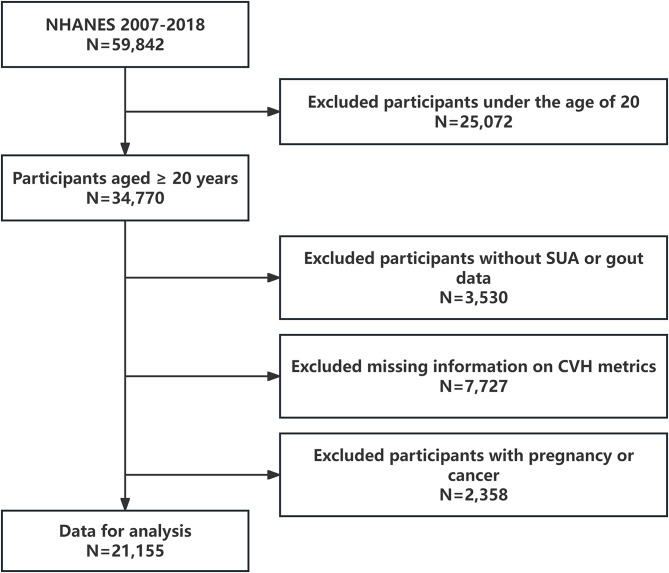
During the NHANES 2007–2018 period, a total of 59,842 participants took part in the project. This study included only 34,770 subjects aged 20 years and older (those under 20 were excluded due to the lack of most medical history reports). Other exclusion criteria were as follows: (1) lack of complete serum uric acid or gout information (n = 3,530); (2) missing data on CVH defining metrics (n = 7,727); (3) history of cancer or pregnancy (n = 2,358). Ultimately, the study included 21,155 US adults (Fig. 1).
Fig. 1.
Flow chart of the screening process for the selection of the study population. NHANES, national health and nutrition examination surveys; SUA, serum uric acid; CVH, cardiovascular health
Definitions of LE8 metrics
LE8 metrics includes four health behaviors (diet, physical activity, nicotine exposure, and sleep health) and four health factors (body mass index [BMI], non-high-density lipoprotein cholesterol, blood glucose, and blood pressure), serving as a means to quantitatively assess CVH [14]. A comprehensive algorithm for computing scores of the eight metrics in LE8 score using NHANES data can be found in Supplementary Table 1 [16]. In summary, each of the LE8 metrics was evaluated on a scale ranging from 0 to 100 points. The total LE8 score was computed as the unweighted average of these eight metrics. The AHA recommended that participants with LE8 score of 80–100, 50–79, and 0–49 were categorized as having high, moderate, and low CVH, respectively [17].
The diet metric was evaluated utilizing the 2015 Healthy Eating Index (HEI-2015) [18]. The HEI-2015 score was calculated by combining participants’ dietary intake (obtained from two 24-hour dietary recall interviews of NHANES) with USDA food pattern equivalency data. Nicotine exposure, sleep duration, physical activity, history of diabetes, and medication usage were all assessed via self-reported questionnaires. Blood pressure, height, and weight of the participants were assessed during the medical examination. BMI was computed by dividing weight in kilograms by the square of height in meters. Participants’ blood samples were sent to a central laboratory where non-high-density lipoprotein cholesterol, plasma glucose, and glycosylated hemoglobin were measured. Participants were divided into three groups (low CVH, moderate CVH, and high CVH groups) based on LE8 score.
Diagnosis of HUA and gout
Serum samples were shipped to the National Center for Environmental Health and stored at -30 °C, where they underwent testing for serum uric acid levels. HUA was defined according to established diagnostic criteria as a uric acid level > 7 mg/dL in men and > 6 mg/dL in women [12]. During the home interviews, all participants were asked the question “Has a doctor or other healthcare professional ever told you that you have gout?” Those who answered “yes” were defined as having gout [4, 12].
Covariates assessment
In this study, the following covariates were included based on NHANES home interview, examination, laboratory, and questionnaire data: age (20–39 years, 40–59 years, or ≥ 60 years), sex (Male and Female), race (Mexican American, non-Hispanic white, non-Hispanic black, or other races), marital status (married/living with a partner, divorced/separated/widowed, never married), education level (less than school, high school, or more than high school), poverty-to-income ratio (PIR), insurance status (Yes and No), alcohol consumption status, family history of cardiovascular disease (HCVD) (Yes and No), and chronic kidney disease (CKD) [19, 20]. Additionally, PIR was the ratio of monthly household income to the poverty level, categorized into three groups: <1.3 (low income), 1.3–3.5 (middle income) and > 3.5 (high income). Alcohol consumption status was categorized into four groups: current heavier drinkers (women: >1 drink per day on average in the past year, men: >2 drinks per day on average in the past year), current mild-moderate drinkers (women: ≤1 drink per day on average in the past year, men: ≤2 drinks per day on average in the past year), former drinkers (≥ 12 drinks in 1 year and no drinks in the past year, or no drinks in the past year but ≥ 12 drinks in their lifetime), and non-drinkers (< 12 drinks in their lifetime) [21]. As defined by recent guidelines, CKD is characterized by an estimated glomerular filtration rate of < 60 mL/min/1.73 m2 or a urinary albumin-creatinine ratio of ≥ 30 mg/g, or a combination of both [22].
Statistical analysis
All data in this study were analyzed in accordance with the NHANES Data Analysis Guidelines and Weighting Design [23]. In the baseline characterization involving the categorization of ideal CVH, continuous variables are expressed as the weighted means ± standard deviation (SD), and categorical variables are presented as weighted percentages (95% confidence interval, 95% CI). To assess disparities in baseline characteristics among the low, moderate, and high CVH groups, one-way ANOVA was applied to examine variations in weighted means of continuous variables. Additionally, the Rao-Scott chi-square test was employed to investigate distinctions in weighted percentages of categorical variables.
The weighted multivariable logistic regression was also applied to investigate the association between LE8 and its components with the presence of HUA and gout. Furthermore, restricted cubic spline (RCS) regression with 4 knots positioned at the 5th, 35th, 65th, and 95th percentiles was employed to examine the potential nonlinear relationship between LE8 and its subscales and HUA and gout. The crude model did not adjust for any potential confounders. The adjusted model adjusted for potential confounders such as age, sex, marital status, education, alcohol consumption status, insurance status, PIR, HCVD, and CKD based on previously published studies [19, 20].
To further investigate the association between LE8 and HUA and gout in different populations, subgroup analyses by age, sex, race, and CKD were performed in fully adjusted models. Additionally, the likelihood ratio test was employed to assess multiplicative interactions. In this study, missing values for covariates were imputed using the MissForest software package [24]. Supplementary Table 2 presents the overall data for the missing covariates. Sensitivity analyses adhered to two criteria: (1) inclusion of only participants with complete covariate data and (2) exclusion of participants with CVD. R version 4.2.2 software (R Foundation for Statistical Computing, Vienna, Austria) was used to conduct all statistical analyses. Statistical tests were two-sided, and the assumption of statistical significance was set at P < 0.05.
Results
Baseline characteristics
The sample in this study comprised 21,155 participants, weighted to represent 150,488,523 non-institutionalized adults aged 20 years and older in the United States. Table 1 presents the baseline characteristics of the study population, categorized based on low, moderate, and high CVH. The mean (SD) age of the surveyed population was 46.35 ± 0.25 years. Among the participants, 51.29% (95% CI, 48.74–53.84%) were female, and 67.20% (95% CI, 61.57–72.82%) were non-Hispanic white. Participants with low and moderate CVH were more likely to be older, male, non-Hispanic black, widowed/divorced/separated, more likely to consume alcohol, had lower levels of insurance coverage, education and PIR, as well as a higher prevalence of CKD and HCVD compared to participants with high CVH. As CVH levels advance (from the low CVH group to the high CVH group), the prevalence of HUA and gout noticeably diminishes.
Table 1.
General characteristics of the included participants (n = 21,155) in the NHANES 2007–2018
| Characteristics | Overall (n = 21,155) |
Low CVH (0–49) (n = 2,729) |
Moderate CVH (50–79) (n = 14,023) |
High CVH (80–100) (n = 4,403) |
P Value |
|---|---|---|---|---|---|
| Age, year | 46.35 ± 0.25 | 53.28 ± 0.36 | 47.48 ± 0.28 | 40.55 ± 0.43 | <0.0001 |
| Age, year | <0.0001 | ||||
| 20–39 | 37.51(35.62–39.40) | 19.28(17.11–21.46) | 34.53(33.05–36.02) | 52.85(50.10–55.60) | |
| 40–59 | 39.54(37.09–42.00) | 45.01(42.13–47.90) | 40.93(39.72–42.14) | 33.64(31.20–36.09) | |
| ≥ 60 | 22.95(21.30–24.60) | 35.71(33.05–38.36) | 24.54(23.28–25.80) | 13.51(11.71–15.30) | |
| Sex | <0.0001 | ||||
| Male | 48.71(46.23–51.19) | 51.85(50.91–52.79) | 51.85(50.91–52.79) | 40.82(38.84–42.80) | |
| Female | 51.29(48.74–53.84) | 52.05(49.45–54.65) | 48.15(47.21–49.09) | 59.18(57.20–61.16) | |
| Race | <0.0001 | ||||
| Non-Hispanic White | 67.20(61.57–72.82) | 64.80(60.87–68.73) | 66.57(63.59–69.55) | 69.82(66.89–72.76) | |
| Non-Hispanic Black | 10.88(9.68–12.08) | 16.87(14.11–19.62) | 11.69(10.15–13.22) | 6.28(5.30–7.26) | |
| Mexican American | 8.59(7.20–9.97) | 7.85(5.94–9.76) | 9.00(7.40-10.61) | 7.80(6.42–9.17) | |
| Others | 13.34(12.26–14.41) | 10.48(8.68–12.28) | 12.74(11.45–14.02) | 16.09(14.11–18.08) | |
| Marital | <0.0001 | ||||
| Married/Living with Partner | 64.22(60.32–68.12) | 60.18(57.44–62.93) | 64.37(63.00–65.74) | 65.52(63.34–67.69) | |
| Widowed/Divorced/Separated | 16.93(15.88–17.99) | 26.06(23.84–28.28) | 18.46(17.64–19.29) | 9.14(8.14–10.14) | |
| Never married | 18.85(17.6-20.06) | 13.75(11.81–15.70) | 17.17(15.90–18.44) | 25.34(23.20–27.48) | |
| Education | <0.0001 | ||||
| Less than high school | 14.57(13.34–15.80) | 26.03(23.54–28.52) | 15.56(14.30–16.81) | 7.23(6.17–8.28) | |
| High school | 23.19(21.55–24.83) | 32.27(29.29–35.25) | 25.59(24.27–26.91) | 13.15(11.91–14.38) | |
| Above high school | 62.24(58.55–65.94) | 41.70(38.61–44.79) | 58.85(56.85–60.86) | 79.63(77.71–81.54) | |
| Poverty-income ratio | <0.0001 | ||||
| <1.3 | 19.52(18.29–20.74) | 30.93(28.18–33.68) | 19.92(18.50–21.35) | 13.71(12.08–15.34) | |
| 1.3–3.5 | 40.01(37.73–42.29) | 45.75(43.13–48.36) | 41.13(39.60-42.66) | 34.70(32.46–36.94) | |
| >3.5 | 40.48(37.26–43.70) | 23.33(20.19–26.46) | 38.95(36.84–41.05) | 51.59(48.76–54.43) | |
| Insurance | <0.0001 | ||||
| No | 17.65(16.43–18.88) | 18.60(16.88–20.33) | 18.60(17.20–20.00) | 14.79(13.30–16.28) | |
| Yes | 82.35(77.86–86.83) | 81.40(79.67–83.12) | 81.40(80.00–82.80) | 85.21(83.72–86.70) | |
| Alcohol consumption status | <0.0001 | ||||
| Never | 10.67(9.66–11.68) | 9.03(7.74–10.32) | 10.29(9.53–11.04) | 12.36(10.23–14.48) | |
| Former | 11.81(10.82–12.81) | 23.12(21.21–25.04) | 12.14(11.21–13.06) | 6.27(5.38–7.15) | |
| Mild-moderate | 37.38(35.08–39.68) | 29.56(26.88–32.24) | 36.63(35.28–37.99) | 42.58(40.19–44.97) | |
| Heavy | 40.14(38.01–42.26) | 38.29(35.65–40.92) | 40.94(39.52–42.37) | 38.80(36.72–40.87) | |
| HCVD | <0.0001 | ||||
| No | 87.75(83.61–91.89) | 79.18(77.11–81.24) | 87.52(86.66–88.37) | 91.91(90.89–92.93) | |
| Yes | 12.25(11.30–13.21) | 20.82(18.76–22.89) | 12.48(11.63–13.34) | 8.09(7.07–9.11) | |
| CKD | <0.0001 | ||||
| No | 87.43(83.05–91.81) | 72.86(70.72–75.00) | 87.36(86.62–88.11) | 93.65(92.64–94.65) | |
| Yes | 12.57(11.77–13.37) | 27.14(25.00–29.28) | 12.64(11.89–13.38) | 6.35(5.35–7.36) | |
| LE8 score | 68.89 ± 0.27 | 42.20 ± 0.18 | 66.25 ± 0.11 | 86.88 ± 0.12 | <0.0001 |
| Health factors score | 70.61 ± 0.26 | 45.71 ± 0.35 | 67.49 ± 0.22 | 89.11 ± 0.21 | <0.0001 |
| Health behaviors score | 67.18 ± 0.34 | 38.69 ± 0.45 | 65.01 ± 0.24 | 84.65 ± 0.22 | <0.0001 |
| Serum uric acid (mg/dL) | 5.42 ± 0.02 | 5.88 ± 0.04 | 5.54 ± 0.02 | 4.90 ± 0.03 | <0.0001 |
| Hyperuricemia | <0.0001 | ||||
| No | 82.80(78.76–86.83) | 71.31(69.03–73.59) | 80.96(79.89–82.03) | 92.36(91.29–93.44) | |
| Yes | 17.20(16.05–18.36) | 28.69(26.41–30.97) | 19.04(17.97–20.11) | 7.64(6.56–8.71) | |
| Gout | <0.0001 | ||||
| No | 96.42(91.76–101.09) | 92.33(90.96–93.70) | 96.18(95.71–96.65) | 98.77(98.28–99.26) | |
| Yes | 3.58(3.13–4.02) | 7.67(6.30–9.04) | 3.82(3.35–4.29) | 1.23(0.74–1.72) |
LE8, Life’s Essential 8; CKD, chronic kidney disease; HCVD, family history of cardiovascular disease; CVH, cardiovascular health; Continuous variables are presented as the weighted mean ± standard deviation, and were compared using the weighted one-way ANOVA test; Categorical variables are presented as weighted percentages (95% confidence interval), and were compared using the Rao-Scott chi-square test
Association between LE8 and its components with HUA
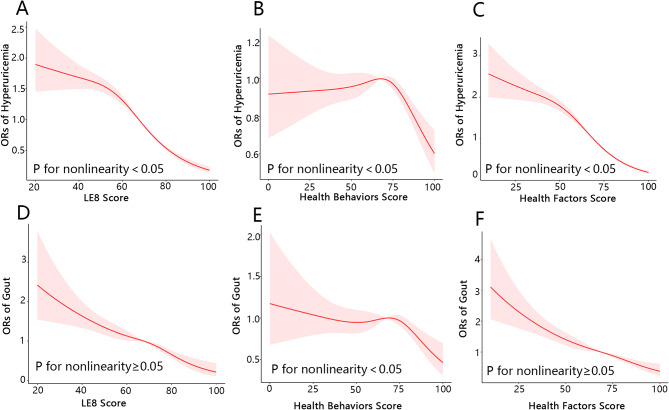
As presents in Table 2, within the fully adjusted weighted multivariable logistic regression model, the odds ratio (OR) for HUA associated with each 1-point increment in LE8 score was 0.968 (95% CI, 0.964–0.972). The OR for HUA was 0.65 (95% CI, 0.56–0.75) in the moderate CVH group and 0.25 (95% CI, 0.20–0.31) in the high CVH group when compared to the low CVH group. Tests for trend revealed a significant and substantial reduction in the risk of HUA among participants grouped by CVH (P for trend < 0.0001). Meanwhile, the results of the multivariable-adjusted RCS analysis indicated a significant nonlinear relationship between LE8 score and HUA (P for nonlinearity < 0.05; Fig. 2A).
Table 2.
Weighted logistic regression coefficients (ORs) and 95% confidence intervals (CIs) for the association between LE8 and its subscale scores and hyperuricemia: the United States, 2007–2018
| Crude Model | Adjusted Model | |||
|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | |
| LE8 score | ||||
| Continuous | 0.966(0.962–0.969) | < 0.0001 | 0.968(0.964–0.972) | < 0.0001 |
| Low (0–49) | Reference | Reference | ||
| Moderate (50–79) | 0.58(0.51–0.67) | < 0.0001 | 0.65(0.56–0.75) | < 0.0001 |
| High (80–100) | 0.21(0.17–0.25) | < 0.0001 | 0.25(0.20–0.31) | < 0.0001 |
| P for trend | < 0.0001 | < 0.0001 | ||
| Health behaviors score | ||||
| Continuous | 0.994(0.991–0.996) | < 0.0001 | 0.997(0.994–1.000) | 0.027 |
| Low (0–49) | Reference | Reference | ||
| Moderate (50–79) | 0.89(0.79–1.00) | 0.044 | 0.98(0.86–1.12) | 0.763 |
| High (80–100) | 0.70(0.61–0.81) | < 0.0001 | 0.83(0.71–0.98) | 0.030 |
| P for trend | < 0.0001 | 0.021 | ||
| Health factors score | ||||
| Continuous | 0.966(0.963–0.968) | < 0.0001 | 0.966(0.963–0.969) | < 0.0001 |
| Low (0–49) | Reference | Reference | ||
| Moderate (50–79) | 0.55(0.48–0.61) | < 0.0001 | 0.60(0.53–0.68) | < 0.0001 |
| High (80–100) | 0.17(0.15–0.20) | < 0.0001 | 0.19(0.15–0.23) | < 0.0001 |
| P for trend | < 0.0001 | < 0.0001 | ||
Crude Model: Unadjusted model; Adjusted Model: Adjusted for age, sex, race, education, marital status, poverty income ratio, insurance status, alcohol consumption status, family history of cardiovascular disease, and chronic kidney disease. LE8, Life’s Essential 8
Fig. 2.
The nonlinear relationships between LE8 and its subscale scores with both hyperuricemia and gout. (A) LE8 score and hyperuricemia; (B) health behaviors score and hyperuricemia; (C) health factors score and hyperuricemia; (D) LE8 score and gout; (E) health behaviors score and gout; (F) health factors score and gout. Adjusted for age, sex, race, education, marital status, PIR, insurance status, alcohol consumption status, HCVD, and CKD.LE8, Life’s Essential 8; OR, odds ratio
In adjusted model, health behavior and health factor score exhibited negative associations with HUA. When the above health behavior score and health factor score were included as continuous variables in the weighted logistic regression, their ORs with HUA were 0.997 (95% CI, 0.994–1.000) and 0.966 (95% CI, 0.963–0.969), respectively. RCS analysis demonstrated significant nonlinear associations between both health behavior score (P for nonlinearity < 0.005; Fig. 2B) and health factor score (P for nonlinearity < 0.05; Fig. 2C) with HUA. Supplementary Table 3 results indicate that the HEI-2015 diet score, physical activity score, blood pressure score, BMI score, blood lipids score, and blood glucose score are all significantly negatively associated with HUA risk.
Association between LE8 and its components with gout
As indicated in Table 3, in the fully adjusted weighted multivariable logistic regression model, the OR associated with gout was 0.977 (95% CI 0.969–0.984) for each 1-point increment in LE8 score. Additionally, the OR for gout was 0.66 (95% CI, 0.53–0.81) for the moderate CVH group and 0.32 (95% CI, 0.20–0.50) for the high CVH group compared with the low CVH group. The trend test revealed a significantly and substantially reduced risk of gout among participants stratified by CVH (P for trend < 0.0001). Furthermore, multivariable-adjusted RCS analysis showed a significant linear relationship between LE8 score and gout (P for nonlinearity ≥ 0.05; Fig. 2D).
Table 3.
Weighted logistic regression coefficients (ORs) and 95% confidence intervals (CIs) for the association between LE8 and its subscale scores and gout: the United States, 2007–2018
| Crude Model | Adjusted Model | |||
|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | |
| LE8 score | ||||
| Continuous | 0.963(0.957–0.969) | < 0.0001 | 0.977(0.969–0.984) | < 0.0001 |
| Low (0–49) | Reference | Reference | ||
| Moderate (50–79) | 0.48(0.39–0.58) | < 0.0001 | 0.66(0.53–0.81) | < 0.0001 |
| High (80–100) | 0.15(0.10–0.23) | < 0.0001 | 0.32(0.20–0.50) | < 0.0001 |
| P for trend | < 0.0001 | < 0.0001 | ||
| Health behaviors score | ||||
| Continuous | 0.991(0.986–0.996) | < 0.001 | 0.994(0.989–0.999) | 0.025 |
| Low (0–49) | Reference | Reference | ||
| Moderate (50–79) | 0.86(0.68–1.07) | 0.171 | 0.99(0.79–1.25) | 0.960 |
| High (80–100) | 0.57(0.42–0.76) | < 0.001 | 0.69(0.50–0.94) | 0.018 |
| P for trend | < 0.0001 | 0.012 | ||
| Health factors score | ||||
| Continuous | 0.965(0.961–0.970) | < 0.0001 | 0.979(0.973–0.985) | < 0.0001 |
| Low (0–49) | Reference | Reference | ||
| Moderate (50–79) | 0.43(0.35–0.52) | < 0.0001 | 0.57(0.46–0.70) | < 0.0001 |
| High (80–100) | 0.16(0.11–0.23) | < 0.0001 | 0.38(0.25–0.56) | < 0.0001 |
| P for trend | < 0.0001 | < 0.0001 | ||
Crude Model: Unadjusted; Adjusted Model: Adjusted for age, sex, race, education, marital status, poverty income ratio, insurance status, alcohol consumption status, family history of cardiovascular disease, and chronic kidney disease. LE8, Life’s Essential 8
In the adjusted model, health behavior and health factor scores showed inverse associations with gout. When incorporating the health behavior score and health factor score as continuous variables in the weighted logistic regression, their ORs with gout were 0.994 (95% CI, 0.989–0.999) and 0.979 (95% CI, 0.973–0.985), respectively. The RCS analysis revealed a significant nonlinear association between health behavior score and gout (P for nonlinearity < 0.05; Fig. 2E). Conversely, a significant linear association was observed between health factor score and gout (P for nonlinearity ≥ 0.05; Fig. 2F). Supplementary Table 3 results show that the components of the LE8 score, including sleep health score, blood pressure score, BMI score, and blood glucose score, are significantly negatively correlated with gout risk.
Subgroup and sensitivity analysis
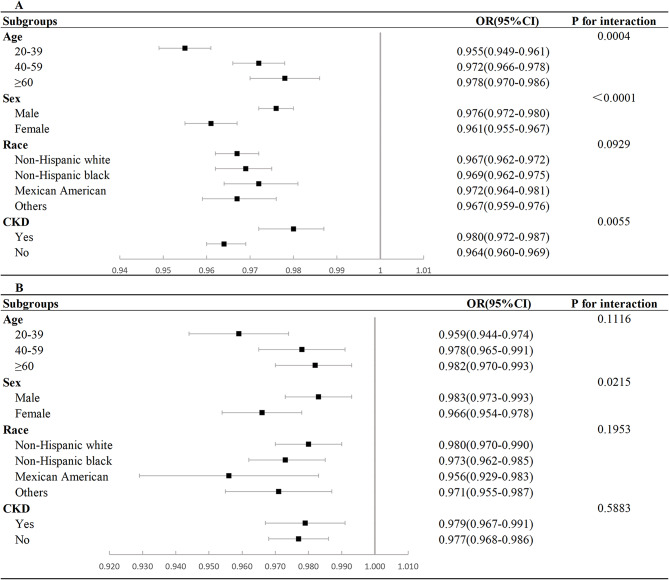
Subgroup analyses were conducted to investigate the influence of age, sex, race, and CKD on the association between LE8 score and HUA and gout. In the case of HUA, an interaction was observed between LE8 score and age, sex, and CKD (P for interaction < 0.05; Fig. 3A). Regarding gout, an interaction was detected exclusively between LE8 and sex (P for interaction < 0.05; Fig. 3B). The negative association between LE8 and HUA showed higher significance in younger, female, non-Hispanic white or other race participants. In addition, the negative association between LE8 score and gout showed higher significance exclusively in female participants.
Fig. 3.
Subgroup analysis of the association of LE8 score and hyperuricemia and gout. (A) Subgroup analysis of the association of LE8 score and hyperuricemia; (B) Subgroup analysis of the association of LE8 score and gout. CKD, chronic kidney disease; OR, odds ratio
Overall, the results of the sensitivity analyses were found to be robust and reliable. Firstly, upon excluding participants with missing covariates from the sensitivity analyses, the fully adjusted multivariate logistic regression models exhibited consistent results with those of the main analysis (Supplementary Table 4). Secondly, upon excluding participants with a history of CVD from the sensitivity analyses, the fully adjusted multivariable logistic regression model demonstrated that the remaining findings were largely consistent with those of the main analysis, except for the absence of an association between gout and health behavior score (Supplementary Table 5).
Discussion
This cross-sectional research is the first to unveil the relationship between CVH, as quantified by the LE8 score, and HUA and gout among a representative US adult population. Notably, our findings revealed that the risk of HUA and gout decreased with increasing LE8 score. Furthermore, we found that this correlation was evident in both the health behavior score and the health factors score. Subgroup analyses indicated that the negative association between LE8 score and HUA was more significant among participants who were younger, female, and non-Hispanic white or of other races. Meanwhile, the negative association between LE8 and gout was stronger in female participants. The correlations remained significant even after excluding participants with CVD or missing covariates.
As a precursor to the LE8, the LS7 demonstrated limitations in practical application. For example, LS7 demonstrated reduced sensitivity to interindividual variability and proved inadequate in evaluating dose-response associations. The updated LE8 effectively addresses the shortcomings of LS7 [14], quantifies the concept of CVH, promotes public self-assessment of CVH, and plays a crucial role in preventive care and the management of cardiovascular disease. Recent research evidence suggests that adherence to optimal CVH significantly lowers the risk of all-cause mortality and CVD-specific mortality in adults [25], highlighting the significance of maintaining ideal CVH for public health and healthcare. Currently, our findings suggest an inverse dose-response relationship between CVH assessed by LE8 score and HUA and gout. The OR associated with HUA exhibited a slower decline in the lower range of LE8 scores, but a more rapid decline in the higher range, suggesting that maintaining optimal CVH may be more effective in reducing the risk of developing HUA. Additionally, a linear dose-response relationship was observed between LE8 score and the gout. The risk of gout may decrease linearly as the LE8 score increases, consistent with findings from a study conducted in a European population [26].
Although the mechanisms linking LE8 to HUA and gout are not yet fully understood, numerous studies have highlighted the critical role of metabolic syndrome and lifestyle factors in the onset and progression of HUA and gout [27, 28], all of which may serve as potential indicators of health factors and health behaviors in LE8. Metabolic syndrome is a group of physiologic and metabolic abnormalities including obesity, abnormal glucose metabolism, dyslipidemia, and hypertension, in which hyperinsulinemia and insulin resistance (IR) play an important role [29]. Research has shown that hyperinsulinemia exacerbates impaired renal uric acid excretion, a process mediated by proximal tubular sodium reabsorption, ultimately resulting in HUA [30]. In the metabolic process, IR diminishes the activity of a crucial enzyme, 3-phosphate Glyceraldehyde dehydrogenase, involved in the production of uric acid. This reduction in enzymatic activity contributes to elevated serum uric acid levels, subsequently leading to the development of HUA [31]. Additionally, IR enhances the liver’s production of very low-density lipoproteins and promotes lipolysis, leading to an increase in triacylglycerol levels. Lipolysis releases a substantial quantity of free fatty acids, metabolized through beta-oxidation. In the metabolic process of these fatty acids, there is a concurrent increase in the production of reduced nicotinamide adenine dinucleotide phosphate, subsequently metabolized into uric acid, resulting in HUA [32]. Recently, a Mendelian randomization study furnished evidence indicating that IR unequivocally leads to HUA and gout [33]. The current approach to managing HUA and gout involves combining lifestyle modifications with the reduction of risk factors and the use of medication. Studies have shown that a healthy lifestyle, including limiting smoking and alcohol consumption, dietary modification, regular moderate exercise, healthy sleep patterns, and weight control, has the potential to diminish serum uric acid concentrations and manage the initiation and progression of HUA and gout to some extent [34, 35]. Therefore, these findings might provide a plausible mechanistic explanation for the link between LE8 and HUA and gout.
The health factor score showed stronger correlations with HUA and gout than the health behavior score. When analyzing the relationship between health behaviors and HUA, only ideal diet and physical activity were associated with a lower risk of HUA. However, health factors such as ideal BMI, lipids, blood glucose, and blood pressure were all negatively associated with HUA. A similar relationship was observed when examining the association between the components of LE8 and gout. When analyzing the relationship between health behaviors and gout, only ideal physical activity and sleep health were found to be negatively associated with gout. Additionally, health factors such as ideal BMI, blood glucose, and blood pressure scores were all negatively associated with gout. All these findings are consistent with the results of a previous study. A 2022 study established a relationship between the HEI-2015 score and HUA and gout [36]. However, our study revealed only a significant negative correlation between HEI-2015 score and HUA. Although the negative correlation between HEI-2015 score and gout was not statistically significant, we observed a trend of decreasing risk of gout with increasing HEI-2015 score. Previous research indicates that adequate physical activity can modulate the synthesis and excretion of uric acid, thereby decreasing the risk of HUA and gout [9, 27]. Research from the UK Biobank indicates that adhering to a healthy sleep routine lowers the risk of gout, with 7–9 h of sleep per day deemed optimal, consistent with the criteria and outcomes of our study [7]. However, research on sleep duration and HUA was limited and presented inconclusive findings. A study from Korea demonstrated a U-shaped relationship between sleep duration and the risk of HUA, identifying 7 h of sleep as the threshold for the lowest risk [37]. Moreover, certain studies failed to detect a correlation between sleep duration and HUA [20, 38], consistent with our own findings. Obesity, hypertension, and hyperglycemia collectively contribute to blood uric acid production and impair uric acid excretion via various mechanisms, ultimately leading to HUA and gout. Conversely, elevated levels of blood uric acid also aggravate the manifestation of obesity, hypertension, and hyperglycemia, resulting in their mutual exacerbation [39]. Our findings indicated that non-HDL cholesterol is linked to HUA, while showing no association with gout. A paucity of studies exists that correlate non-HDL cholesterol with HUA or gout, necessitating further research for correlative analysis in future studies. Previous studies have concentrated on the association of a single factor with HUA or gout and have lacked a comprehensive analysis of the people’s overall situation. The LE8 score, as a comprehensive multifactorial index, is capable of thoroughly and accurately assessing an individual’s condition, making it highly suitable for clinical practice.
Subgroup analyses revealed a robust inverse relationship between LE8 score and HUA in young, female, non-CKD participants. Additionally, the inverse relationship between the LE8 score and gout exhibited increased statistical significance among female participants. Epidemiological studies have shown that the prevalence of HUA and gout escalates with advancing age [5]. Concerning HUA, this trend is observable across the male lifespan, with a notable increase in women after menopause, likely attributable to the pro-uric acid excretion effect of estrogen [40]. CKD-related impaired uric acid clearance is linked to the heightened risk of HUA [41]. These findings point to the fact that LE8 score not only enhances the methodology for quantitative assessment of CVH, but also increases the sensitivity of scores to differences between individuals and groups.
From the perspective of community health needs assessment, ideal CVH is crucial as it can significantly reduce the incidence of chronic diseases such as HUA and gout, thereby alleviating the medical burden and improving the overall quality of life for residents [42]. Furthermore, good CVH can decrease healthcare costs, enhance economic benefits, and promote the dissemination of health education and awareness, providing data support for policy formulation and ensuring the rational allocation of resources. These factors collectively contribute to the sustainable development of the community and the improvement of health levels [43].
Strengths and limitations
The data for this study were obtained from a nationally representative sample of US adults, enabling better generalization of the findings to the population as a whole. Additionally, we examined the dose-response relationship between CVH and HUA and gout while accounting for potential confounders. The findings of this study offer important guidance for the prevention and management of HUA and gout. However, several potential limitations must be considered inherent to this study. Firstly, the utilization of a cross-sectional study design constrained the ability of this study to establish causal relationships, and further longitudinal studies should be conducted to validate our findings. Secondly, the assessment of certain indicators relied on self-report questionnaires, which are susceptible to recall bias and potential statistical error. Thirdly, while we considered dietary factors in the CVH metric, we were unable to estimate and exclude the potential effect of certain medications on serum uric acid levels. This inability may impact the diagnosis of HUA, potentially leading to erroneous conclusions. Finally, our ability to dynamically monitor changes in cardiovascular health and uric acid levels is significantly limited.
Conclusion
In this study involving a representative sample of US adults, we found that LE8 score was negatively associated with the risk of HUA and gout. Moreover, health factors exhibited a stronger association with both HUA and gout compared to health behaviors. The results of RCS analyses revealed a nonlinear relationship between LE8 score and the risk of HUA, along with a linear relationship with gout risk. These findings suggest that the LE8 score is a practical and validated composite index suitable for clinical practice. Additionally, maintaining an ideal cardiovascular health (CVH) may potentially decrease the risk of HUA and gout. However, further studies are necessary to provide robust evidence for establishing causality.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- CVH
Cardiovascular health
- AHA
American Heart Association
- LS7
Life’s Simple 7
- LE8
Life’s Essential 8
- CVD
Cardiovascular disease
- NHANES
National Health and Nutrition Examination Survey
- NCHS
National Center for Health Statistics
- BMI
Body mass index
- HUA
Hyperuricemia
- HEI-2015
2015 Healthy Eating Index
- PIR
Poverty-income ratio
- HCVD
Family history of cardiovascular disease
- CKD
Chronic kidney disease
- SD
Standard deviation
- CI
Confidence interval
- RCS
Restricted cubic spline
- OR
Odds ratio
- IR
Insulin resistance
Author contributions
PF-L, KK, and CY-C contributed to the conception and design, acquisition, analysis, interpretation of the data, and drafting of the manuscript or critical revision for important intellectual content. XL-P, WQ-H, Ai-A and AA completed the software analysis and data visualization. QW, MA, XZ-L and YT-M collected and organized data. XM contributed to the conception and design and reviewing of the manuscript or critical revision for important intellectual content. All authors approved the final version, and agree to be accountable for all aspects of the work.
Funding
This study was supported by the key research projects in Xinjiang Uygur Autonomous Region (Grant No. 2022B03022-3) and national natural science foundation of China (Grant No. 82360090).
Data availability
This study is a secondary analysis based on a publicly available database and the raw data can be found on the website: https://www.cdc.gov/nchs/nhanes/index.htm.
Declarations
Ethics approval and consent to participate
The study protocols of NHANES were approved by the NCHS Research Ethics Review Board and participant written informed consent was obtained (https://www.cdc.gov/nchs/nhanes/irba98.htm). The additional ethical review was no longer required for the present study due to the usage of publicly available data without identifiable personal information.
Consent for publication
No applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pengfei Liu, Kaisaierjiang Kadier and Chunying Cui contributed equally to this work and share first authorship.
Contributor Information
Kaisaierjiang Kadier, Email: ks_kdr@yeah.net.
Xiang Ma, Email: maxiangxj@yeah.net.
References
- 1.Dai Y, Lee C-H. Transport mechanism and structural pharmacology of human urate transporter URAT1. Cell Res. 2024. 10.1038/s41422-024-01023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castaneda J, et al. 2018 updated European League against Rheumatism evidence-based recommendations for the diagnosis of gout. Ann Rheum Dis. 2020;79:31–8. [DOI] [PubMed] [Google Scholar]
- 3.Pascart T, Lioté F. Gout: state of the art after a decade of developments. Rheumatology (Oxford). 2019;58:27–44. [DOI] [PubMed] [Google Scholar]
- 4.Chen-Xu M, Yokose C, Rai SK, Pillinger MH, Choi HK. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019;71:991–9. [DOI] [PMC free article] [PubMed]
- 5.Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. 2020;16:380–90. [DOI] [PubMed] [Google Scholar]
- 6.Yokose C, McCormick N, Choi HK. The role of diet in hyperuricemia and gout. Curr Opin Rheumatol. 2021;33:135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Q, He P, Ye Z, Zhou C, Liu M, Yang S, et al. Sleep patterns, genetic susceptibility, and risk of new-onset gout: the UK Biobank prospective cohort study. J Psychosom Res. 2023;170:111381. [DOI] [PubMed] [Google Scholar]
- 8.Gee Teng G, Pan A, Yuan J-M, Koh W-P. Cigarette smoking and the risk of incident gout in a prospective cohort study. Arthritis Care Res (Hoboken). 2016;68:1135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng X, Huang J, Shen T, Xu Y, Yan X, Li Q, et al. Nonlinear dose-response association of moderate-to-vigorous physical activity with hyperuricemia in US adults: NHANES 2007–2018. PLoS ONE. 2024;19(5):e0302410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortada I, Hyperuricemia. Type 2 diabetes Mellitus, and hypertension: an Emerging Association. Curr Hypertens Rep. 2017;19:69. [DOI] [PubMed] [Google Scholar]
- 11.Liang J, Jiang Y, Huang Y, Song W, Li X, Huang Y, et al. The comparison of dyslipidemia and serum uric acid in patients with gout and asymptomatic hyperuricemia: a cross-sectional study. Lipids Health Dis. 2020;19:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mao T, He Q, Yang J, Jia L, Xu G. Relationship between gout, hyperuricemia, and obesity-does central obesity play a significant role?-a study based on the NHANES database. Diabetol Metab Syndr. 2024;16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of Cardiovascular Health: a Presidential Advisory from the American Heart Association. Circulation. 2022;146:e18–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: guidelines forreporting observational studies. Lancet. 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd-Jones DM, Ning H, Labarthe D, Brewer L, Sharma G, Rosamond W, et al. Status of Cardiovascular Health in US adults and Children Using the American Heart Association’s New Life’s essential 8 Metrics: Prevalence Estimates from the National Health and Nutrition Examination Survey (NHANES), 2013 through 2018. Circulation. 2022;146:822–35. [DOI] [PubMed] [Google Scholar]
- 17.Ma H, Wang X, Xue Q, Li X, Liang Z, Heianza Y, et al. Cardiovascular Health and Life Expectancy among adults in the United States. Circulation. 2023;147:1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. 2018;118:1591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadier K, Liu P, Dilixiati D, Peng X, Ainiwaer A, Kadier D, et al. Maintaining ideal cardiovascular health is associated with higher serum anti-aging protein klotho in the middle-aged and older populations. J Nutr Health Aging. 2024;28:100224. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Meng Q, Zhang X, Baima K, Chen L, Dai Y, et al. Life’s essential 8, Life’s simple 7 and the odds of hyperuricaemia: results from the China multi-ethnic cohort study. Rheumatol Adv Pract. 2024;8:rkae009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadier K, Dilixiati D, Ainiwaer A, Liu X, Lu J, Liu P, et al. Analysis of the relationship between sleep-related disorder and systemic immune-inflammation index in the US population. BMC Psychiatry. 2023;23(1):773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kidney Disease. Improving global outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the management of glomerular diseases. Kidney Int. 2021;100:S1–276. [DOI] [PubMed] [Google Scholar]
- 23.Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat 2. 2013;1–24. [PubMed]
- 24.Stekhoven DJ, Bühlmann P. MissForest–non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28:112–8. [DOI] [PubMed] [Google Scholar]
- 25.Sun J, Li Y, Zhao M, Yu X, Zhang C, Magnussen CG, et al. Association of the American Heart Association’s new Life’s essential 8 with all-cause and cardiovascular disease-specific mortality: prospective cohort study. BMC Med. 2023;21:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Y, Sun Y, Yu Y, Wang Y, Chen C, Tan X, et al. Life’s essential 8 and risk of non-communicable chronic diseases: outcome-wide analyses. Chin Med J (Engl). 2024;137:1553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kakutani-Hatayama M, Kadoya M, Okazaki H, Kurajoh M, Shoji T, Koyama H, et al. Nonpharmacological management of gout and hyperuricemia: hints for Better Lifestyle. Am J Lifestyle Med. 2015;11:321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eun Y, Han K, Lee SW, Kim K, Kang S, Lee S, et al. Altered risk of incident gout according to changes in metabolic syndrome status: a Nationwide, Population-based Cohort Study of 1.29 million Young men. Arthritis Rheumatol. 2023;75:806–15. [DOI] [PubMed] [Google Scholar]
- 29.Han Y, Han X, Yin Y, Cao Y, Di H, Wu J, et al. Dose-response relationship of uric acid with fasting glucose, insulin, and Insulin Resistance in a United States Cohort of 5,148 non-diabetic people. Front Med (Lausanne). 2022;9:905085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokose C, McCormick N, Abhishek A, Dalbeth N, Pascart T, Lioté F, et al. The clinical benefits of sodium-glucose cotransporter type 2 inhibitors in people with gout. Nat Rev Rheumatol. 2024;20:216–31. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Zhang H, Chang D, Guo F, Pan H, Yang Y. Metabolomics approach by 1H NMR spectroscopy of serum reveals progression axes for asymptomatic hyperuricemia and gout. Arthritis Res Ther. 2018;20:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simão ANC, Lozovoy MAB, Dichi I. The uric acid metabolism pathway as a therapeutic target in hyperuricemia related to metabolic syndrome. Expert Opin Ther Targets. 2012;16:1175–87. [DOI] [PubMed] [Google Scholar]
- 33.McCormick N, O’Connor MJ, Yokose C, Merriman TR, Mount DB, Leong A, et al. Assessing the Causal relationships between insulin resistance and hyperuricemia and gout using bidirectional mendelian randomization. Arthritis Rheumatol. 2021;73:2096–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castañeda-Sanabria J, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76:29–42. [DOI] [PubMed] [Google Scholar]
- 35.Vargas-Santos AB, Castelar-Pinheiro G, da Coutinho R, Schumacher ESF, Singh HR Jr, Schlesinger JA. Adherence to the 2012 American College of Rheumatology (ACR) guidelines for management of gout: a Survey of Brazilian rheumatologists. PLoS ONE. 2015;10:e0135805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nie J, Deng M-G, Wang K, Liu F, Xu H, Feng Q, et al. Higher HEI-2015 scores are associated with lower risk of gout and hyperuricemia: results from the national health and nutrition examination survey 2007–2016. Front Nutr. 2022;9:921550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee Y-C, Son D-H, Kwon Y-J. U-Shaped Association between Sleep Duration, C-Reactive protein, and Uric Acid in Korean Women. Int J Environ Res Public Health. 2020;17:2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maloberti A, Bombelli M, Facchetti R, Barbagallo CM, Bernardino B, Rosei EA, et al. Relationships between diuretic-related hyperuricemia and cardiovascular events: data from the URic acid right for heArt Health study. J Hypertens. 2021;39:333–40. [DOI] [PubMed] [Google Scholar]
- 39.Thottam GE, Krasnokutsky S, Pillinger MH. Gout and metabolic syndrome: a tangled web. Curr Rheumatol Rep. 2017;19:60. [DOI] [PubMed] [Google Scholar]
- 40.Cho SK, Winkler CA, Lee S-J, Chang Y, Ryu S. The prevalence of Hyperuricemia sharply increases from the late menopausal transition stage in middle-aged women. J Clin Med. 2019;8:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anders H-J, Li Q, Steiger S. Asymptomatic hyperuricaemia in chronic kidney disease: mechanisms and clinical implications. Clin Kidney J. 2023;16:928–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J, Jang S, Kim C S. Patterns and determinants of health and social care service needs among community-dwelling older adults. Geriatric Nursing. 2023;51:69–75. [DOI] [PubMed]
- 43.Abuzied Y, Deeb A, AlAnizy L, Al-Amer R, AlSheef M. Improving venous thromboembolism Prophylaxis through Service Integration, Policy Enhancement, and Health Informatics. Glob J Qual Saf Healthc. 2024;7:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study is a secondary analysis based on a publicly available database and the raw data can be found on the website: https://www.cdc.gov/nchs/nhanes/index.htm.