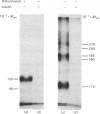
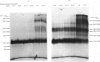
Abstract
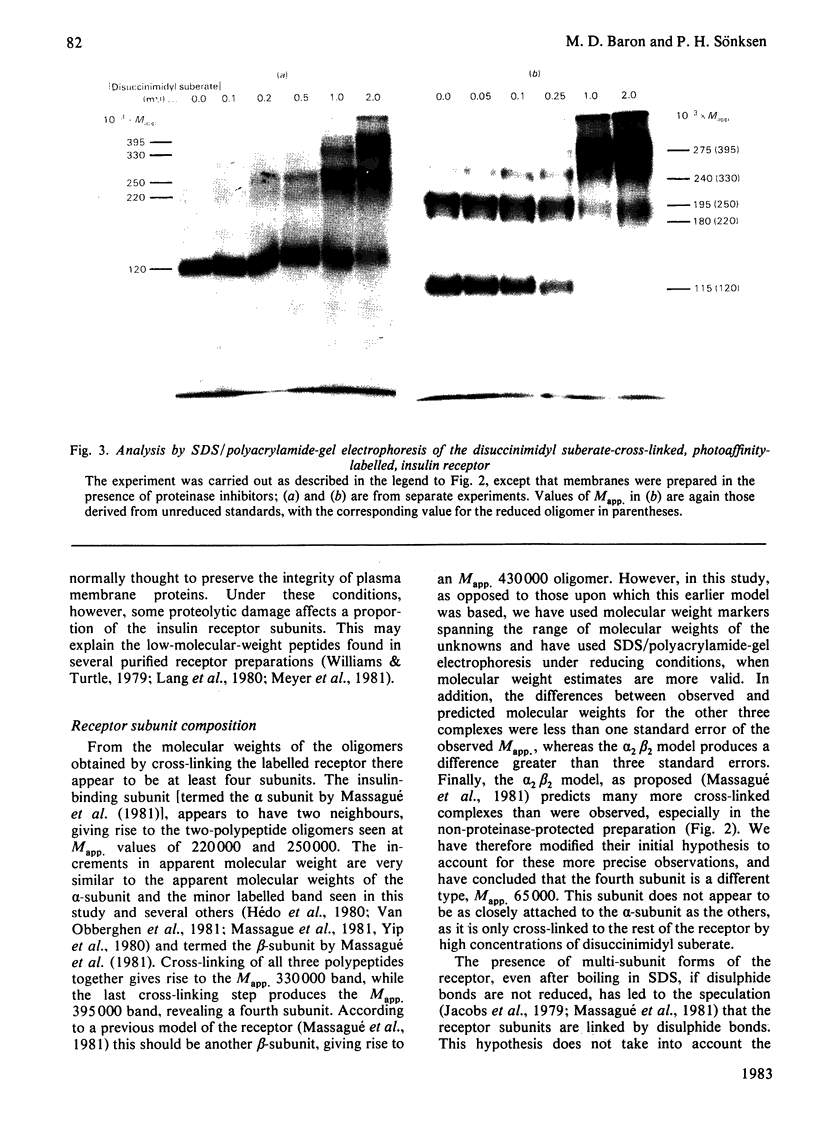
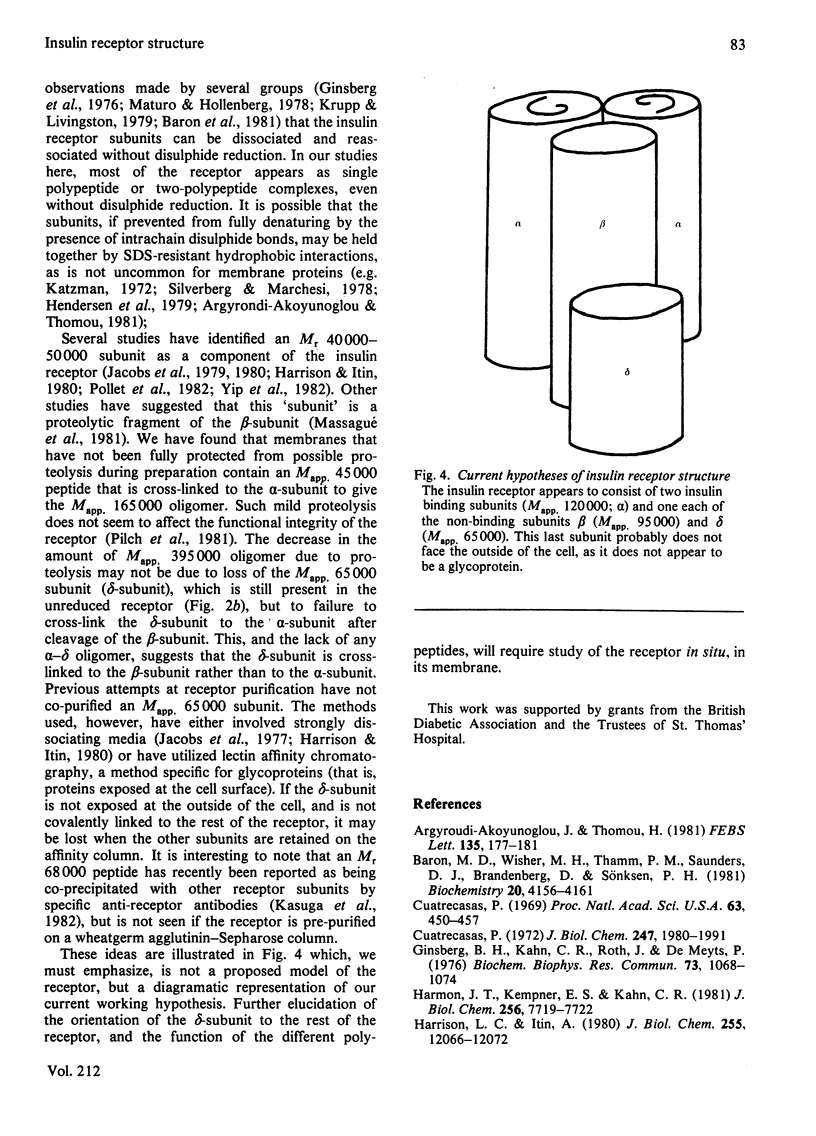
Photoreactive insulin analogues specifically label predominantly one polypeptide in the insulin receptor of rat liver plasma membranes. We have used the bifunctional reagent disuccinimidyl suberate to cross-link this polypeptide to its neighbouring, but not necessarily labelled, subunits. The results of these studies show that (1) there are at least three types of subunit in the receptor, with apparent Mr (Mapp.) values of 65 000, 95 000 and 120 000; (2) the receptor appears to consist of two Mapp. 120 000, one Mapp. 95 000 and one Mapp. 65 000 subunits; (3) the Mapp. 65 000 subunit, which has not been previously reported, may be only loosely attached to the receptor, and does not interact directly with the insulin-binding subunit (M app. 120 000).
Full text
PDF
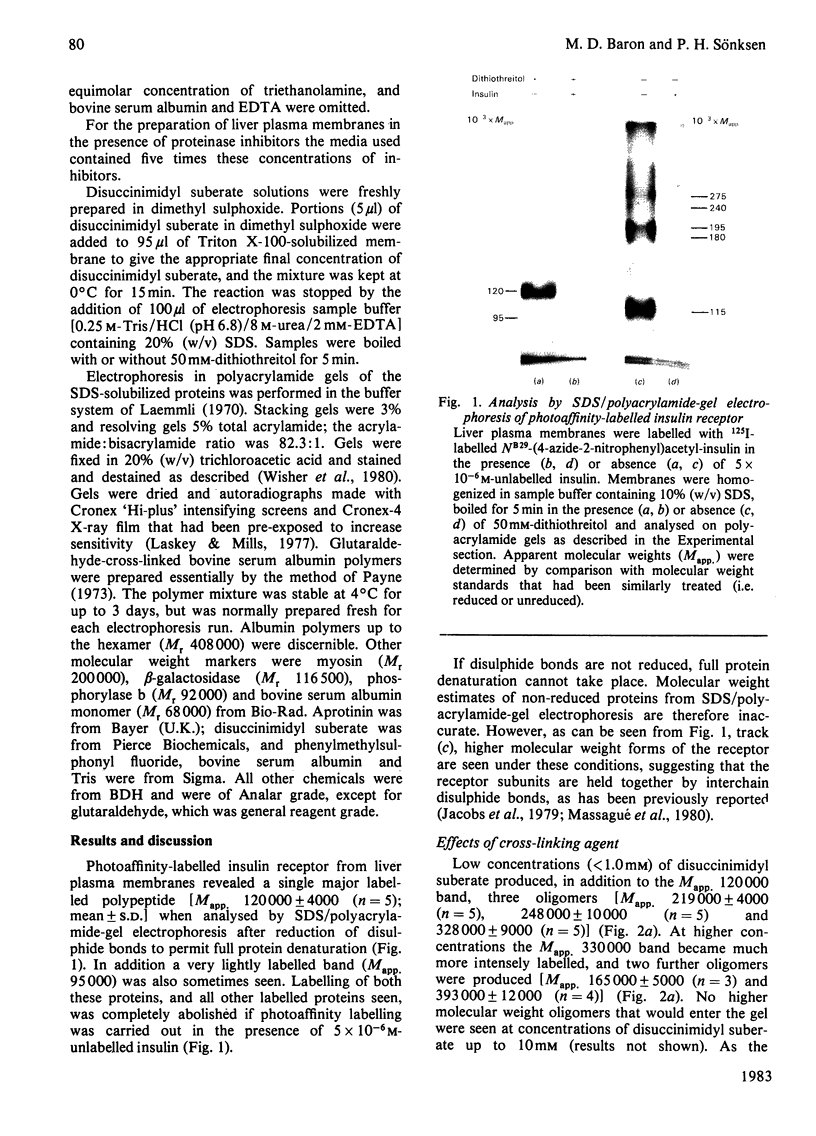
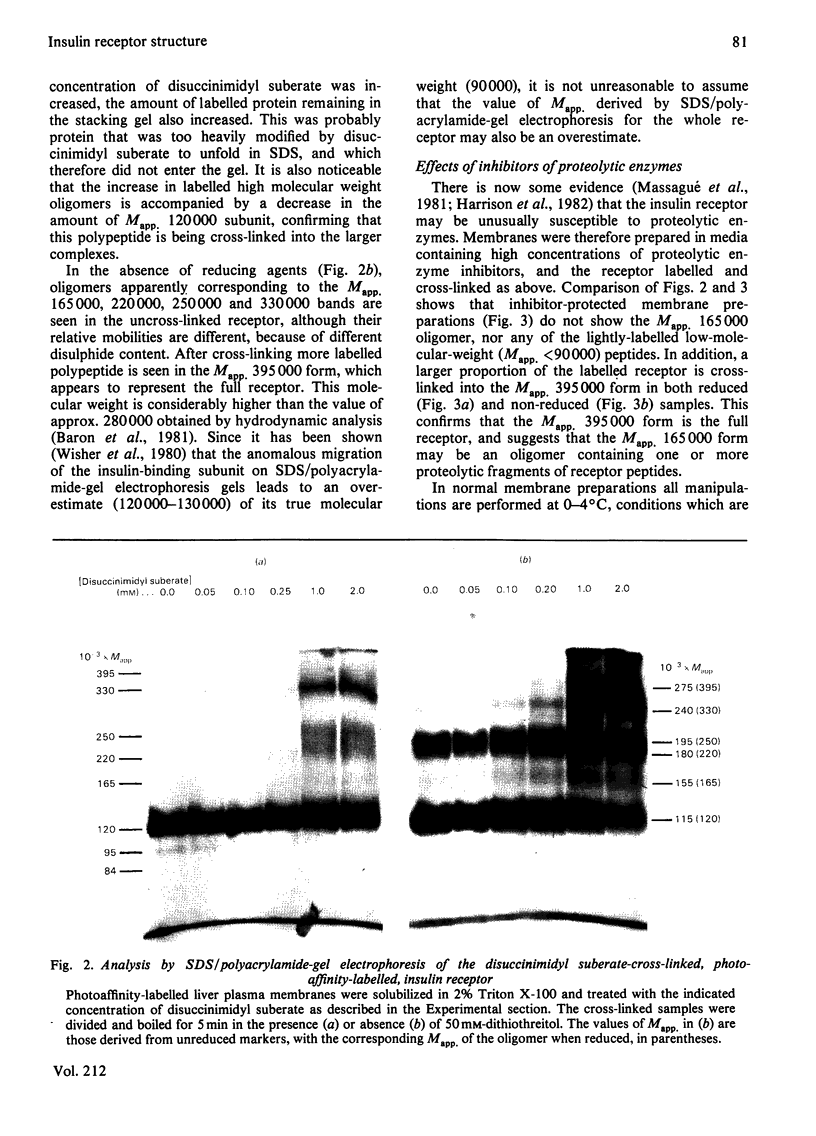

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron M. D., Wisher M. H., Thamm P. M., Saunders D. J., Brandenburg D., Sönksen P. H. Hydrodynamic characterization of the photoaffinity-labeled insulin receptor solubilized in Triton X-100. Biochemistry. 1981 Jul 7;20(14):4156–4161. doi: 10.1021/bi00517a032. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of insulin with the cell membrane: the primary action of insulin. Proc Natl Acad Sci U S A. 1969 Jun;63(2):450–457. doi: 10.1073/pnas.63.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Properties of the insulin receptor isolated from liver and fat cell membranes. J Biol Chem. 1972 Apr 10;247(7):1980–1991. [PubMed] [Google Scholar]
- Ginsberg B. H., Kahn C. R., Roth J., De Meyts P. Insulin-induced dissociation of its receptor into subunits: possible molecular concomitant of negative cooperativity. Biochem Biophys Res Commun. 1976 Dec 20;73(4):1068–1074. doi: 10.1016/0006-291x(76)90232-1. [DOI] [PubMed] [Google Scholar]
- Harmon J. T., Kempner E. S., Kahn C. R. Demonstration by radiation inactivation that insulin alters the structure of the insulin receptor in rat liver membranes. J Biol Chem. 1981 Aug 10;256(15):7719–7722. [PubMed] [Google Scholar]
- Harrison L. C., Itin A., Kasuga M., Van Obberghen E. The insulin receptor on the human lymphocyte: insulin-induced down-regulation of 126,000 and 90,000 glycosylated subunits. Diabetologia. 1982 Apr;22(4):233–238. doi: 10.1007/BF00281297. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Itin A. Purification of the insulin receptor from human placenta by chromatography on immobilized wheat germ lectin and receptor antibody. J Biol Chem. 1980 Dec 25;255(24):12066–12072. [PubMed] [Google Scholar]
- Hedo J. A., Kasuga M., Van Obberghen E., Roth J., Kahn C. R. Direct demonstration of glycosylation of insulin receptor subunits by biosynthetic and external labeling: evidence for heterogeneity. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4791–4795. doi: 10.1073/pnas.78.8.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D., Eibl H., Weber K. Structure and biochemistry of mouse hepatic gap junctions. J Mol Biol. 1979 Aug 5;132(2):193–218. doi: 10.1016/0022-2836(79)90391-7. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Cuatrecasas P. The subunit structure of rat liver insulin receptor. Antibodies directed against the insulin-binding subunit. J Biol Chem. 1980 Jul 25;255(14):6937–6940. [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Shechter Y., Cuatrecasas P. Insulin receptor: covalent labeling and identification of subunits. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4918–4921. doi: 10.1073/pnas.76.10.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Shechter Y., Bissell K., Cuatrecasas P. Purification and properties of insulin receptors from rat liver membranes. Biochem Biophys Res Commun. 1977 Aug 8;77(3):981–988. doi: 10.1016/s0006-291x(77)80074-0. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Katzman R. L. The inadequacy of sodium dodecyl sulfate as a dissociative agent for brain proteins and glycoproteins. Biochim Biophys Acta. 1972 Apr 14;266(1):269–272. doi: 10.1016/0005-2736(72)90141-1. [DOI] [PubMed] [Google Scholar]
- Krupp M. N., Livingston J. N. Effects of insulin on insulin-binding components extracted from rat fat cell membranes. Nature. 1979 Mar 1;278(5699):61–62. doi: 10.1038/278061a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang U., Kahn C. R., Harrison L. C. Subunit structure of the insulin receptor of the human lymphocyte. Biochemistry. 1980 Jan 8;19(1):64–70. doi: 10.1021/bi00542a010. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Linde S., Hansen B., Sonne O., Holst J. J., Gliemann J. Tyrosine A14[125I]monoiodoinsulin: Preparation, Biologic Properties, and long-term stability. Diabetes. 1981 Jan;30(1):1–8. doi: 10.2337/diab.30.1.1. [DOI] [PubMed] [Google Scholar]
- Massague J., Pilch P. F., Czech M. P. A unique proteolytic cleavage site on the beta subunit of the insulin receptor. J Biol Chem. 1981 Apr 10;256(7):3182–3190. [PubMed] [Google Scholar]
- Massague J., Pilch P. F., Czech M. P. Electrophoretic resolution of three major insulin receptor structures with unique subunit stoichiometries. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7137–7141. doi: 10.1073/pnas.77.12.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturo J. M., 3rd, Hollenberg M. D. Insulin receptor: interaction with nonreceptor glycoprotein from liver cell membranes. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3070–3074. doi: 10.1073/pnas.75.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H. E., Bubenzer H. J., Herbertz L., Kuehn L., Reinauer H. Purification of the insulin receptor protein from porcine liver membranes. Hoppe Seylers Z Physiol Chem. 1981 Dec;362(12):1621–1629. doi: 10.1515/bchm2.1981.362.2.1621. [DOI] [PubMed] [Google Scholar]
- Payne J. W. Polymerization of proteins with glutaraldehyde. Soluble molecular-weight markers. Biochem J. 1973 Dec;135(4):867–873. doi: 10.1042/bj1350867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. The subunit structure of the high affinity insulin receptor. Evidence for a disulfide-linked receptor complex in fat cell and liver plasma membranes. J Biol Chem. 1980 Feb 25;255(4):1722–1731. [PubMed] [Google Scholar]
- Pollet R. J., Kempner E. S., Standaert M. L., Haase B. A. Structure of the insulin receptor of the cultured human lymphoblastoid cell IM-9. Evidence suggesting that two subunits are required for insulin binding. J Biol Chem. 1982 Jan 25;257(2):894–898. [PubMed] [Google Scholar]
- Roth J. Methods for assessing immunologic and biologic properties of iodinated peptide hormones. Methods Enzymol. 1975;37:223–233. doi: 10.1016/s0076-6879(75)37018-3. [DOI] [PubMed] [Google Scholar]
- Silverberg M., Marchesi V. T. The anomalous electrophoretic behavior of the major sialoglycoprotein from the human erythrocyte. J Biol Chem. 1978 Jan 10;253(1):95–98. [PubMed] [Google Scholar]
- Van Obberghen E., Ksauga M., Le Cam A., Hedo J. A., Itin A., Harrison L. C. Biosynthetic labeling of insulin receptor: studies of subunits in cultured human IM-9 lymphocytes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1052–1056. doi: 10.1073/pnas.78.2.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. F., Turtle J. R. Purification of the insulin receptor from human placental membranes. Biochim Biophys Acta. 1979 Aug 28;579(2):367–374. doi: 10.1016/0005-2795(79)90064-3. [DOI] [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Functional polarity of the rat hepatocyte surface membrane. Isolation and characterization of plasma-membrane subfractions from the blood-sinusoidal, bile-Canalicular and contiguous surfaces of the hepatocyte. Biochem J. 1975 Feb;146(2):375–388. doi: 10.1042/bj1460375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip C. C., Moule M. L., Yeung C. W. Subunit structure of insulin receptor of rat adipocytes as demonstrated by photoaffinity labeling. Biochemistry. 1982 Jun 8;21(12):2940–2945. doi: 10.1021/bi00541a021. [DOI] [PubMed] [Google Scholar]
- Yip C. C., Yeung C. W., Moule M. L. Photoaffinity labeling of insulin receptor of rat adiopocyte plasma membrane. J Biol Chem. 1978 Mar 25;253(6):1743–1745. [PubMed] [Google Scholar]
- Yip C. C., Yeung C. W., Moule M. L. Photoaffinity labeling of insulin receptor proteins of liver plasma membrane preparations. Biochemistry. 1980 Jan 8;19(1):70–76. doi: 10.1021/bi00542a011. [DOI] [PubMed] [Google Scholar]