Abstract
Purpose
Retraction of the hepatic left lateral segment (HLLS) is a crucial maneuver for surgical field exposure during laparoscopic gastrectomy with systematic lymphadenectomy. Though various methods of retraction are available, there is no perfect solution. Here, we report the results of our initial 42 cases with HLLS inversion method and discuss the feasibility, safety, effectiveness and technical aspects of this method.
Methods
The intraoperative and postoperative short-term outcomes of 42 patients who underwent HLLS inversion during laparoscopic total gastrectomy and proximal gastrectomy in our department September, 2023 to January, 2024 were reviewed. HLLS inversion was performed by mobilizing the HLLS and inverting it to the right supra-hepatic space through an incision at the falciform ligament.
Results
42 patients underwent HLLS inversion successfully with an average time of 13.9 min. 7 patients needed re-inversion due to slipping back of the HLLS during operation. Optimal exposure of the surgical field was achieved in all patients. No intra-operative complications occurred, except for 1 patient presented with mild intraoperative hepatic hemorrhage requiring electrocoagulation for hemostasis. Alanine aminotransferase and glutamine aminotransferase elevated in some patients on postoperative day 1(POD1), but declined to preoperative levels on the 7th postoperative day. There were no Clavien-Dindo II grade or higher digestive complications after surgery. In 5 patients with preservation the hepatic branch of the vagus nerve, the contractile function of the gall bladder was intact or slightly impaired 2 weeks after operation.
Conclusion
For laparoscopic proximal gastrectomy (LPG) and laparoscopic total gastrectomy (LTG), HLLS inversion is a feasible method for optimizing visualization of the surgical field with preservation of the function of the hepatic branch of the vagus nerve. It is safe and acceptable as to the manipulation time. Re-inversion is easy and effective even in case of failure of inversion. HLLS inversion seems to be a promising technique for retraction of the liver during laparoscopic gastrectomy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12893-024-02635-5.
Keywords: Liver traction, Esophagogastric union, Laparoscopic proximal gastrectomy, Radical gastrectomy, Hepatic left lateral segment inversion
Introduction
Proper exposure of the surgical field is a prerequisite for accomplishing high-quality laparoscopic gastrectomy with systematic lymph node dissection for gastric cancer and esophagogastric junction cancer. Suspension of the left lateral segment of the live represents the most important step for better visualization of the lesser curvature and peri-hiatal region, which is even more mandatory during LPG and LTG. Though a series of methods have been designed for liver retraction, such as retraction of the liver with Nathanson retractor or silicone disk, suspension of the hepatogastric ligament with suture and sticking the liver with the diaphragm with glue, etc. [1–4], there is no perfect solution [5–8].
Nakamura et al. first reported HLLS inversion in 2020. They completely isolated HLLS from the operative field by the dissection of the round ligament, falciform ligament, coronary ligament, left deltoid ligament, hepatogastric ligament superficial to the duct of Arantius and the physiologic adhesion between segments S3-4 [9]. The modified method by Harada et al. spared the the round ligament and shortened the manipulation time to about 16 min [5]. Here, we report the first 42 cases of HLLS inversion with Harada’s method.
Materials and methods
Patients
In this series, 42 patients who underwent HLLS inversion at the Department of Gastrointestinal Surgery, the Second Affiliated Hospital of Soochow University from September, 2023 to January, 2024 were included. The characteristics of these cases were summarized in Table 1. All patients were diagnosed as gastric cancer or esophagogastric junction cancer preoperatively and received LTG or LPG. 18 patients were diagnosed as Siewert II adenocarcinoma of the esophagogastric junction (AEG) [10].
Table 1.
Characteristics of the cases with modified HLLS inversion (n=42)
| Characteristic Variables | Value/Number |
|---|---|
| Age (years), m ± sd | 67.4 ± 10.8 |
| BMI (kg/m2), m ± sd | 24.1 ± 3.3 |
| >25, n (%) | 18(42%) |
| Sex, Male/Female | 32/10 |
| Liver cirrhosis | |
| No | 40(95.2%) |
| Hepatitis B Related | 1(2.4%) |
| Schistosomiasis-related | 1(2.4%) |
| Tumor location | |
| Siewert II AEG | 18 |
| Upper part of the stomach | 24 |
| Postoperative pathological staging | |
| I | 12 |
| II | 19 |
| III | 11 |
| Surgical procedure | |
| Routine LPG + ROSF | 14(33.3%) |
| Routine LTG + Roux-en-Y reconstruction | 23(54.8%) |
| Reduced-port LPG + ROSF | 4(9.5%) |
| Reduced-port LTG + Roux-en-Y reconstruction | 1(2.4%) |
Abbreviations:
BMI: body mass index; AEG: adenocarcinoma of the esophagogastric junction; LPG: laparoscopic proximal gastrectomy; LTG: laparoscopic total gastrectomy; ROSF: right-sided overlap with single flap valvuloplasty
Surgical Procedure
The indications for LPG were early gastric cancer in the upper third of the stomach or T1 − 3 esophagogastric junction cancer less than 4 cm in diameter. The extent of lymphadenectomy was based on the Japanese Guideline for the Treatment of Gastric Cancer (6th edition) [11]. 37 patients received routine laparoscopic procedure with 5 incisions and 5 patients received reduced-port procedure. For reduced-port laparoscopic gastrectomy, a 4 cm incision in the umbilicus and two 5 mm incisions in the right abdomen were made. For LPG, right-sided overlap with single flap valvuloplasty (ROSF) was adopted for reconstruction [6]. Reduced-port LPG with ROSF reconstruction was performed in 4 patients and reduced-port LTG was performed in 1 patient.
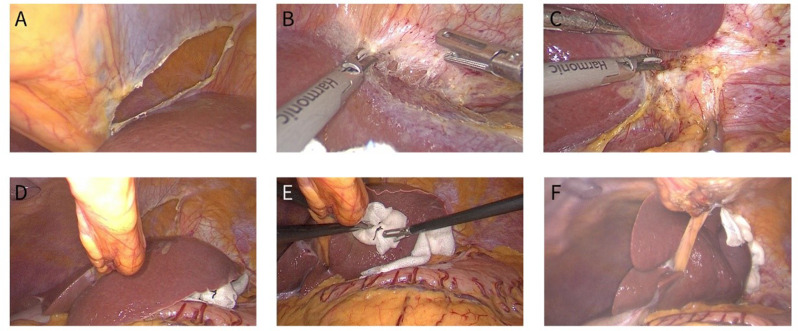
The HLLS inversion was performed as follows: At the cephalad side of the round ligament, the falciform ligament was incised with electrocautery at the avascular zone (Fig. 1A). The coronary ligament was dissected without laceration of the veins from the diaphragm (Fig. 1B). The left deltoid ligament was dissected (Fig. 1C). A 2 − 0 purse-string suture was inserted into the abdominal cavity on the right side of the falciform ligament caudal to the xiphoid process, and the suture was passed through the round ligament proximal to the liver and then drawn out of the abdominal wall at the left side of the falciform ligament (Fig. 1D). Under the protection of gauze, HLLS was inverted through the incision of the falciform ligament and pushed into the right suprahepatic region (Fig. 1E). One to two pieces of gauzes were placed between the inverted HLLS and the abdominal wall to prevent it from rebounding (Fig. 1F). The purse-string is tightened externally to block the HLLS inverted between the abdominal wall and the liver. If HLLS was not easy to fix, the falciform ligament was pulled down and fixed to the round ligament with Hemlock (Fig. 5). If the inverted HLLS slipped back and interfered with manipulation, HLLS re-inversion was performed.
Fig. 1.
Procedure of the modified HLLS inversion method. A The falciform ligament was incised with electrocautery at the avascular zone. B The coronary ligament was dissected without laceration of the veins from the diaphragm. C The left deltoid ligament was dissected. D The suture was passed through the round ligament proximal to the liver and then drawn out of the abdominal wall at the left side of the falciform ligament. E HLLS was inverted through the incision of the falciform ligament and pushed into the right suprahepatic region. F One to two pieces of gauzes were placed between the inverted HLLS and the abdominal wall to prevent it from rebounding
At the end of the procedure, the purse string was cut and the inverted HLLS was carefully repositioned.
Post-operative management
The postoperative management of the patients was based on the concept of enhanced recovery after surgery (ERAS) with exclusion of gastric tube insertion, early removal of the catheters and early ambulation. On postoperative day (POD) 1, 3 and 7, laboratory tests were performed, with special attention paid to liver functions. Plain CT of the abdomen was performed before discharge. In early AEG patients with preservation of the vagus nerve, gallbladder contractile function was assessed by ultrasound at 2 weeks postoperatively.
Results
HLLS inversion was successfully performed in all 42 patients with a mean operation time of 13.9 ± 3.3 min (Table 2). 7 patients underwent intraoperative re-retraction. No patients needed a third re-inversion. The left accessory hepatic artery was ligated in 8 patients (19%). 1 patient experienced minor intraoperative hemorrhage during dissection of the left deltoid ligament. No intraoperative liver injury was observed.
Table 2.
Intraoperative and postoperative results of 42 patients after modified HLLS inversion
| Variables | Value/Number |
|---|---|
| Inversion operation time (min), m ± sd (range) | 13.9 ± 3.3(10–21) |
| Ligation of the left collateral hepatic artery | |
| Yes/No | 8/34 |
| Intraoperative liver injury/bleeding | |
| Yes/No | 1/41 |
| Intraoperative retraction (effective remedy) | |
| Yes/No | 7/35 |
| Preservation the hepatic branch of the vagus nerve | |
| Yes/No | 5/37 |
| Physiological adhesions between S3 and S4 | 18/42 |
|
Membranous adhesion Parenchymal adhesions |
10 8 |
| Postoperative hospitalization (days), median (IQR) | 11.2 (6–20) |
| Short-term postoperative complications | |
| No | 40 |
| Digestive system | 0 |
| Pleural effusion (Clavien-Dindo class I) | 2 (No specific intervention treatment) |
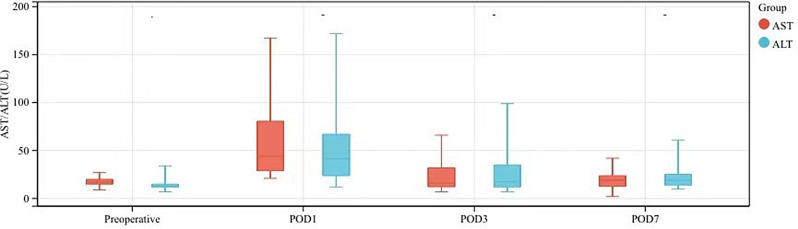
The median postoperative hospitalization time of 42 patients was 11.2 days, and no Clavien-Dindo grade II or higher complications occurred after operation (Table 2). The postoperative changes in alanine aminotransferase (ALT) and alanine aminotransferase (AST) are shown in Fig. 2.
Fig. 2.
Postoperative changes in liver function in 42 patients after modified HLLS inversion (m ± sd)
Discussion
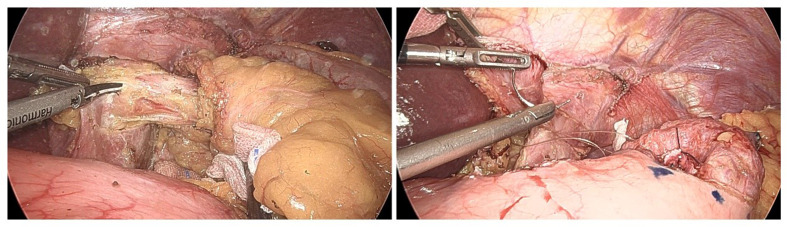
In laparoscopic gastrectomy, surgical field visualization greatly affects the difficulty, quality and duration of the operation. Effective retraction of HLLS is crucial for exposure of the lesser curvature side and the peri-hiatal region. In our series, HLLS inversion completely isolated HLLS and provided excellent view in 35 patients. Though HLLS slipped back from the falciform ligament incision in 7 patients and needed re-inversion, satisfactory view was acquired thereafter. It is noteworthy that HLLS inversion provided adequate field visualization even in reduced port LPG and LTG (Fig. 3). In 5 early AEG cases, the anterior trunk and hepatic branch of the vagus nerve were spared and the contractile function of the gall bladder was intact or slightly impaired 2 weeks after operation as demonstrated by Ultrasound. HLLS inversion seemed to be a feasible method for preservation of the function of the hepatic branch of the vagus nerve.
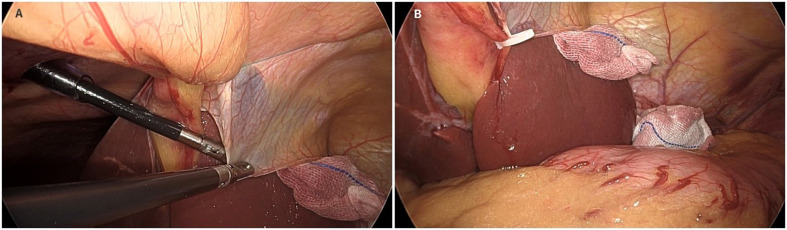
Fig. 3.
Clear visualization of the cardia region during reduced-port ROSF
Impairment of hepatic function after laparoscopic gastrectomy and fundoplication was reported in literature [8, 12], which may be related to extrusion of the liver during exposure or ligation of the left accessory hepatic artery. Nakamura et al. found that the HLLS inversion method resulted in only mild hepatic function abnormalities, and the postoperative ALT and AST were significantly lower than patients using the Nathanson liver retractor [9]. Mild elevation of ALT and AST was similarly observed in our series, which may be explained by the fact that HLLS was only inverted and there was no compression or extrusion by external forces. Overall, mean liver enzymes of the patients were elevated in patients on POD 1, but decreased significantly on POD3, and then to normal levels on POD7. Only 1 patient with schistosomiasis and ligation of the left accessory hepatic artery had a more pronounced elevation (Fig. 2). But neither ALT nor AST exceeded 180 U/L and fell back to preoperative levels on POD 7. Although in other patients, the left accessory hepatic artery was ligated, no obvious elevation of liver enzymes was observed, similar to that reported in the literature [5]. A patient with hepatitis B and liver cirrhosis didn’t experienced elevation of liver enzymes. Though HLLS inversion was safe even in patients with left accessory hepatic artery ligation and liver cirrhosis as seen in our study, the safety of HLLS inversion in such patients and those with hepatic dysfunction is still to be investigated.
Several factors may impact the successful inversion of HLLS, including the morphology of the liver, the existence of cirrhosis or fibrosis and the presence of physiological adhesion between S3 and S4. Short and thick HLLS tends to be difficult to invert and easy to slip out of the incision of the falciform ligament, leading to failure of inversion or need for re-inversion. Theoretically, hepatic cirrhosis or fibrosis may cause difficulty of HLLS inversion. But in our series, HLLS inversion was successful even in such patients, though the extent of hepatic cirrhosis or fibrosis should have impact on the success rate of inversion. Technical contraindications of HLLS inversion are still to be determined.
Nakamura et al. first reported the physiologic adhesion between segments S3 and S4 in the HLLS inversion method [9]. In our study, we classified such physiological adhesions into parenchymal and membranous adhesions (Fig. 4). Though in the initial cases, we dissected the membranous adhesions, we found such adhesions did not affect inversion and dissection was not always necessary (Fig. 4B). Parenchymal adhesions are more likely to cause inversion failure (Fig. 4A).
Fig. 4.
Parenchymal and membranous adhesions between S3 and S4
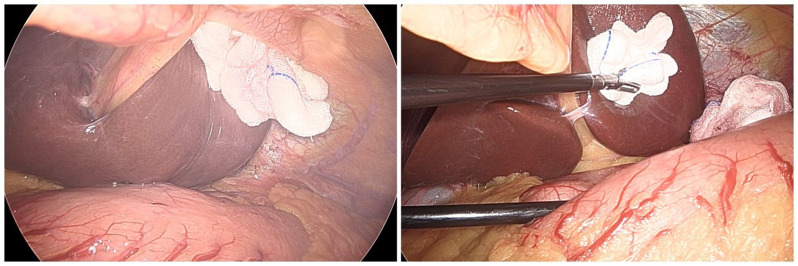
During operation, the inverted HLLS slipped back in 7 cases and needed re-inversion. In 4 patients, re-inversion was fulfilled by putting the HLLS into the falciform ligament incision, placing 2 pieces of gauze in between and fastening the purse string. The lower edge of S3 tucked in falciform ligament incision facilitated durable inversion (Fig. 1F). In 3 other patients, re-inversion was performed by pulling down the incised, crumpled falciform ligament (Fig. 5A) and fixing it to the round ligament with Hemlock and placing one or two pieces of gauze between HLLS and the falciform ligament to increase friction (Fig. 5B). No patients needed a third inversion.
Fig. 5.
Effective remedies for the modified HLLS inversion
For safe inversion of HLLS, prevention of bleeding is important. In our series, minor bleeding occurred during dissection of the left deltoid ligament in 1 patient. It was caused by cutting into the parenchyma proximal to the ligament. Besides the gentle manipulation of the liver, special attention should be paid to the left subphrenic vein. Loukas et al. reported that the left subphrenic vein can be classified into 5 types according to the sites of inflow [13]: L-1 (flowing into the inferior vena cava subphrenic segment, 37% incidence); L-2 (flowing into the left adrenal vein, 25% incidence); L-3 (flowing into the left renal vein, 15% incidence); L-4( flowing into the left hepatic vein, 14% incidence); L-5 (flowing into the inferior vena cava and the left adrenal vein simultaneously in two branches, 9% incidence). Irrespective of the types, good visualization and cautious dissection of the coronary ligament guarantee safety. Finally, this study is only a single-center study with a limited number of cases. Surgeons may encounter other complex liver conditions not mentioned in this article that are not amenable to this technique.
Conclusion
For LPG and LTG, HLLS inversion is a feasible method for optimizing visualization of the surgical field with preservation of the function of the hepatic branch of the vagus nerve. It is safe and acceptable as to the manipulation time. Re-inversion is easy and effective even in case of failure of inversion. HLLS inversion seems to be a promising technique for retraction of the liver during laparoscopic gastrectomy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
All authors contributed to the study conception and design. W.Y proposed the idea for the article and the first draft of the manuscript was written by T.Y. and C.M. T.Y. and C.M. contribute equally to this work and share first authorship. S.Q and T.Y. collected and analyzed the data. All authors commented and critically revised on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by Clinical Medical Team Introduction Program of Suzhou (grant number SZYJTD201804).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
All procedures performed involving human participants were in accordance with the ethical standards of the institution and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by The Ethics Committee of Second Affiliated Hospital of Soochow University. Informed consent was obtained from all participants included in the study.
Consent for publication
All authors read and approved the final manuscript and publication.
Clinical trial number
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuan Tian and Ming Cheng contributed equally to this work and share first authorship.
Contributor Information
Yongyou Wu, Email: wuyongyou72@126.com.
Souya Nunobe, Email: souya.nunobe@jfcr.or.jp.
References
- 1.Kinjo Y, Okabe H, Obama K, et al. Elevation of liver function tests after laparoscopic gastrectomy using a Nathanson liver retractor. World J Surg. 2011;35(12):2730–8. [DOI] [PubMed] [Google Scholar]
- 2.Shabbir A, Lee JH, Lee MS, et al. Combined suture retraction of the falciform ligament and the left lobe of the liver during laparoscopic total gastrectomy. Surg Endosc. 2010;24(12):3237–40. [DOI] [PubMed] [Google Scholar]
- 3.Takemura M, Ikebe T, Mayumi K, et al. A novel liver retraction technique for lateral lobe of the liver during laparoscopic surgery using silicone disk. J Laparoendosc Adv Surg Tech A. 2011;21(8):729–32. [DOI] [PubMed] [Google Scholar]
- 4.Wu S, Yu H, Fan Y, et al. Liver retraction using n-butyl-2-cyanoacrylate glue during single- incision laparoscopic upper abdominal surgery. Br J Surg. 2014;101(5):546–9. [DOI] [PubMed] [Google Scholar]
- 5.Harada H, Hayami M, Makuuchi R, et al. A sandwiching method that simplifies hepatic left lateral segment inversion to secure an optimal surgical view around the esophageal hiatus in laparoscopic and robotic gastrectomy for upper gastric and esophagogastric junction cancers. Langenbecks Arch Surg. 2023;408(1):159. [DOI] [PubMed] [Google Scholar]
- 6.Peng W, Yan S, Huang Y, et al. Laparoscopic proximal gastrectomy with right-sided overlap and single-flap valvuloplasty (ROSF): a case-series study. BMC Surg. 2023;23(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiramatsu K, Aoba T, Kamiya T, et al. Novel use of the Nathanson liver retractor to prevent postoperative transient liver dysfunction during laparoscopic gastrectomy. Asian J Endosc Surg. 2020;13(3):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris-Stiff G, Jones R, Mitchell S, et al. Retraction transaminitis: an inevitable but benign complication of laparoscopic fundoplication. World J Surg. 2008;32(12):2650–4. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura K, Suda K, Shibasaki S, et al. The hepatic left lateral segment inverting Method Offering a wider operative field of View during Laparoscopic Proximal Gastrectomy. J Gastrointest Surg. 2020;24(10):2395–403. [DOI] [PubMed] [Google Scholar]
- 10.Siewert JR, Hölscher AH, Becker K, et al. [Cardia cancer: attempt at a therapeutically relevant classification]. Chirurg. 1987;58(1):25–32. [PubMed] [Google Scholar]
- 11.Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition) [J]. Gastric Cancer. 2023, 26(1):1–25. [DOI] [PMC free article] [PubMed]
- 12.Katai H, Mizusawa J, Katayama H, et al. Single-arm confirmatory trial of laparoscopy-assisted total or proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan Clinical Oncology Group study JCOG1401. Gastric Cancer. 2019;22(5):999–1008. [DOI] [PubMed] [Google Scholar]
- 13.Loukas M, Louis RG Jr, Hullett J, Loiacano M, Skidd P, Wagner T. An anatomical classification of the variations of the inferior phrenic vein. Surg Radiol Anat. 2005;27(6):566–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.