Abstract
Peripheral artery disease (PAD) affects 200 million individuals worldwide. In the United States, certain demographic groups experience a disproportionately higher prevalence and clinical effect of PAD. The social and clinical effect of PAD includes higher rates of individual disability, depression, minor and major limb amputation along with cardiovascular and cerebrovascular events. The reasons behind the inequitable burden of PAD and inequitable delivery of care are both multifactorial and complex in nature, including systemic and structural inequity that exists within our society. Herein, we present an overview statement of the myriad variables that contribute to PAD disparities and conclude with a summary of potential novel solutions.
Keywords: AHA Scientific Statements, ankle brachial index, chronic limb-threatening ischemia, health status disparities, healthcare disparities, peripheral arterial disease
Peripheral artery disease (PAD) is present in >12 million Americans and 200 million individuals worldwide. PAD has a differential prevalence and clinical effect across demographic groups in the United States.
In patients who have PAD, limb symptoms may reduce quality of life, and the devastating outcome of limb amputation produces social and economic burdens for patient and family. Appropriate identification of PAD in affected individuals allows for the timely initiation of guideline-directed medical therapy for secondary prevention of cardiovascular disease, cerebrovascular disease, and limb events.
The purpose of this scientific statement is to summarize the disparities in PAD across key elements, including the epidemiology, medical management, and interventional outcomes and how social determinants of health intersect with the development of PAD. We identify existing knowledge gaps and future investigational research targets, and we offer potential structural solutions.
DISPARITIES IN THE PREVALENCE OF PAD
The prevalence of PAD in the United States varies across sex, race, and ethnicity, contributing to differential disease burden. Overall prevalence estimates indicate that there are 7 to 12 million affected individuals in the United States and 200 million people worldwide.1-3 The prevalence of PAD depends on definitions, because the inclusion of only symptomatic patients tends to underestimate disease burden. PAD rates rise rapidly with age with the highest prevalence in adults >70 years of age.4,5 Severe PAD with critical limb ischemia (CLI)/chronic limb-threatening ischemia (CLTI) that may lead to amputation affects 1.3% of the adults in the United States.2,6
When PAD is defined by an ankle brachial index (ABI) ≤0.9, many studies have found that the prevalence of PAD is equivalent between sexes (≈3%–4.5% among those ≥40 years), or slightly higher in women (2.5% prevalence in men versus 3.5% prevalence in women ≥40 years).1 In a majority of studies of symptomatic PAD, defined as symptoms of intermittent claudication, the prevalence and incidence appear to be higher in men.
In studies evaluating multiethnic populations, Black American patients are disproportionally affected by PAD.1,7 Evaluating data from 7 community-based studies (Cardiovascular Health Study, Honolulu Heart Program, Multiethnic Study of Atherosclerosis, National Health and Nutrition Examination Survey, San Diego PAD Study, San Diego Population Study, and the Strong Heart Study), Black men ≥50 years of age had the highest rates of prevalent PAD (Figure 1).1 Prevalence at ages 50 to 59 years was ≈5%, 13.2% at ages 60 to 69 years, and 59% at ≥80 years, compared with ≈1.9%, 5.4%, and 22.6% in non-Hispanic White individuals at similar ages. Likewise, the rates of PAD were much higher in Black women than in other racial and ethnic groups; although these higher rates were observed a decade later compared with men (starting ≈60 years of age).
Figure 1. Prevalence of peripheral artery disease by race in men (A) and women (B).
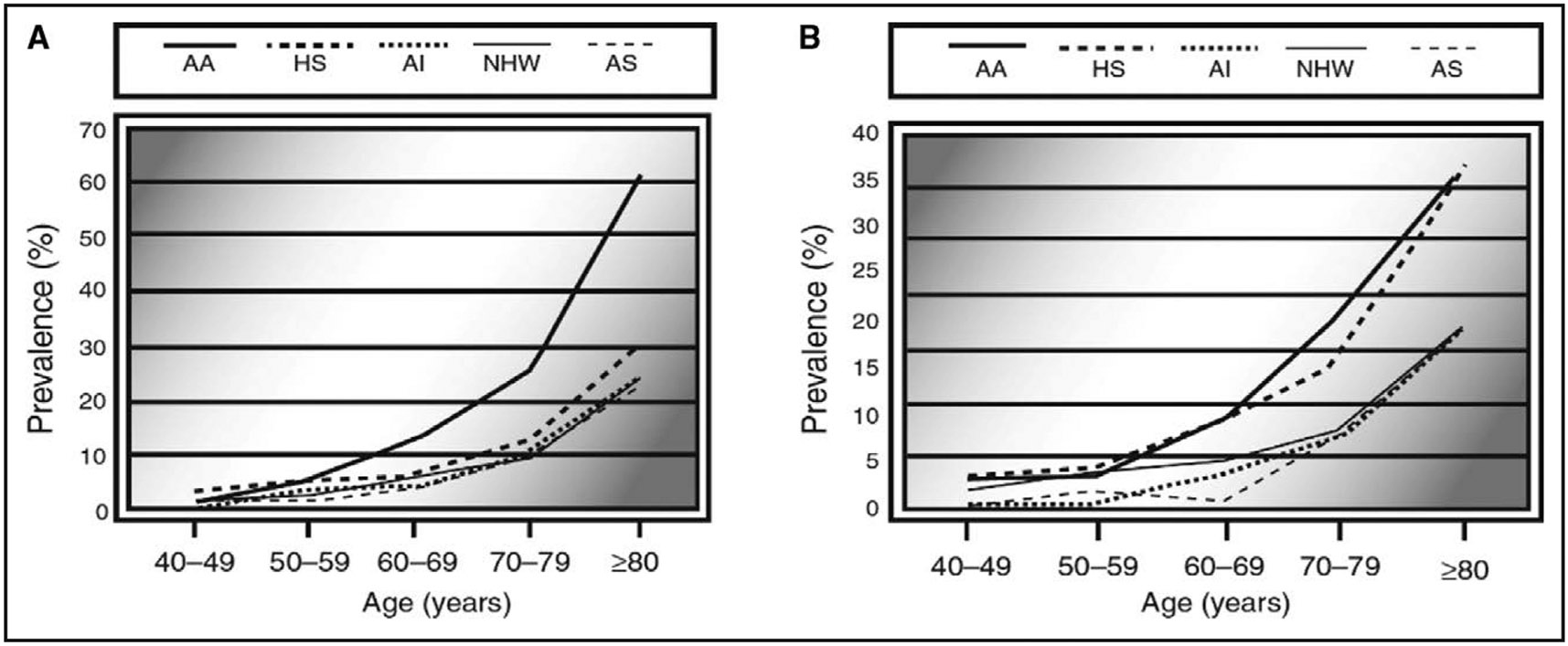
AA indicates African American; AI, American Indian; AS, Asian American; HS, Hispanic American; and NHW, non-Hispanic White. Reprinted from Allison et al.1 Copyright 2007, with permission from American Journal of Preventive Medicine, published by Elsevier, Inc.
Differences in PAD prevalence have also been explored among Hispanic subgroups. In one study looking at an ethnically diverse population of Hispanic individuals, and after adjusting for multiple risk factors, Cuban American patients were found to have the highest risk of PAD (odds ratio, 2.9 [95% CI, 1.9–4.4]), compared with Mexican American patients, followed by Puerto Rican American patients, those with a Central American background, and Dominican American patients.8
In a self-referred screening study, American Indian adults had higher rates of presenting with both mild-to-moderate and severe PAD compared with White adults even when accounting for the presence of traditional risk factors.9
Estimates of the lifetime risk suggest that 30% of Black men and 27% of Black women will develop PAD defined as low ABI compared with 22% of Hispanic men and women and 19% of White men and women.4 Differences in the prevalence of key risk factors for PAD, including smoking, diabetes, hypertension, and dyslipidemia, may be important contributors to differential rates of asymptomatic and symptomatic PAD across race and ethnicity. However, patterns of higher PAD prevalence in Black American patients persist even when accounting for traditional risk factors.
CORONARY AND CEREBROVASCULAR DISEASE IN PAD
Atherosclerosis is a chronic, reparative inflammatory process that affects the arterial tree systemically. Individuals diagnosed with lower extremity PAD may have concomitant disease in other vascular territories.5 Previous studies have demonstrated a relatively high prevalence of coronary and cerebrovascular disease among patients with PAD. For example, in the United States, primary care evaluation showed that 50% of patients with PAD had concomitant coronary artery disease or cerebrovascular disease.5 Among patients in general practice with PAD (by ABI criteria) >65 years of age living in Germany, the odds ratios for prevalent cerebrovascular and coronary artery disease were 1.5 and 1.8, respectively, compared with those without PAD.5 Similar results have been published from the Cardiovascular Health Study using a symptom-based definition of PAD. There appear to be significant differences in these rates by race and ethnicity. Using data from the REACH registry (Reduction of Atherothrombosis for Continued Health), the prevalence of “multisystem disease” among US residents who self-reported as Black, Hispanic, or White were as follows: (1) PAD: 45%, 28%, 29%; (2) PAD+coronary artery disease: 31%, 48%, 48%; (3) PAD+cerebrovascular disease: 11%, 4%, 7%; and (4) PAD+coronary artery disease+cerebrovascular disease: 14%, 20%, 17%. These results suggest that Black American patients have the highest rates of PAD and PAD with cerebrovascular disease, whereas Hispanic and White American patients have the highest rates of PAD with coronary artery disease, and PAD with both coronary and cerebrovascular disease, as well.10,11 It is important to note that the REACH registry reflects clinically diagnosed disease, and there may be relative underdiagnosis that could vary across race and ethnicity groups.
DIABETIC FOOT CARE
Lower extremity complications of diabetes continue to exact a substantial burden on all patients. These complications, including diabetic foot ulceration, resultant infection, and amputation occur because of a combination of neuropathy, PAD, and repetitive trauma on the at-risk limb.12 Five-year mortality after amputation is comparable with many cancers: ≈45% of people with foot-level amputation and >60% for people with more proximal amputations. High risk of amputation and resultant mortality in PAD is enhanced in people of color. Native American, Black, and Hispanic patients carry a disproportionately increased risk for neuropathy, PAD, diabetic foot ulceration, and amputation than do their White counterparts.13
Social determinants appear to play a central role in the observed outcome disparities across patients with diabetes and PAD. Native American, Black American, Hispanic American, rural, and low-income patients are at greater risk of being uninsured, an independent risk factor for amputation.14
Limited access to preventative podiatric and interdisciplinary foot care is correlated with an amputation risk as has occurred during economic downturns. Interventions that improve access to foot care reduce amputation rates. Expansion of Medicaid eligibility for podiatric, vascular, and other interdisciplinary care may reduce diabetes-related amputation particularly in racial and ethnic underrepresented groups. Access to interdisciplinary limb preservation and training programs unfortunately remains inadequate across the United States. Given the known benefits for amputation reduction along with reduced resource use, enhanced access to podiatry and interdisciplinary care will be a key approach to limit disparities in PAD outcomes.
Advances in mobile health and remote patient monitoring are an emerging advance to expand access and lower reulceration rates in patients who have achieved in diabetic foot remission. Remote diabetic foot monitoring is, unfortunately, presently available to only a small percentage of patients at risk, nationwide. We call on health systems and health science programs to consider the development of initiatives focused on expanding access to remote monitoring by interdisciplinary teams in an effort to reduce the disproportionately high number of ulcers and amputations throughout the United states, in general, and for underserved communities, in particular.
NONPROCEDURAL MANAGEMENT/OPTIMIZATION
Medical Management and Comorbid States
Medical management of PAD is recommended to reduce the risk of myocardial infarction, stroke, amputation, and cardiovascular death. It entails antiplatelet therapy, lipid reduction therapy, low-dose anticoagulation, peripheral vasodilators, blood pressure control, smoking cessation, diet, and exercise therapy (Table 1). Although the evidence for aspirin and lipid-lowering therapy in patients with symptomatic PAD is strong, the data are less clear for those with subclinical PAD. Despite the robust evidence that statins reduce incident cardiovascular events, and acute limb events, as well, Black American patients continue to have lower rates of prescriptions for statins even in the presence of established PAD.11 Likewise, there is strong evidence supporting the use of antithrombotic agents and blood pressure control in the treatment of PAD for the reduction of cardiovascular events, but non-White patients, especially Black patients with PAD, are less likely to be taking aspirin and angiotensin-converting enzyme inhibitors than their White counterparts.15 In Hispanic American patients with known PAD, there are low rates of antiplatelet (31%), lipid-lowering (26%), and antihypertensive therapies (57%).16 Compounding this problem is the fact that Black, Native, and Hispanic American patients are underrepresented in the large clinical trials that serve as the basis for clinical associations’ recommendations for the medical management of PAD.
Table 1.
Lifestyle and Pharmaceutical Management of Peripheral Artery Disease17
| Medical therapy | Proposed intervention |
|---|---|
| Antiplatelets | Aspirin (81 mg) daily or clopidogrel 75 mg daily Dual antiplatelet therapy (clopidogrel 75 mg+aspirin 81 mg) for 1 to 6 mo after endovascular revascularization |
| Anticoagulation | Rivaroxaban 2.5 mg twice daily+low-dose aspirin (81 mg) reduced cardiovascular event rates compared with aspirin in people with stable atherosclerosis, including those with PAD. |
| Lipid-lowering therapy | All patients with PAD should be treated with high-intensity statin therapy. Further lipid-lowering therapy may be necessary in patients taking a maximum dose of statin or who are statin-intolerant, namely ezetimibe and PCSK9 inhibitors, to achieve low-density lipoprotein goals as documented in the American College of Cardiology/American Heart Association 2018 lipid management guidelines. Statins: Atorvastatin 40–80 mg or rosuvastatin 20–40 mg once daily PCSK9 inhibitor: Evolucomab 140 mg SC or alirocumab 150 mg SC every 2 wk Ezetimibe 10 mg once daily |
| Antihypertensive agents | Patients with PAD who have hypertension should have blood pressure treated as recommended by current hypertension guidelines. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may have advantages for patients with PAD. |
| Peripheral vasodilators | Cilostazol is effective in improving symptoms and increasing walking distance in patients with claudication. |
| Smoking cessation | Patients with PAD who smoke cigarettes should be advised to quit smoking and offered pharmacotherapy to assist with smoking cessation (including varenicline, bupropion or nicotine replacement therapy, or both) at every clinical visit. |
| Exercise therapy | Patients with PAD who have claudication should undergo supervised exercise therapy to improve functional status, improve quality of life, and reduce leg symptoms. Home-based exercise programs with behavioral change therapy can improve functional status and improve walking ability. |
| Diet therapy | Patients with PAD should eat a diet high in fruits and vegetables, which contain flavonoids and polyphenols to reduce risk of atherosclerosis and inflammation and to improve endothelial function and vitamin K that may decrease arterial calcification. A high-fiber diet lowers total and low-density lipoprotein cholesterol and is associated with lower PAD risk. Diets should limit processed and higher fat cuts of meats and saturated and trans fats. Mediterranean and DASH style diets incorporate PAD diet therapy recommendations and can be beneficial. Vitamin D can upregulate nitric oxide, and lower circulating 25-hydroxyvitamin D is associated with endothelial dysfunction and lower prevalence of PAD. |
| Antidiabetic Therapy | Consider initiation of therapy to lower cardiovascular risk and treat hyperglycemia in patients with PAD who have concomitant diabetes. Sodium-glucose cotransporter 2 inhibitors: empagliflozin, canagliflozin, dapagliflozin Glucagon-like peptide 1 agonists: dulaglutide, exenatide, liraglutide, semaglutide |
DASH indicates Dietary Approaches to Stop Hypertension; PAD, peripheral artery disease; and PCSK9, proprotein convertase subtilisin/kexin type 9.
Physical Activity/Exercise
Racial disparities seen in physical activity and health are complicated by factors, including poverty, education, and socioeconomic status. Differences in functional limitations in Black compared with White older adults are lower after accounting for disparities in income and education. Hypertension and diabetes, two of the major risk factors associated with the development of PAD, are more prevalent in highly segregated areas.18 Black American patients who live in more walkable communities have better control of diabetes.19
Black American patients have been found to have poorer lower extremity function than non–Black Americans, including poorer performance on the 6-minute walk test and the short physical performance battery. However, these differences were mitigated when accounting for clinical and social factors, including age, sex, level of education, ABI, and leg symptoms.20 Likewise, Black American patients have been shown to have an increased decline in physical function over time compared with non–Black American patients; however, these differences are largely explained by socioeconomic status and physical activity levels.21
The small subset of clinical trials that control for sociodemographic variables report that physical functioning improvement in response to exercise therapy does not differ by race, education, or socioeconomic status.22
Lifestyle Behaviors
Lifestyle behaviors such as diet, obesity, and smoking can contribute to risk of PAD and are important in its treatment. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and high fruit and vegetable dietary patterns incorporate most of the foods and nutrients recommended to decrease the risk of PAD (Table 1) and are associated with weight reduction and reduced cardiovascular risk.23
Although few Americans have diets that are consistent with these healthful patterns, the differences in income, education, and food access among different racial and ethnic groups make healthy eating and weight management more challenging. Black and Hispanic American patients in the Multiethnic Cohort are less likely to follow a Mediterranean-style or DASH dietary pattern, and both have higher rates of obesity compared with White Americans.24
Various interventions aim to improve diet and weight loss, but newer interventions target differences in access. “Food is medicine” interventions often partner with community-based organizations like food banks to increase healthful food availability for patients with chronic diseases in health care settings. Food is medicine strategies include medically tailored meals delivered to patients who cannot prepare their own meals or medically tailored groceries for those who can cook. Food “farmacies” provide produce and sometimes other healthful foods like lean meats and low-fat dairy to patients in a clinical setting. These programs can increase fruit and vegetable intake and reduce hospital admissions and health care costs.25 Community gardening projects and introduction of new retailers to food deserts may also improve accessibility of healthful foods.
Smoking is the most important risk factor for PAD, and smoking cessation is critical for reducing morbidity and mortality in patients with PAD. American Indian/Alaska Native American patients have higher rates of smoking than other racial and ethnic groups and, although smoking rates have decreased overall, the quit ratio for Black American and American Indian patients has been lower.26 The quantity and time interval of tobacco use associates directly with the development and evolution of PAD in Black American patients, and those who continue to smoke experience increased mortality and higher amputation rates.27,28
DISEASE PRESENTATION
Given comparable PAD severity and comorbidities, Black patients with PAD present with a disproportionately increased risk of amputation across socioeconomic strata compared with other groups.29 Beyond access to care/resources and health care professional bias, other explanations for racial differences in disease distribution include the disproportionate representation of diabetes, kidney disease, metabolic syndrome, and inflammation.29
Black patients present with more advanced CLI/CLTI, including gangrene or foot sepsis, compared with White patients with CLI/CLTI who tend to present with ulcers or rest pain.29-31 Black patients tend to have lower ABIs, increased arterial stiffness, small vessel disease, and more severe infrapopliteal disease. In the inpatient setting, American Indian patients have the highest prevalence of presenting with PAD with CLI/CLTI; whereas Black and Hispanic patients have the higher rate of amputation.32 Even while adjusting for PAD severity and comorbidities, Black patients with CLI/CLTI are less likely to undergo limb salvage revascularization and instead are more likely to undergo major lower extremity amputation compared with White patients.29,30
VASCULAR INTERVENTION
Arterial Bypass
Disparities in the use and outcomes of invasive lower extremity arterial procedures have been a significant barrier to providing effective treatments for patients with PAD across many regions of the United States. Surgical treatments such as lower extremity arterial bypass are effective in limiting amputation, but only if these treatments are offered in a timely fashion and are accompanied by appropriate, guideline-directed medical therapy. However, large datasets examining national practice patterns have shown that patients who live in certain regions of the United States, especially Black, Hispanic, and Native American populations, are less likely to be offered invasive treatments.33 Furthermore, when these treatments are performed, these racial and ethnic groups have 10% to 30% higher rates of complications and are often at higher risk for limb loss in the period immediately after surgery and at 3 years.33
Percutaneous Treatment
Percutaneous treatment has been used to prevent amputation and improve quality of life in patients with symptomatic PAD. A recent study using the Vascular Quality Initiative from 2003 and 2017 demonstrated that there are disparities for patients who are Black compared with patients who are White in infrainguinal revascularization procedures and their clinical outcomes.34 The authors observed that Black patients were at increased risk for 30-day mortality, long-term mortality, and major adverse limb events, and were often younger and had higher rates of diabetes, hypertension, and chronic obstructive pulmonary disease than their White counterparts. In a study of patients who underwent peripheral arterial interventions in California, between 2005 and 2009, Hispanic and Black patients had worse amputation-free survival than non-Hispanic White patients after endovascular therapy. In addition, Hispanic and Black patients were more likely to undergo lower extremity arterial re-interventions than non-Hispanic White patients. Lower income areas and Black race is actually associated with higher rates of endovascular therapy for claudication.35,36 In patients with claudication, differences in risk factor burden appear to drive disparities in outcomes after endovascular therapy.35
Limb Loss From Diabetes and CLI/CLTI
The combination of diabetes and PAD leads to higher complication rates than either disease process alone.37 The synergistic effect of diabetes and PAD especially affects Native American populations, who have amputation rates that are greater than those of similar non–Native American populations (Figure 1). In patients undergoing revascularization for CLI/CLTI, Black and Hispanic patients had higher rates of amputation even accounting for anatomic and risk factor burden.38 Rates of major lower extremity amputation are higher in areas with a higher proportion of Black adults and lower median household income.39 Adjuncts to prevent amputations, such as hemoglobin A1c testing, vascular assessment of circulation, and diabetic foot examinations, are used less commonly in these racial and ethnic groups.
Amputation Outcomes
When patients are treated with amputation, these same challenged racial and ethnic groups, African American, Hispanic, and Native American, continue to experience similar disparities. Patients in these groups are less likely to receive a minor amputation or a below-knee amputation compared with a major above-knee amputation. Likewise, survival after amputation and quality of life is worse in these groups (ie, they are less likely to walk with a prosthesis and more likely to reside in a nursing home).31 Improving these outcomes will be challenging, because many of the structural aspects of care that led to the amputation manifests itself in these later outcomes.
Differences in the risk factors, clinical presentation, management, and outcomes across race and ethnicity in patients with PAD are summarized in Table 2.
Table 2.
Health Disparities in PAD Risk Factors, Treatment, and Outcomes
| Polyvascular disease | Black American patients have the highest rates of PAD with cerebrovascular disease. Hispanic and White American patients have the highest rates of PAD with coronary artery disease and PAD with both coronary artery disease and cerebrovascular disease. |
| Diabetic foot care | Native American, Black, and Hispanic patients have a higher risk of neuropathy, diabetic foot ulcers, and amputation. Native American, Black, Hispanic, rural, and low-income patients are at greater risk of being uninsured, an independent risk factor for amputation. |
| Medical management | Black American patients with PAD have lower rates of statin, aspirin, and angiotensin-converting enzyme inhibitor prescriptions. Hispanic American patients with PAD have low rates of statin, antiplatelet, and antihypertensive treatment. |
| Physical activity | Black American patients who live in more walkable communities have better control of their diabetes. Black American patients have an increased physical decline over time, largely explained by socioeconomic status and physical activity levels. |
| Lifestyle behaviors | Black and Hispanic American patients are less likely to follow a Mediterranean style or DASH dietary pattern. Black and Hispanic American patients have higher rates of obesity. American Indian and Alaska Native American patients have higher rates of smoking. Social determinants of health influence the presentation of patients. |
| Disease presentation | Black American patients with PAD have an increased risk of amputation than other groups with comparable PAD severity and comorbidities. Black American patients present with more advanced CLI/CLTI compared with White American patients. Black patients have lower ankle brachial indexes, increased arterial stiffness, and more small vessel disease. For inpatients, American Indian patients have the highest prevalence of presenting with CLI/CLTI. Black patients with CLI/CLTI are less likely to undergo limb salvage revascularization as opposed to major amputation. |
| Vascular interventions | Black, Hispanic, and Native American patients who live in certain regions of the United States are less likely to be offered invasive treatments and have higher rates of complications. For patients undergoing endovascular intervention, Black and Hispanic patients have an increased risk of mortality and poor outcomes. Black and Hispanic patients have higher rates of amputation, even after accounting for anatomic and risk factor burden. Black, Hispanic, and Native American patients are less likely to receive minor versus major amputations and walk with a prosthesis. |
CLI indicates critical limb ischemia; CLTI, chronic limb-threatening ischemia; DASH, Dietary Approaches to Stop Hypertension; and PAD, peripheral artery disease.
MECHANISMS MEDIATING THE RACIAL DISPARITIES IN PAD
Vascular Health Measures
Evidence suggests pathophysiological drivers of the clinical expression of PAD differ across self-reported race and ethnicity.40 Several studies document accentuation of vascular aging with reduced endothelial function, increased arterial stiffness, and increased oxidative stress in Black American patients (and in some studies of Hispanic American patients).41 In healthy adults, Black American women display reduced skeletal muscle mitochondrial function that may reflect increased oxidative injury. Systemic inflammation associates with poor functional capacity in PAD and biomarkers, including C-reactive protein and fibrinogen, are elevated in Black compared with White Americans.42 Microvascular disease is a key predictor of amputation risk and is more prevalent in Black than in White Americans in the Veterans Aging Cohort Study.43 Racial differences in vascular health reflects, in part, a higher burden of cardiovascular risk factors, including diabetes, smoking, and hypertension. Emerging evidence also links social determinants of health to alternations in vascular health.44 Limited data suggest that genetic determinants of PAD may be distinct across ancestry, but more information is needed from diverse samples.45
Social Determinants of Health
Social determinants of health are linked to the development of PAD. Prevalence studies show higher rates of PAD with lower income, lower education levels, and less social support with higher risk for amputation with lower socioeconomic status.29,46 Current frameworks link structural health elements, including economic status, built environment, education access, health care access, food quality, and community resilience to chronic stressors and downstream adverse physiological effects, including chronic inflammation.44-47 Racial discrimination is associated with elevated biomarkers of systemic inflammation and vasoconstrictor responses in Black American men related to higher sympathetic nerve activity, potentially reflecting influence of chronic stress.48 Accelerated vascular aging related to adverse social determinants of health may also be driven by epigenetic alterations, including DNA methylation and shortened telomere length.44 Public health interventions targeted at the social determinants of health are likely to improve the clinical status of patients with PAD across diverse racial groups.
OPPORTUNITIES TO MITIGATE PAD DISPARITIES
Overall strategies to reduce disparities in PAD are summarized in Table 3 and Figure 2.
Table 3.
Strategies to Reduce Health Care Disparities in PAD
| Systems-based interventions | Optimization of cost-effective approaches to mitigate structural differences in how health care is provided. More efficient delivery of low-cost preventative measures (ie, hemoglobin A1c testing, ankle brachial index testing, and diabetic foot examinations). Leveraging of the electronic medical record to support peripheral artery disease–specific clinical decision support tools for the early identification and management of high-risk patients. Assessing and addressing social determinants of health to reduce bias. |
| Physician workforce interventions | Creation of a racially, ethnically, and culturally diverse workforce. Early engagement of young adults from racial, ethnic, and low-socioeconomic groups to science-based careers. Promotion of evidence-based approaches to address cultural competency. Development of international expertise in peripheral artery disease management. |
| Community education models | Public health efforts aimed at peripheral artery disease education integrated into the community (ie, the “barbershop” model). Increased clinical trials and community-based research to understand why disparities exist and to create avenues to increase health equity. |
PAD indicates peripheral artery disease.
Figure 2. Schematic depicting potential solutions to overcoming health disparities in peripheral artery disease (PAD).
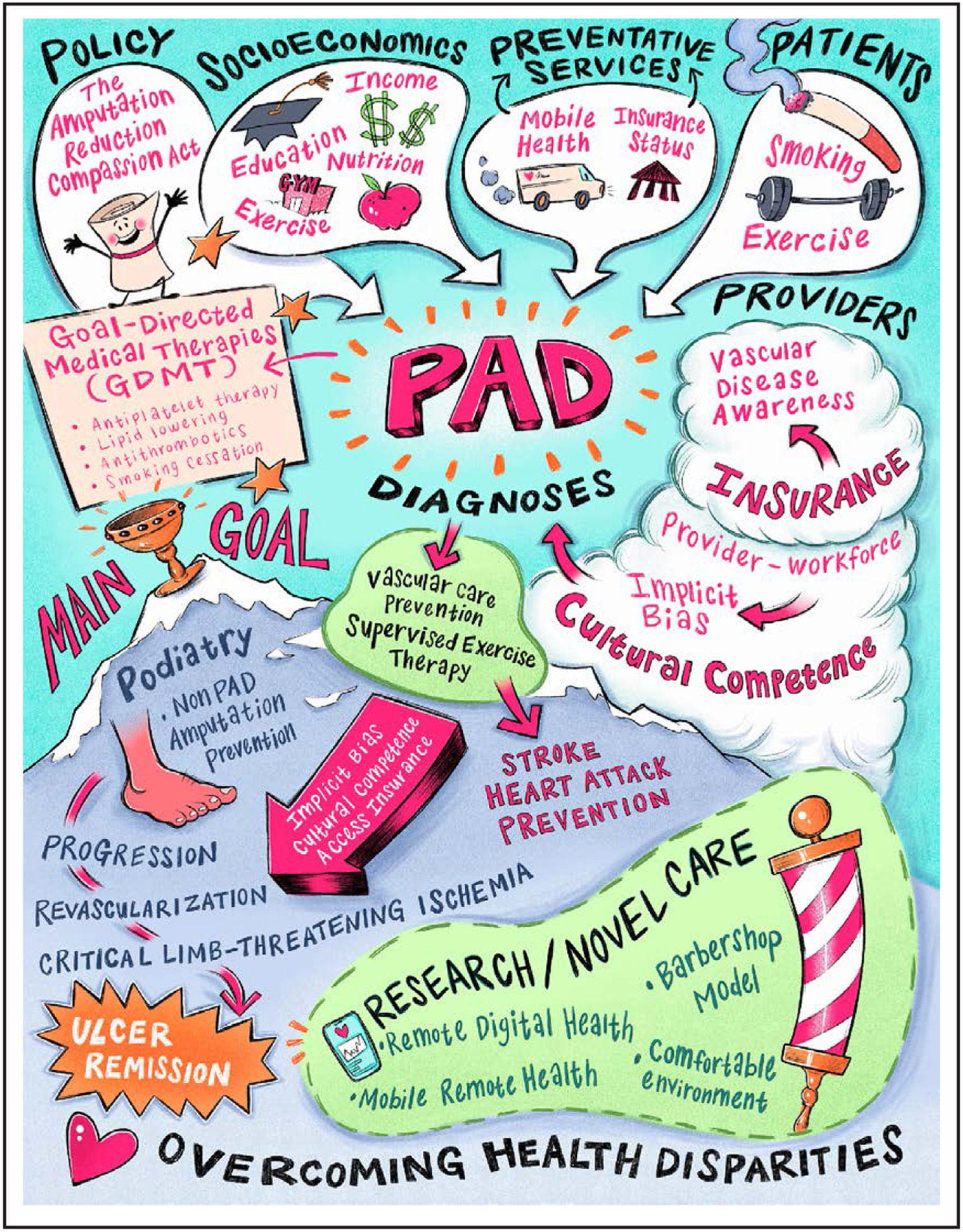
System-Based Differences
Mitigating the effects of differences in the outcomes of PAD is a multifaceted challenge and needs to begin with the structural differences in the way health care is provided. The health care organizations that provide care to patients with PAD, from primary care to subspecialty evaluation to revascularization to recovery, need to optimize cost-effective interventions at every step. For example, patients with PAD should routinely receive low-cost preventive measures, such as hemoglobin A1c testing, ABI testing, and diabetic foot examinations. However, these adjuncts sometimes arrive years after a PAD diagnosis and this challenge exists across all racial groups.11 More efficient delivery of these low-cost mechanisms may help to limit overall PAD spending in the long run by preventing problems before they occur. The electronic medical record has yet to be leveraged in PAD in the way that it has with the use of pooled cohort risk equations for the use of statins and antihypertensive therapy to reduce cardiovascular complications. Continued research exploring the role that clinical decision support tools have in the early identification and management of patients at high risk for PAD is necessary.
Physician Workforce
Various studies have validated the benefit of physician-patient racial concordance in health care outcomes. Improved medication adherence, research trial enrollment, and patient experience are variables confirmed to be positively affected.49 The effect of creating a racially, ethnically, and culturally diverse physician workforce is essential in a country in which non-White racial and ethnic groups will collectively become the majority by 2050 on the basis of US census data. Efforts focused on early engagement of young adults from racial, ethnic, and low-socioeconomic groups to science and mathematics are essential to close the achievement gap that begins as early as preschool and may, in some instances, continue throughout formal education.50 Such efforts are necessary to enhance diversity in the pool of physicians underrepresented in medicine who are more likely to return to practice in diverse and underresourced communities. Evidenced-based approaches to address cultural competency should be routinely used to bridge the gaps in the care of our diverse patient population.51
Community Education Models
Public health efforts aimed at educating the public and communities about PAD may improve general awareness in the community. Several studies have laid the ground-work for barbershops as a venue for health outreach among Black men. Most recently, studies have shown that barbershop-based screening and follow-up is effective at lowering blood pressure and promoting healthy behaviors in Black men. In a pilot study for PAD screening in Cleveland barbershops, screening using the ABI, and an educational video on PAD, as well, significantly increased performance on a PAD awareness survey representing knowledge about the disease.52 More clinical trials and community-based research studies are needed to help understand where and why disparities exist and help create more avenues to diminish their existence and increase health equity.
Conclusions
A confluence of social, economic, and health variables contribute to the disproportionate prevalence of PAD in the heretofore described demographic groups. Distinguishing the precise contribution of these respective factors is not possible. Suffice it to say that a holistic view of this disorder is necessary to craft the appropriate complement of tiered strategic measures if we are to successfully address the burden of those most susceptible to developing PAD that affects Americans most vulnerable to its consequences.
Footnotes
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on January 24, 2023, and the American Heart Association Executive Committee on February 22, 2023. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, or Meredith.Edelman@wolterskluwer.com
The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.”
Disclosures
| Writing group member |
Employment | Research grant | Other research support |
Speakers’ bureau/ honoraria |
Expert witness |
Ownership interest |
Consultant/ advisory board |
Other |
|---|---|---|---|---|---|---|---|---|
| Matthew A. Allison | University of California, San Diego | None | None | None | None | None | None | None |
| David G. Armstrong | Keck School of Medicine of USC Keck Medical Center of USC | NIH (This study is partially supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Award Number 1R01124789-01A1)†; NSF (This study is partially supported by NSF CNS Award Number 2052578)† | None | None | None | None | None | None |
| Philip P. Goodney | Dartmouth Hitchcock Medical Center | None | None | None | None | None | None | None |
| Naomi M. Hamburg | Boston University School of Medicine | None | None | None | None | None | Sanifit* | None |
| Lee Kirksey | The Cleveland Clinic | None | None | None | None | None | None | None |
| Kristie J. Lancaster | NYU Steinhardt | None | None | None | None | None | Council on Black Health* | None |
| Carlos I. Mena-Hurtado | Yale University School of Medicine | None | None | None | None | None | Abbott†; Cook†; Penumbra* | |
| Sanjay Misra | Mayo Clinic | None | Medtronic (consultant/research)*; NHLBI (R01 grant-HL098967)†; RMM (research funding)† | None | None | None | None | None |
| Diane J. Treat-Jacobson | University of Minnesota School of Nursing | NIH (PROVE trial site PI, COCOA trial site PI, INTERCEDE trial site PI)† | None | None | None | None | None | None |
| Khendi T. White Solaru | University Hospitals Cleveland Medical Center Harrington Heart and Vascular Institute | None | None | None | None | None | None | None |
| Reviewer | Employment | Research grant |
Other research support |
Speakers’ bureau/ honoraria |
Expert witness |
Ownership interest |
Consultant/ advisory board |
Other |
|---|---|---|---|---|---|---|---|---|
| Olamide Alabi | Emory University School of Medicine | None | None | None | None | None | None | None |
| Daniel J. Bertges | University of Vermont Medical Center | None | None | None | None | None | None | None |
| Parveen K. Garg | University of Southern California | None | None | None | None | None | None | None |
| Andrew M. Goldsweig | Baystate Medical Center | None | None | None | None | None | None | None |
| Rajesh Gupta | University of Toledo Medical Center | None | None | None | None | None | None | None |
| Francesco Torella | Liverpool University Hospitals (United Kingdom) | None | None | None | None | None | None | None |
REFERENCES
- 1.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–333. doi: 10.1016/j.amepre.2006.12.010 [DOI] [PubMed] [Google Scholar]
- 2.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 3.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0 [DOI] [PubMed] [Google Scholar]
- 4.Matsushita K, Sang Y, Ning H, Ballew SH, Chow EK, Grams ME, Selvin E, Allison M, Criqui M, Coresh J, et al. Lifetime risk of lower-extremity peripheral artery disease defined by ankle-brachial index in the United States. J Am Heart Assoc 2019;8:e012177. doi: 10.1161/jaha.119.012177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aday AW, Matsushita K. Epidemiology of peripheral artery disease and polyvascular disease. Circ Res. 2021;128:1818–1832. doi: 10.1161/CIRCRESAHA.121.318535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nehler MR, Duval S, Diao L, Annex BH, Hiatt WR, Rogers K, Zakharyan A, Hirsch AT. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2014;60:686–95.e2. doi: 10.1016/j.jvs.2014.03.290 [DOI] [PubMed] [Google Scholar]
- 7.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0 [DOI] [PubMed] [Google Scholar]
- 8.Allison MA, Gonzalez F, Raij L, Kaplan R, Ostfeld RJ, Pattany MS, Heiss G, Criqui MH. Cuban Americans have the highest rates of peripheral arterial disease in diverse Hispanic/Latino communities. J Vasc Surg. 2015;62:665–672. doi: 10.1016/j.jvs.2015.03.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baxter AR, Jacobowitz GR, Guo Y, Maldonado T, Adelman MA, Berger JS, Rockman CB. Increased prevalence of moderate and severe peripheral arterial disease in the American Indian (AI)/Alaskan Native (AN) population; a study of 96,000 AI/AN. Ann Vasc Surg. 2017;38:177–183. doi: 10.1016/j.avsg.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 10.Newman AB, Naydeck BL, Sutton-Tyrrell K, Polak JF, Kuller LH; Cardiovascular Health Study Research Group. The role of comorbidity in the assessment of intermittent claudication in older adults. J Clin Epidemiol. 2001;54:294–300. doi: 10.1016/S0895-4356(00)00308-5 [DOI] [PubMed] [Google Scholar]
- 11.Meadows TA, Bhatt DL, Hirsch AT, Creager MA, Califf RM, Ohman EM, Cannon CP, Eagle KA, Alberts MJ, Goto S, et al. ; REACH Registry Investigators. Ethnic differences in the prevalence and treatment of cardiovascular risk factors in US outpatients with peripheral arterial disease: insights from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Am Heart J. 2009;158:1038–1045. doi: 10.1016/j.ahj.2009.09.014 [DOI] [PubMed] [Google Scholar]
- 12.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. New Engl J Med. 2017;376:2367–2375. doi: 10.1056/NEJMra1615439 [DOI] [PubMed] [Google Scholar]
- 13.Tan TW, Armstrong DG, Concha-Moore KC, Marrero DG, Zhou W, Calhoun E, Chang CY, Lo-Ciganic WH. Association between race/ethnicity and the risk of amputation of lower extremities among Medicare beneficiaries with diabetic foot ulcers and diabetic foot infections. BMJ Open Diab Res Care. 2020;8:e001328. doi: 10.1136/bmjdrc-2020-001328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margolis DJ, Hoffstad O, Nafash J, Leonard CE, Freeman CP, Hennessy S, Wiebe DJ. Location, location, location: geographic clustering of lower-extremity amputation among Medicare beneficiaries with diabetes. Diabetes Care. 2011;34:2363–2367 doi: 10.2337/dc11-0807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pande RL, Perlstein TS, Beckman JA, Creager MA. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011;124:17–23. doi: 10.1161/CIRCULATIONAHA.110.003954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hua S, Isasi CR, Kizer JR, Matsushita K, Allison MA, Tarraf W, Qi Q, Ponce SG, Daviglus M, Kaplan RC. Underuse of cardiovascular medications in individuals with known lower extremity peripheral artery disease: HCHS/SOL. J Am Heart Assoc 2020;9:e015451. doi: 10.1161/jaha.119.015451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726–e779. doi: 10.1161/CIR.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le-Scherban F, Ballester L, Castro JC, Cohen S, Melly S, Moore K, Buehler JW. Identifying neighborhood characteristics associated with diabetes and hypertension control in an urban African-American population using geo-linked electronic health records. Prev Med Rep. 2019;15:100953. doi: 10.1016/j.pmedr.2019.100953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mena C, Sepulveda C, Ormazabal Y, Fuentes E, Palomo I. Impact of walk-ability with regard to physical activity in the prevention of diabetes. Geospat Health. 2017;12:595. doi: 10.4081/gh.2017.595 [DOI] [PubMed] [Google Scholar]
- 20.Rucker-Whitaker C, Greenland P, Liu K, Chan C, Guralnik JM, Criqui MH, Taylor L, Pearce WH, McGraeMcDermott M. Peripheral arterial disease in African Americans: clinical characteristics, leg symptoms, and lower extremity functioning. J Am Geriatr Soc. 2004;52:922–930. doi: 10.1111/j.1532-5415.2004.52259.x [DOI] [PubMed] [Google Scholar]
- 21.McDermott MM, Polonsky TS, Kibbe MR, Tian L, Zhao L, Pearce WH, Gao Y, Guralnik JM. Racial differences in functional decline in peripheral artery disease and associations with socioeconomic status and education. J Vasc Surg. 2017;66:826–834. doi: 10.1016/j.jvs.2017.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel K, Polonsky TS, Kibbe MR, Guralnik JM, Tian L, Ferrucci L, Criqui MH, Sutit R, Leeuwenburgh C, Zhang D, et al. Clinical characteristics and response to supervised exercise therapy of people with lower extremity peripheral artery disease. J Vasc Surg. 2021;73:608–625. doi: 10.1016/j.jvs.2020.04.498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delaney CL, Smale MK, Miller MD. Nutritional considerations for peripheral arterial disease: a narrative review. Nutrients. 2019;11:1219. doi: 10.3390/nu11061219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harmon BE, Boushey CJ, Shvetsov YB, Ettienne R, Reedy J, Wilkens LR, Le Marchand L, Henderson BE, Kolonel LN. Associations of key diet-quality indexes with mortality in the Multiethnic Cohort: the Dietary Patterns Methods Project. Am J Clin Nutr. 2015;101:587–597 doi: 10.3945/ajcn.114.090688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Downer S, Berkowitz SA, Harlan TS, Olstad DL, Mozaffarian D. Food is medicine: actions to integrate food and nutrition into healthcare. BMJ 2020;369:m2482. doi: 10.1136/bmj.m2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bandi P, Minihan AK, Siegel RL, Islami F, Nargis N, Jemal A, Fedewa SA. Updated review of major cancer risk factors and screening test use in the United States in 2018 and 2019, with a focus on smoking cessation. Cancer Epidemiol Biomarkers Prev. 2021;30:1287–1299. doi: 10.1158/1055-9965.EPI-20-1754 [DOI] [PubMed] [Google Scholar]
- 27.Armstrong EJ, Wu J, Singh GD, Dawson DL, Pevec WC, Amsterdam EA, Laird JR. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J Vasc Surg. 2014;60:1565–1571. doi: 10.1016/j.jvs.2014.08.064 [DOI] [PubMed] [Google Scholar]
- 28.Clark D 3rd, Cain LR, Blaha MJ, DeFilippis AP, Mentz RJ, Kamimura D, White WB, Butler KR, Robertson RM, Bhatnagar A, et al. Cigarette smoking and subclinical peripheral arterial disease in Blacks of the Jackson Heart Study. J Am Heart Assoc 2019;8:e010674. doi: 10.1161/JAHA.118.010674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arya S, Binney Z, Khakharia A, Brewster LP, Goodney P, Patzer R, Hockenberry J, Wilson PWF. Race and socioeconomic status independently affect risk of major amputation in peripheral artery disease. J Am Heart Assoc. 2018;7:e007425. doi: 10.1161/JAHA.117.007425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holman KH, Henke PK, Dimick JB, Birkmeyer JD. Racial disparities in the use of revascularization before leg amputation in Medicare patients. J Vasc Surg. 2011;54:420–426, 426.e1. doi: 10.1016/j.jvs.2011.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soden PA, Zettervall SL, Deery SE, Hughes K, Stoner MC, Goodney PP, Vouyouka AG, Schermerhorn ML; Society for Vascular Surgery Vascular Quality Initiative. Black patients present with more severe vascular disease and a greater burden of risk factors than white patients at time of major vascular intervention. J Vasc Surg. 2018;67:549–556.e3. doi: 10.1016/j.jvs.2017.06.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen LT, Zhang DL, Shi L, Kalbaugh CA. Disparities in peripheral artery disease hospitalizations identified among understudied race-ethnicity groups. Front Cardiovasc Med. 2021;8:692236. doi: 10.3389/fcvm.2021.692236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anjorin AC, Marcaccio CL, Patel PB, Wang SX, Rowe V, Aulivola B, Wyers MC, Schermerhorn ML. Racial and ethnic disparities in 3-year outcomes following infrainguinal bypass for chronic limb-threatening ischemia. J Vasc Surg. 2022;76:1335–1346.e7. doi: 10.1016/j.jvs.2022.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Donnell TFX, Powell C, Deery SE, Darling JD, Hughes K, Giles KA, Wang GJ, Schermerhorn ML. Regional variation in racial disparities among patients with peripheral artery disease. J Vasc Surg. 2018;68:519–526. doi: 10.1016/j.jvs.2017.10.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krawisz AK, Natesan S, Wadhera RK, Chen SY, Song Y, Yeh RW, Jaff MR, Giri J, Julien H, Secemsky EA. Differences in comorbidities explain black-white disparities in outcomes after femoropopliteal endovascular intervention. Circulation. 2022;146:191–200. doi: 10.1161/CIRCULATIONAHA.122.058998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hicks CW, Wang PQ, Bruhn WE, Abularrage CJ, Lum YW, Perler BA, Black JH, Makary MA. Race and socioeconomic differences associated with endovascular peripheral vascular interventions for newly diagnosed claudication. J Vasc Surg. 2020;72:611–621.e5. doi: 10.1016/j.jvs.2019.10.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnes JA, Eid MA, Creager MA, Goodney PP. Epidemiology and risk of amputation in patients with diabetes mellitus and peripheral artery disease. Arteriosci Throm Vasc Biol 2020;40:1808–1817. doi: 10.1161/Atvbaha.120.314595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathlouthi A, Zarrintan S, Khan MA, Malas M, Barleben A. Contemporary outcomes of limb-salvage procedures using Vascular Quality Initiative-Medicare-linked data: racial and ethnic disparities persist. J Vasc Surg. 2022;75:2013–2018. doi: 10.1016/j.jvs.2022.01.120 [DOI] [PubMed] [Google Scholar]
- 39.Fanaroff AC, Yang L, Nathan AS, Khatana SAM, Julien H, Wang TY, Armstrong EJ, Treat-Jacobson D, Glaser JD, Wang G, et al. Geographic and socioeconomic disparities in major lower extremity amputation rates in metropolitan areas. J Am Heart Assoc 2021;10:e021456. doi: 10.1161/JAHA.121.021456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamburg NM, Creager MA. Pathophysiology of intermittent claudication in peripheral artery disease. Circ J. 2017;81:281–289. doi: 10.1253/circj.CJ-16-1286 [DOI] [PubMed] [Google Scholar]
- 41.Brothers RM, Fadel PJ, Keller DM. Racial disparities in cardiovascular disease risk: mechanisms of vascular dysfunction. Am J Physiol Heart Circ Physiol. 2019;317:H777–H789. doi: 10.1152/ajpheart.00126.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hackler E, Lew J, Gore MO, Ayers CR, Atzler D, Khera A, Rohatgi A, Lewis A, Neeland I, Omland T, et al. Racial differences in cardiovascular biomarkers in the general population. J Am Heart Assoc. 2019;8:e012729. doi: 10.1161/jaha.119.012729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beckman JA, Duncan MS, Damrauer SM, Wells QS, Barnett JV, Wasserman DH, Bedimo RJ, Butt AA, Marconi VC, Sico JJ, et al. Microvascular disease, peripheral artery disease, and amputation. Circulation. 2019;140:449–458. doi: 10.1161/CIRCULATIONAHA.119.040672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powell-Wiley TM, Baumer Y, Baah FO, Baez AS, Farmer N, Mahlobo CT, Pita MA, Potharaju KA, Tamura K, Wallen GR. Social determinants of cardiovascular disease. Circ Res. 2022;130:782–799. doi: 10.1161/CIRCRESAHA.121.319811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klarin D, Tsao PS, Damrauer SM. Genetic determinants of peripheral artery disease. Circ Res. 2021;128:1805–1817. doi: 10.1161/CIRCRESAHA.121.318327 [DOI] [PubMed] [Google Scholar]
- 46.Pande RL, Creager MA. Socioeconomic inequality and peripheral artery disease prevalence in US adults. Circ Cardiovasc Qual Outcomes. 2014;7:532–539. doi: 10.1161/CIRCOUTCOMES.113.000618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, Davey-Smith G, Dennison-Himmelfarb CR, Lauer MS, Lockwood DW, et al. ; on behalf of the American Heart Association Council on Quality of Care and Outcomes Research, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, Council on Lifestyle and Cardiometabolic Health, and Stroke Council. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132:873–898. doi: 10.1161/CIR.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 48.Panza GA, Puhl RM, Taylor BA, Zaleski AL, Livingston J, Pescatello LS. Links between discrimination and cardiovascular health among socially stigmatized groups: a systematic review. Plos One. 2019;14:e0217623. doi: 10.1371/journal.pone.0217623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takeshita J, Wang SY, Loren AW, Mitra N, Shults J, Shin DB, Sawinski DL. Association of racial/ethnic and gender concordance between patients and physicians with patient experience ratings. JAMA Netw Open. 2020;3:e2024583. doi: 10.1001/jamanetworkopen.2020.24583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones AC, Nichols AC, McNicholas CM, Stanford FC. Admissions is not enough: the racial achievement gap in medical education. Acad Med. 2021;96:176–181. doi: 10.1097/ACM.0000000000003837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Churchwell K, Elkind MSV, Benjamin RM, Carson AP, Chang EK, Lawrence W, Mills A, Odom TM, Rodriguez CJ, Rodriguez F, et al. ; on behalf of the American Heart Association. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142:E454–E468. doi: 10.1161/Cir.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 52.Solaru KTW, Coy T, DeLozier S, Brinza E, Ravenell J, Longenecker CT, Wright JT, Gornik HL. Findings of a novel barbershop-based peripheral artery disease screening program for Black men. J Am Heart Assoc. 2022;11:e026347. doi: 10.1161/JAHA.122.026347 [DOI] [PMC free article] [PubMed] [Google Scholar]


