Abstract
Background
The burden and functional significance of autonomic dysfunction among survivors of childhood cancer is unknown.
Objectives
We evaluated the prevalence, risk factors, and functional relevance of autonomic dysfunction in survivors.
Methods
We conducted a cross-sectional prospective evaluation of 1,041 adult survivors of childhood cancer treated with anthracyclines (31.1%), chest-directed radiation (13.5%), both (19.5%), or neither (35.9%), and 286 community control subjects enrolled in the SJLIFE (St Jude Lifetime Cohort Study). Four measures of autonomic dysfunction were evaluated: elevated resting heart rate, decreased heart rate reserve, decreased systolic blood pressure response to exercise, and delayed heart rate recovery. Logistic regression tested associations with impaired cardiorespiratory fitness (peak Vo2 < 80% predicted).
Results
Survivors (50.7% female) were 9.0 ± 5.8 years at cancer diagnosis and 35.5 ± 8.9 years at evaluation. Prevalence (survivors vs control subjects) of elevated resting heart rate (17.9% vs 7.0%), decreased heart rate reserve (21.7% vs 9.1%), decreased systolic blood pressure response to exercise (25.3% vs 12.6%), and delayed heart rate recovery (24.3% vs 10.6%) was more than 2-fold higher among survivors (P < 0.001 for all). Carboplatin (adjusted OR: 2.50; 95% CI: 1.42-4.40; P = 0.001), chest-directed radiation therapy (adjusted OR: 2.06; 95% CI: 1.52-2.75; P < 0.001), and cranial radiation (adjusted OR: 1.49; 95% CI: 1.08-2.05; P = 0.015) were associated with an increased likelihood of having ≥2 measures of autonomic dysfunction. Survivors with ≥2 measures of autonomic dysfunction were at increased risk for impaired cardiorespiratory fitness (adjusted OR: 2.71; 95% CI: 1.82-4.02; P < 0.001).
Conclusions
Survivors of childhood cancer manifest a higher prevalence of autonomic dysfunction associated with impaired cardiorespiratory fitness.
Key Words: autonomic dysfunction, autonomic function, childhood cancer survivors, cardio-oncology, impaired cardiorespiratory fitness
Central Illustration
With contemporary therapy, more than 85% of children and adolescents diagnosed with a malignancy will become 5-year survivors.1,2 However, the greater than half-million survivors of childhood cancers estimated to be living in the United States today are at high risk for early onset of chronic health conditions and premature mortality.3,4 The risk of cardiovascular mortality among survivors is up to 7 times higher than that of the general population.5 In addition, exercise limitation, which is associated with increased risk for mortality, is observed in over one-half of this population.6 Beyond the well-established associations with anthracycline chemotherapy and chest-directed radiotherapy,7,8 the mechanisms underlying exercise limitation and adverse cardiovascular outcomes in childhood cancer survivors are incompletely understood.
The autonomic nervous system is increasingly recognized as a key regulator of cardiopulmonary health and its role in the development and progression of cardiopulmonary disease is an important under-recognized research focus.9 In survivors of adult cancers, autonomic dysfunction is associated with exercise intolerance and an increase in cardiovascular mortality, and may be due to the interplay of cancer, cardiotoxic chemotherapy, radiation therapy, cancer-associated lifestyle disturbances, and cancer-independent comorbidities.10 These exposures are also highly relevant to childhood cancer survivors. We recently described the potential contribution of autonomic dysfunction to both exercise limitation11,12 and mortality12 in a small population of survivors of childhood acute lymphoblastic leukemia and Hodgkin lymphoma limited to only 2 measures of autonomic function. However, our current understanding of the burden of autonomic dysfunction among survivors of more diverse diagnoses, and its functional significance, is unknown. Improved characterization of autonomic dysfunction in survivors may identify the autonomic nervous system as a therapeutic target for prevention of future cardiac disease.
The objectives of this study were to comprehensively assess the total burden of and risk factors for autonomic dysfunction in a large population of clinically phenotyped childhood cancer survivors, using community control subjects for comparison, and to explore associations with echocardiographic and cardiopulmonary exercise test measurements. Four measures of autonomic function evaluated by exercise testing were considered in this study: resting heart rate, heart rate reserve, systolic blood pressure (SBP) response to exercise, and heart rate recovery. Abnormalities in these measures of autonomic function either alone or in combination have prognostic relevance, given their reported associations with a range of adverse outcomes, which include cardiovascular events,13,14 cardiovascular mortality,15,16 and all-cause mortality13,15 in predominantly cancer-free cohorts.
Methodology
Study Population
All participants were members of the SJLIFE (St. Jude Lifetime Cohort Study) treated for childhood cancer at St. Jude Children’s Research Hospital between 1962 and 2007; all participants consented to the study, and the institutional review boards at the St Jude Children's Research Hospital approved the study. Eligibility criteria included: 1) childhood malignancy; 2) survival ≥10 years from diagnosis; 3) assessment age ≥18 years; and 4) participation in echocardiographic and cardiopulmonary exercise testing. A detailed description of the overall study design has been published.17 Eligible survivors were stratified before recruitment by exposure (chest-directed radiation and anthracyclines; only chest-directed radiation; only anthracyclines; neither chest-directed radiation nor anthracyclines), and recruited in random order for participation. A community control cohort consisting of individuals without a history of cancer, frequency-matched for race/ethnicity, age, and sex were recruited from the same general geographic area as the SJLIFE survivor population.
Demographic and Clinical Variables
All participants underwent detailed clinical evaluation. The following demographic and clinical variables were collected: cancer diagnosis, age at diagnosis and at assessment, race/ethnicity, sex, body mass index (BMI), body surface area, health habits, and educational attainment. Total anthracycline dose (mg/m2) was the sum of doxorubicin, daunorubicin, epirubicin, idarubicin, and mitoxantrone in doxorubicin-equivalent doses.18 Radiotherapy records were centrally reviewed. Exposure to the chest was categorized as yes if at least part of the chest was in the treatment field; maximum total dose was determined by summing total delivered dose from all overlapping treatment fields.19
Cardiopulmonary Exercise Testing
Maximal cardiopulmonary exercise testing used a Modified Bruce Treadmill Protocol.20 Oxygen consumption (Vo2) was acquired breath by breath using a ventilatory expired gas analysis system (Ultima CardiO2, MGC Diagnostics) as per standard practice. The Wasserman formula was used to calculate predicted peak Vo2; impaired cardiorespiratory fitness was defined as peak Vo2 <80% predicted.21 Heart rate and SBP were measured before, throughout, and at peak exercise, and during recovery using a 12-lead electrocardiogram and automatic sphygmomanometer, respectively.
Other Clinical Measurements
Echocardiograms were obtained using a Vivid 7 machine (GE Medical Systems). Echocardiographic indices were measured following the American Society of Echocardiography standards.22,23 Left ventricular (LV) ejection fraction was measured using the Simpson’s Biplane method. Pulsed-wave peak E and A velocities of mitral inflow were used to calculate the E/A ratio, and pulsed-wave tissue Doppler velocities were obtained from both medial (septal e′) and lateral (lateral e′) mitral annuli.22 The average of septal e′ and lateral e′ were used to calculate E/e′ ratio. A septal e′, lateral e′, and average E/e′ ratio of <7 cm/s, <10 cm/s, and >14, respectively, were defined as abnormal.22 LV global longitudinal strain (GLS) was assessed using speckle tracking–based strain imaging software (EchoPAC PC version 10.0),23 and values ≥1.5 SD above sex-, age-, and vendor-specific means were considered reduced. All echocardiograms were centrally evaluated by a core echocardiography laboratory at the Baylor College of Medicine. Interobserver variability for echocardiographic measures has previously been reported.24
Pre-bronchodilator spirometry was used to determine forced expiratory volume in 1 second (FEV1).25 FEV1 <80% of race and sex predicted was considered impaired.26
Isokinetic quadriceps strength was evaluated in a seated position (Biodex System 4, Biodex Medical Systems).27 Participants performed 15 maximal knee extensions with each leg at 300°/s.28 Peak torque for body weight (Nm/kg) was converted to age- and sex-specific z-scores (based on control values) for analysis.
Peripheral sensorimotor function was evaluated with the modified total neuropathy scale.29 Participants were queried for sensory/motor symptoms, and tested for pin sensation, vibration, distal strength, and deep tendon reflexes. Scores ≥5 (of 24) were considered impaired.29
Measures of Autonomic Dysfunction
Elevated resting heart rate was defined as resting heart rate >80 beats/min,12 obtained from 12-lead electrocardiogram reading before exercise testing, after 10 minutes of quiet sitting. Heart rate reserve was calculated using the formula (heart rate at peak exercise − resting heart rate)/(age-predicted maximum heart rate − resting heart rate) · 100, and was defined as abnormal if <80% (or <62% for subjects taking beta-blockers).30 Decreased SBP response to exercise was defined as an exercise-induced decrease in SBP below standing baseline value or failure to increase SBP by ≥20 mm Hg from baseline during exercise.31 Delayed heart rate recovery was defined as a difference between heart rate at peak exercise and heart rate measured 2 minutes into recovery of ≤42 beats/min.32 Each subject was placed into 1 of 4 overall burden groups defined by the aggregate number of abnormal autonomic function measures (0, 1, 2, and ≥3).
Statistical Analysis
Descriptive statistics were used to characterize survivors and control subjects including mean ± SD or median with 25th-75th percentiles (Q1-Q3) for continuous variables and count (percentages) for categorical variables; group comparisons were performed using a t-test, chi-square test, or Fisher exact test, as appropriate. Frequency of each marker of autonomic dysfunction and the distribution of overall burden groups (0, 1, 2, and ≥3) for all survivors were compared with control subjects using the chi-square or Fisher exact test. Univariable analyses were used to develop a shortlist of candidate variables (Supplemental Table 1) from which final variables were selected based on clinical reasoning for a multivariable stepwise selection regression model that employed a significance level of 0.10 to identify independent predictors of ≥2 measures of autonomic dysfunction; a similar approach was used to determine independent predictors of impaired cardiorespiratory fitness defined as peak Vo2 of <80% predicted (Supplemental Table 2), reduced GLS (data not shown), and elevated E/e′ (data not shown). No collinearity was observed in models presented. Model associations are presented as adjusted OR (aOR) with 95% CI. Cardiopulmonary exercise test and echocardiographic parameters were compared across survivors categorized according to the aggregate number of markers of autonomic dysfunction, that is, 0, 1, 2, and ≥3, using analysis of variance, chi-square test, or Fisher exact test, as appropriate. SAS version 9.4 software (SAS Institute) was used for all analysis, and a P value of <0.05 was considered statistically significant.
Results
Characteristics of participants
The mean ± SD age of survivors at time of cancer diagnosis and time of evaluation was 9.0 ± 5.8 years and 35.5 ± 8.9 years, respectively (Table 1). The mean interval between cancer diagnosis and evaluation was 27.3 ± 9.2 (range 10.6-51.1) years. Compared with control subjects, survivors were slightly older at time of evaluation (35.5 ± 8.9 years vs 34.5 ± 10.0 years; P = 0.01). Radiation exposures to chest, abdomen/pelvis, and cranium were present in 33.0%, 32.5%, and 27.5% of survivors, respectively. Distributions of treatment exposures are provided in Table 1.
Table 1.
Demographic and Treatment Characteristics of Childhood Cancer Survivors and Control Subjects
| Survivors (n = 1,041) | Community Control Subjects (n = 286) | P Value | |
|---|---|---|---|
| Age at evaluation, y | 35.5 ± 8.9 | 34.5 ± 10.0 | 0.01 |
| Age at diagnosis, y | 9.0 ± 5.8 | — | — |
| Time since diagnosis, y | 27.3 ± 9.2 | — | — |
| Sex | 0.84 | ||
| Female | 528 (50.7) | 147 (51.4) | |
| Male | 513 (49.3) | 139 (48.6) | |
| Race/Ethnicity | <0.001 | ||
| Non-Hispanic White | 886 (85.2) | 258 (90.2) | |
| Non-Hispanic Black | 130 (12.5) | 14 (4.9) | |
| Hispanic | 8 (0.8) | 11 (3.9) | |
| Other | 16 (1.5) | 3 (1.0) | |
| Primary cancer diagnosis | — | — | |
| Leukemia | 273 (26.2) | ||
| Hodgkin lymphoma | 194 (18.6) | ||
| Central nervous system tumor | 157 (15.1) | ||
| Bone tumor | 90 (8.6) | ||
| Wilms tumor | 85 (8.2) | ||
| Soft tissue sarcoma | 55 (5.3) | ||
| Neuroblastoma | 52 (5.0) | ||
| Non-Hodgkin lymphoma | 43 (4.1) | ||
| Retinoblastoma | 39 (3.8) | ||
| Other solid tumors | 53 (5.1) | ||
| Treatment exposure | |||
| Anthracycline | 324 (31.1) | ||
| Chest-directed radiation | 140 (13.5) | ||
| Anthracycline + chest-directed radiation | 203 (19.5) | ||
| Neither anthracycline nor chest-directed radiation | 374 (35.9) | ||
| Radiation therapy | — | — | |
| Chest radiation, % | 343 (33.0) | ||
| Dose of chest radiation, Gy | 26 (20-35) | ||
| Cranial radiation | 286 (27.5) | ||
| Dose of cranial radiation, Gy | 26 (24-54) | ||
| Abdomen/pelvis radiation | 338 (32.5) | ||
| Dose of abdomen/pelvis radiation, Gy | 25 (20-35) | ||
| Anthracycline therapy | — | — | |
| Any anthracycline exposure | 527 (50.6) | ||
| Dose,a mg/m2 | 201 (151-323) | ||
| <100 mg/m2 | 44 (4.2) | ||
| 100-249 mg/m2 | 288 (27.7) | ||
| ≥250 mg/m2 | 194 (18.6) | ||
| Other chemotherapies | — | — | |
| Vincristine | 657 (63.1) | ||
| Alkylating agents | 590 (56.7) | ||
| Glucocorticoids | 386 (37.1) | ||
| Methotrexate | 381 (36.6) | ||
| Mercaptopurine | 268 (25.7) | ||
| Asparaginase | 212 (20.4) | ||
| Cisplatin | 96 (9.2) | ||
| Bleomycin | 77 (7.4) | ||
| Carboplatin | 62 (6.0) | ||
| Surgery | — | — | |
| Nephrectomy | 95 (9.1) | ||
| Amputation | 51 (4.9) | ||
| Thoracotomy | 44 (4.2) | ||
| Anthropometrics | |||
| Body mass index, kg/m2 | 28.4 ± 7.3 | 27.9 ± 6.7 | 0.39 |
| Percent fat mass, % | 37.7 ± 9.3 | 26.0 ± 8.9 | <0.001 |
| Percent lean mass, % | 62.3 ± 9.4 | 74.0 ± 8.9 | <0.001 |
| Health habits | |||
| Smoking, pack-years | 2.7 ± 7.3 | 2.8 ± 7.4 | 0.70 |
| Moderate or vigorous physical activity, min/wk | 407 ± 716 | 406 ± 548 | 0.97 |
| Risky alcohol consumption | 36 (3.5) | 16 (5.6) | 0.11 |
| Baseline cardiovascular risk factors | |||
| Hypertension | 219 (21.0) | 27 (9.4) | <0.001 |
| Diabetes mellitus | 71 (6.8) | 9 (3.2) | 0.021 |
| Dyslipidemia | 159 (15.3) | 20 (7.0) | <0.001 |
| Baseline cardiovascular medications | |||
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers | 124 (11.9) | 3 (1.1) | <0.001 |
| Beta-blockers | 100 (9.6) | 6 (2.1) | <0.001 |
| Thiazide diuretic agents | 82 (7.9) | 4 (1.4) | <0.001 |
| Calcium channel blockers | 56 (5.4) | 5 (1.8) | 0.009 |
| Loop diuretic agents | 12 (1.2) | 0 | - |
| Aldosterone antagonists | 11 (1.1) | 0 | - |
| Statin therapy | 144 (13.8) | 11 (3.9) | <0.001 |
Values are mean ± SD, n (%), or median (Q1-Q3).
Anthracycline dose was missing for 1 survivor who had been treated with anthracycline.
Burden of autonomic dysfunction
All 4 measures of autonomic dysfunction were observed at higher prevalence among survivors compared with control subjects (Figure 1A, Supplemental Table 3). Elevated resting heart rate was observed in 17.9% of survivors compared with 7.0% of control subjects (P < 0.001) and was most prevalent among survivors with a history of chest-directed radiation (28.3%). Decreased heart rate reserve was twice as prevalent among survivors compared with control subjects (21.7% vs 9.1%; P < 0.001), and was most prevalent among survivors with a history of chest (22.2%) or cranial (28.7%) radiation. Decreased SBP response to exercise occurred in 25.3% of survivors vs 12.6% of control subjects (P < 0.001), which was also most prevalent in the setting of prior chest-directed radiation (31.2%). Delayed heart rate recovery was evident in almost one-quarter of survivors, with a >2-fold frequency compared with control subjects (24.3% vs 10.6%; P < 0.001). The prevalence of this abnormality was highest among survivors with a history of either chest (34.2%) or cranial (30.4%) radiation.
Figure 1.
Measures of Autonomic Dysfunction in Adult Survivors of Childhood Cancer
(A) Prevalence of each measure of autonomic dysfunction (AD) in survivors vs community control subjects. P value for intergroup comparison for each measure of autonomic dysfunction is <0.001. (B) Burden of autonomic dysfunction in survivors vs community control subjects, categorized by the aggregate number of each of the 4 measures present. P value for intergroup comparison for each measure of autonomic dysfunction is <0.001. HR = heart rate; SBP = systolic blood pressure.
Over one-half (55.0%) of survivors had at least 1 measure of autonomic dysfunction compared with only 32.5% of control subjects (P < 0.001), and almost one-quarter (24.3%) of survivors had a burden of 2 or more of these measures compared with only 6.6% of control subjects. The maximum number of abnormal measures of autonomic function observed in any control subject was 2, whereas 8.2% of survivors manifested abnormalities of 3 or all 4 measures (Figure 1B, Supplemental Table 3). Among survivors, the burden of 3 or 4 measures of impaired autonomic function was most prevalent among those with a history of chest-directed radiation (14.0%).
Independent predictors of measures of autonomic dysfunction
As shown in Table 2, chest-directed radiation therapy (aOR: 2.06; 95% CI: 1.52-2.75; P < 0.001), cranial radiation (aOR: 1.49; 95% CI: 1.08-2.05; P = 0.015), and carboplatin exposure (aOR: 2.50; 95% CI: 1.42-4.40; P = 0.001) were each associated with significantly increased risk for having ≥2 measures of autonomic dysfunction. Other independent predictors included increasing age at time of evaluation and smoking history (Table 2). No independent association was observed with anthracycline therapy (Table 2). Although 18 of 62 (29.0%) of carboplatin-treated survivors had central nervous system (CNS) tumors, the independent association with carboplatin was still observed when survivors with CNS tumors were excluded from analyses (Supplemental Table 4). Similarly, findings did not differ significantly when either diabetes mellitus, hypertension, or dyslipidemia were added to the original model (data not shown). Compared with survivors of leukemia (reference population), survivors of CNS tumors (aOR: 2.22; 95% CI: 1.37-3.60; P = 0.001) (Supplemental Table 5) and Hodgkin lymphoma (aOR: 1.62; 95% CI: 1.06-2.46; P = 0.025) (Supplemental Table 5) were the only specific cancer diagnoses associated with an increased likelihood of autonomic dysfunction.
Table 2.
Independent Predictors of Measures of Autonomic Dysfunction Among Childhood Cancer Survivors
| Adjusted OR (95% CI) for ≥2 Measures of Autonomic Dysfunction | P Value | |
|---|---|---|
| Age at evaluation | 1.03 (1.01-1.05) | <0.001 |
| Smoking history | 1.40 (1.02-1.92) | 0.039 |
| Chest-directed radiation | 2.06 (1.52-2.75) | <0.001 |
| Cranial radiation | 1.49 (1.08-2.05) | 0.015 |
| Carboplatin | 2.50 (1.42-4.40) | 0.001 |
Other variables included in this multivariable model, but not identified as independent predictors, were age at cancer diagnosis, sex, body mass index, alkylating agent, and anthracycline therapy. Note that body mass index was included in the model as a 3-category variable.
Association of autonomic dysfunction with cardiopulmonary fitness and echocardiographic parameters
There was a stepwise reduction in peak oxygen consumption with increasing burden of autonomic dysfunction, decreasing from 28.9 ± 7.9 mL/kg/min in the absence of any marker of autonomic dysfunction successively to 19.2 ± 6.9 mL/kg/min in the presence of ≥3 measures of autonomic dysfunction (Table 3, Figure 2). Survivors with 2 or more measures of autonomic dysfunction demonstrated moderately impaired functional capacity based on peak oxygen consumption of 60% to 70% predicted. The prevalence of impaired cardiorespiratory fitness increased from 34.6% among survivors without measures of autonomic dysfunction to 79.8% among survivors with ≥3 measures.
Table 3.
Influence of Increasing Burden of Measures of Autonomic Dysfunction on Various Echocardiographic and CPET Parameters
| Survivors (n = 1,041) |
|||||
|---|---|---|---|---|---|
| Measures of Autonomic Dysfunction | |||||
| None (n = 468) | Only 1 (n = 320) | 2 (n = 168) | ≥3 (n = 85) | P Value | |
| Cardiopulmonary exercise test parameters | |||||
| Peak oxygen consumption, mL/kg/min | 28.9 ± 7.9 | 26.2 ± 7.4 | 21.2 ± 7.1 | 19.2 ± 6.9 | <0.001 |
| Peak oxygen consumption as a percent of predicted, % | 86.6 ± 18.2 | 79.1 ± 17.8 | 69.1 ± 18.5 | 62.8 ± 18.9 | <0.001 |
| Peak oxygen consumption <80% predicted | 162 (34.6) | 169 (52.8) | 121 (72.0) | 67 (79.8) | <0.001 |
| Echocardiographic parameters | |||||
| Left ventricular ejection fraction, % | 58.6 ± 5.0 | 57.4 ± 5.5 | 56.5 ± 6.3 | 57.3 ± 6.1 | <0.001 |
| Left ventricular ejection fraction <50% | 22 (5.1) | 21 (7.2) | 17 (11.2) | 6 (9.8) | 0.058 |
| Global longitudinal strain, % | −20.1 ± 2.7 | −19.8 ± 2.7 | −19.3 ± 2.6 | −18.7 ± 2.4 | 0.11 |
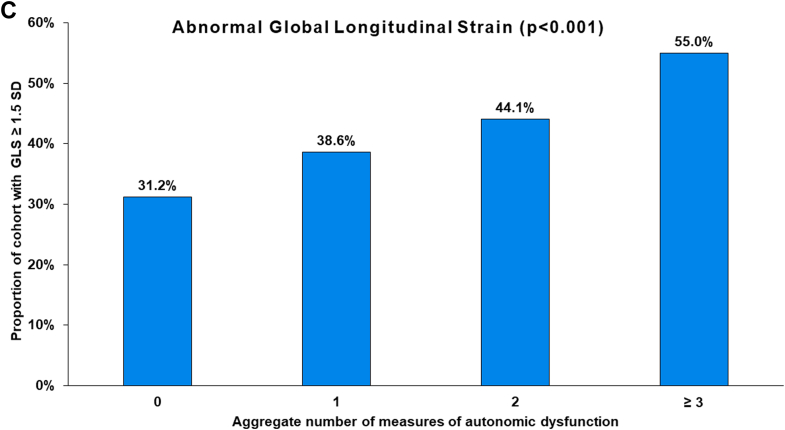
| Global longitudinal strain ≥1.5 SD | 137 (31.2) | 114 (38.6) | 67 (44.1) | 44 (55.0) | <0.001 |
| Septal e′ <7 cm/s | 24 (5.2) | 14 (4.5) | 13 (8.0) | 11 (13.8) | 0.011 |
| Lateral e′ <10 cm/s | 54 (11.7) | 44 (14.1) | 38 (23.2) | 22 (27.5) | <0.001 |
| E/e′ ratio >14 | 65 (14.1) | 45 (14.6) | 34 (21.1) | 24 (30.8) | <0.001 |
Values are mean ± SD or n (%).
CPET = cardiopulmonary exercise test.
Figure 2.
Relationship of Autonomic Dysfunction With Exercise and Echocardiographic Assessments
Relationship of peak oxygen consumption (A), E/e′ (B), and global longitudinal stain (C) to the aggregate number of measures of autonomic dysfunction among adult survivors of childhood cancer.
In multivariable models, 2 or more measures of autonomic dysfunction was associated with a >2-fold increased likelihood of peak Vo2 <80% predicted after controlling for important confounders of fitness including age, BMI, LV function, and reduced quadriceps strength among survivors (aOR: 2.71; 95% CI: 1.82-4.02; P < 0.001) (Table 4). This association remained significant when survivors with CNS tumors were excluded from analysis (Supplemental Table 6). In a similar multivariable model that included type of cancer diagnosis, multiple measures of autonomic dysfunction again emerged as a strong independent predictor of impaired cardiorespiratory fitness (aOR: 2.66; 95% CI: 1.76-4.02; P < 0.001) (Supplemental Table 7).
Table 4.
Independent Predictors of Impaired Cardiorespiratory Fitness Among Childhood Cancer Survivors
| Adjusted OR (95% CI) for Vo2 <80% Predicted | P Value | |
|---|---|---|
| Age at evaluation | 0.98 (0.96-0.99) | 0.043 |
| ≥2 measures of autonomic dysfunction | 2.71 (1.82-4.02) | <0.001 |
| Female | 0.59 (0.43-0.80) | <0.001 |
| MVPA ≥150 min/wk | 0.49 (0.36-0.67) | <0.001 |
| Quadriceps strength, 1 SD decrease | 1.56 (1.34-1.81) | <0.001 |
| FEV1 < 80% | 3.24 (2.27-4.64) | <0.001 |
Other variables included in this multivariable model, but not identified as independent predictors, were: age at diagnosis, body mass index, smoking history, antihypertensive at the time of cardiopulmonary exercise test, modified total neuropathy score (MTNS) ≥ 5, global longitudinal strain ≥1.5 SD, and left ventricular ejection fraction. Note that body mass index was included in the model as a 3-category variable.
FEV1 = forced expiratory volume in 1 minute; MVPA = moderate or vigorous physical activity; Vo2 = oxygen consumption.
The proportion of survivors with worsened GLS progressively increased with increasing burden of measures of autonomic dysfunction, from 31.2% in survivors with no such measures to 55% of survivors with 3 or more measures. Similarly, the proportion of survivors with abnormal septal e′, lateral e′, and E/e′ ratio progressively increased with increasing aggregate number of measures of autonomic dysfunction (Table 3, Figure 2). However, the presence of ≥2 measures of autonomic dysfunction was not associated with either reduced GLS or elevated E/e′ after adjusting for potential confounders in multivariable models (data not shown).
Discussion
Our analysis of a large and diverse adult cohort of childhood cancer survivors indicates a higher prevalence of elevated resting heart rate, decreased heart rate reserve, abnormal SBP response to exercise, and abnormal heart rate recovery, all measures of autonomic dysfunction, as well as a higher cumulative burden of these measures, compared with control subjects (Central Illustration). The current study is, to our knowledge, the largest study to describe the burden of autonomic injury among childhood cancer survivors. We previously described evidence of cardiac autonomic dysfunction, as defined by just 2 measures, elevated resting heart rate or abnormal heart rate recovery, in a small population (N = 388) of survivors of childhood acute lymphoblastic leukemia.11 Similarly, adult survivors of mantle radiation (N = 263) had a greater frequency of elevated resting heart rate (44.5% vs 17.9%; P < 0.001) and abnormal heart rate recovery (31.9% vs 9.3%; P < 0.001) compared with matched control subjects.12 A few studies have previously reported individual measures of autonomic injury among survivors of adult cancers treated with platinum compounds,33,34 vinca alkaloids,35 cranial irradiation,36 neck irradiation,37 and chest-directed radiation.12 With unique insights into the aggregate burden of 4 established measures of autonomic dysfunction among childhood cancer survivors, and by exploring predictors and functional significance of these measures, this current study advances our understanding of the prevalence, associations, and significance of autonomic injury among childhood cancer survivors.
Central Illustration.
Autonomic Dysfunction in Childhood Cancer Survivors vs Control Subjects in SJLIFE
This illustration outlines the 4 measures of autonomic dysfunction evaluated in this analysis and highlights a higher prevalence of autonomic dysfunction among survivors vs control subjects. It provides a summary of the predictors of autonomic dysfunction in survivors and outlines the association of autonomic dysfunction with peak oxygen consumption in survivors. SBP = systolic blood pressure; SJLIFE = St. Jude Lifetime Cohort Study.
We report worsening cardiorespiratory fitness with each increase in the aggregate number of these 4 measures of autonomic dysfunction among childhood cancer survivors, such that the mean peak Vo2 of survivors with ≥2 abnormalities is in a range consistent with moderately impaired cardiorespiratory fitness (peak Vo2 = 50% to 69% predicted). Moreover, after adjusting for important confounders of cardiorespiratory fitness including age, sex, LV function, reduced muscle strength, and pulmonary reserve, the presence of ≥2 measures of autonomic dysfunction emerged as a strong independent predictor of peak Vo2 <80% predicted. Although a stepwise increase in the proportion of survivors who manifest reduced LV GLS (a more sensitive measure of LV systolic function than ejection fraction38) and elevated E/e′ ratio (a surrogate for elevated LV end-diastolic pressure and marker of LV diastolic dysfunction22) was observed with increasing burden of abnormal measures of autonomic function, independent associations were not evident in multivariable analyses. Collectively, these findings suggest that autonomic dysfunction is not only prevalent among childhood cancer survivors, but that its presence is independently associated with impaired cardiorespiratory fitness.
Multiple factors may predispose childhood cancer survivors to autonomic dysfunction. Direct injury to the autonomic nervous system can occur as consequence of malignancy itself, or because of cancer therapies, surgery, and/or radiation therapy.10 In the current analysis, factors independently associated with increased likelihood of ≥2 measures of autonomic dysfunction in survivors were increasing age at time of evaluation, smoking history, chest-directed radiation, cranial radiation, and carboplatin exposure. In addition, the higher risk of autonomic dysfunction among survivors of childhood Hodgkin lymphoma and CNS tumors likely reflects underlying treatment associations, that is, chest-directed radiation therapy used in Hodgkin lymphoma, and cranial radiation and carboplatin used in treatment of CNS tumors.
Carboplatin was the only chemotherapy of several candidate cardiotoxic chemotherapies evaluated to emerge as an independent predictor of autonomic dysfunction in this analysis. It is known that platinum compounds, including carboplatin, are associated with a dose-dependent peripheral neurotoxicity.39 Although 2 small studies (N = 16 and N = 90) of adult cancer survivors suggest relative sparing of autonomic neurons,40,41 other studies have demonstrated evidence of autonomic injury based on a decrease in Valsalva ratio following cisplatin treatment for ovarian adenocarcinoma (n = 11),42 and postural hypotension following cisplatin-based combination chemotherapy for metastatic germ cell cancer (n = 71)33 and oxaliplatin-based chemotherapy for colorectal cancer (n = 36).34 The current study offers the most compelling evidence to date of carboplatin-associated autonomic injury, given that childhood cancer survivors who were exposed to carboplatin were 2.5 times (aOR: 2.50; 95% CI: 1.42-4.40; P = 0.001) more likely to manifest ≥2 measures of autonomic dysfunction. The exact mechanism underlying this potential association is uncertain, but we did not observe any dose-dependent increase in risk in this small subset of survivors. In addition, carboplatin-exposed survivors did not appear to have of a higher prevalence of peripheral neuropathy compared with survivors who were not based on the modified total neuropathy scale (data not shown).
Radiation-mediated injury to the autonomic nervous system is well-described.10,12,36,37 Like radiation-mediated injury to the myocardium, pericardium, valvular apparatus, coronary arteries, and large arteries, autonomic anatomy within the radiation field is vulnerable to injury.10 For example, radiation has been shown to affect Schwann cells, leading to retraction of their processes and the appearance of naked axonal segments in the rat cervical sympathetic trunk.43 Dose-related autonomic injury with progressive and late manifestation from time of radiation therapy has been previously described.12 Mantle radiation was associated with an almost 4-fold higher likelihood of elevated resting heart rate and over a 5-times higher likelihood of delayed heart rate recovery among 263 Hodgkin lymphoma survivors compared with cancer-naive matched control subjects.12 Monotonous heart rate, persistent tachycardia, and blunted hemodynamic response to exercise were prevalent among 48 Hodgkin lymphoma survivors treated with mediastinal radiation.44 Our analyses confirmed that the aggregate burden of abnormal measures of autonomic function was highest among survivors with chest-directed and/or cranial radiation exposures (Supplemental Table 3). In addition, chest-directed and cranial radiation exposures each emerged as independent predictors of increased likelihood of autonomic dysfunction in multivariable analyses. Although the frequencies of individual and aggregate measures of autonomic dysfunction were higher among survivors treated with anthracyclines compared with control subjects, we did not demonstrate an independent association between anthracyclines and likelihood of autonomic dysfunction. Although abnormalities in autonomic testing has been described in adult cancer survivors treated with anthracyclines, particularly when used in high-dose or in conjunction with radiation therapy,45 relatively low doses of epirubicin were not associated with persistent alterations in heart rate variability in breast cancer survivors.46 The median cumulative anthracycline dose in this study was 201 (151-323) mg/m2. However, no association was observed between cumulative anthracycline dose ≥250 mg/m2 and any of the 4 measures of autonomic dysfunction (data not shown).
Study Limitations
Diversity of race/ethnicity is limited in this predominantly non-Hispanic White cohort. Our measures of autonomic function did not include assessment of heart rate variability. However, the 4 measures of autonomic function used in this study are well-validated, practical, inexpensive, and can be measured easily during any exercise test.47 The use of these 4 measures in combination offers a more robust approach to assess autonomic function than any 1 measure used in isolation. Due to the cross-sectional nature of this study, we are unable to assess for causal association between autonomic dysfunction and impaired cardiorespiratory fitness and/or LV function, and vice versa. Prospective studies to determine the temporal relationship of these abnormalities are needed. Similarly, this cross-sectional analysis cannot inform the time course for onset of autonomic injury and progression over time after childhood cancer exposures. Baseline and repeated assessments of autonomic function before and after cancer diagnosis and treatments are needed to define the natural history of autonomic injury in this setting.
Clinical Implications
These study findings have important clinical implications for the growing population of childhood cancer survivors. We provide the most robust evidence to date of persistent autonomic injury among a significant proportion of this cohort that is associated with impaired cardiorespiratory fitness. These findings emphasize the need for clinicians to consider evaluation of autonomic function during work-up of exercise limitation in this cohort. Furthermore, given the well-established association of autonomic dysfunction with cardiovascular morbidity and mortality, the potential contribution of autonomic injury to adverse cardiovascular outcomes among childhood cancer survivors warrants evaluation. Survivors with evidence of autonomic imbalance may represent a higher risk cohort of survivors that may require even more careful cardiovascular risk mitigation from their clinicians. Our findings establish the autonomic nervous system as a potential therapeutic target and provide rationale to support clinical trials of interventions that modulate sympathovagal balance. Indeed, there is preclinical and/or clinical rationale to support evaluation of several pharmacological (eg, ivabradine, beta-blockers, renin-angiotensin-aldosterone system inhibition) and non-pharmacological interventions (exercise training, vagal nerve stimulation, carotid baroreceptor stimulation) in this context.48
Conclusions
Persistent autonomic injury remote from cancer diagnosis/treatment in adult survivors of childhood cancer is suggested by a higher prevalence of elevated resting heart rate, decreased heart rate reserve, abnormal SBP response to exercise, and abnormal heart rate recovery, and the combination of these 4 measures of abnormal autonomic function compared to control subjects. Survivors with carboplatin exposure, cranial radiation, or chest-directed radiation therapy appear particularly predisposed. Autonomic dysfunction is associated with significant impairment of cardiorespiratory fitness, even after adjusting for important confounders including LV function, lung function, muscle strength, age, and BMI. Collectively, these findings implicate autonomic injury in the etiology of exercise limitation that frequently impairs quality of life of childhood cancer survivors. Therapeutic interventions to ameliorate autonomic dysfunction in cancer survivors require evaluation.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Persistent autonomic injury remote from cancer diagnosis/treatment in adult survivors of childhood cancer is suggested by a higher prevalence and aggregate burden of 4 measures of abnormal autonomic function (elevated resting heart rate, decreased heart rate reserve, decreased SBP response to exercise, and delayed heart rate recovery) compared with control subjects. Autonomic dysfunction is independently associated with significant impairment of cardiorespiratory fitness among adult survivors of childhood cancer and should be considered during evaluation of exercise limitation in these patients.
TRANSLATIONAL OUTLOOK: Our findings establish the autonomic nervous system as a potential therapeutic target among adult survivors of childhood cancer with exercise limitation and provide rationale to support clinical trials of interventions that modulate sympathovagal balance, such as structured exercise training.
Funding Support and Author Disclosures
Support to St. Jude Children’s Research Hospital was provided by the National Cancer Institute grants U01 CA195547 (Drs Hudson and Ness) and R01 CA157838 (Dr Armstrong), the Cancer Center Support (CORE) grant P30 CA21765 (Dr C. Roberts), and the American Lebanese-Syrian Associated Charities (ALSAC). Dr Groarke has received research support from Amgen, Inc, was previously employed by Amgen, Inc; and is currently an employee of Pfizer. Dr Nohria has received research support from Bristol Myers Squibb; and consulting fees from AstraZeneca, Bantam Pharmaceuticals, Regeneron Pharmaceuticals, and Takeda Oncology. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Hudson M.M., Oeffinger K.C., Jones K., et al. Age-dependent changes in health status in the Childhood Cancer Survivor cohort. J Clin Oncol. 2015;33:479–491. doi: 10.1200/JCO.2014.57.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader N., Noone A.M., Krapcho M., et al., editors. SEER Cancer Statistics Review, 1975-2014. National Cancer Institute; 2017. https://seer.cancer.gov/csr/1975_2014/ [Google Scholar]
- 3.Armstrong G.T., Chen Y., Yasui Y., et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374:833–842. doi: 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibson T.M., Mostoufi-Moab S., Stratton K.L., et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970-99: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2018;19:1590–1601. doi: 10.1016/S1470-2045(18)30537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong G.T., Liu Q., Yasui Y., et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ness K.K., Plana J.C., Joshi V.M., et al. Exercise intolerance, mortality, and organ system impairment in adult survivors of childhood cancer. J Clin Oncol. 2020;38:29–42. doi: 10.1200/JCO.19.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ness K.K., DeLany J.P., Kaste S.C., et al. Energy balance and fitness in adult survivors of childhood acute lymphoblastic leukemia. Blood. 2015;125:3411–3419. doi: 10.1182/blood-2015-01-621680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stenehjem J.S., Smeland K.B., Murbraech K., et al. Cardiorespiratory fitness in long-term lymphoma survivors after high-dose chemotherapy with autologous stem cell transplantation. Br J Cancer. 2016;115:178–187. doi: 10.1038/bjc.2016.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehra R., Tjurmina O.A., Ajijola O.A., et al. Research opportunities in autonomic neural mechanisms of cardiopulmonary regulation: a report from the National Heart, Lung, and Blood Institute and the National Institutes of Health Office of the Director Workshop. JACC Basic Transl Sci. 2022;7:265–293. doi: 10.1016/j.jacbts.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coumbe B.G.T., Groarke J.D. Cardiovascular autonomic dysfunction in patients with cancer. Curr Cardiol Rep. 2018;20:69. doi: 10.1007/s11886-018-1010-y. [DOI] [PubMed] [Google Scholar]
- 11.Christoffersen L., Gibson T.M., Pui C.H., et al. Cardiac autonomic dysfunction in survivors of childhood acute lymphoblastic leukemia: the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2020;67 doi: 10.1002/pbc.28388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groarke J.D., Tanguturi V.K., Hainer J., et al. Abnormal exercise response in long-term survivors of Hodgkin lymphoma treated with thoracic irradiation: evidence of cardiac autonomic dysfunction and impact on outcomes. J Am Coll Cardiol. 2015;65:573–583. doi: 10.1016/j.jacc.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 13.Qiu S., Cai X., Sun Z., et al. Heart rate recovery and risk of cardiovascular events and all-cause mortality: a meta-analysis of prospective cohort studies. J Am Heart Assoc. 2017;6(5) doi: 10.1161/JAHA.117.005505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox K., Ford I., Steg P.G., et al. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817–821. doi: 10.1016/S0140-6736(08)61171-X. [DOI] [PubMed] [Google Scholar]
- 15.Arbit B., Azarbal B., Hayes S.W., et al. Prognostic contribution of exercise capacity, heart rate recovery, chronotropic incompetence, and myocardial perfusion single-photon emission computerized tomography in the prediction of cardiac death and all-cause mortality. Am J Cardiol. 2015;116:1678–1684. doi: 10.1016/j.amjcard.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 16.Myers J., Tan S.Y., Abella J., Aleti V., Froelicher V.F. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehabil. 2007;14:215–221. doi: 10.1097/HJR.0b013e328088cb92. [DOI] [PubMed] [Google Scholar]
- 17.Howell C.R., Bjornard K.L., Ness K.K., et al. Cohort profile: the St. Jude Lifetime Cohort Study (SJLIFE) for paediatric cancer survivors. Int J Epidemiol. 2021;50:39–49. doi: 10.1093/ije/dyaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feijen E.A., Leisenring W.M., Stratton K.L., et al. Equivalence ratio for daunorubicin to doxorubicin in relation to late heart failure in survivors of childhood cancer. J Clin Oncol. 2015;33:3774–3780. doi: 10.1200/JCO.2015.61.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stovall M., Weathers R., Kasper C., et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 20.Pescatello L.S., American College of Sports Medicine . 9th ed. Wolters Kluwer/Lippincott Williams & Wilkins Health; 2014. ACSM's Guidelines for Exercise Testing and Prescription. [Google Scholar]
- 21.Balady G.J., Arena R., Sietsema K., et al. Clinician's guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 22.Nagueh S.F., Smiseth O.A., Appleton C.P., et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong G.T., Joshi V.M., Ness K.K., et al. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results from the St. Jude Lifetime Cohort Study. J Am Coll Cardiol. 2015;65:2511–2522. doi: 10.1016/j.jacc.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller M.R., Hankinson J., Brusasco V., et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 26.Hudson M.M., Ehrhardt M.J., Bhakta N., et al. Approach for classification and severity grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2017;26:666–674. doi: 10.1158/1055-9965.EPI-16-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feiring D.C., Ellenbecker T.S., Derscheid G.L. Test-retest reliability of the biodex isokinetic dynamometer. J Orthop Sports Phys Ther. 1990;11:298–300. doi: 10.2519/jospt.1990.11.7.298. [DOI] [PubMed] [Google Scholar]
- 28.Neder J.A., Nery L.E., Shinzato G.T., Andrade M.S., Peres C., Silva A.C. Reference values for concentric knee isokinetic strength and power in nonathletic men and women from 20 to 80 years old. J Orthop Sports Phys Ther. 1999;29:116–126. doi: 10.2519/jospt.1999.29.2.116. [DOI] [PubMed] [Google Scholar]
- 29.Wampler M.A., Miaskowski C., Hamel K., Byl N., Rugo H., Topp K.S. The modified total neuropathy score: a clinically feasible and valid measure of taxane-induced peripheral neuropathy in women with breast cancer. J Support Oncol. 2006;4:W9–W16. [Google Scholar]
- 30.Khan M.N., Pothier C.E., Lauer M.S. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol) Am J Cardiol. 2005;96:1328–1333. doi: 10.1016/j.amjcard.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 31.Olivotto I., Maron B.J., Montereggi A., Mazzuoli F., Dolara A., Cecchi F. Prognostic value of systemic blood pressure response during exercise in a community-based patient population with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1999;33:2044–2051. doi: 10.1016/s0735-1097(99)00094-7. [DOI] [PubMed] [Google Scholar]
- 32.Cole C.R., Foody J.M., Blackstone E.H., Lauer M.S. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med. 2000;132:552–555. doi: 10.7326/0003-4819-132-7-200004040-00007. [DOI] [PubMed] [Google Scholar]
- 33.Richardson P., Cantwell B.M. Autonomic neuropathy after cisplatin based chemotherapy. BMJ. 1990;300:1466–1467. doi: 10.1136/bmj.300.6737.1466-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dermitzakis E.V., Kimiskidis V.K., Eleftheraki A., et al. The impact of oxaliplatin-based chemotherapy for colorectal cancer on the autonomous nervous system. Eur J Neurol. 2014;21:1471–1477. doi: 10.1111/ene.12514. [DOI] [PubMed] [Google Scholar]
- 35.Roca E., Bruera E., Politi P.M., et al. Vinca alkaloid-induced cardiovascular autonomic neuropathy. Cancer Treat Rep. 1985;69:149–151. [PubMed] [Google Scholar]
- 36.Kamath M.V., Halton J., Harvey A., Turner-Gomes S., McArthur A., Barr R.D. Cardiac autonomic dysfunction in survivors of acute lymphoblastic leukemia in childhood. Int J Oncol. 1998;12:635–640. doi: 10.3892/ijo.12.3.635. [DOI] [PubMed] [Google Scholar]
- 37.Sharabi Y., Dendi R., Holmes C., Goldstein D.S. Baroreflex failure as a late sequela of neck irradiation. Hypertension. 2003;42:110–116. doi: 10.1161/01.HYP.0000077441.45309.08. [DOI] [PubMed] [Google Scholar]
- 38.Liu J.E., Barac A., Thavendiranathan P., Scherrer-Crosbie M. Strain imaging in cardio-oncology. JACC CardioOncol. 2020;2:677–689. doi: 10.1016/j.jaccao.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Screnci D., McKeage M.J. Platinum neurotoxicity: clinical profiles, experimental models and neuroprotective approaches. J Inorg Biochem. 1999;77:105–110. doi: 10.1016/s0162-0134(99)00135-x. [DOI] [PubMed] [Google Scholar]
- 40.Krarup-Hansen A., Helweg-Larsen S., Schmalbruch H., Rorth M., Krarup C. Neuronal involvement in cisplatin neuropathy: prospective clinical and neurophysiological studies. Brain. 2007;130:1076–1088. doi: 10.1093/brain/awl356. [DOI] [PubMed] [Google Scholar]
- 41.Nuver J., Smit A.J., Sleijfer D.T., et al. Left ventricular and cardiac autonomic function in survivors of testicular cancer. Eur J Clin Invest. 2005;35:99–103. doi: 10.1111/j.1365-2362.2005.01460.x. [DOI] [PubMed] [Google Scholar]
- 42.Boogerd W., ten Bokkel Huinink W.W., Dalesio O., Hoppenbrouwers W.J., van der Sande J.J. Cisplatin induced neuropathy: central, peripheral and autonomic nerve involvement. J Neurooncol. 1990;9:255–263. doi: 10.1007/BF02341156. [DOI] [PubMed] [Google Scholar]
- 43.Aguayo A.J., Bray G.M., Terry L.C., Sweezey E. Three dimensional analysis of unmyelinated fibers in normal and pathologic autonomic nerves. J Neuropathol Exp Neurol. 1976;35:136–151. doi: 10.1097/00005072-197603000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Adams M.J., Lipsitz S.R., Colan S.D., et al. Cardiovascular status in long-term survivors of Hodgkin's disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–3148. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 45.Viniegra M., Marchetti M., Losso M., et al. Cardiovascular autonomic function in anthracycline-treated breast cancer patients. Cancer Chemother Pharmacol. 1990;26:227–231. doi: 10.1007/BF02897205. [DOI] [PubMed] [Google Scholar]
- 46.Meinardi M.T., van Veldhuisen D.J., Gietema J.A., et al. Prospective evaluation of early cardiac damage induced by epirubicin-containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J Clin Oncol. 2001;19:2746–2753. doi: 10.1200/JCO.2001.19.10.2746. [DOI] [PubMed] [Google Scholar]
- 47.Lahiri M.K., Kannankeril P.J., Goldberger J.J. Assessment of autonomic function in cardiovascular disease: physiological basis and prognostic implications. J Am Coll Cardiol. 2008;51:1725–1733. doi: 10.1016/j.jacc.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 48.Chatterjee N.A., Singh J.P. Novel interventional therapies to modulate the autonomic tone in heart failure. JACC Heart Fail. 2015;3:786–802. doi: 10.1016/j.jchf.2015.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.