Abstract
Since their introduction in the 1960s, anthracyclines have been a significant breakthrough in oncology, introducing dramatic changes in the treatment of solid and hematologic malignancies. Although new-generation targeted drugs and cellular therapies are revolutionizing contemporary oncology, anthracyclines remain the cornerstone of treatment for lymphomas, acute leukemias, and soft tissue sarcomas. However, their clinical application is limited by a dose-dependent cardiotoxicity that can reduce cardiac performance and eventually lead to overt heart failure. The field of cardio-oncology has emerged to safeguard the cardiovascular health of cancer patients receiving these therapies. It focuses on controlling risk factors, implementing preventive strategies, ensuring appropriate surveillance, and managing complications. This state-of-the-art review summarizes the current indications for anthracyclines in modern oncology, explores recent evidence on pathophysiology and epidemiology, and discusses advances in cardioprotection measures in the anthracycline-treated patient. Additionally, it highlights key clinical challenges and research gaps in this area.
Key Words: anthracycline, biomarkers, cancer survivorship, diagnosis, heart failure
Central Illustration
Highlights
-
•
Anthracyclines serve as an essential therapy for many solid and hematologic malignancies, but their use is burdened by cardiac complications that negatively affect patient outcomes and may limit optimal cancer treatment.
-
•
Appropriate surveillance before, during, and after anthracycline exposure is crucial to prevent the progression of related cardiotoxicity toward overt heart failure.
-
•
Despite our knowledge on anthracycline cardiotoxicity, many unanswered questions still need to be addressed through properly designed basic/translational research and randomized clinical trials.
-
•
The establishment of a multidisciplinary cardio-oncology team is crucial to optimize oncologic outcomes without compromising cardiovascular health.
Since the 1980s, anthracyclines, including doxorubicin, daunorubicin, epirubicin, and idarubicin, have been central to treating various hematologic and solid malignancies, such as Hodgkin lymphoma (HL), non-Hodgkin lymphoma (nHL), acute leukemias, breast cancer (BC), ovarian cancer, and sarcomas.1,2 In clinical practice, the application of anthracyclines is limited by a dose-dependent cardiotoxicity, leading to both systolic and diastolic cardiac dysfunction and, in less common cases, overt heart failure (HF).1, 2, 3 Despite a decline in anthracyclines over recent years driven by the availability of less cardiotoxic or tumor-targeted alternatives,4 many patients continue to receive anthracycline-based regimens,5 thereby incurring a risk of treatment-related cardiotoxicity.
Anthracycline-related cardiotoxicity can be categorized based on the temporal relationship to drug administration.6 Acute cardiotoxicity may develop anytime during or shortly after the anthracycline treatment and is generally reversible upon drug discontinuation; it is rare, occurring in <1% of cases. Early onset cardiotoxicity can appear within the first year after treatment, which is often associated with an asymptomatic decline in left ventricular ejection fraction (LVEF); this form represents 98% of the total cardiotoxicity cases in a large cohort of anthracycline-treated patients.6,7 Late onset cardiotoxicity, which becomes clinically evident more than 1 year after exposure, typically presents as hypokinetic and/or dilated cardiomyopathy with overt HF symptoms.
A 2013 meta-analysis of 22,815 patients treated with anthracyclines reported a significant decline in LVEF, indicating cardiotoxicity in 6% of patients at a median follow-up of 9 years, with subclinical effects described in 18% of the cohort.8 Similarly, a recent retrospective population-based case-control study linked anthracycline exposure to an increased risk of HF, which begins 1 year after treatment and can persist for up to 20 years.9 Thus, anthracycline-related cardiotoxicity emerges as a unique pathophysiologic entity that builds on subclinical myocardial cell injury,1,2,10 progressing to an asymptomatic decline in LVEF and, if not addressed, culminating in overt HF. Such a continuum likely begins early in the course of anthracycline treatment, as suggested by the release of troponin soon after an anthracycline infusion.7,11 Clinical manifestations may occur at any point after treatment as a consequence of the heart’s inability to compensate for the initial damage.12
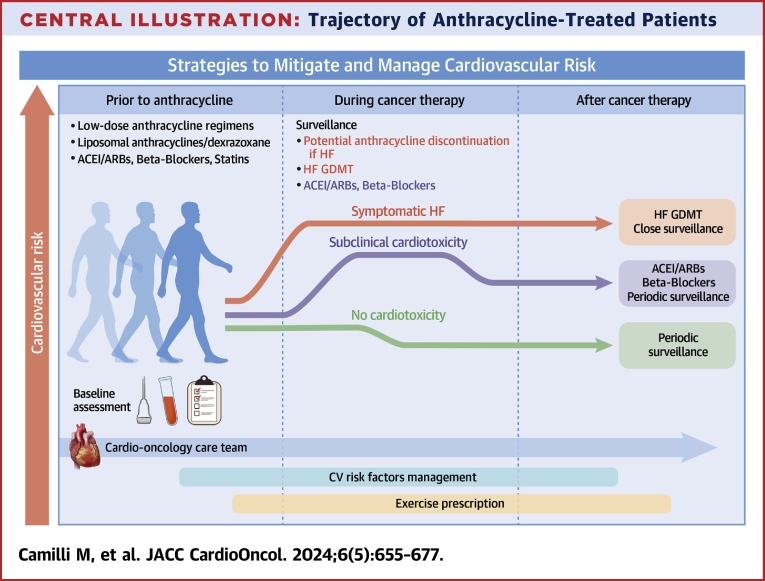
Considering the additive cardiotoxic effects of newer cancer drugs and/or radiotherapy (RT) alongside the growing number of cancer survivors exposed to cardiotoxic agents, a more proactive approach is crucial. This approach should identify the at-risk patient and implement strategies for preventing or treating cardiotoxicity (Central Illustration).
Central Illustration.
Trajectory of Anthracycline-Treated Patients
The risk of cardiotoxicity is dynamic and changes over time, influenced by the cumulative anthracyclines and concomitant cardiotoxic therapies. Pre-existing cardiovascular disease may amplify acute and long-term effects of anthracyclines on the heart. A dedicated cardio-oncology team can attenuate the risk through continuous refinement of risk factors, the establishment of cardioprotection measures, and exercise prescriptions. Despite these efforts, some patients may still develop overt heart failure (red line), necessitating chemotherapy adjustments or cessation and guideline-directed medical therapy. More commonly, patients experience subclinical cardiotoxicity is more frequent (green line), requiring prescription of cardiovascular drugs and close surveillance. ACEI = angiotensin-converting enzyme inhibitors; ARB = angiotensin 2 receptor blocker; CV = cardiovascular; GDMT = guideline-directed medical therapy; HF = heart failure.
In this state-of-the-art review, we examine the current clinical indications for anthracycline-based therapy in the adult cancer population. We also discuss the pathophysiological concepts of cardiovascular toxicity induced by anthracyclines and the cardiac surveillance strategies used before, during, and after chemotherapy framed within the context of recently published guidelines.6 Additionally, we explore the role of cardioprotective strategies in mitigating cardiac damage and the application of HF therapy in this population.
Current Anthracycline Indications and Regimens
Over the last 4 decades, anthracyclines have formed the backbone of many chemotherapy regimens.1,2 The risk of cardiotoxicity led to the development of anthracycline analogs, such as liposomal doxorubicin, which possess less toxic features.4,6,13 This concern also led to the incorporation of non–anthracycline-containing regimens (usually involving taxanes) in everyday practice.14 Additionally, clinical trials have explored the efficacy and safety of decreasing the cumulative dose of anthracyclines, mainly in the setting of early breast cancer (eBC).2,4,14 Table 1 outlines anthracycline-containing regimens and cumulative doses15 according to the underlying malignancy.
Table 1.
Principal Anthracycline-containing regimens used in clinical practice according to cancer type, timing and schedule, and number of cycles
| Cancer and Regimen | Anthracycline Used | Timing and Schedule | Number of Cycles | Doxorubicin Equivalent Dose15 |
|---|---|---|---|---|
| Breast Cancer | ||||
| Early Breast Cancer | ||||
| Neoadjuvant/Adjuvant Setting | 4 | |||
| AC/EC | Adriamycin | 60 mg/m2 day 1-every 21 days | 240 mg/m2 | |
| Epirubicin | 90 mg/m2 day 1-every 21 days | ≈240 mg/m2 | ||
| A/E->CMF | Adriamycin | 75 mg/m2 day 1-every 21 days | 4 | 300 mg/m2 |
| Epirubicin | 90 mg/m2 day 1-every 21 days | ≈240 mg/m2 | ||
| CAF/CEF | Adriamycin | 30 mg/m2, days 1, 8-every 28 days | 6 | 360 mg/m2 |
| Epirubicin | 60 mg/m2, days 1, 8-every 28 days | ≈480 mg/m2 | ||
| FAC/FEC | Adriamycin | 50-60 mg/m2, day 1-every 21 days | 6 | 360 mg/m2 |
| Epirubicin | 75-100 mg/m2, day 1-every 21 days | ≈400 mg/m2 | ||
| AC/EC/FEC->weekly Paclitaxel (12 weeks) | 4 | 240 mg/m2 | ||
| AC->weekly Docetaxel (4 weeks) | 4 | 240 mg/m2 | ||
| FEC100 > Docetaxel | Epirubicin | 100 mg/m2, day 1-every 21 days | 3 | ≈200 mg/m2 |
| TAC | Adriamycin | 50 mg/m2, day 1-every 21 days | 6 | 300 mg/m2 |
| Advanced/Metastatic Breast Cancer | ||||
| Anthracyclines+Taxanes | The association Anthracyclines (epirubicin, adriamycin)+Taxanes (paclitaxel, docetaxel, nabpaclitaxel) is preferred in patients with advanced PD-L1-, gBRCA1/2- TNBC, in particular in those without previous exposition to anthracyclines or with long disease-free interval. | |||
| Cancer and Regimen | Anthracycline Used | Timing and Schedule | Number of Cycles | Doxorubicin Equivalent Dose |
|---|---|---|---|---|
| Acute Myeloid Leukemia | ||||
| Ara-C+ Daunorubicin/Idarubicin |
Daunorubicin | 45 mg/m2, days 1 to 3 | ≈110 mg/m2 | |
| Idarubicin | 12 mg/m2, days 1 to 3 | ≈180 mg/m2 | ||
| Acute Lymphoblastic Leukemia | ||||
| CALGB 8811 | Daunorubicin | 45 mg/m2, days 1 to 3 | ≈110 mg/m2 | |
| Adriamycin for late intensification | 30 mg/m2, days 1, 8, 15 | 90 mg/m2 | ||
| hyper-CVAD | Adriamycin | 50 mg/m2, day 4 | 50 mg/m2 | |
| Hodgkin Lymphoma | ||||
| ABVD | Adriamycin | 25 mg/m2, day 1-15, every 28 days | 3-6 | 150 mg/m2-300 mg/m2 |
| BEACOPP | 8 6 |
|||
| Standard | Adriamycin | 25 mg/m2, day 1, every 21 days | 200 mg/m2 | |
| Dose-Escalated | Adriamycin | 35 mg/m2, day 1 every 21 days | 210 mg/m2 | |
| Diffuse Large B Cell Lymphoma | ||||
| R-CHOP | Adriamycin | 50 mg/m2, day 1, every 21 days | 6-8 | 300-400 mg/m2 |
| Sarcomasa | ||||
| STS | ||||
| Adriamycin alone or in combination with Ifosfamideb | Adriamycin | 75 mg/m2, day 1, every 14/21 days | 6 | 450 mg/m2 |
| Osteosarcoma | ||||
| High-dose ifosfamide, methotrexate, cisplatin and adriamycin | Adriamycin | 75 mg/m2 in neoadjuvant setting 90 mg/m2 |
2 | 255 mg/m2 |
| Ewing Sarcoma | ||||
| Vincristine, cyclophosphamide, ifosfamide, etoposide and adriamycin | Adriamycin | 60 mg/m2 (20 mg/m2 day 1, 2, 3 repeated every 14 days) | 6 | 360 mg/m2 |
A = adriamycin; ABVD = doxorubicin + bleomycin + vinblastine + dacarbazine; AC = adriamycin + cyclophosphamide; BEACOPP = bleomycin + etoposide + doxorubicin + cyclophosphamide + vincristine + procarbazine + prednisone; CAF = cyclophosphamide + adriamycin + 5-fluorouracil; CALGB 8811 = daunorubicin + vincristine + prednisone + pegaspargase + cyclophosphamide; CEF = cyclophosphamide + epirubicin + fluorouracil; CMF = cyclophosphamide + methotrexate + fluorouracil; E = epirubicin; EC = epirubicin+cyclophosphamide; FAC = fluorouracil + adriamycin + cyclophosphamide; FEC = fluorouracil + epirubicin + cyclophosphamide; hyper-CVAD = fractionated cyclophosphamide + vincristine + doxorubicin + dexamethasone; PD-L1 = programmed death-ligand 1; R-CHOP = rituximab-cyclophosphamide + doxorubicin + vincristine + prednisone; STS = soft tissue sarcoma; TAC = docetaxel + Adriamycin + cyclophosphamide; TNBC = triple-negative breast cancer.
With due exceptions, in patients affected by sarcoma, dose intensification by interval compression has demonstrated increased benefit than standard 3-week intervals, with no increase in toxicity.
Regimen used in advanced or metastatic STS. For anthracycline toxicity equivalence ratio15, doxorubicin: 1, daunorubicin: 0.833, idarubicin: 5, epirubicin: 0.67.
Breast cancer
Female BC is the most common cancer worldwide,16 with an estimated 2.3 million new cases in 2020, accounting for 11.7% of all new malignancies.16 BC is a highly heterogeneous disease, and treatment strategies vary according to the stage, baseline patient characteristics, and molecular features.16,17 For prognostic prediction and treatment decision making, breast tumors are classified into subtypes based on estrogen and progesterone receptors and human epidermal growth factor receptor 2 (HER2) expression.17 Other biomarkers, such as programmed death ligand 1 and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha, guide therapeutic choices in advanced cases.17
The role of anthracyclines in treating BC, particularly in the early stages, has continued to evolve over the last decade, with strategies ranging from the omission of this drug (Figure 1) to the reduction of the cumulative exposure. HER2-targeted therapies such as trastuzumab, pertuzumab, lapatinib, and neratinib are prescribed for both early and advanced BC.17 These therapies are associated with left ventricular (LV) dysfunction, typically presenting as an asymptomatic drop in LVEF, and less commonly as overt HF, particularly affecting fewer than 5% of cases.18 This risk increases when these therapies are used sequentially to anthracyclines.19
Figure 1.
Current Landscape of eBC Treatment
Main treatment opportunities for early breast cancer (eBC) are depicted, differentiating between neoadjuvant or adjuvant settings. Current pharmacologic options are diverse based on estrogen and progesterone receptors, human epidermal growth factor receptor 2 (HER2) expression, and BRCA mutations. Numerous chemotherapy schemes, increasingly excluding anthracyclines, are used in clinical practice. AC-T = adriamycin, cyclophosphamide followed by paclitaxel; AI = aromatase inhibitor; APT = adjuvant paclitaxel + trastuzumab; CDK4/6 = cyclin-dependent kinase 4 and 6; CMF = cyclophosphamide, methotrexate, 5-fluorouracil; eBC = early breast cancer; ER = estrogen receptor; ET = endocrine therapy; H = trastuzumab; NAT = neoadjuvant; OS = ovarian suppression; P = pertuzumab; PARP = polymeric adenosine diphosphate polymerase; pCR = pathologic complete response; PR = progesterone receptor; TC = docetaxel, cyclophosphamide; TCHP = docetaxel, carboplatin, trastuzumab, pertuzumab; T-DM1 = trastuzumab emtansine.
Research on anthracycline-sparing regimens has primarily focused on this context. The Breast Cancer International Research Group 006 trial20 demonstrated that omitting anthracyclines does not compromise clinical efficacy in women with HER2-positive eBC. Rates of HF and cardiac dysfunction (declines in LVEF >10%) were significantly higher in the anthracycline-containing regimen compared with a regimen of trastuzumab, carboplatin, and docetaxel.
Dual HER2 blockade with pertuzumab and trastuzumab has been shown to be more efficacious compared with trastuzumab alone, without significantly increasing the risk of cardiotoxicity in patients with lymph node–positive or high-risk lymph node–negative disease (tumors >2 cm or high-grade BC).21,22 This dual blockade is now offered before surgery.
In the neoadjuvant regimen, the combination of Doxorubicin and cyclophosphamide followed by paclitaxel with trastuzumab and pertuzumab has been effective. However, the docetaxel, carboplatin, trastuzumab and pertuzumab regimen has increasingly been adopted in clinical practice because it is similarly efficacious but less cardiotoxic.
For patients with lower-risk HER2-positive BC (<2 cm and lymph node negative), a regimen of weekly paclitaxel and trastuzumab for 12 weeks followed by trastuzumab for 40 weeks23 has shown a very low risk of eBC recurrence and no significant cardiotoxicity. Consequently, this regimen has been widely adopted. In summary, for patients with HER2-positive disease, the use of anthracyclines is not justified for low-risk patients and should only be considered for patients with a high risk of recurrence.
A number of studies have evaluated non–anthracycline-containing regimens in the HER2-negative eBC population. The U.S. Oncology Research Trial 9735 found that, at 7 years of follow-up, 4 cycles of docetaxel and cyclophosphamide (TC) resulted in superior disease-free survival and overall survival (OS) compared with 4 cycles of Adriamycin and cyclophosphamide in women with eBC.24 Similarly, the Western German Plan B trial25 found excellent outcomes for patients with T1 to T4 and node-positive disease or high-risk node-negative BC treated with 6 cycles of TC compared with 4 cycles of epirubicin/cyclophosphamide followed by 4 cycles of docetaxel. However, the incidence of HF or cardiac-related death was similar between the 2 groups.25
Finally, a pooled analysis of the ABC (Anthracyclines in Early Breast Cancer) trials,26 which included more than 2,000 HER2-negative eBC patients, investigated whether TC was noninferior to various anthracycline/taxane sequential regimen variants. However, this study failed to demonstrate noninferiority, instead highlighting a statistically significant improvement in disease-free survival with the administration of anthracyclines. No significant differences were observed between the 2 strategies in terms of relevant cardiac disorders.26 For patients with estrogen receptor–positive/HER2-negative eBC, anthracyclines should be considered for those cases at high risk of recurrence, acknowledging that there are efficacious non–anthracycline-containing options available, such as 6 cycles of TC.
In triple-negative breast cancer (TNBC), anthracyclines remain a cornerstone in treatment. There is evidence suggesting a significant benefit of anthracyclines for the TNBC subgroup,24 although the WSG (West German Study PlanB Trial) Plan B study25 demonstrated no additional benefit compared with non–anthracycline-containing regimens. The PATTERN (Adjuvant Platinum and Taxane in Triple-negative Breast Cancer) trial demonstrated a greater benefit of a platinum/taxane regimen over an anthracycline/taxane sequential regimen27; however, limitations have prevented its adoption in clinical practice. Similarly, the NeoSTOP (Randomized Phase II Trial of Anthracycline-free and Anthracycline-containing Neoadjuvant Carboplatin Chemotherapy Regimens in Stage I-III Triple-negative Breast Cancer) study investigated the efficacy of carboplatin/docetaxel vs carboplatin/paclitaxel followed by doxorubicin/cyclophosphamide. It showed similar treatment response and survival outcomes between the groups, with a more favorable toxicity profile for the sequential scheme.28 Although anthracyclines and taxanes appear to be needed for the vast majority of TNBC cases, some have postulated the possibility of an anthracycline-free regimen for stage 1 TNBC patients.29
For patients with metastatic disease, accurate molecular characterization is essential for selecting appropriate treatments, especially considering the recent favorable results from trials of targeted therapies. Anthracyclines may still serve as the primary therapy for those with TNBC who do not express programmed death ligand 1 or have BRCA1/2 germline mutations.30,31 Both pegylated and non–pegylated liposomal doxorubicin formulations are also approved in this setting.30,31
RT remains an essential component of BC therapy across all disease stages, from in situ to advanced.32 Despite improvements in RT techniques, planning, and delivery, myocardial damage and dysfunction can occur in a dose-dependent manner, with no safe dose identified.32 Additionally, concomitant anthracycline exposure can amplify the risk of cardiac disease regardless of the cardiac volume and radiation dose involved.32
Hematologic malignancies
Acute leukemias and lymphomas present disease scenarios in which the efficacy of anthracycline chemotherapy is indisputable. Indeed, anthracyclines play a crucial role in the treatment of leukemias because of the high proliferative rate of tumor cells (blasts), which necessitate the potent cytotoxic effects that anthracyclines provide.33,34
Daunorubicin, combined with cytosine arabinoside, has been the standard induction therapy for acute myeloid leukemia for over 3 decades.33,34 The cumulative doses generally depend on patients’ age and performance status; a high dose of daunorubicin (90 mg/m2 vs the standard dose of 45 mg/m2) is considered to improve OS in young adults without unfavorable cytogenetics.35 However, reducing the dose to 60 mg/m2 during the induction phase has been largely adapted in clinical practice.36
Similarly, dose-intense approaches have demonstrated survival in adults with acute lymphoblastic leukemia in whom induction regimens include anthracycline together with vincristine and corticosteroids.37 Initially, significant cardiovascular toxicity was not observed,37 but a higher cumulative dose of anthracyclines may lead to delayed cardiotoxicity, which is of particular importance in a young population with a potentially curable condition.
HL and the majority of nHLs with aggressive behavior rely on anthracycline-containing treatment schemes.38,39 With few exceptions, Adriamycin, bleomycin, vinblastine, and dacarbazine and rituximab-cyclophosphamide, Adriamycin, vincristine, and prednisone have been the most commonly prescribed chemotherapies for HL and diffuse large B-cell lymphomas, respectively, with few available frontline alternatives.38,39 All in all, advanced age and comorbidities may lead clinicians to opt for non–pegylated liposomal doxorubicin.13
All applied schemes include anthracyclines, which, in conjunction with RT,32 introduce a long-term risk of cardiac dysfunction and HF in survivors.1, 2, 3, 4 These issues continue to place anthracycline-related cardiotoxicity at the forefront of cardio-oncology, especially considering the increasing survival rates of this population.1, 2, 3, 4,6,12
Gynecologic malignancies
Gynecologic cancers, predominantly carcinomas of the ovary, endometrium, and cervix, account for approximately 13% of all cancers in women.40,41 Although recent advancements in targeted therapies have improved clinical outcomes, anthracyclines continue to play a role in managing gynecologic malignancies, particularly in advanced or recurrent ovarian and endometrial cancers.40,41 Pegylated liposomal doxorubicin, used in combination with carboplatin, is a preferred treatment for ovarian cancer, endometrial cancer, and uterine sarcomas.41 This formulation is notably associated with fewer cardiac sequelae, even at cumulative doses considerably higher than those typically considered safe. Cardiovascular toxicity has been investigated in a limited manner in this population.41
Bone and soft tissue sarcomas
Bone sarcomas and soft tissue sarcomas are rare tumors in adults.42,43 Because of their variable presentation, diagnosis is often delayed, leading many patients to require systemic treatment at advanced disease stages.42,43
Anthracyclines are the cornerstone of treatment both in the neoadjuvant/adjuvant setting and for metastatic cases.42,43 High doses of chemotherapy, particularly those containing anthracyclines and ifosfamide, have been shown to achieve higher response rates, possibly correlating with prolonged OS. Additionally, patients with large volume tumors, poor response after therapy, lung metastasis, or inadequate surgical margins may require adjuvant RT. This treatment further increases the risk of cardiotoxicity.
While we await approved therapies tailored to specific molecular targets, particularly tyrosine kinase inhibitors and immune checkpoint inhibitors, this population remains vulnerable to very high doses of anthracyclines and the risk of cardiovascular complications, especially in the absence of cardioprotective measures.44
Key Points
-
•
Anthracyclines continue to play an important role in the treatment of various solid and hematologic malignancies.
-
•
Despite efforts to reduce cumulative doses in the treatment of cancer, the use of liposomal formulations, and the omission of anthracyclines from chemotherapy regimens, many cancer patients are still treated with these drugs, which increases their risk of cardiac dysfunction.
Pathophysiology
The molecular mechanisms of anthracycline cardiotoxicity can be broadly divided into 3 main areas45, 46, 47: oxidative stress, alterations of cell death pathways, and epigenetic changes. Concerning oxidative stress, anthracyclines both generate reactive oxygen species (ROS)10,48, 49, 50 and mobilize iron,50,51 which facilitates the iron-catalyzed free radical deterioration of mitochondria and sarcoplasmic reticulum. This damage may lead to dysregulation of energy metabolism and calcium homeostasis. However, the cause-and-effect relations between the 2 processes remain unclear in many respects.45
Regarding alterations in cell death pathways, anthracyclines are known to induce myocyte apoptosis through both intrinsic and extrinsic pathways. Recent studies have also highlighted how pyroptosis and ferroptosis contribute to the loss of viable myocytes, primarily through the up-regulation of terminal differentiation-induced noncoding RNA, which leads to iron dysregulation and lipid peroxidation, respectively.45,50,51 Notably, oxidative stress and ferroptosis share clear biochemical mechanisms. Additionally, anthracyclines dysregulate autophagy, resulting in the accumulation of undegraded autophagosomes and autolysosomes. This accumulation eventually leads to death rather than allowing for the physiological degradation and recycling of cellular components.45
Epigenetics represents a new frontier of anthracycline cardiotoxicity. Anthracyclines can down-regulate DNA methylation, which results in altered mitochondrial gene expression, and up-regulate histone deacetylation, leading to deacetylation of proteins such as α-tubulin. Moreover, anthracyclines can both up-regulate and down-regulate numerous microRNAs, although the downstream consequences of these changes are only partially understood.52
At this point in time, identifying a single prevailing mechanism of cardiotoxicity amid what seems to be a constellation of potential contributors (or confounders) is overly ambitious. For example, attempts to prevent clinical manifestations of anthracycline cardiotoxicity with antioxidants have been unsuccessful,50 casting doubts on the role of oxidative stress and related aspects of ferroptosis. Dexrazoxane, which was long believed to prevent cardiotoxicity by chelating iron, has more recently been shown to prevent DNA double-strand breaks induced by anthracyclines through topoisomerase IIβ, which is constitutively expressed in cardiomyocytes.2,46 By doing so, dexrazoxane may also prevent downstream alterations of transcriptomics and mitochondrial biogenesis.53
Although the molecular mechanisms of cardiotoxicity remain uncertain in many respects, 2 pharmacokinetic determinants of cardiotoxicity rest on more solid evidence: the cumulative dose of anthracyclines10 and the extent of their accumulation in cardiac tissue.49 Both animal models and clinical data indicate a dose range below which the risk of cardiomyopathy is very low. This range presumably reflects the cardiomyocytes’ capacity to repair or compensate for the damage inflicted by anthracyclines. However, this equilibrium is disrupted when ongoing anthracycline administration overwhelms cellular defenses.10,48,49
On the other hand, the significance of the cumulative dose aligns with what we know about anthracycline accumulation in cardiac tissue.48 Anthracyclines are incompletely cleared from cardiomyocytes, leading to a cardiac pool that increases with each infusion and begins to induce cardiotoxicity.48 Morphologic and functional damage eventually occur when the cumulative dose causes the size of these cardiac anthracycline pools to exceed the detoxifying capacity of cardiomyocytes.48 With doxorubicin, the threshold between low and high HF risk because of anthracycline accumulation is defined at 250 to 300 mg/m2.6,46,50
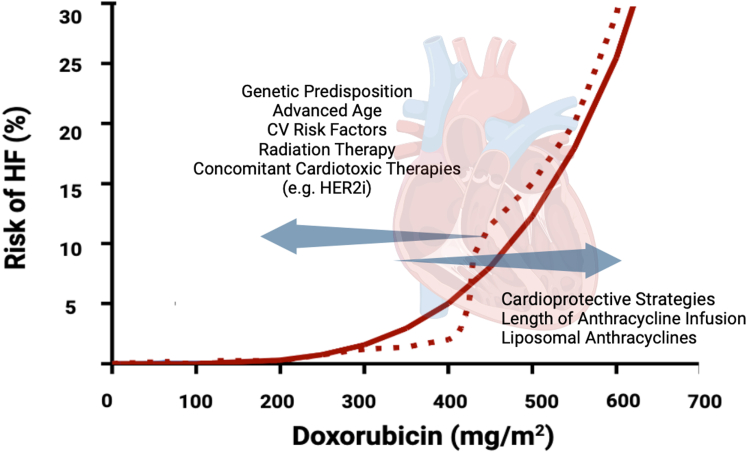
Cause-and-effect relationships between cumulative dose, anthracycline accumulation, and the risk of cardiomyopathy should not be viewed in isolation. Low cumulative doses and small anthracycline pools still induce cardiotoxicity if genetic variants, comorbidities, cardiac RT, or concomitant administration of other potentially cardiotoxic drugs magnify cardiac vulnerability.6,44,54 Conversely, cardioprotective strategies may shift cardiotoxicity to occur at higher cumulative doses. These concepts are important because they underline how various factors can eventually modify dose-risk relationships in anthracycline-treated patients (Figure 2).
Figure 2.
Determinants and Modifiers of Anthracycline Cardiotoxicity
The risk of cardiomyopathy and heart failures (HF) increases with the cumulative dose of doxorubicin (depicted by a dashed line). A mathematical model predicts the cause-and-effect relationship between cumulative dose and HF, incorporating the size of anthracycline pools that accumulate in cardiac tissue as exposure to doxorubicin increases. Cardiotoxicity occurs at lower doses with smaller anthracycline pools or at higher doses if the patient has predisposing risk factors or undergoes cardioprotective strategies. Adapted from Minotti et al.75 CV = cardiovascular; HER2i = human epidermal growth factor receptor 2 inhibitor.
On a different note, much of the uncertainty surrounding the molecular mechanisms of anthracycline cardiotoxicity reflects the inadequacy of experimental models. These models only occasionally use genetically conditioned or comorbid laboratory animals and even more rarely examine cardiotoxicity induced by multiagent cancer regimens.55
Furthermore, systemic inflammation, which occurs in cancer patients, may well compound the cardiotoxicity induced by anthracyclines.56 The extent of inflammation can vary widely across both solid and hematologic malignancies and even within tumor subtypes, introducing more variables to consider.56
Cause-and-effect relations between cardiac anthracycline accumulation and HF risk can also be exploited to devise cardioprotective strategies. Liposomal doxorubicin formulations, because of their size, do not easily diffuse through the regular endothelium of coronary vessels, resulting in reduced delivery of anthracycline to cardiomyocytes.6,13,46,50 Likewise, slow or prolonged infusions generate plasma anthracycline levels too low to promote significant anthracycline accumulation in cardiac tissue,46 yet tumors remain highly sensitive to continued anthracycline exposure. Thus, both liposomal formulations and slow or prolonged infusions can be used to mitigate the risk of cardiotoxicity while maintaining antitumor activity.
Definition of Cancer Therapy–Related Cardiac Dysfunction
Anthracycline administration can result in myocardial injury, dysfunction, and HF, with definitions of HF including new onset HF symptoms and/or a decline in estimated cardiac contractility, generally detected through echocardiography.6,46,57, 58, 59 However, numerous definitions of anthracycline-related cardiotoxicity have been recommended across consensus papers, observational reports, and clinical trials (Table 2).6,46,57, 58, 59 This heterogeneity has hampered the efforts to accurately determine the true incidence of anthracycline-related cardiotoxicity and its clinical outcomes.
Table 2.
Cancer Therapy–Related Cardiac Dysfunction Definitions According to Scientific Societies
| ASE/EACVI 201458 |
ESC Position Paper 201646 |
ESMO 202059 |
|---|---|---|
| Decrease in LVEF of >10% from baseline to LVEF <53% Relative drop in GLS >15% from baseline |
Decrease in LVEF of >10% from baseline to LVEF <50% Relative decrease in GLS of >15% from baseline |
LVEF drop by ≥10%-15% or LVEF <50% HF symptoms regardless of LVEF |
| IC-OS 202160 | ||
| ||
| ||
| ||
| ESC Guidelines 20226 | ||
| Asymptomatic | ||
| ||
| ||
| ||
| Symptomatic | ||
| ||
| ||
| ||
| ||
ASE = American Society of Echocardiography; BNP = brain natriuretic peptide; EACVI = European Association of Cardiovascular Imaging; ESC = European Society of Cardiology; ESMO = European Society of Medical Ooncology; GLS = global longitudinal strain; HF = heart failure; IC-OS = International Cardio-Oncology Society; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro–B-type natriuretic peptide.
In the seminal study by Cardinale et al,7 which followed a prospective cohort of 2,625 BC patients treated with anthracyclines over 5.2 years, the overall incidence of cancer therapy–related cardiac dysfunction (CTRCD), defined as a decrease in LVEF by more than 10 absolute points to below 50%, was found to be 9%. Most cases occurred during the first year of chemotherapy and were considered partially reversible after neurohormonal therapy.7 However, recent studies exploring the potential benefit of primary prevention in cancer patients receiving anthracyclines suggest that the incident rate of CTRCD can be attenuated.60,61
Defining CTRCD has become increasingly complex with the broader use of cardiac magnetic resonance (CMR)62 and the introduction of speckle-tracking global longitudinal strain (GLS), permitting more sensitive detection of LV dysfunction.6,57, 58, 59,63 Traditionally, the incidence of significant changes in LV function has been reported as <10%.6,7,46 However, the use of subclinical indexes of myocardial function has led to the diagnosis of CTRCD in up to 40% of patients enrolled in clinical studies.63 In a 2019 meta-analysis,63 both relative changes in GLS during treatment and reduced absolute GLS values showed adequate sensitivity and specificity as prognostic indexes of CTRCD.
In 2014, the American Society of Echocardiography and the European Association of Cardiovascular Imaging defined CTRCD as a drop in LVEF >10% and a decline of LVEF below 53%.57 However, a threshold for GLS decrement was not established, and modifications in cardiac biomarkers were not included. Aiming to harmonize terminology with available evidence, the International Cardio-Oncology Society (IC-OS) published a consensus document endorsed by the European Society of Cardiology (ESC) cardio-oncology guidelines.6,59 The term CTRCD, also adopted by the American Heart Association and the American College of Cardiology,59,64 now encompasses alterations in clinical, laboratory, and imaging biomarkers during or after anthracycline administration.6,59 This definition incorporates not only LVEF but also significant changes of GLS (>15% decrement from baseline), any new rise in blood biomarkers such as troponins and natriuretic peptides (NPs), and symptom occurrence.6,59
The clinical application of this integrated definition has led to increased rates of CTRCD diagnosis, including cases characterized only by troponin elevations, defined as “mild” cardiotoxicity.65 However, the clinical significance of such rises in troponins is a matter of debate. In a prospective, observational study of more than 300 BC patients followed for approximately 4 years, troponin elevation at the end of anthracycline therapy was associated with a 2-fold increased risk of CTRCD, defined as LVEF declining by 10% to a value below 50%.66 Additionally, a meta-analysis including 5,691 cancer patients showed that elevated troponin was common after treatment. Despite these limitations caused by high heterogeneity among studies, patients with high troponin values appeared to be at higher risk for LV dysfunction.67
The recent CardiaCARE (High-Sensitivity Cardiac Troponin I–Guided Combination Angiotensin Receptor Blockade and Beta Blocker Therapy to Prevent Cardiac Toxicity in Cancer Patients Receiving Anthracycline Chemotherapy) trial, an open-label, blinded endpoint trial involving 175 patients with BC or nHL, produced different results, showing no association between troponin I levels and changes in LVEF.68 However, it is important to note that troponin values were often below the reference limit typically used to define myocardial cell injury and identify high-risk patients in most clinical settings.69 Moreover, recent prospective data showed that biomarker-based CTRCD, diagnosed according to the IC-OS definition at the end of anthracycline therapy, did not predict reduced systolic function at the extended follow-up.65
Further prospective studies are needed to probe the prognostic significance of the integrated definition of CTRCD in terms of both LVEF reduction and cardiovascular and overall mortality. These studies should be powered by patients’ stratification based on anthracycline dose, include adequate follow-up, and use uniformly defined thresholds of high-sensitivity troponins.
CTRCD can manifest as asymptomatic drops in LVEF and, less frequently, as overt HF,6,46 with a prognosis comparable to other forms of nonischemic cardiomyopathies.70 This calls for effective management and diligent follow-up of cancer patients. Specifically, the application of guideline-recommended HF therapy71,72 may provide significant benefits for cancer patients who are usually excluded from HF clinical trials.
A recent retrospective analysis from the Women’s Health Initiative, which included older BC survivors, highlighted a higher incidence of hospitalizations for HF with preserved ejection fraction compared with HF with reduced ejection fraction.73,74 This may suggest that anthracycline cardiotoxicity may follow different pathophysiological trajectories, including early changes in diastolic relaxation.75 Additional data are needed to formally integrate HF with preserved ejection fraction into the current definitions of anthracycline cardiomyopathy.
Key Points
-
•
Anthracycline cardiotoxicity represents the paradigm of CTRCD.
-
•
Variability in defining anthracycline cardiotoxicity limits precise determinations of its incidence, progression, and prognostic value.
-
•
The definition of CTRCD, endorsed by multiple societies, now integrates LVEF and GLS decrements as well as troponin elevations and new onset HF symptoms.
-
•
The epidemiology and clinical outcomes of anthracycline-related cardiomyopathy should be re-evaluated in light of new CTRCD definitions, pathophysiological trajectories, and responses to HF therapies.
Surveillance of Cancer Patients Exposed to Anthracyclines
The risk of anthracycline-induced CTRCD is dynamic and evolves during patients’ journeys.6 It is influenced by several modifiable and nonmodifiable conditions such as age, sex, genetic factors, previous oncologic therapies, and pre-existing cardiovascular risk factors or diseases as well as by the type, duration, and intensity of the scheduled cancer therapies.6 Consequently, a comprehensive baseline risk assessment is recommended for all patients undergoing treatment with anthracyclines. This aims to mitigate cardiovascular risk, consider cardioprotective therapy, and ensure surveillance by a cardio-oncology team.6
In 2020, cardiovascular risk proformas, developed for some of the most common cancer treatments, were jointly released by the Heart Failure Association and IC-OS.76 Their use is endorsed by the 2022 ESC guidelines6 and have been evaluated in retrospective studies,77,78 although prospective validation in large-scale populations is still pending. Patients’ baseline assessment should include judicious anamnesis, physical examination, electrocardiogram, and cardiac function evaluation by echocardiography as well as troponin/NP dose when a high risk of CTRCD is expected. These cardiovascular risk proformas76 can help to stratify patients into low, intermediate, high, and very high risk categories for cardiotoxicity. However, individual variability because of genetic predisposition or other unidentified factors may still lead to cardiotoxicity regardless of the baseline risk assessment.
Current role and possibilities for gene testing in the anthracycline-treated patient
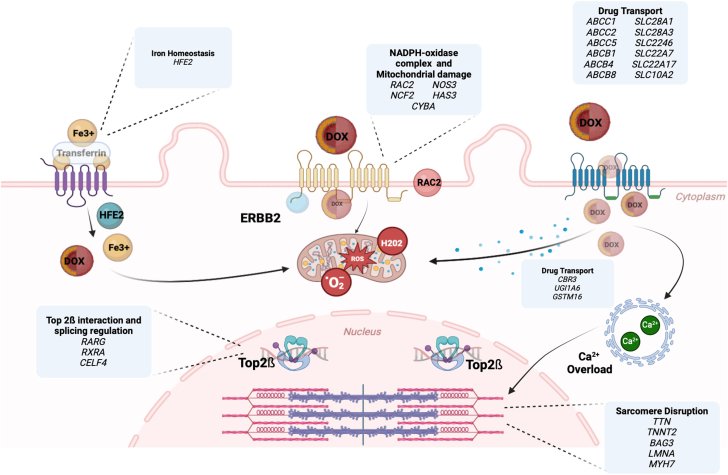
Genetic variants can alter a patient’s vulnerability to anthracyclines.54 Both genes encoding structural and functional proteins as well as noncoding regulatory DNA sequences may influence individual susceptibility.54,79 The main categories of genes involved in anthracycline cardiotoxicity are depicted in Figure 3. These include genetic variants linked to well-known cardiomyopathy conditions, such as titin, as well as genes that regulate the production or detoxification of ROS and those involved in drug metabolism and disposition.54,79,80 Regulatory microRNAs play an important role in mediating these genetic effects.52
Figure 3.
Graphical Representation of Main Cellular Pathways and Genes Involved in Susceptibility to Anthracycline Cardiotoxicity
Genetic alterations that predispose individuals to anthracycline cardiotoxicity may affect drug transport across the cell membrane or its clearance from the cell. Inside mitochondria, the reduction of anthracyclines forms a superoxide anion. Polymorphism in nicotinamide adenine dinucleotide phosphate (NADPH) oxidase subunits may cause overproduction of ROS, which can result from alterations in iron homeostasis. At the nucleus level, DNA topoisomerase I and II relieve tension in tightly wound DNA by introducing a DNA break. Anthracyclines target the Top2b-cleaved DNA complex, causing accumulation of double-strand DNA breaks and subsequent apoptosis. Genetic variants with inherited dilated cardiomyopathy also play a role. DOX = doxorubicin; Fe = iron; HFE2 = hemojuvelin 2; ROS = reactive oxygen species; Top2 = topoisomerase 2.
Traditional genetic association studies have primarily focused on single-nucleotide polymorphisms (SNPs).81 These studies involve childhood cancer survivors who were exposed to anthracyclines and subsequently developed cardiac dysfunction.82 However, an approach considering multiple SNPs within a gene and their interactions at the gene level could unveil associations that might otherwise be overlooked.
Several limitations have hindered the incorporation of specific gene-association study findings into clinical practice.6,81 As mentioned previously, the majority of available reports are event-driven retrospective studies of childhood cancer survivors, which precludes generalizations to adult patients. Moreover, despite numerous genome-wide association studies and candidate gene association studies identifying almost 80 genes with SNPs significantly linked to anthracycline-induced cardiotoxicity, only 1 variant locus has been functionally validated and independently confirmed.83
Emerging studies using human-induced pluripotent stem cell–derived cardiomyocytes may offer novel opportunities to characterize genetic determinants of anthracycline cardiotoxicity.84 To advance this field, translational research supported by proof-of-concept clinical studies is needed to build personalized approaches for patients undergoing anthracycline-containing regimens who are at risk of CTRCD.55
Cardiovascular imaging in the anthracycline-treated patient
Imaging is the cornerstone of baseline evaluation for candidates of cardiotoxic therapies like CTRCD,57,62,64 with echocardiography as the first-choice modality to evaluate anthracycline-treated patients.57,62,64 Baseline LVEF, whether estimated by the 2-dimensional or 3-dimensional approach, is the most widely used index of systolic function and a predictor of future HF in patients exposed to anthracyclines.64,85 Patients with LVEF at the lower limits of normal are at an increased risk of incident HF.85
In cases of reduced LV function, HF medications should be initiated according to ESC guidelines.71,72 Additionally, a multidisciplinary discussion involving the oncologist and cardiologist should define the patient’s care journey, including a joint risk-benefit analysis of using an anthracycline-containing oncologic regimen.6 Baseline determination of GLS may assist risk assessment for patients presenting at treatment with an LVEF between 50% and 59%.86 GLS <16% or a relative modification >15% from baseline are considered risk markers,61,63,86 indicating the potential need to start cardioprotective drugs before LVEF decrements eventually occur.6,60
The SUCCOUR (Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy) trial investigated guiding patient management on the basis of GLS,60 randomizing patients to start beta-blockers and angiotensin-converting enzyme inhibitors (ACEIs) according to modifications of LVEF or GLS. In a low-risk population, mainly represented by women with BC, the primary outcome of LVEF decrements at 1 and 3 years showed no difference between the 2 arms. Additionally, the rate of patients with LVEF <55% was similar despite more frequent prescriptions of cardioprotective therapy in those randomized to the GLS arm. Moreover, in the GLS-guided arm, there were more cases of delayed or discontinued cancer therapy, suggesting that an emphasis on GLS may lead to heightened concerns about the potential onset of clinical cardiotoxicity. The very low rate of cardiovascular events observed in both arms did not result in practice-changing indications87 but raised questions about the role and interpretation of GLS changes in these patients.
Diastolic dysfunction (DD) can occur in patients undergoing treatment with anthracyclines.75,88 The largest prospective study on DD in cancer patients, which included 362 women with eBC, showed that LVEF decrements were associated with longitudinal alterations of diastolic function during therapy.88 Therefore, DD may represent a promising tool for early detection of CTRCD. However, diagnostic challenges and hemodynamic confounders unique to cancer patients must be carefully considered.
Myocardial work and LV-arterial coupling, noninvasive metrics that assess chamber stiffness and arterial load, have an established prognostic role in the general HF population.89,90 These metrics might provide insights into alterations of diastolic performance after exposure to anthracycline, although ad hoc studies in the cancer population are still needed. On the other hand, myocardial work indexes did not demonstrate any incremental value over GLS or clinical risk factors in identifying CTRCD in 136 HER2-positive BC patients treated with anthracyclines and trastuzumab.91
As for other potential indexes to explore, limited but persuasive evidence suggests that the right ventricle is also prone to anthracycline damage.6 Three-dimensional echocardiography and strain imaging can be used to evaluate right ventricular function in the anthracycline-treated patient.6
Although CMR is not always available in community centers, introduces cost-related considerations, and challenges patient compliance, it is not routinely used for everyday cancer patient monitoring. However, its higher reproducibility in estimating LVEF and biventricular volume or mass, along with its unique capacity for tissue characterization,6,62,65,92,93 has led to increased use in clinical trials.93 CTRCD is defined by CMR as LVEF reduction of more than 10% to a value below 53%.92 Strain can be also assessed through algorithms such as displacement encoding with stimulated echoes or Fast-SENC (MyoStrain),92,93 although definitive thresholds for toxicity have not been established.
CMR can detect microvascular obstruction, tissue iron overload, and diffuse interstitial fibrosis (via native T1 mapping and calculation of the extracellular volume fraction), whereas myocardial edema can be quantified via T2 mapping.62,65,92,93 These techniques have recently shown increased diagnostic and prognostic ability in limited studies of patients undergoing anthracycline-based therapy94 and have gained credibility to integrate late gadolinium enhancement quantification to evaluate focal fibrosis.92,93
In clinical practice, CMR is mainly used for evaluating LVEF when echocardiography is limited by a poor acoustic window or provides borderline values or when a clinical suspicion of myocarditis requires diagnostic refinement.62,65,92,93 CMR is also useful for diagnosing pericardial disease, which is common in patients exposed to mediastinal irradiation and occasionally occurs after anthracycline treatment.62,65,92,93 The lengthy scan time poses a challenge for patient compliance, particularly for debilitated patients. New CMR techniques, such as single breath-hold 3-dimensional sequences, have shorter scan times and might prove useful in these cases.95
Cardiac computed tomography (CCT) has long been used to diagnose hemodynamically significant coronary artery disease (CAD) or pericardial disease and to guide interventions in high-risk patients with valvular disease.6,62,65,96 Recent advancements have also made CCT useful for tissue characterization,96 expanding its application in cardio-oncology.
In the realm of nuclear medicine, multigated acquisition scintigraphy can quantify LVEF using the patient’s own radiolabeled erythrocytes without relying on geometric assumptions.6,62,65,97 However, the serial use of multigated acquisitions introduces a burden of radiation exposure, which has led to their gradual replacement by serial echocardiograms. However, nuclear imaging continues to play a key role in detecting physical or pharmacologically induced ischemia, whether at baseline in high-risk patients or in patients with recurrent symptoms.6,62,65,97 Moreover, new radiotracers are being developed that may help characterize myocardial metabolomic and proteomic profiles, possibly serving as novel biomarkers of cardiotoxicity.97
Serum biomarkers in the anthracycline-treated patient
Circulating biomarkers offer sensitive, personalized, and cost-effective surveillance in patients undergoing anthracycline therapy.98 Troponins, which are cardiac-specific circulating proteins, become indicative of myocardial injury when their levels exceed the 99th percentile in the general population.99 The currently available high-sensitivity assays are able to detect cardiac damage up to 7 times more frequently than previous generations.98 This enhancement supports their application in diagnosing CTRCD. Troponins have also demonstrated predictive and prognostic value in assessing cardiotoxicity (Supplemental Table 1).
Moreover, the role of troponins in guiding cardioprotective interventions was tested in 2 randomized controlled trials. The first trial, involving 114 patients exposed to high-dose chemotherapy, showed that enalapril provided 100% protection against decrements in LVEF after significant troponin increases.100 The hypothesis of a “troponin-triggered” strategy was then probed in the IC-OS-one trial in which 273 patients were randomly assigned to receive enalapril either before chemotherapy or only after an increase in troponin during or after anthracycline treatment.101 The participants in this trial were at low risk for cardiotoxicity, both in terms of baseline characteristics and cumulative anthracycline doses, resulting in very low event rates and no significant difference between the 2 groups.
Overall, although early studies suggested troponin-triggered cardiovascular therapy may represent a readily available cardioprotective measure, particularly for patients exposed to high-dose chemotherapy, the varying performance between troponin I and T and the absence of results stratified by sex-specific thresholds have historically limited the interpretation of troponin-based studies.66,102 In addition, significant heterogeneity in the timing of blood testing across clinicians and institutions complicates the comparison of study results, making interpretations challenging.
NPs, including B-type natriuretic peptide and N-terminal pro–B-type natriuretic peptide (NT-proBNP), are cardiac hormones critical for diagnosis and managing HF.71,72 NPs were extensively investigated in CTRCD prediction, but the interpretation of results must consider confounders such as kidney function, body weight, use of angiotensin receptor neprilysin inhibitors, and cardiac rhythm disorders.71,72 Although several studies have demonstrated increases in B-type natriuretic peptide patients treated with anthracyclines and persistent elevation of NT-proBNP after BC treatment, correlations between these laboratory findings and the prediction of cardiotoxicity endpoints across multiple studies have been variable.67 Moreover, the Cardiac-Oncology Toxicity registry did not find baseline NT-proBNP to be a strong predictor of the degree of cardiac dysfunction detected after chemotherapy.103 Consequently, although NP elevations are often observed at baseline and during chemotherapy in patients who may eventually develop CTRCD,6,98 the predictive value of such elevations across studies remains controversial.
The multifactorial nature of anthracycline cardiotoxicity suggests that circulating markers of oxidative stress (eg, myeloperoxidase), inflammation (eg, C-reactive protein and interleukin-6), and fibrosis (eg, soluble suppressor of tumorigenicity 2 and galectin-3) might be useful in predicting cardiotoxicity.51,56,104 Profiling a patient’s genomics, transcriptomics, proteomics, and metabolomics could explain the diverse cardiovascular vulnerabilities across patients. Additionally, novel biomarkers should be tailored to specific cancer types to avoid false positives caused by the cancer itself.104 Although developing biomarkers that can unmistakably diagnose CTRCD is attractive, it is crucial to consider how the mechanisms of cardiotoxicity overlap with cancer biology and comorbidities.
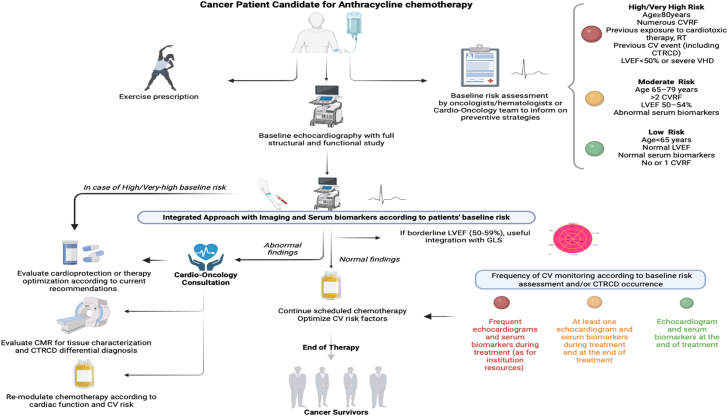
In summary, evaluating cardiac biomarkers before chemotherapy can play a role in stratifying the baseline risk of cardiotoxicity.6,104 Biomarker-assisted surveillance during anthracycline administration should also be considered, particularly for individuals at higher risk or if symptoms occur.6,76 However, laboratory findings must be integrated with imaging and electrocardiographic data (Figure 4).6 Although the cutoffs that define a clinically significant event are yet to be established, troponins have shown a high negative predictive value for cardiotoxicity.11,98 This allows for the identification of low-risk patients who may not require intensive cardiologic surveillance or cardioprotective strategies.
Figure 4.
Cardiovascular Monitoring in Cancer Patients Undergoing Anthracycline-Containing Regimens According to Risk Assessment
Surveillance should be guided by baseline cardiovascular risk stratification (indicated by red, yellow, and green bullets) and any abnormal findings during follow-up. These findings may necessitate further diagnostic investigations, refinement of cardioprotective therapy, or modifications to the oncologic treatment. CMR = cardiac magnetic resonance; CTRCD = cancer therapy–related cardiac dysfunction; CV = cardiovascular; CVRF = cardiovascular risk factor; GLS = global longitudinal strain; LVEF = left ventricular ejection fraction; RT = radiotherapy; VHD = valvular heart disease.
NPs may be useful at baseline evaluation for revealing pre-existing but unrecognized cardiac conditions and guiding patients toward more intensive surveillance. Additionally, assessing NPs during and particularly after anthracycline treatment may be valuable in identifying (diagnosing) asymptomatic cardiac dysfunction.105 Based on these considerations, recent ESC guidelines6 suggest using cardiac biomarkers throughout a cancer patient’s treatment pathway, adjusting the intensity according to estimated baseline cardiovascular risk. More frequent assessments are suggested only for patients considered at high and very high risk for CTRCD (Class of Recommendation 1, Level of Evidence: B).
Key Points
-
•
Baseline risk stratification tools are essential for guiding both treatment and surveillance of cancer patients during and after chemotherapy.
-
•
Multimodality imaging allows for early diagnosis of CTRCD, with echocardiography playing a pivotal role in the management of cancer patients.
-
•
Cardiac biomarkers can complement imaging and electrocardiographic evaluations, but the interpretation of these studies is often limited by small sample sizes and the lack of adequate standardization.
Cardioprotective Strategies in the Anthracycline-Treated Patient
Protective measures against CTRCD include strategies that address patient-related and treatment-related risk factors.6,106 Cardioprotective strategies can be divided into pharmacologic and nonpharmacologic categories, the latter of which includes interventions such as aerobic physical exercise.6,106,107 Participating in exercise programs is known to have significant cardiac health benefits and is particularly important for cancer patients at risk of cardiotoxicity.107
The American Heart Association’s Cardio-Oncology Rehabilitation approach extends traditional cardiology rehabilitation programs to oncology patients.107 Primary prevention strategies such as smoking habit cessation and maintaining a healthy diet are also crucial.6 Other modifiable risk factors, such as diabetes, hypertension, and dyslipidemia, should be systematically investigated and managed before, during, and after anthracycline treatment.6
The number of cardiovascular risk factors defines the baseline risk of CTRCD,6,76 which necessitates the optimization of cardiovascular medications. Neurohormonal antagonists, including ACEIs, angiotensin receptor blockers, and beta-blockers, have been extensively investigated with the aim of preserving LVEF during cardiotoxic therapy.106,108 Although effective against neurohormonal activation induced by LV dysfunction,71 these drugs may be less effective in preventing the cardiotoxic effects of anthracyclines, which induce only moderate neurohormonal activation.
Randomized clinical trials involving low- to moderate-risk patients treated with anthracyclines have not shown significant cardioprotective effects from carvedilol (in the CECCY [Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity] trial),109 candesartan (in the PRADA [Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy] trial),110 or combinations of candesartan and carvedilol (in the CardiacCARE trial).68 However, interestingly, an interim analysis of the placebo-controlled Cardiotoxicity Prevention in Breast Cancer Patients Treated With Anthracyclines and/or Trastuzumab trial,111 which randomized 174 low-risk patients to bisoprolol, ramipril, or both, showed that cardioprotective therapy could prevent changes in LVEF and GLS, particularly in patients receiving both ramipril and bisoprolol. The observation of more frequent LVEF decrements >10% in the placebo arm reinforced the potential value of cardioprotection, even in low-risk patients. Neurohormonal blockade may be more effective in high-risk to very high–risk patients; however, in general, these patients often begin cancer treatment already taking prescribed cardiovascular drugs. Therefore, therapy should be optimized before starting chemotherapy.6
The cardioprotective efficacy of statins has been investigated in cancer patients at varying risks of CTRCD.112, 113, 114 In particular, the placebo-controlled STOP-CA (Atorvastatin for Anthracycline-Associated Cardiac Dysfunction) trial,114 which included patients diagnosed with lymphoma receiving doxorubicin doses exceeding 300 mg/m2 in more than 50% of cases, showed that 40 mg atorvastatin prevented decrements in LVEF 1 year later. In contrast, the SPARE-HF (Statins for the Primary Prevention of Heart Failure in Patients Receiving Anthracycline Pilot Study)111 and PREVENT (Preventing Anthracycline Cardiovascular Toxicity With Statins)113 trials, primarily involving BC patients randomized to 40 mg atorvastatin or placebo, did not demonstrate a significant effect of atorvastatin on LVEF as measured by CMR. The inconsistency in responses is likely because BC patients typically receive lower cumulative doses of anthracycline and have a lower risk of CTRCD115 compared with lymphoma patients. Lymphoma patients experience a higher inflammatory burden, which may respond well to the pleiotropic effects of statins. Therefore, statins could be considered for preventing CTRCD in high-risk lymphoma patients.
Additionally, ongoing clinical studies of angiotensin receptor neprilysin inhibitors116 and preclinical data on sodium glucose co-transporter 2 inhibitors117 are exploring novel cardioprotective strategies. Other potential candidates for cardioprotection include ranolazine, an inhibitor of the inward INa+ current118; trimetazidine, an anti-ischemic metabolic agent106; and ivabradine, which inhibits the funny current.119
Dexrazoxane remains the only approved drug for preventing anthracycline-related CTRCD given the lack of conclusive evidence supporting the efficacy of primary prevention with neurohormonal therapies.6,44,105,118 Clinical trials and Cochrane analyses120,121 consistently show dexrazoxane’s efficacy in reducing HF risk in patients exposed to anthracyclines.6 However, its clinical use has been limited because of isolated concerns about potential interferences with anthracycline activity or an increased risk of secondary malignancies.44
Dexrazoxane provides substantial protection when initiated concurrently with the first anthracycline infusion. However, regulatory agencies recommend its use only for patients with advanced BC who have received a cumulative doxorubicin dose of 300 mg/m2 and may benefit from continued anthracycline treatment.6 Liposomal doxorubicin is a viable alternative, particularly for older patients, those with pre-existing LV dysfunction, or those with significant comorbidities,6,13,106 and is formally recommended by ESC guidelines for CTRCD prevention.6 Slow infusion methods,122 once more common, have fallen out of favor because of challenges such as length of hospitalization and aggravation of hematologic and nonhematologic toxicities.
Collaboration between oncologists and cardiologists through the establishment of cardio-oncology services123 is essential for managing patients at risk of CTRCD. Adjusting anthracycline doses or replacing anthracyclines with less cardiotoxic drugs involves a multidisciplinary risk-benefit assessment, similar to the optimization of treatment for pre-existing cardiovascular conditions.
Key Points
-
•
The optimal cardioprotective strategy for patients undergoing anthracycline therapy is yet to be clearly defined.
-
•
Neurohormonal blockade is recommended for preventing CTRCD in patients who receive high-dose anthracyclines (≥250 mg/m2) and/or those with pre-existing cardiovascular conditions. Statins may be beneficial for lymphoma patients scheduled for high-dose anthracyclines.
-
•
Appropriate patient education is recommended, including advice on maintaining a healthy lifestyle and engaging in aerobic physical activity.
Management of Anthracycline-Induced CTRCD
Despite its association with poor prognosis, anthracycline-induced CTRCD should not be considered irreversible or intractable. Two significant reports7,124 have demonstrated the effectiveness of an ACEI (enalapril) and beta-blockers (carvedilol/bisoprolol) in reversing anthracycline-induced CTRCD. These reports documented an inverse relationship between the time elapsed from the end of chemotherapy to the commencement of HF therapy and the improvements in LVEF. Specifically, complete recovery from LVEF ≤45% occurred in 64% of patients when HF therapy was initiated within 2 months postchemotherapy. In contrast, no complete recovery or essentially no improvement was noted when cardiovascular drugs were started at 6 or 12 months, respectively.124
Furthermore, clinical benefits were more evident in asymptomatic or mildly symptomatic patients. In a cohort of 2,625 consecutive patients exposed to anthracycline,7 quarterly echocardiography during the first year after chemotherapy allowed for the early detection of most cases of CTRCD. Prompt initiation of enalapril, carvedilol, or bisoprolol enabled LVEF normalization in 82% of cases.7
The ESC guidelines on cardio-oncology6 recommend treating CTRCD according to the current guidelines for nononcologic HF.71,72 Therefore, the use of ACEIs, angiotensin receptor blockers, angiotensin receptor neprilysin inhibitors, beta-blockers, sodium glucose co-transporter 2 inhibitors, and mineralocorticoid receptor antagonists is advised, tailored to LVEF and NP levels.6,71,72 Uptitration to target doses is advocated.6,71,72 If CTRCD occurs during treatment, the continuation of anthracyclines is allowed only in patients exhibiting mild asymptomatic dysfunction, pending the establishment of pharmacologic therapy and a multidisciplinary team discussion.6
Key Points
-
•
CTRCD can be reversible if detected early and treated appropriately.
-
•
ESC guidelines on cardio-oncology recommend managing CTRCD according to current HF recommendations.
-
•
Discussions within a cardio-oncology multidisciplinary team are crucial when managing LV dysfunction and determining the oncologic necessity for anthracycline treatment.
Cancer Survivorship
Survivors of anthracycline-treated cancer incur a 2-fold to 5-fold higher risk of mortality because of cardiovascular disease compared with the general population, requiring careful evaluation and management of long-term cardiovascular risk.6,124 Several known factors contribute to the risk of long-term CTRCD including aging; concomitant chest RT; high doses of anthracycline; and the presence of pre-existing or de novo cardiovascular conditions such as hypertension, diabetes, dyslipidemia, CAD, and abnormal LVEF.125 Risk assessment tools have been proposed to predict the risk of HF, ischemic heart disease, and stroke in survivors of childhood cancer.126
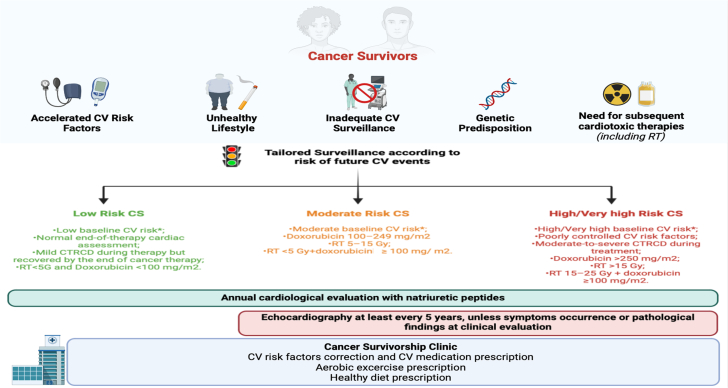
Recent recommendations have emphasized the importance of using the coronary artery calcium score, which is obtained through CCT, for identifying the burden of CAD.96 The ESC guidelines advocate for an integrated risk assessment that includes refining cardiovascular risk factors within the first year after therapy. This assessment should include clinical evaluations, echocardiograms, and serum biomarkers as needed. Extended follow-up is recommended and should be tailored based on incident pathological findings during the initial assessment6 (Figure 5).
Figure 5.
Adult Cancer Survivors: Comorbidities, Risk Stratification, and CV Care Necessities
A patient is considered a cancer survivor from the time of diagnosis until the end of life. With improvements in early detection and treatment, the population of cancer survivors has grown steadily. These survivors face a higher burden of CV disease compared with the general population, necessitating long-term care based on risk stratification. Establishing clinics for cancer survivors can address these needs through the prescription of healthy lifestyles and the early identification and treatment of CV comorbidities. CS = cancer survivor; other abbreviations as in Figure 4.
The lack of adequate surveillance significantly contributes to late occurrences of CTRCD, allowing cardiovascular diseases to progress unchecked. However, although there is a need for dedicated clinics for cancer survivors,127 this must be considered in light of the resource constraints of health care institutions. It is crucial to support strategic models of cancer survivorship care that include primary care providers. Equally important is the need to improve cancer survivors’ awareness of the risk-benefit ratio of the oncologic treatment they have received. Educating patients about possible long-term complications and the benefits of maintaining an adequate lifestyle are essential for minimizing future health risks.
Key Points
-
•
Cancer survivors experience a higher risk of cardiovascular diseases than the general population.
-
•
Dedicated clinics for survivors should be established to periodically reassess cardiovascular risks, manage potential cardiovascular complications, and offer tailored rehabilitation programs.
Future Perspectives and Conclusions
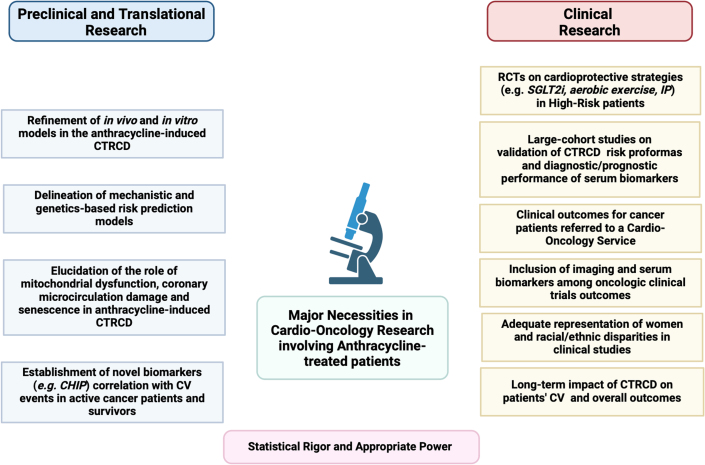
Anthracycline-related CTRCD and cardiomyopathy continue to pose significant challenges in contemporary cardio-oncology, with many unanswered questions about the underlying mechanisms and clinical consequences of these conditions (Figure 6). Further translational research is mandatory to identify and validate early and sensitive biomarkers of cardiotoxicity that are robust and predictive.
Figure 6.
Major Preclinical/Translational/Clinical Necessities in Cardio-Oncology Research Involving Anthracycline-Treated Patients and Survivors
This figure highlights the key preclinical and clinical research needs for patients treated with anthracyclines. Despite years of study, CV toxicity induced by anthracyclines still has many pathophysiological aspects uncovered. Additionally, much of clinical practice lacks support from high-quality evidence. This underscores the need for randomized controlled trials (RCTs) and collaboration efforts between clinicians and preclinical experts to address these gaps. CHIP = clonal hematopoiesis of indeterminate potential; IP = ischemic preconditioning; SGLT2i = sodium glucose co-transporter 2 inhibitor; other abbreviations as in Figure 4.
Genetic variants that modify individual susceptibility to CTRCD require thorough investigation, but this must be weighed against the cost-effectiveness of pre-emptive tests. Randomized controlled trials should validate risk proformas and cardioprotective strategies for high-risk populations using innovative approaches that address sex and racial disparities. Moreover, the optimal management of anthracycline-induced cardiotoxicity must be refined to consider more than the typical clinical outcomes observed in the general population.
The care of patients with cancer is complex. To optimize oncologic outcomes without compromising cardiovascular health, the establishment of a multidisciplinary cardio-oncology team (Figure 7) is essential. There is a crucial need for educational programs across various disciplines—nursing, internal medicine, and pharmacy—and at all training levels, from medical students to fellows, to ensure health care providers are well equipped with the knowledge needed to treat cancer patients effectively.
Figure 7.
The Multidisciplinary Cardio-Oncology Team
The expected increase in the number of cancer patients at risk of developing/worsening cardiovascular disease (CVD) necessitates an integrative multidisciplinary approach and care by dedicated specialists. Multidisciplinary cardio-oncology teams are essential for addressing the full spectrum of prevention, detection, monitoring, and treatment of cancer patients at risk of cardiotoxicity and those with concomitant CVDs. CV = cardiovascular; CTR-CVT = cancer treatment–related cardiovascular toxicity.
In today’s era, individuals diagnosed with cancer are living longer, which necessitates proportionally extended longer periods of surveillance compared to the past. As health care professionals, we need to guarantee that these survival rates are not negated by increased cardiovascular morbidity and mortality.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors acknowledge Biorender.com for preparation of the images.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental table, please see the online version of this paper.
Appendix
References
- 1.McGowan J.V., Chung R., Maulik A., Piotrowska I., Walker J.M., Yellon D.M. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31(1):63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minotti G., Menna P., Salvatorelli E., Cairo G., Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56(2):185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 3.Bloom M.W., Hamo C.E., Cardinale D., et al. cancer therapy-related cardiac dysfunction and heart failure: part 1: definitions, pathophysiology, risk factors, and imaging. Circ Heart Fail. 2016;9(1) doi: 10.1161/CIRCHEARTFAILURE.115.002661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levis B.E., Binkley P.F., Shapiro C.L. Cardiotoxic effects of anthracycline-based therapy: what is the evidence and what are the potential harms? Lancet Oncol. 2017;18(8):e445–e456. doi: 10.1016/S1470-2045(17)30535-1. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . World Health Organization; 2023. World Health Organization Model List of Essential Medicines-23rd List, 2023. [Google Scholar]
- 6.Lyon A.R., López-Fernández T., Couch L.S., et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur Heart J. 2022;43(41):4229–4361. doi: 10.1093/eurheartj/ehac244. [DOI] [PubMed] [Google Scholar]
- 7.Cardinale D., Colombo A., Bacchiani G., et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 8.Lotrionte M., Biondi-Zoccai G., Abbate A., et al. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol. 2013;112(12):1980–1984. doi: 10.1016/j.amjcard.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 9.Larsen C.M., Garcia Arango M., Dasari H., et al. Association of anthracycline with heart failure in patients treated for breast cancer or lymphoma, 1985-2010. JAMA Netw Open. 2023;6(2) doi: 10.1001/jamanetworkopen.2022.54669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang S., Liu X., Bawa-Khalfe T., et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 11.Cardinale D., Sandri M.T., Martinoni A., et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol. 2000;36(2):517–522. doi: 10.1016/s0735-1097(00)00748-8. [DOI] [PubMed] [Google Scholar]
- 12.Camilli M., Del Buono M.G., Crea F., Minotti G. Acute heart failure 29 years after treatment for childhood cancer. JACC CardioOncol. 2020;2(2):316–319. doi: 10.1016/j.jaccao.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rigacci L., Annibali O., Kovalchuk S., et al. Nonpeghylated liposomal doxorubicin combination regimen (R-COMP) for the treatment of lymphoma patients with advanced age or cardiac comorbidity. Hematol Oncol. 2020;38(4):478–486. doi: 10.1002/hon.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Anthracycline-containing and taxane-containing chemotherapy for early-stage operable breast cancer: a patient-level meta-analysis of 100 000 women from 86 randomised trials. Lancet. 2023;401(10384):1277–1292. doi: 10.1016/S0140-6736(23)00285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feijen E.A.M., Leisenring W.M., Stratton K.L., et al. Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity. JAMA Oncol. 2019;5(6):864–871. doi: 10.1001/jamaoncol.2018.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold M., Morgan E., Rumgay H., et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 2022;66:15–23. doi: 10.1016/j.breast.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rakha E.A., Tse G.M., Quinn C.M. An update on the pathological classification of breast cancer. Histopathology. 2023;82(1):5–16. doi: 10.1111/his.14786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng H., Mahmood S.S., Khalique O.K., Zhan H. Trastuzumab-induced cardiotoxicity: when and how much should we worry? JCO Oncol Pract. 2024;20(8):1055–1063. doi: 10.1200/OP.23.00816. [DOI] [PubMed] [Google Scholar]
- 19.Cardinale D., Colombo A., Torrisi R., et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28(25):3910–3916. doi: 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 20.Slamon D., Eiermann W., Robert N., et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Minckwitz G., Procter M., de Azambuja E., et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneeweiss A., Chia S., Hickish T., et al. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur J Cancer. 2018;89:27–35. doi: 10.1016/j.ejca.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Tolaney S.M., Tarantino P., Graham N., et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol. 2023;24(3):273–285. doi: 10.1016/S1470-2045(23)00051-7. [DOI] [PubMed] [Google Scholar]
- 24.Jones S.E., Savin M.A., Holmes F.A., et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24(34):5381–5387. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 25.Nitz U., Gluz O., Clemens M., et al. West German Study PlanB trial: adjuvant four cycles of epirubicin and cyclophosphamide plus docetaxel versus six cycles of docetaxel and cyclophosphamide in HER2-negative early breast cancer. J Clin Oncol. 2019;37(10):799–808. doi: 10.1200/JCO.18.00028. [DOI] [PubMed] [Google Scholar]
- 26.Blum J.L., Flynn P.J., Yothers G., et al. Anthracyclines in early breast cancer: the ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology) J Clin Oncol. 2017;35(23):2647–2655. doi: 10.1200/JCO.2016.71.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu K.D., Ye F.G., He M., et al. Effect of adjuvant paclitaxel and carboplatin on survival in women with triple-negative breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2020;6(9):1390–1396. doi: 10.1001/jamaoncol.2020.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma P., Kimler B.F., O’Dea A., et al. Randomized phase II trial of anthracycline-free and anthracycline-containing Neoadjuvant Carboplatin Chemotherapy Regimens in Stage I-III Triple-negative Breast Cancer (NeoSTOP) Clin Cancer Res. 2021;27(4):975–982. doi: 10.1158/1078-0432.CCR-20-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guarneri V., de Azambuja E. Anthracyclines in the treatment of patients with early breast cancer. ESMO Open. 2022;7(3) doi: 10.1016/j.esmoop.2022.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gennari A., André F., Barrios C.H., et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–1495. doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Dent S.F., Moore H., Raval P., Alder L., Guha A. How to manage and monitor cardiac dysfunction in patients with metastatic HER2-positive breast cancer. JACC CardioOncol. 2022;4(3):404–408. doi: 10.1016/j.jaccao.2022.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergom C., Bradley J.A., Ng A.K., et al. Past, present, and future of radiation-induced cardiotoxicity: refinements in targeting, surveillance, and risk stratification. JACC CardioOncol. 2021;3(3):343–359. doi: 10.1016/j.jaccao.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson S.A. Anthracycline-induced cardiotoxicity in adult hematologic malignancies. Semin Oncol. 2006;33(suppl 8):S22–S27. doi: 10.1053/j.seminoncol.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 34.Johnson S.A., Richardson D.S. Anthracyclines in haematology: pharmacokinetics and clinical studies. Blood Rev. 1998;12(1):52–71. doi: 10.1016/s0268-960x(98)90030-3. [DOI] [PubMed] [Google Scholar]
- 35.Luskin M.R., Lee J.W., Fernandez H.F., et al. Benefit of high-dose daunorubicin in AML induction extends across cytogenetic and molecular groups. Blood. 2016;127(12):1551–1558. doi: 10.1182/blood-2015-07-657403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dombret H., Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood. 2016;127(1):53–61. doi: 10.1182/blood-2015-08-604520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terwilliger T., Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7(6) doi: 10.1038/bcj.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ullah F., Dima D., Omar N., Ogbue O., Ahmed S. Advances in the treatment of Hodgkin lymphoma: current and future approaches. Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1067289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kambhampati S., Herrera A.F., Rhee J.W. How to treat diffuse large B-cell lymphoma: oncologic and cardiovascular considerations. JACC CardioOncol. 2023;5(3):281–291. doi: 10.1016/j.jaccao.2023.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vergote I., Gonzalez-Martin A., Lorusso D., et al. Clinical research in ovarian cancer: consensus recommendations from the Gynecologic Cancer InterGroup. Lancet Oncol. 2022;23(8):e374–e384. doi: 10.1016/S1470-2045(22)00139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parashar S., Akhter N., Paplomata E., et al. Cancer treatment-related cardiovascular toxicity in gynecologic malignancies: JACC: CardioOncology state-of-the-art review. JACC CardioOncol. 2023;5(2):159–173. doi: 10.1016/j.jaccao.2023.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gómez J., Tsagozis P. Multidisciplinary treatment of soft tissue sarcomas: an update. World J Clin Oncol. 2020;11(4):180–189. doi: 10.5306/wjco.v11.i4.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strauss S.J., Frezza A.M., Abecassis N., et al. Bone sarcomas: ESMO-EURACAN-GENTURIS-ERN PaedCan clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(12):1520–1536. doi: 10.1016/j.annonc.2021.08.1995. [DOI] [PubMed] [Google Scholar]
- 44.Benjamin R.S., Minotti G. Doxorubicin-dexrazoxane from day 1 for soft-tissue sarcomas: the road to cardioprotection. Clin Cancer Res. 2021;27(14):3809–3811. doi: 10.1158/1078-0432.CCR-21-1376. [DOI] [PubMed] [Google Scholar]
- 45.Qiu Y., Jiang P., Huang Y. Anthracycline-induced cardiotoxicity: mechanisms, monitoring, and prevention. Front Cardiovasc Med. 2023;10 doi: 10.3389/fcvm.2023.1242596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zamorano J.L., Lancellotti P., Rodriguez Muñoz D., et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37(36):2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 47.Rawat P.S., Jaiswal A., Khurana A., Bhatti J.S., Navik U. Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed Pharmacother. 2021;139 doi: 10.1016/j.biopha.2021.111708. [DOI] [PubMed] [Google Scholar]
- 48.Salvatorelli E., Menna P., Chello M., Covino E., Minotti G. Low-dose anthracycline and risk of heart failure in a pharmacokinetic model of human myocardium exposure: analog specificity and role of secondary alcohol metabolites. J Pharmacol Exp Ther. 2018;364(2):323–331. doi: 10.1124/jpet.117.246140. [DOI] [PubMed] [Google Scholar]
- 49.Russo M., Della Sala A., Tocchetti C.G., Porporato P.E., Ghigo A. Metabolic aspects of anthracycline cardiotoxicity. Curr Treat Options Oncol. 2021;22(2):18. doi: 10.1007/s11864-020-00812-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishi M., Wang P.Y., Hwang P.M. Cardiotoxicity of cancer treatments: focus on anthracycline cardiomyopathy. Arterioscler Thromb Vasc Biol. 2021 Nov;41(11):2648–2660. doi: 10.1161/ATVBAHA.121.316697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sawicki K.T., De Jesus A., Ardehali H. Iron metabolism in cardiovascular disease: physiology, mechanisms, and therapeutic targets. Circ Res. 2023;132(3):379–396. doi: 10.1161/CIRCRESAHA.122.321667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boen H., Cherubin M., Franssen C., et al. Circulating microRNA as biomarkers of anthracycline-induced cardiotoxicity: JACC: CardioOncology state-of-the-art review. JACC CardioOncol. 2024;6(2):183–199. doi: 10.1016/j.jaccao.2023.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murabito A., Hirsch E., Ghigo A. Mechanisms of anthracycline-induced cardiotoxicity: is mitochondrial dysfunction the answer? Front Cardiovasc Med. 2020;7:35. doi: 10.3389/fcvm.2020.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhatia S. Genetics of anthracycline cardiomyopathy in cancer survivors: JACC: CardioOncology state-of-the-art review. JACC CardioOncol. 2020;2(4):539–552. doi: 10.1016/j.jaccao.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salloum F.N., Tocchetti C.G., Ameri P., et al. Priorities in cardio-oncology basic and translational science: GCOS 2023 Symposium proceedings: JACC: CardioOncology state-of-the-art review. JACC CardioOncol. 2023;5(6):715–731. doi: 10.1016/j.jaccao.2023.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fabiani I., Aimo A., Grigoratos C., et al. Oxidative stress and inflammation: determinants of anthracycline cardiotoxicity and possible therapeutic targets. Heart Fail Rev. 2021;26(4):881–890. doi: 10.1007/s10741-020-10063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plana J.C., Galderisi M., Barac A., et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15(10):1063–1093. doi: 10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Curigliano G., Lenihan D., Fradley M., et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31(2):171–190. doi: 10.1016/j.annonc.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herrmann J., Lenihan D., Armenian S., et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. 2022;43(4):280–299. doi: 10.1093/eurheartj/ehab674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thavendiranathan P., Negishi T., Somerset E., et al. Strain-guided management of potentially cardiotoxic cancer therapy. J Am Coll Cardiol. 2021;77(4):392–401. doi: 10.1016/j.jacc.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 61.Ruane L., Prasad S., Atherton J. Straining for more evidence. JACC CardioOncol. 2023;5(5):711–714. doi: 10.1016/j.jaccao.2023.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baldassarre L.A., Ganatra S., Lopez-Mattei J., et al. Advances in multimodality imaging in cardio-oncology: JACC state-of-the-art review. J Am Coll Cardiol. 2022;80(16):1560–1578. doi: 10.1016/j.jacc.2022.08.743. [DOI] [PubMed] [Google Scholar]
- 63.Oikonomou E.K., Kokkinidis D.G., Kampaktsis P.N., et al. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. 2019;4(10):1007–1018. doi: 10.1001/jamacardio.2019.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Addison D., Neilan T.G., Barac A., et al. cardiovascular imaging in contemporary cardio-oncology: a scientific statement from the American Heart Association. Circulation. 2023;148(16):1271–1286. doi: 10.1161/CIR.0000000000001174. [DOI] [PubMed] [Google Scholar]
- 65.Mecinaj A., Gulati G., Ree A.H., et al. Impact of the ESC cardio-oncology guidelines biomarker criteria on incidence of cancer therapy-related cardiac dysfunction. JACC CardioOncol. 2024;6(1):83–95. doi: 10.1016/j.jaccao.2023.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Demissei B.G., Hubbard R.A., Zhang L., et al. Changes in cardiovascular biomarkers with breast cancer therapy and associations with cardiac dysfunction. J Am Heart Assoc. 2020;9(2) doi: 10.1161/JAHA.119.014708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Michel L., Mincu R.I., Mahabadi A.A., et al. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail. 2020;22(2):350–361. doi: 10.1002/ejhf.1631. [DOI] [PubMed] [Google Scholar]
- 68.Henriksen P.A., Hall P., MacPherson I.R., et al. Multicenter, prospective, randomized controlled trial of high-sensitivity cardiac troponin I-guided combination angiotensin receptor blockade and beta-blocker therapy to prevent anthracycline cardiotoxicity: the CardiacCARE Trial. Circulation. 2023;148(21):1680–1690. doi: 10.1161/CIRCULATIONAHA.123.064274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cardinale D., Lyon A.R., López-Fernández T. Letter by Cardinale et al regarding article, “Multicenter, Prospective, Randomized Controlled Trial of High-Sensitivity Cardiac Troponin I-Guided Combination Angiotensin Receptor Blockade and Beta-Blocker Therapy to Prevent Anthracycline Cardiotoxicity: The Cardiac CARE Trial.”. Circulation. 2024;149(22):e1219–e1220. doi: 10.1161/CIRCULATIONAHA.123.068069. [DOI] [PubMed] [Google Scholar]
- 70.Fornaro A., Olivotto I., Rigacci L., et al. Comparison of long-term outcome in anthracycline-related versus idiopathic dilated cardiomyopathy: a single centre experience. Eur J Heart Fail. 2018;20(5):898–906. doi: 10.1002/ejhf.1049. [DOI] [PubMed] [Google Scholar]
- 71.McDonagh T.A., Metra M., Adamo M., et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44(37):3627–3639. doi: 10.1093/eurheartj/ehad195. [DOI] [PubMed] [Google Scholar]
- 72.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):1757–1780. doi: 10.1016/j.jacc.2021.12.011. [DOI] [PubMed] [Google Scholar]
- 73.Reding K.W., Cheng R.K., Vasbinder A., et al. Lifestyle and cardiovascular risk factors associated with heart failure subtypes in postmenopausal breast cancer survivors. JACC CardioOncol. 2022;4(1):53–65. doi: 10.1016/j.jaccao.2022.01.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Camilli M., Ferdinandy P., Salvatorelli E., Menna P., Minotti G. Anthracyclines, diastolic dysfunction and the road to heart failure in cancer survivors: an untold story. Prog Cardiovasc Dis. Published online July 16, 2024 doi: 10.1016/j.pcad.2024.07.002. [DOI] [PubMed] [Google Scholar]
- 75.Minotti G., Reggiardo G., Camilli M., Salvatorelli E., Menna P. From cardiac anthracycline accumulation to real-life risk for early diastolic dysfunction: a translational approach. JACC CardioOncol. 2022;4(1):139–140. doi: 10.1016/j.jaccao.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lyon A.R., Dent S., Stanway S., et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22(11):1945–1960. doi: 10.1002/ejhf.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Battisti N.M.L., Andres M.S., Lee K.A., et al. Incidence of cardiotoxicity and validation of the Heart Failure Association-International Cardio-Oncology Society risk stratification tool in patients treated with trastuzumab for HER2-positive early breast cancer. Breast Cancer Res Treat. 2021;188(1):149–163. doi: 10.1007/s10549-021-06192-w. [DOI] [PubMed] [Google Scholar]
- 78.Tini G., Cuomo A., Battistoni A., et al. Baseline cardio-oncologic risk assessment in breast cancer women and occurrence of cardiovascular events: the HFA/ICOS risk tool in real-world practice. Int J Cardiol. 2022;349:134–137. doi: 10.1016/j.ijcard.2021.11.059. [DOI] [PubMed] [Google Scholar]
- 79.Al-Otaibi T.K., Weitzman B., Tahir U.A., Asnani A. Genetics of anthracycline-associated cardiotoxicity. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.867873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wong-Siegel J.R., Kim Y., Stitziel N.O., Javaheri A. Genetic testing in evaluating risk of anthracycline cardiomyopathy: are we there yet? JACC CardioOncol. 2023;5(3):406–408. doi: 10.1016/j.jaccao.2023.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bernstein D., Hayden M.R., Amstutz U., Carleton B.C., et al. Recommendations for genetic testing to reduce the incidence of anthracycline-induced cardiotoxicity. Br J Clin Pharmacol. 2016;82(3):683–695. doi: 10.1111/bcp.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chow E.J., Leger K.J., Bhatt N.S., et al. Paediatric cardio-oncology: epidemiology, screening, prevention, and treatment. Cardiovasc Res. 2019;115(5):922–934. doi: 10.1093/cvr/cvz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fonoudi H., Jouni M., Cejas R.B., et al. Functional validation of doxorubicin-induced cardiotoxicity-related genes. JACC CardioOncol. 2024;6(1):38–50. doi: 10.1016/j.jaccao.2023.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burridge P.W., Li Y.F., Matsa E., et al. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med. 2016;22(5):547–556. doi: 10.1038/nm.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L., Tan T.C., Halpern E.F., et al. Major cardiac events and the value of echocardiographic evaluation in patients receiving anthracycline-based chemotherapy. Am J Cardiol. 2015;116(3):442–446. doi: 10.1016/j.amjcard.2015.04.064. [DOI] [PubMed] [Google Scholar]
- 86.Liu J.E., Barac A., Thavendiranathan P., Scherrer-Crosbie M. Strain imaging in cardio-oncology. JACC CardioOncol. 2020;2(5):677–689. doi: 10.1016/j.jaccao.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moslehi J.J., Witteles R.M. Global longitudinal strain in cardio-oncology. J Am Coll Cardiol. 2021;77(4):402–404. doi: 10.1016/j.jacc.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Upshaw J.N., Finkelman B., Hubbard R.A., et al. Comprehensive assessment of changes in left ventricular diastolic function with contemporary breast cancer therapy. JACC Cardiovasc Imaging. 2020;13(1 Pt 2):198–210. doi: 10.1016/j.jcmg.2019.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marzlin N., Hays A.G., Peters M., et al. Myocardial work in echocardiography. Circ Cardiovasc Imaging. 2023;16(2) doi: 10.1161/CIRCIMAGING.122.014419. [DOI] [PubMed] [Google Scholar]
- 90.Ky B., French B., May Khan A., et al. Ventricular-arterial coupling, remodeling, and prognosis in chronic heart failure. J Am Coll Cardiol. 2013;62:1165–1172. doi: 10.1016/j.jacc.2013.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Calvillo-Argüelles O., Thampinathan B., Somerset E., et al. Diagnostic and prognostic value of myocardial work indices for identification of cancer therapy-related cardiotoxicity. JACC Cardiovasc Imaging. 2022;15(8):1361–1376. doi: 10.1016/j.jcmg.2022.02.027. [DOI] [PubMed] [Google Scholar]
- 92.O’Quinn R., Ferrari V.A., Daly R., et al. Cardiac magnetic resonance in cardio-oncology: advantages, importance of expediency, and considerations to navigate pre-authorization. JACC CardioOncol. 2021;3(2):191–200. doi: 10.1016/j.jaccao.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saunderson C.E.D., Plein S., Manisty C.H. Role of cardiovascular magnetic resonance imaging in cardio-oncology. Eur Heart J Cardiovasc Imaging. 2021;22(4):383–396. doi: 10.1093/ehjci/jeaa345. [DOI] [PubMed] [Google Scholar]
- 94.Muehlberg F., Kornfeld M., Zange L., et al. Early myocardial oedema can predict subsequent cardiomyopathy in high-dose anthracycline therapy. ESC Heart Fail. 2023;10(1):616–627. doi: 10.1002/ehf2.14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gómez-Talavera S., Fernandez-Jimenez R., Fuster V., et al. Clinical validation of a 3-dimensional ultrafast cardiac magnetic resonance protocol including single breath-hold 3-dimensional sequences. JACC Cardiovasc Imaging. 2021;14(9):1742–1754. doi: 10.1016/j.jcmg.2021.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lopez-Mattei J.C., Yang E.H., Ferencik M., Baldassarre L.A., Dent S., Budoff M.J. Cardiac computed tomography in cardio-oncology: JACC: CardioOncology primer. JACC CardioOncol. 2021;3(5):635–649. doi: 10.1016/j.jaccao.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mikail N., Chequer R., Imperiale A., et al. Tales from the future-nuclear cardio-oncology, from prediction to diagnosis and monitoring. Eur Heart J Cardiovasc Imaging. 2023;24(9):1129–1145. doi: 10.1093/ehjci/jead168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pudil R., Mueller C., Čelutkienė J., et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur J Heart Fail. 2020;22(11):1966–1983. doi: 10.1002/ejhf.2017. [DOI] [PubMed] [Google Scholar]
- 99.Welsh P., Preiss D., Hayward C., et al. Cardiac troponin T and troponin I in the general population. Circulation. 2019;139(24):2754–2764. doi: 10.1161/CIRCULATIONAHA.118.038529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cardinale D., Colombo A., Sandri M.T., et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114(23):2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 101.Cardinale D., Ciceri F., Latini R., et al. Anthracycline-induced cardiotoxicity: a multicenter randomised trial comparing two strategies for guiding prevention with enalapril: The International CardioOncology Society-one trial. Eur J Cancer. 2018;94:126–137. doi: 10.1016/j.ejca.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 102.De Michieli L., Jaffe A. Cancer therapy–related cardiac dysfunction: expanding the horizon of cardiac troponin in clinical practice. JACC CardioOncol. 2024;6(1):96–98. doi: 10.1016/j.jaccao.2024.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.López-Sendón J., Álvarez-Ortega C., Zamora Auñon P., et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J. 2020;41(18):1720–1729. doi: 10.1093/eurheartj/ehaa006. [DOI] [PubMed] [Google Scholar]
- 104.Murtagh G., Januzzi J.L., Scherrer-Crosbie M., et al. Circulating cardiovascular biomarkers in cancer therapeutics-related cardiotoxicity: review of critical challenges, solutions, and future directions. J Am Heart Assoc. 2023;12(21) doi: 10.1161/JAHA.123.029574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dixon S.B., Howell C.R., Lu L., et al. Cardiac biomarkers and association with subsequent cardiomyopathy and mortality among adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort. Cancer. 2021;127(3):458–466. doi: 10.1002/cncr.33292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Omland T., Heck S.L., Gulati G. The role of cardioprotection in cancer therapy cardiotoxicity: JACC: CardioOncology state-of-the-art review. JACC CardioOncol. 2022;4(1):19–37. doi: 10.1016/j.jaccao.2022.01.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wilson R.L., Christopher C.N., Yang E.H., et al. Incorporating exercise training into cardio-oncology care: current evidence and opportunities: JACC: CardioOncology state-of-the-art review. JACC CardioOncol. 2023;5(5):553–569. doi: 10.1016/j.jaccao.2023.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vaduganathan M., Hirji S.A., Qamar A., et al. Efficacy of neurohormonal therapies in preventing cardiotoxicity in patients with cancer undergoing chemotherapy. JACC CardioOncol. 2019;1(1):54–65. doi: 10.1016/j.jaccao.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Avila M.S., Ayub-Ferreira S.M., de Barros Wanderley M.R., Jr., et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol. 2018;71(20):2281–2290. doi: 10.1016/j.jacc.2018.02.049. [DOI] [PubMed] [Google Scholar]
- 110.Heck S.L., Mecinaj A., Ree A.H., et al. Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA): extended follow-up of a 2×2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Circulation. 2021;143(25):2431–2440. doi: 10.1161/CIRCULATIONAHA.121.054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Livi L., Barletta G., Martella F., et al. Cardioprotective strategy for patients with nonmetastatic breast cancer who are receiving an anthracycline-based chemotherapy: a randomized clinical trial. JAMA Oncol. 2021;7(10):1544–1549. doi: 10.1001/jamaoncol.2021.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thavendiranathan P., Houbois C., Marwick T.H., et al. Statins to prevent early cardiac dysfunction in cancer patients at increased cardiotoxicity risk receiving anthracyclines. Eur Heart J Cardiovasc Pharmacother. 2023;9(6):515–525. doi: 10.1093/ehjcvp/pvad031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hundley W.G., D’Agostino R., Jr., Crotts T., et al. Statins and left ventricular ejection fraction following doxorubicin treatment. NEJM Evid. 2022;1(9) doi: 10.1056/evidoa2200097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Neilan T.G., Quinaglia T., Onoue T., et al. Atorvastatin for anthracycline-associated cardiac dysfunction: the STOP-CA randomized clinical trial. JAMA. 2023;330(6):528–536. doi: 10.1001/jama.2023.11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee A.R.Y.B., Yau C.E., Low C.E., et al. Natural progression of left ventricular function following anthracyclines without cardioprotective therapy: a systematic review and meta-analysis. Cancers (Basel) 2023;15(2):512. doi: 10.3390/cancers15020512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mecinaj A., Gulati G., Heck S.L., et al. Rationale and design of the PRevention of cArdiac Dysfunction during Adjuvant breast cancer therapy (PRADA II) trial: a randomized, placebo-controlled, multicenter trial. Cardiooncology. 2021;7(1):33. doi: 10.1186/s40959-021-00115-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Camilli M., Viscovo M., Maggio L., et al. Sodium-glucose cotransporter 2 inhibitors and the cancer patient: from diabetes to cardioprotection and beyond. Basic Res Cardiol. Published online June 27, 2024 doi: 10.1007/s00395-024-01059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Minotti G. Pharmacology at work for cardio-oncology: ranolazine to treat early cardiotoxicity induced by antitumor drugs. J Pharmacol Exp Ther. 2013;346(3):343–349. doi: 10.1124/jpet.113.204057. [DOI] [PubMed] [Google Scholar]
- 119.Čiburienė E., Aidietienė S., Ščerbickaitė G., et al. Ivabradine for the prevention of anthracycline-induced cardiotoxicity in female patients with primarily breast cancer: a prospective, randomized, open-label clinical trial. Medicina (Kaunas) 2023;59(12):2140. doi: 10.3390/medicina59122140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Macedo A.V.S., Hajjar L.A., Lyon A.R., et al. Efficacy of dexrazoxane in preventing anthracycline cardiotoxicity in breast cancer. JACC CardioOncol. 2019;1(1):68–79. doi: 10.1016/j.jaccao.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Baat E.C., Mulder R.L., Armenian S., et al. Dexrazoxane for preventing or reducing cardiotoxicity in adults and children with cancer receiving anthracyclines. Cochrane Database Syst Rev. 2022;9(9) doi: 10.1002/14651858.CD014638.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van Dalen E.C., van der Pal H.J., Kremer L.C. Different dosage schedules for reducing cardiotoxicity in people with cancer receiving anthracycline chemotherapy. Cochrane Database Syst Rev. 2016;3(3) doi: 10.1002/14651858.CD005008.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.López-Fernández T., Farmakis D., Ameri P., et al. European Society of Cardiology core curriculum for cardio-oncology. Eur J Heart Fail. 2024;26(4):754–771. doi: 10.1002/ejhf.3102. [DOI] [PubMed] [Google Scholar]
- 124.Cardinale D., Colombo A., Lamantia G., et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55(3):213–220. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 125.Zullig L.L., Sung A.D., Khouri M.G., et al. Cardiometabolic comorbidities in cancer survivors: JACC: CardioOncology state-of-the-art review. JACC CardioOncol. 2022;4(2):149–165. doi: 10.1016/j.jaccao.2022.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chow E.J., Chen Y., Kremer L.C., et al. Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol. 2015;33(5):394–402. doi: 10.1200/JCO.2014.56.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Akhter N., Dent S. Cardio-oncology rehabilitation programs-the next phase in improving care for cancer survivors. JAMA Cardiol. 2023;8(12):1128–1130. doi: 10.1001/jamacardio.2023.3568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.