Abstract
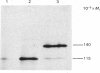
The biosynthesis of small-intestinal aminopeptidase N (EC 3.4.11.2) was studied in a cell-free translation system derived from rabbit reticulocytes. When dog pancreatic microsomal fractions were present during translation, most of the aminopeptidase N synthesized was found in a membrane-bound rather than a soluble form, indicating that synthesis of the enzyme takes place on ribosomes attached to the rough endoplasmic reticulum. The microsomal fractions process the Mr-115 000 polypeptide, which is the primary translation product of aminopeptidase N, to a polypeptide of Mr 140 000. This was found to be sensitive to the action of endo-beta-N-acetylglucosaminidase H (EC 3.2.1.96), showing that aminopeptidase N undergoes transmembrane glycosylation during synthesis. The position of the signal sequence in aminopeptidase N was determined by a synchronized translation experiment. It was found that microsomal fractions should be added before about 25% of the polypeptide was synthesized to ensure processing to the high-mannose glycosylated form. This suggests that the signal sequence is situated in the N-terminal part of the aminopeptidase N. The size of the cell-free translation product in the absence of microsomal fractions was found to be similar to that on one of the forms of the enzyme obtained from tunicamycin-treated organ-cultured intestinal explants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Nun S., Kreibich G., Adesnik M., Alterman L., Negishi M., Sabatini D. D. Synthesis and insertion of cytochrome P-450 into endoplasmic reticulum membranes. Proc Natl Acad Sci U S A. 1980 Feb;77(2):965–969. doi: 10.1073/pnas.77.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braell W. A., Lodish H. F. Ovalbumin utilizes an NH2-terminal signal sequence. J Biol Chem. 1982 Apr 25;257(8):4578–4582. [PubMed] [Google Scholar]
- Braell W. A., Lodish H. F. The erythrocyte anion transport protein is contranslationally inserted into microsomes. Cell. 1982 Jan;28(1):23–31. doi: 10.1016/0092-8674(82)90371-3. [DOI] [PubMed] [Google Scholar]
- Browning T. H., Trier J. S. Organ culture of mucosal biopsies of human small intestine. J Clin Invest. 1969 Aug;48(8):1423–1432. doi: 10.1172/JCI106108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M. Biosynthesis of intestinal microvillar proteins. Pulse-chase labelling studies on aminopeptidase N and sucrase-isomaltase. Biochem J. 1982 Jun 15;204(3):639–645. doi: 10.1042/bj2040639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Norén O., Sjöström H. Biosynthesis of intestinal microvillar proteins. Translational evidence in vitro that aminopeptidase N is synthesized as a Mr-115000 polypeptide. Biochem J. 1982 Apr 15;204(1):323–327. doi: 10.1042/bj2040323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Sjöström H., Norén O., Bro B., Dabelsteen E. Biosynthesis of intestinal microvillar proteins. Characterization of intestinal explants in organ culture and evidence for the existence of pro-forms of the microvillar enzymes. Biochem J. 1982 Mar 15;202(3):647–654. doi: 10.1042/bj2020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Skovbjerg H., Norén O., Sjöström H. Biosynthesis of intestinal microvillar proteins. Nature of precursor forms of microvillar enzymes from Ca2+-precipitated enterocyte membranes. FEBS Lett. 1981 Sep 28;132(2):197–200. doi: 10.1016/0014-5793(81)81159-3. [DOI] [PubMed] [Google Scholar]
- Jackson R. C., Blobel G. Post-translational cleavage of presecretory proteins with an extract of rough microsomes from dog pancreas containing signal peptidase activity. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5598–5602. doi: 10.1073/pnas.74.12.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny A. J., Maroux S. Topology of microvillar membrance hydrolases of kidney and intestine. Physiol Rev. 1982 Jan;62(1):91–128. doi: 10.1152/physrev.1982.62.1.91. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lodish H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977 Oct 27;269(5631):775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider M. D., Robbins P. W. Transmembrane organization of protein glycosylation. Mature oligosaccharide-lipid is located on the luminal side of microsomes from Chinese hamster ovary cells. J Biol Chem. 1982 Jun 25;257(12):6796–6801. [PubMed] [Google Scholar]