Abstract
Exposure of isolated rat hepatocytes (approx. 2 x 10(7)--5 x 10(7) cells/10ml of incubation mixture) to 0.5 mg of the mycotoxin sporidesmin for 30--60 min at 37 degrees C produced loss of plasma-membrane microvilli with some disruption of organelle distribution in the sub-surface region. There was accompanying inhibition of [14C]cholate and [14C]taurocholate transport, but bile acid conjugation was not altered. Inhibition of cholate uptake was maximal after exposure of hepatocytes to sporidesmin for 1 min, and was not reversed by washing cells free of extracellular sporidesmin. N-Ethylmaleimide (0.1 mM) or dithiothreitol (1 mM) partially protected hepatocytes from sporidesmin inhibition of bile acid uptake. Significant protection was not given by other thiols or by zinc sulphate, cholesterol, ascorbate or alpha-tocopherol. The results are discussed in terms of sporidesmin action on cell membranes and the toxin's effect on bile secretion.
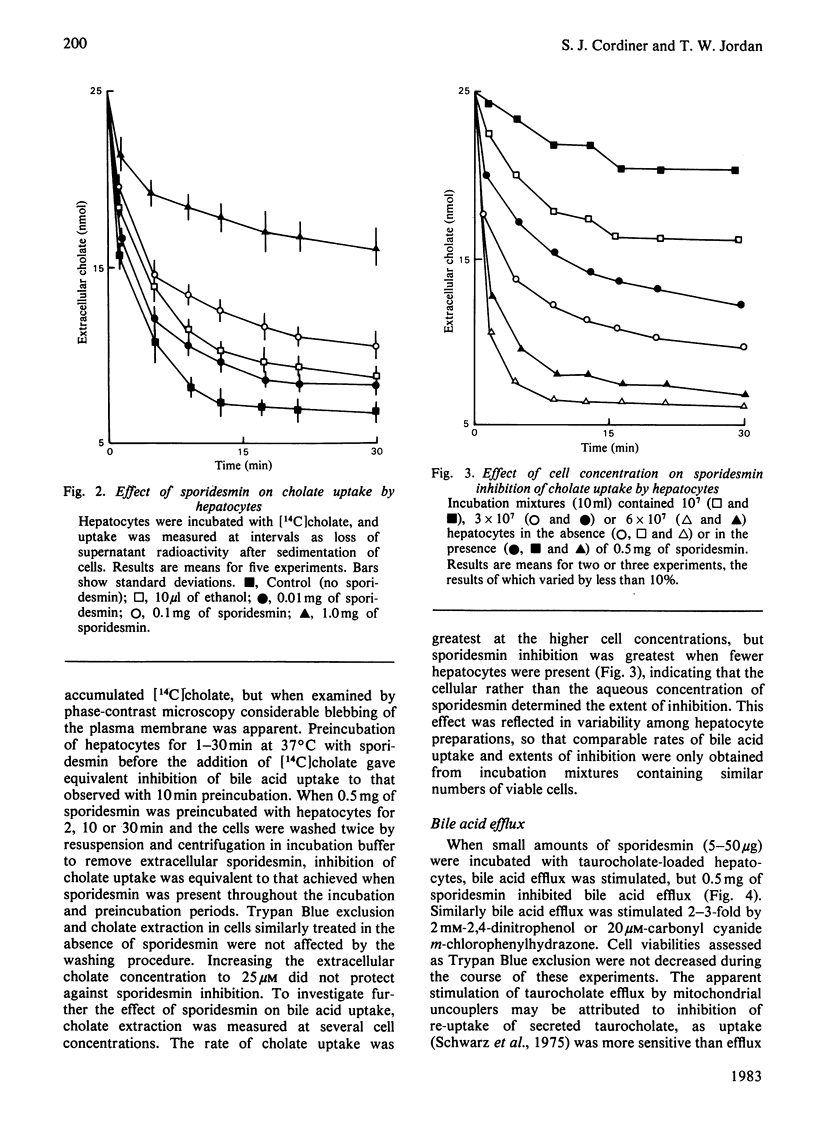
Full text
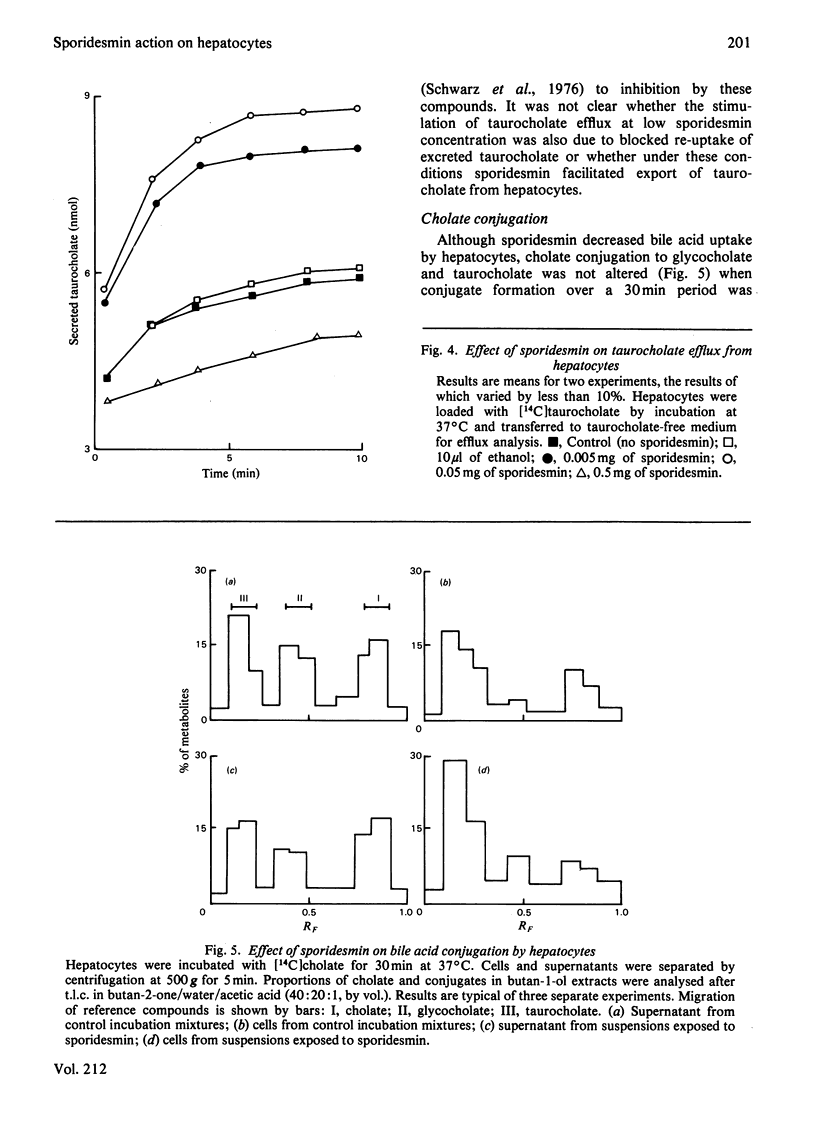
PDF




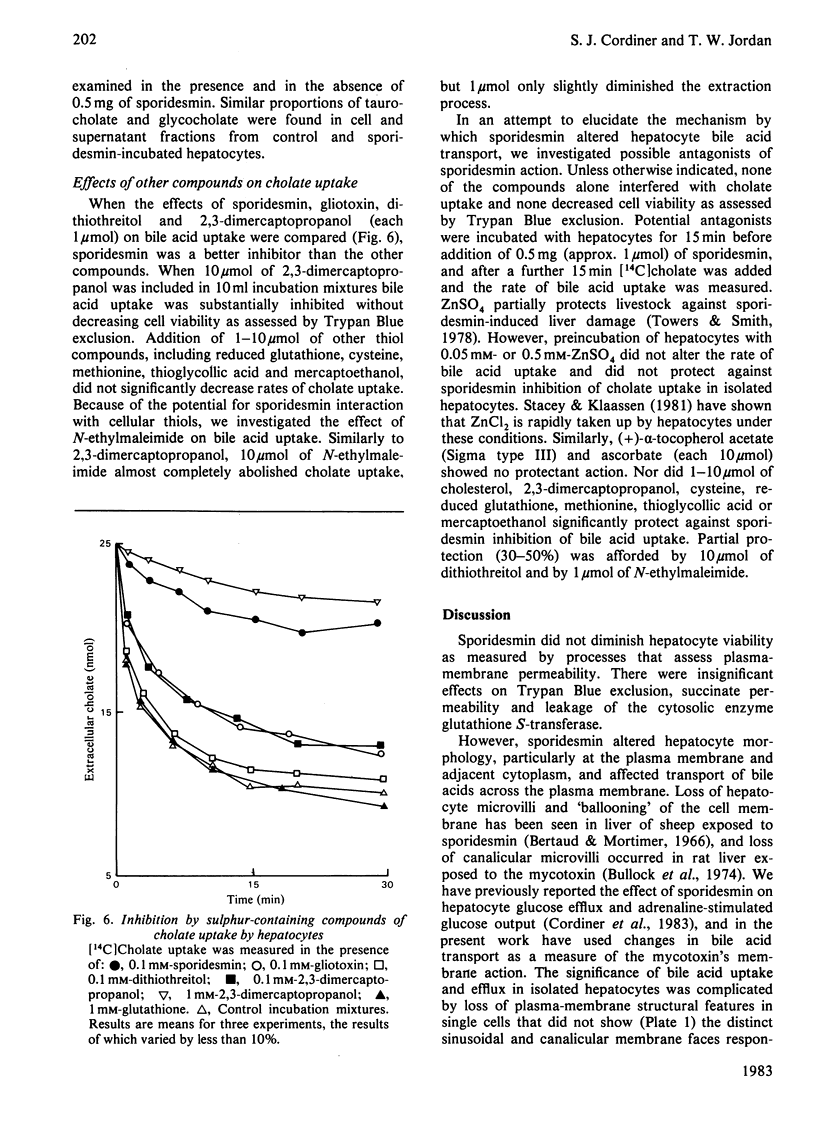


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer B. L., Boyer J. L. Cellular mechanisms of bile formation. Gastroenterology. 1982 Feb;82(2):346–357. [PubMed] [Google Scholar]
- Bullock G., Eakins M. N., Sawyer B. C., Slater T. F. Studies on bile secretion with the aid of the isolated perfused rat liver. I. Inhibitory action of sporidesmin and icterogenin. Proc R Soc Lond B Biol Sci. 1974 Jul 30;186(1085):333–356. doi: 10.1098/rspb.1974.0053. [DOI] [PubMed] [Google Scholar]
- Dipple I., Houslay M. D. The activity of glucagon-stimulated adenylate cyclase from rat liver plasma membranes is modulated by the fluidity of its lipid environment. Biochem J. 1978 Jul 15;174(1):179–190. doi: 10.1042/bj1740179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakins M. N. The effect of three triterpene acids and sporidesmin on the enzyme activities of rat liver plasma membranes. Chem Biol Interact. 1978 Apr;21(1):117–124. doi: 10.1016/0009-2797(78)90072-8. [DOI] [PubMed] [Google Scholar]
- GALLAGHER C. H. THE EFFECT OF SPORIDESMIN ON LIVER ENZYME SYSTEMS. Biochem Pharmacol. 1964 Jul;13:1017–1026. doi: 10.1016/0006-2952(64)90098-x. [DOI] [PubMed] [Google Scholar]
- Keeffe E. B., Blankenship N. M., Scharschmidt B. F. Alteration of rat liver plasma membrane fluidity and ATPase activity by chlorpromazine hydrochloride and its metabolites. Gastroenterology. 1980 Aug;79(2):222–231. [PubMed] [Google Scholar]
- Kroker R., Anwer M. S., Hegner D. Effect of sporidesmin on bile acid metabolism in isolated rat hepatocytes. Zentralbl Veterinarmed A. 1977 Mar;24(3):205–209. doi: 10.1111/j.1439-0442.1977.tb01565.x. [DOI] [PubMed] [Google Scholar]
- Lim W. C., Jordan T. W. Subcellular distribution of hepatic bile acid-conjugating enzymes. Biochem J. 1981 Sep 1;197(3):611–618. doi: 10.1042/bj1970611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton M. C. Effects of the mycotoxin sporidesmin on swelling and respiration of liver mitochondria. Biochem Pharmacol. 1974 Feb 15;23(4):801–810. doi: 10.1016/0006-2952(74)90210-x. [DOI] [PubMed] [Google Scholar]
- Middleton M. C. The involvement of the disulphide group of sporidesmin in the action of the toxin on swelling and respiration of liver mitochondria. Biochem Pharmacol. 1974 Feb 15;23(4):811–820. doi: 10.1016/0006-2952(74)90211-1. [DOI] [PubMed] [Google Scholar]
- Mortimer P. H., Collins B. S. The in vitro toxicity of the sporidesmins and related compounds to tissue-culture cells. Res Vet Sci. 1968 Mar;9(2):136–142. [PubMed] [Google Scholar]
- Mortimer P. H., Stanbridge T. A. Changes in biliary secretion following sporidesmin poisoning in sheep. J Comp Pathol. 1969 Apr;79(2):267–275. doi: 10.1016/0021-9975(69)90016-4. [DOI] [PubMed] [Google Scholar]
- Mortimer P. H., Stanbridge T. A. The excretion of sporidesmin given by mouth to sheep. J Comp Pathol. 1968 Oct;78(4):505–512. doi: 10.1016/0021-9975(68)90050-9. [DOI] [PubMed] [Google Scholar]
- Munday R. Studies on the mechanism of toxicity of the mycotoxin, sporidesmin. I. Generation of superoxide radical by sporidesmin. Chem Biol Interact. 1982 Sep;41(3):361–374. doi: 10.1016/0009-2797(82)90112-0. [DOI] [PubMed] [Google Scholar]
- Prentki M., Chaponnier C., Jeanrenaud B., Gabbiani G. Actin microfilaments, cell shape, and secretory processes in isolated rat hepatocytes. Effect of phalloidin and cytochalasin D. J Cell Biol. 1979 Jun;81(3):592–607. doi: 10.1083/jcb.81.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichen J., Berman M. D., Berk P. D. The role of microfilaments and microtubules in taurocholate uptake by isolated rat liver cells. Biochim Biophys Acta. 1981 Apr 22;643(1):126–133. doi: 10.1016/0005-2736(81)90224-8. [DOI] [PubMed] [Google Scholar]
- Schwarz L. R., Burr R., Schwenk M., Pfaff E., Greim H. Uptake of taurocholic acid into isolated rat-liver cells. Eur J Biochem. 1975 Jul 15;55(3):617–623. doi: 10.1111/j.1432-1033.1975.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Schwarz L. R., Schwenk M., Pfaff E., Greim H. Cholestatic steroid hormones inhibit taurocholate uptake into isolated rat hepatocytes. Biochem Pharmacol. 1977 Dec 15;26(24):2433–2437. doi: 10.1016/0006-2952(77)90453-1. [DOI] [PubMed] [Google Scholar]
- Schwarz L. R., Schwenk M., Pfaff E., Greim H. Excretion of taurocholate from isolated hepatocytes. Eur J Biochem. 1976 Dec 11;71(2):369–373. doi: 10.1111/j.1432-1033.1976.tb11123.x. [DOI] [PubMed] [Google Scholar]
- Smith M. T., Loveridge N., Wills E. D., Chayen J. The distribution of glutathione in the rat liver lobule. Biochem J. 1979 Jul 15;182(1):103–108. doi: 10.1042/bj1820103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey N. H., Klaassen C. D. Zinc uptake by isolated rat hepatocytes. Biochim Biophys Acta. 1981 Feb 6;640(3):693–697. doi: 10.1016/0005-2736(81)90099-7. [DOI] [PubMed] [Google Scholar]
- Towers N. R., Smith B. L. The protective effect of zinc sulphate in experimental sporidesmin intoxication of lactating dairy cows. N Z Vet J. 1978 Aug;26(8):199–202. doi: 10.1080/00480169.1978.34540. [DOI] [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Preparation of plasma-membrane subfractions from isolated rat hepatocytes. Biochem J. 1977 May 15;164(2):415–422. doi: 10.1042/bj1640415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahlten R. N., Stratman F. W. The isolation of hormone-sensitive rat hepatocytes by a modified enzymatic technique. Arch Biochem Biophys. 1974 Aug;163(2):600–608. doi: 10.1016/0003-9861(74)90519-0. [DOI] [PubMed] [Google Scholar]