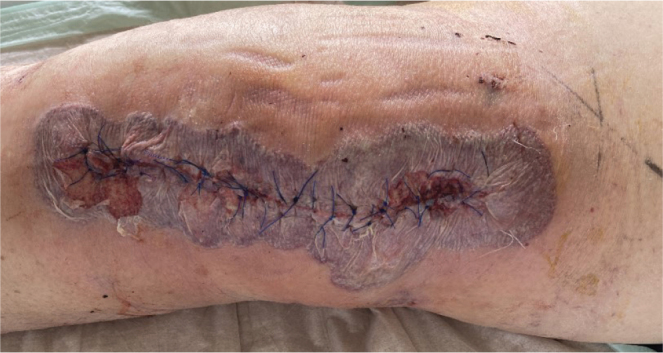
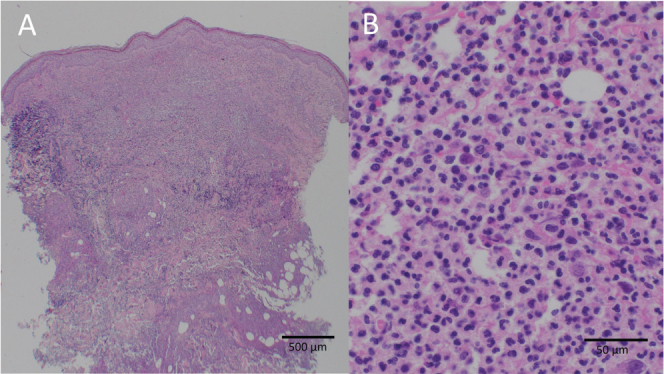
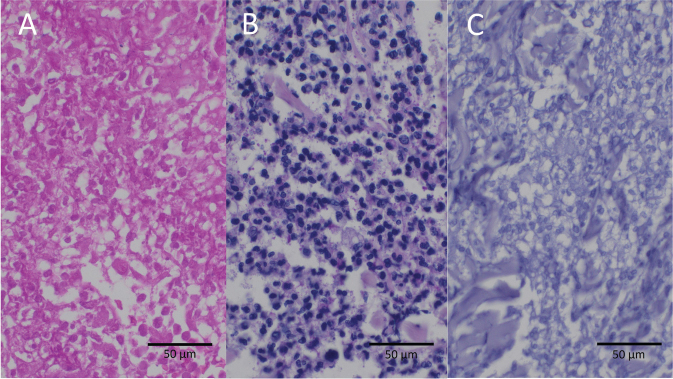
Diagnosis: Bullous pyoderma gangrenosum
Antibiotic therapy was changed to imipenem and clindamycin intravenously and concomitant high-dose systemic steroid therapy was initiated. Under this treatment regimen, the patient’s clinical state improved rapidly. All microbial cultures and eubacterial polymerase chain reaction (PCR) remained negative.
Five days after escalating antibiotic therapy it was discontinued, while sustaining systemic high-dose steroid therapy. The patient’s condition remained stable, and wound conditions improved. Subsequently, surgical reconstruction of the affected knee joint and tissue defect was performed over several months. As part of a continuous steroid reduction regimen for systemic immunosuppression, tumour necrosis factor (TNF)-alpha inhibitors were introduced. The patient tolerated all subsequent surgical interventions well. Immunosuppressive therapy was eventually able to be stopped after 8 months.
Pyoderma gangrenosum (PG) is a rare dermatological condition with most therapy recommendations based on clinical experience and case reports/series. It is mostly a clinical diagnosis supported by histological findings of deep dermal neutrophilic infiltrate (1, 2). Atypical (bullous) pyoderma gangrenosum (APG) is an even rarer sub-variant (1–3). Often initially misdiagnosed as an infectious process, it presents with bullous and ulcerative skin changes, sometimes with signs of necrosis (4–6). Diagnosis is made by excluding other disease entities. Generally, surgical interventions in this condition pose a risk of recurrence, leading to the pathergy phenomenon. PG has been known to manifest for the first time after surgical interventions, particularly in breast and orthopaedic surgery (5–8). In this context diagnosis is often delayed by mimicking postoperative wound infections, posing a peculiar challenge in postoperative care and management (5, 7–10).
Atypical pyoderma gangrenosum should be considered when anti-infective therapy yields insufficient improvement and dermatological evaluation should be sought. Perioperative management can be challenging and requires interdisciplinary cooperation. Often long-term systemic immunosuppression is necessary to achieve remission.
ACKNOWLEDGEMENT
Patient informed written consent was obtained regarding the anonymous publication of their medical information and images.
REFERENCES
-
1.Powell FC, Collins S. Pyoderma gangrenosum. Clin Dermatol
2000; 18: 283–293. 10.1016/S0738-081X(99)00119-4
[DOI] [PubMed] [Google Scholar]
-
2.Bennett ML, Jackson JM, Jorizzo JL, Fleischer ABJ, White WL, Callen JP. Pyoderma gangrenosum: a comparison of typical and atypical forms with an emphasis on time to remission. Case review of 86 patients from 2 institutions. Medicine (Baltimore)
2000; 79: 37–46. 10.1097/00005792-200001000-00004
[DOI] [PubMed] [Google Scholar]
-
3.Callen JP, Jackson JM. Pyoderma gangrenosum: an update. Rheum Dis Clin North Am
2007; 33: 787–802, vi. 10.1016/j.rdc.2007.07.016
[DOI] [PubMed] [Google Scholar]
-
4.Koester G, Tarnower A, Levisohn D, Burgdorf W. Bullous pyoderma gangrenosum. J Am Acad Dermatol
1993; 29: 875–878. 10.1016/0190-9622(93)70261-Q
[DOI] [PubMed] [Google Scholar]
-
5.Tolkachjov SN, Fahy AS, Cerci FB, Wetter DA, Cha SS, Camilleri MJ. Postoperative pyoderma gangrenosum: a clinical review of published cases. Mayo Clin Proc
2016; 91: 1267–1279. 10.1016/j.mayocp.2016.05.001
[DOI] [PubMed] [Google Scholar]
-
6.Ruebhausen MR, Mendenhall SD, Neumeister MW, Berry NN. Postsurgical pyoderma gangrenosum following carpal tunnel release: a rare disease following a common surgery. Eplasty
2017; 17: e10.
[PMC free article] [PubMed] [Google Scholar]
-
7.Gulyas K, Kimble FW. Atypical pyoderma gangrenosum after breast reduction. Aesthetic Plast Surg
2003; 27: 328–331. 10.1007/s00266-003-3017-y
[DOI] [PubMed] [Google Scholar]
-
8.Ebrad S, Severyns M, Benzakour A, Roze B, Derancourt C, Odri G-A, et al. Pyoderma gangrenosum after orthopaedic or traumatologic surgery: a systematic revue of the literature. Int Orthop
2018; 42: 239–245. 10.1007/s00264-017-3672-2
[DOI] [PubMed] [Google Scholar]
-
9.Ramamurthi A, Adamson KA, Yang KJ, Sanger J, Ling-LeBlanc JP, Wilson B, et al. Management of postsurgical pyoderma gangrenosum following deep inferior epigastric perforator flap breast reconstruction: a role for a dermal regeneration template. Wounds Compend Clin Res Pract
2021; 33: E67–74. 10.25270/wnds/2021.e6774 [DOI] [PubMed] [Google Scholar]
-
10.To D, Wong A, Montessori V. Atypical pyoderma gangrenosum mimicking an infectious process. Case Rep Infect Dis
2014; 2014: 589632. 10.1155/2014/589632
[DOI] [PMC free article] [PubMed] [Google Scholar]