Abstract
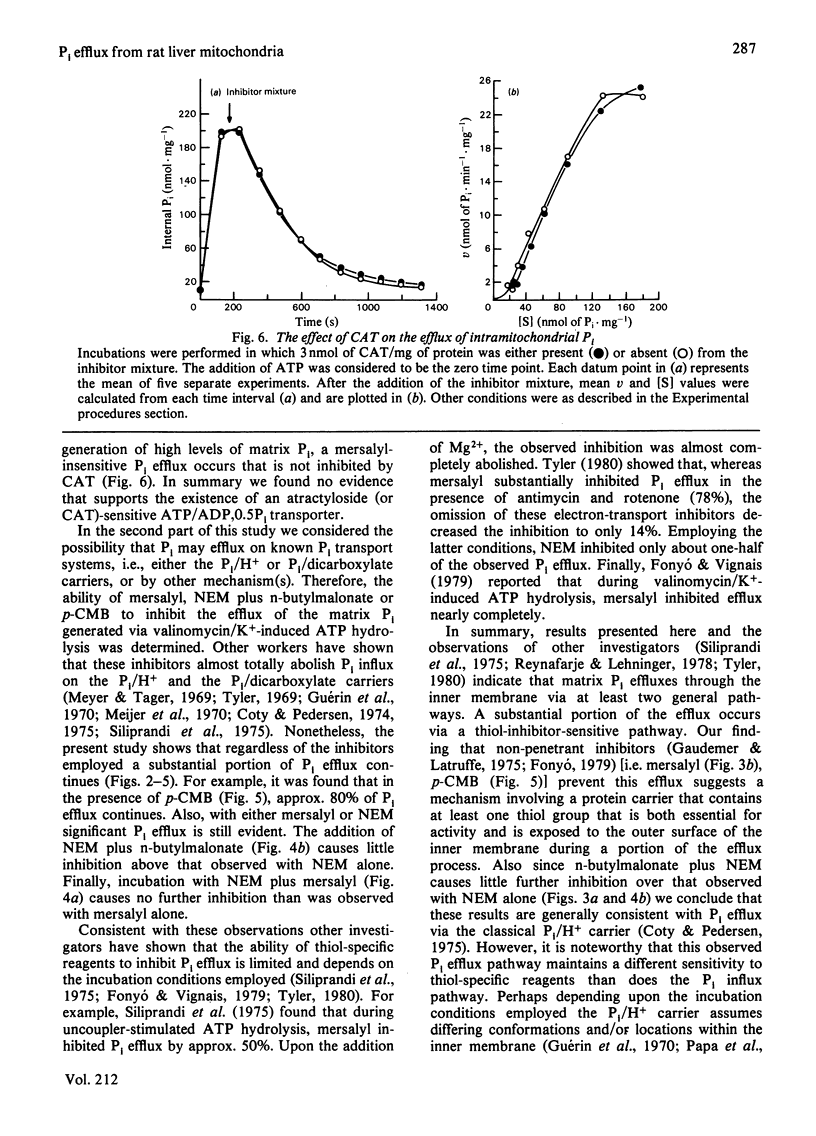
ATP hydrolysis catalysed by the H+-ATPase of intact mitochondria can be induced by addition of ATP in the presence of valinomycin and KCl. This leads to an increase in intramitochondrial Pi and therefore allows investigation of potential Pi efflux pathways in intact mitochondria. Combining this approach with the direct measurement of both internal and external Pi, we have attempted to determine whether Pi efflux occurs via an atractyloside-sensitive transporter, by the classical operation of the Pi/H+ and Pi/dicarboxylate carriers, and/or by other mechanisms. Initial experiments re-examined the evidence that led to the current view that one efflux pathway for Pi is an atractyloside-sensitive ATP/ADP,0.5Pi transporter. No evidence was found in support of this efflux pathway. Rather, atractyloside-sensitivity of the low rate of Pi efflux observed in previous studies (oligomycin present) was accounted for by ATP entry on the well known ATP/ADP transport system followed by hydrolysis of ATP and subsequent Pi efflux. Thus, under these conditions, where ATP hydrolysis is not completely inhibited, Pi efflux becomes atractyloside sensitive most likely because this inhibitor blocks ATP entry, not because it directly inhibits Pi efflux. Substantial efflux of Pi from rat liver mitochondria is observed on generation of high levels of matrix Pi by ATP hydrolysis induced by valinomycin and K+ (oligomycin absent). A portion of this efflux can be inhibited by thiol-specific reagents at concentrations that normally inhibit the Pi/H+ and Pi/dicarboxylate carriers. However, a significant fraction of efflux continues even in the presence of p-chloromercuribenzoate, N-ethylmaleimide plus n-butylmalonate or mersalyl. The mersalyl-insensitive Pi efflux, which is also insensitive to carboxyatractyloside, is a saturable process, thus suggesting carrier mediation. During this efflux the mitochondrial inner membrane retains considerable impermeability to other low-molecular-weight anions (i.e., malate, 2-oxoglutarate). In conclusion, results presented here rule out an atractyloside-sensitive ATP/ADP,0.5Pi transport system as a mechanism for Pi efflux in rat liver mitochondria. Rather Pi efflux appears to occur on the classical Pi/H+ transport system as well as via a mersalyl-insensitive saturable process. The inhibitor-insensitive Pi efflux may occur on a portion of the Pi/H+ carrier molecules that exist in a state different from that normally catalysing Pi influx. Alternatively, a separate Pi efflux carrier may exist.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzone G. F., Massari S., Pozzan T. Mechanism of active shrinkage in mitochondria. I. Coupling between weak electrolyte fluxes. Biochim Biophys Acta. 1976 Jan 15;423(1):15–26. doi: 10.1016/0005-2728(76)90097-9. [DOI] [PubMed] [Google Scholar]
- Brdiczka D., Pette D., Brunner G., Miller F. Kompartimentierte Verteilung von Enzymen in Rattenlebermitochondrien. Eur J Biochem. 1968 Jul;5(2):294–304. doi: 10.1111/j.1432-1033.1968.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Coty W. A., Pedersen P. L. Phosphate transport in rat liver mitochondria. Kinetics and energy requirements. J Biol Chem. 1974 Apr 25;249(8):2593–2598. [PubMed] [Google Scholar]
- Coty W. A., Pedersen P. L. Phosphate transport in rat liver mitochondria. Kinetics, inhibitor sensitivity, energy requirements, and labeled components. Mol Cell Biochem. 1975 Nov 14;9(2):109–124. doi: 10.1007/BF01732202. [DOI] [PubMed] [Google Scholar]
- Fonyo A., Vignais P. V. Phosphate carrier of liver mitochondria: the reaction of its SH groups with mersalyl, 5,5'-dithio-bis-nitrobenzoate, and N-ethylmaleimide and the modulation of reactivity by the energy state of the mitochondria. J Bioenerg Biomembr. 1980 Aug;12(3-4):137–149. doi: 10.1007/BF00744679. [DOI] [PubMed] [Google Scholar]
- Fonyó A., Vignais P. V. Phosphate retention and release during ATP hydrolysis in liver mitochondria. FEBS Lett. 1979 Jun 15;102(2):301–305. doi: 10.1016/0014-5793(79)80023-x. [DOI] [PubMed] [Google Scholar]
- Gaudemer Y., Latruffe N. Evidence for penetrant and non-penetrant thiol reagents and their use in the location of rat liver mitochondrial D(-)-beta-hydroxybutyrate dehydrogenase. FEBS Lett. 1975 Jun 1;54(1):30–34. doi: 10.1016/0014-5793(75)81061-1. [DOI] [PubMed] [Google Scholar]
- Guérin Bernard, Guérin Martine, Klingenberg Martin. Differential inhibition of phosphate efflux and influx and a possible discrimination between an inner and outer location of the phosphate carrier in mitochondria. FEBS Lett. 1970 Oct 16;10(4):265–268. doi: 10.1016/0014-5793(70)80644-5. [DOI] [PubMed] [Google Scholar]
- Le Quoc D., Le Quoc K., Gaudemer Y. Influence of the energetic state of rat liver mitochondria on the sensitivity of the phosphate carrier towards SH reagents. Biochim Biophys Acta. 1977 Oct 12;462(1):131–140. doi: 10.1016/0005-2728(77)90195-5. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Secemski I. I. P 1 ,P 5 -Di(adenosine-5')pentaphosphate, a potent multisubstrate inhibitor of adenylate kinase. J Biol Chem. 1973 Feb 10;248(3):1121–1123. [PubMed] [Google Scholar]
- Meijer A. J., Groot G. S.P., Tager J. M. Effect of sulphydryl-blocking reagents on mitochondrial anion-exchange reactions involving phosphate. FEBS Lett. 1970 May 11;8(1):41–44. doi: 10.1016/0014-5793(70)80220-4. [DOI] [PubMed] [Google Scholar]
- Naftalin R. J. A model for sugar transport across red cell membranes without carriers. Biochim Biophys Acta. 1970 Jul 7;211(1):65–78. doi: 10.1016/0005-2736(70)90124-0. [DOI] [PubMed] [Google Scholar]
- Papa S., Kanduc D., Lofrumento N. E. Phosphate transport in mitochondria action of mersalyl on the binding and transport of inorganic phosphate. FEBS Lett. 1973 Oct 1;36(1):9–11. doi: 10.1016/0014-5793(73)80325-4. [DOI] [PubMed] [Google Scholar]
- Pedersen P. L., Greenawalt J. W., Reynafarje B., Hullihen J., Decker G. L., Soper J. W., Bustamente E. Preparation and characterization of mitochondria and submitochondrial particles of rat liver and liver-derived tissues. Methods Cell Biol. 1978;20:411–481. doi: 10.1016/s0091-679x(08)62030-0. [DOI] [PubMed] [Google Scholar]
- Pfaff E., Klingenberg M., Heldt H. W. Unspecific permeation and specific exchange of adenine nucleotides in liver mitochondria. Biochim Biophys Acta. 1965 Jun 15;104(1):312–315. doi: 10.1016/0304-4165(65)90258-8. [DOI] [PubMed] [Google Scholar]
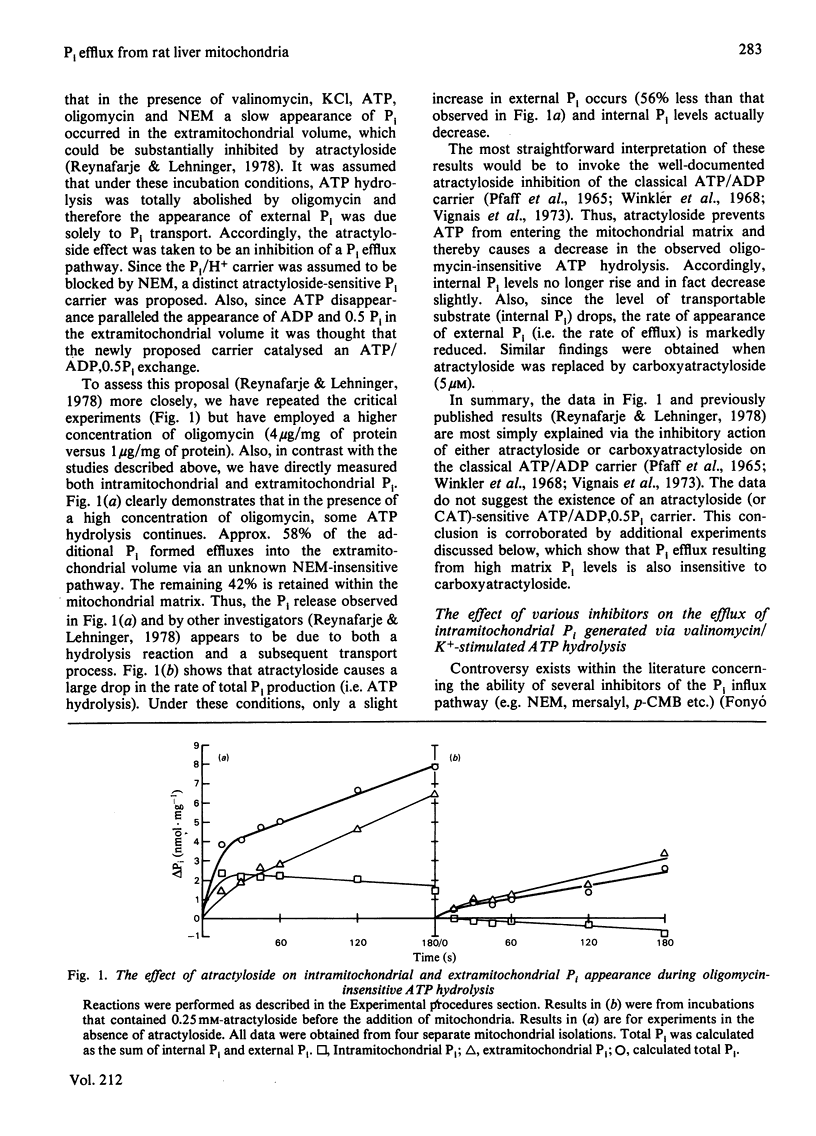
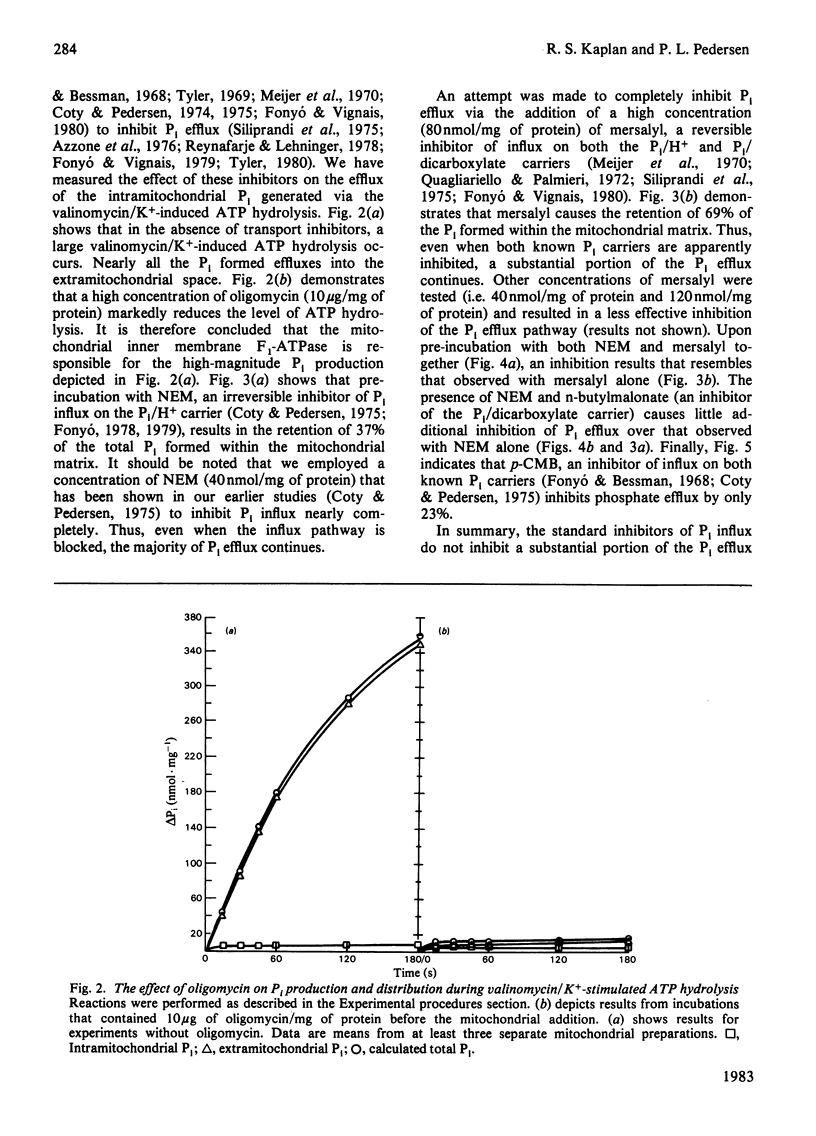
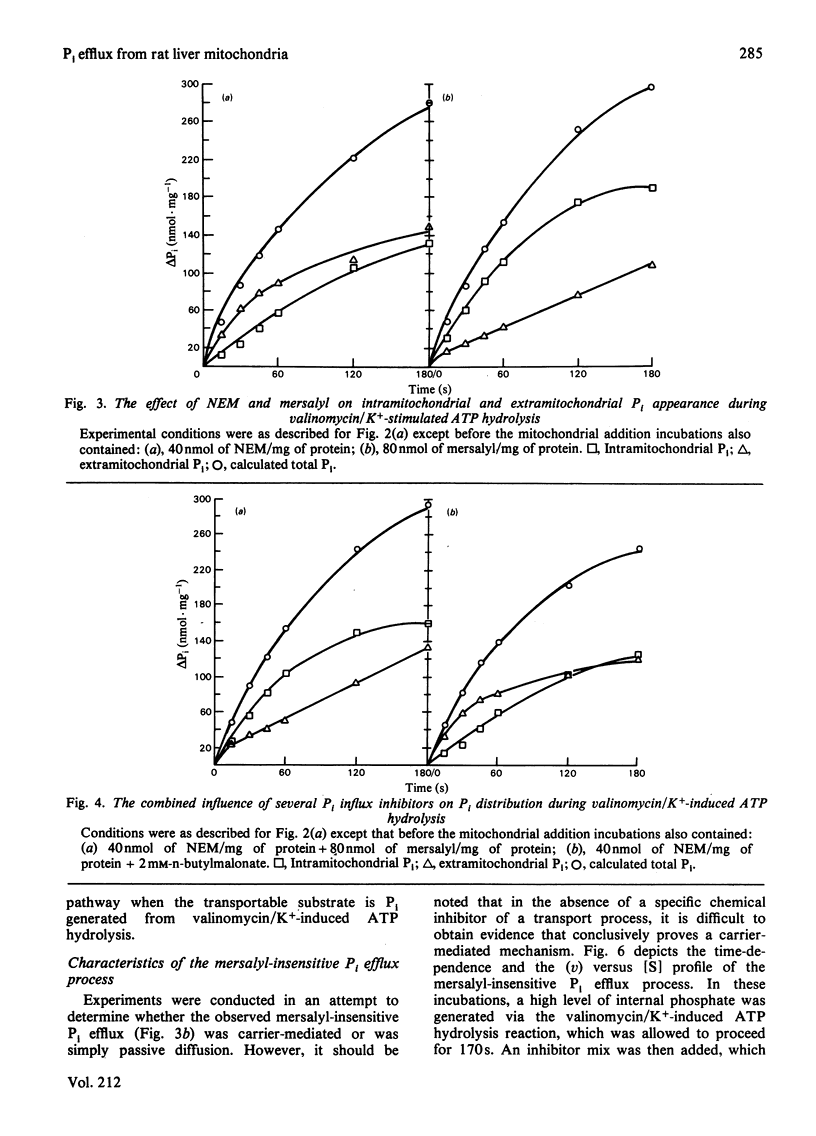
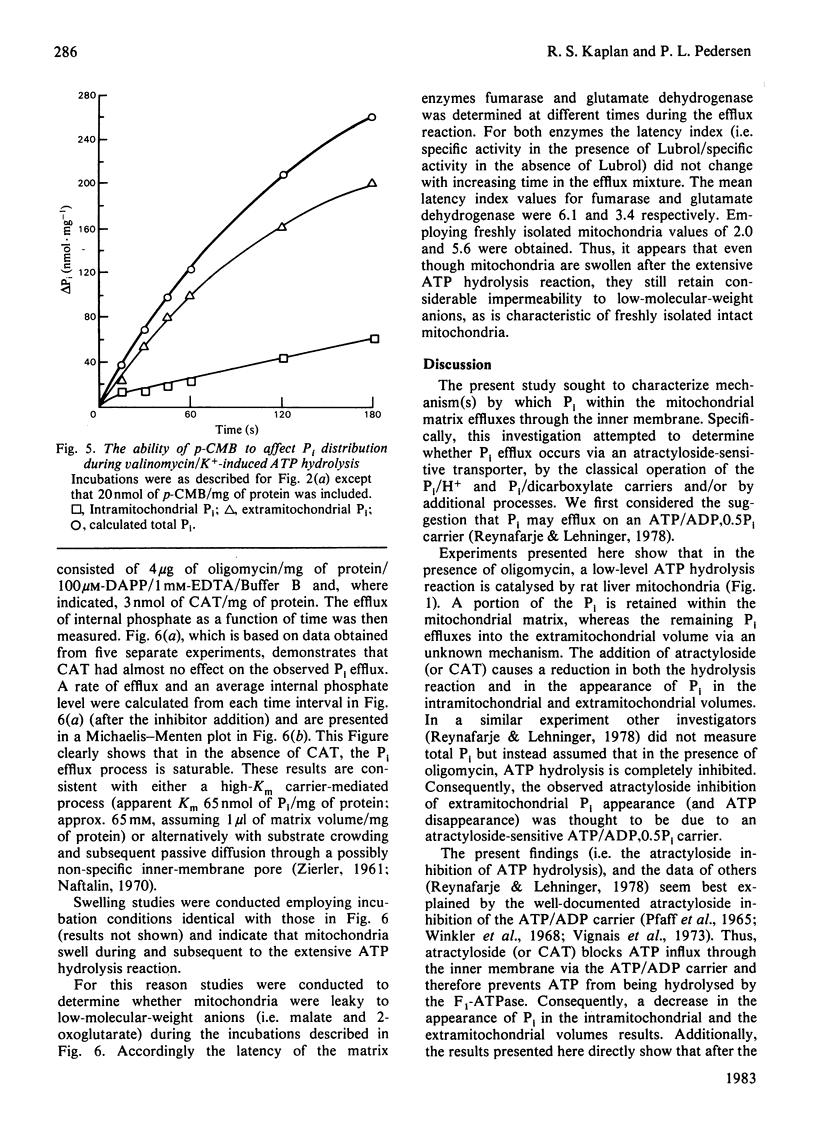
- Reynafarje B., Lehninger A. L. An alternative membrane transport pathway for phosphate and adenine nucleotides in mitochondria and its possible function. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4788–4792. doi: 10.1073/pnas.75.10.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliprandi D., Toninello A., Zoccarato F., Bindoli A. Phosphate transport across the mitochondrial membrane: the influence of thiol oxidation and of Mg++ on inhibition by mercurials. FEBS Lett. 1975 Mar 1;51(1):15–17. doi: 10.1016/0014-5793(75)80844-1. [DOI] [PubMed] [Google Scholar]
- Tyler D. D. Evidence of a phosphate-transporter system in the inner membrane of isolated mitochondria. Biochem J. 1969 Mar;111(5):665–678. doi: 10.1042/bj1110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler D. D. The pathway of inorganic-phosphate efflux from isolated liver mitochondria during adenosine triphosphate hydrolysis. Biochem J. 1980 Dec 15;192(3):821–828. doi: 10.1042/bj1920821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignais P. V., Vignais P. M., Defaye G. Adenosine diphosphate translocation in mitochondria. Nature of the receptor site for carboxyatractyloside (gummiferin). Biochemistry. 1973 Apr 10;12(8):1508–1519. doi: 10.1021/bi00732a007. [DOI] [PubMed] [Google Scholar]
- Wehrle J. P., Cintrón N. M., Pedersen P. L. Phosphate transport in rat liver mitochondria. Energy-dependent accumulation of phosphate by inverted inner membrane vesicles. J Biol Chem. 1978 Dec 10;253(23):8598–8603. [PubMed] [Google Scholar]
- Wehrle J. P., Pedersen P. L. Phosphate transport in rat liver mitochondria: location of sulfhydryl groups essential for transport activities. J Bioenerg Biomembr. 1981 Dec;13(5-6):285–294. doi: 10.1007/BF00743206. [DOI] [PubMed] [Google Scholar]
- Winkler H. H., Bygrave F. L., Lehninger A. L. Characterization of the atractyloside-sensitive adenine nucleotide transport system in rat liver mitochondria. J Biol Chem. 1968 Jan 10;243(1):20–28. [PubMed] [Google Scholar]
- ZIERLER K. L. A model of a poorly-permeable membrane as an alternative to the carrier hypothesis of cell membrane penetration. Bull Johns Hopkins Hosp. 1961 Jul;109:35–48. [PubMed] [Google Scholar]



