Abstract
Background
To evaluate the accuracy of artificial intelligence (AI)-based technology in recognizing tessellated fundus in students aged 7–14 years.
Methods
A retrospective study was conducted to collect consecutive fundus photographs for visual function screening of students aged 7–14 years old in Haikou City from June 2018 to May 2019, and 1907 cases were included in the study. Among them, 949 cases were male and 958cases were female. The results were manually analyzed by two attending ophthalmologists to ensure the accuracy of the results. In case of discrepancies between the results analyzed by the two methods, the manual results were used as the standard. To assess the sensitivity and specificity of AI in recognizing tessellated fundus, a Kappa consistency test was performed comparing the results of manual recognition with those of AI recognition.
Results
Among 1907 cases, 1782 cases, or 93.4%, were completely consistent with the recognition results of manual and AI; 125 cases, or 6.6%, were analyzed with differences. The diagnostic rates of manual and AI for tessellated fundus were 26.1% and 26.4%, respectively. The sensitivity, specificity and area of the ROC curve (AUC) of AI for recognizing tessellated fundus in students aged 7–14 years were 88.0%, 95.4% and 0.917, respectively. The results of test showed that that the manual and AI identification results were highly consistent (κ = 0.831, P = 0.000).
Conclusion
AI analysis has high specificity and sensitivity for tessellated fundus identification in students aged 7–14 years, and it is feasible to apply artificial intelligence to visual function screening in students aged 7–14 years.
Keywords: Tessellated fundus, Artificial intelligence, Visual function screening
Background
Myopia is one of the most common eye diseases in the world [1–3], and studies have suggested that the global prevalence of myopia was 28.3% in 2010, and is projected to reach 49.8% in 2050 [4]. In China, the prevalence of myopia among schoolchildren ranks first in the world [5]. Tesselated fundus (TF), characterized by the clear visibility of large choroidal vessels surrounding the macular central recess and the vascular arch, is an important early manifestation of pathologic myopia [6, 7]. Pathological myopia can lead to myopic macular degeneration (MMD) and posterior scleral staphyloma, resulting in irreversible vision loss [8]. Therefore, screening children with fundus tessellation, even in the early stages of pathologic myopia where visual impairment is not yet present, is crucial for several reasons. It allows for early detection and intervention, which can help slow the progression of myopia and reduce the risk of severe complications later in life. Additionally, screening facilitates risk stratification, enabling personalized management for those at higher risk. It also supports public health planning by providing data on the prevalence of early-stage myopia, aiding in resource allocation and the development of preventive education programs. Furthermore, early screening contributes to longitudinal research, enhancing our understanding of myopia’s progression and informing future treatment strategies [9].
With the advantages of large data resources, precision, accuracy and stability beyond human ability, as well as the ability to save human resources, artificial intelligence analysis has become increasingly popular in clinical medical research applications. In recent years, AI techniques represented by deep convolutional neural networks (DCNN) have been successfully applied to the automatic identification and quantitative segmentation tasks of various retinopathies, such as diabetic retinopathy (DR) [10, 11], retinopathy of prematurity (ROP) [12, 13], age-related macular degeneration (AMD) [14], glaucoma [15–17], and other ophthalmic diseases in screening. Research on the identification of TF, particularly its association with pathologic myopia, has been gaining attention in recent years. In the context of myopia, AI technologies have been applied to detect myopic maculopathy and other related retinal conditions. For instance, studies by Tan et al. (2021) [18] and Lu et al. (2021) [19] demonstrated that AI could accurately identify myopic maculopathy and pathologic myopia using fundus photographs, with performance metrics indicating high sensitivity and specificity. More recently, deep learning algorithms (DL) have been reported to be applied to automatically detect myopic macular degeneration and pathologic myopic fundus images [20–22]. In this study, we used manual and artificial intelligence to identify the TF changes shown by fundus photography for visual function screening of students aged 7–14 years old, and compared the consistency of the two approaches, with a view to providing a faster and more effective means of screening TF in primary and secondary school students, as well as to compensate for the defect of misdiagnosis due to the inexperience of physicians to a certain extent. The results are reported as follows.
Objects and methods
Study participants
This was a retrospective study approved by the Ethics Committee of Haikou People’s Hospital (approval number: 2020 − 232). All patients signed a written informed consent.
During the visual function screening of primary and secondary school students in Haikou City from June 2018 to May 2019, a total of 15,633 cases of 31,266 eyes were referred to the Department of Ophthalmology of Haikou Hospital, affiliated to Xiangya School of Medicine, Central South University. For those with refraction not in the range of ± 1.50 DS in either eye, further specialty examinations were conducted. These included fundus photography, pupil dilation optometry, and biometric measurements. Excluding those with poor picture quality, incomplete information and age greater than 14 years or less than 7 years, a total of 1907 cases were included in this study. Considering the variability of both eyes for the leopard-like manifestation, the right eyes were selected for this study.
Detection method
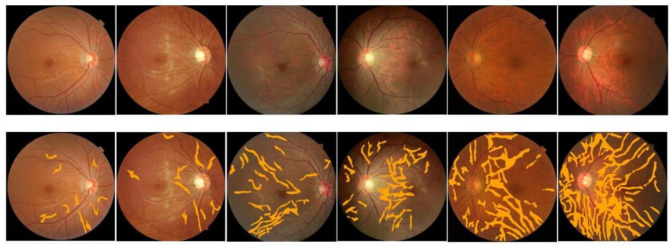
All patients underwent fundus examination using an automatic pupil-less Upstate Fundus Camera (SK-650 A, Chongqing, China), which captured 45° color fundus images of the patients centered on the macula. Artificial intelligence software (Airdoc, Beijing, China) was used to automatically detect leopard-like fundus changes, and the results of image detection were automatically recognized. This AI software utilizes deep learning algorithms to analyze the fundus images, identifying patterns and anomalies that may indicate the presence of leopard-like changes. The software version used in this application operates on a classification principle, tagging images with different disease labels. Similar to a doctor’s image interpretation, the software makes judgments based on visual features. It includes an image enhancement module designed to minimize the impact of varying light intensities or refractive media on the results. The output of the deep neural network is a vector, and based on the value of the heat map output vector, the quantitative description of the TF was realized by region segmentation, and the AI software gave the prediction of the TF (Fig. 1).
Fig. 1.
Representative heat maps generated by artificial intelligence for different severities of TF
Manual analysis was performed separately by two physicians with the title of attending physician or above to ensure the accuracy of the manual analysis. These physicians were selected based on their extensive experience and expertise in fundus examination and diagnosis. Ophthalmologists typically grade TF based on the extent of exposure of large choroidal vessels in the fundus: Grade 0, no choroidal vessel exposure (no TF); Grade 1, visible large choroidal vessels (mild TF); Grade 2, visible large and medium choroidal vessels (moderate TF); Grade 3, extensive visibility of large and medium choroidal vessels (severe TF). In case of discrepancies between the results of the two analysis methods, the manual analysis results were used as the standard. For cases where the two physicians made different judgments, they discussed only the differing images to reach a consensus, ensuring that each case was thoroughly evaluated and agreed upon. The agreement rate of the two analysis methods was calculated and compared. This comparison helps in understanding the reliability and accuracy of the AI software in real-world clinical settings. Consistency rate = (number of eyes with the same diagnostic result/total number of valid eyes collected) × 100%. A high consistency rate indicates a strong agreement between the AI and manual analysis, validating the effectiveness of the AI software.
Statistical analysis
SPSS 23.0 software was used for statistical analysis. Kappa consistency test was performed on the results of manual analysis and AI analysis, and Kappa values were judged by the following criteria: 0.0 ≤ κ < 0.2 was a very poor degree of consistency; 0.2 ≤ κ < 0.4 was a general consistency; 0.4 ≤ κ < 0.6 was a moderate degree of consistency; 0.6 ≤ κ < 0.8 was a good degree of consistency; 0.8 ≤ κ < 1 was a strong degree of consistency. The p < 0.05 was considered as the difference was statistically significant. The sensitivity and specificity of the computational AI analysis for TF changes in students aged 7–14 years.
Results
This study included 1907 cases of primary and secondary school students, including 949 males (49.8%) and 958 females (50.2%). Their ages ranged from 7 to 14 years old, with a mean age of 10.6 ± 2.33 years. The mean age of male and female subjects was 10.7 ± 2.31 and 10.5 ± 2.35 years old, respectively, and the difference was not statistically significant when comparing the mean age of those of different genders (t = 1.805, p = 0.071). Their right eyes were used for further analysis.
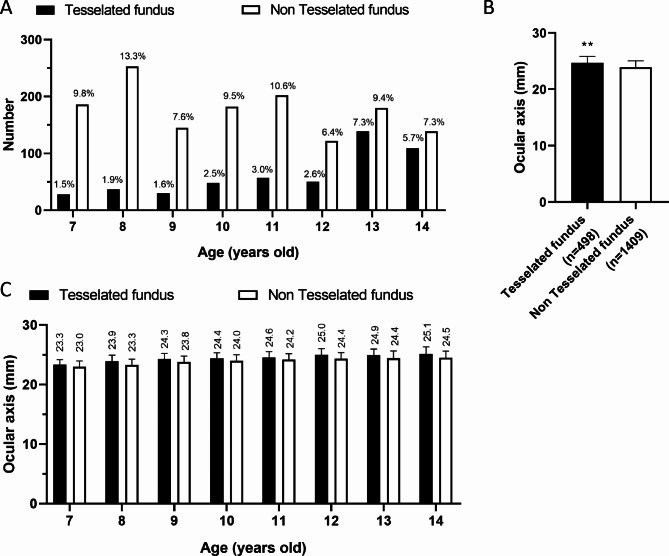
Among 1907 right eyes, 498 eyes (26.1%) were diagnosed by the ophthalmologist as having TF, including 264 males and 234 females. The distribution of all TF of eye in different ages is shown in Fig. 2A, the results showed that 28 eyes were from 7 years old, 37 eyes were from 8 years old, 30 eyes were from 9 years old, 48 eyes were from 10 years old, 57 eyes were from 11 years old, 50 eyes were from 12 years old, 139 eyes were from 13 years old, and 109 eyes were from 14 years old patients. Meanwhile, the axial length of the right eye in the leopard fundus group was significantly larger than that in the non-leopard fundus group at different ages (Fig. 2B/C).
Fig. 2.
Comparison of age distribution and mean ocular axis between leopard and non-TF. A, Age distribution of TF and non-TF in primary and secondary school students. B-C. Comparison of mean ocular axis of TF and non-TF in whole (B) or different ages(C).
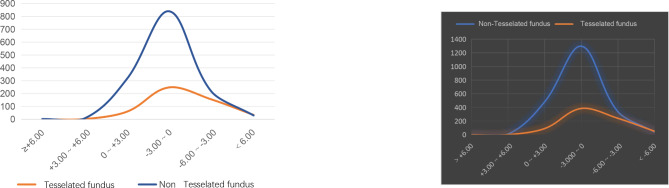
In addition, there were 0 and 2 leopard and non-leopard eyes with refractions greater than + 6.00D; 2 and 7 leopard and non-leopard eyes with refractions in the range of + 3.00D to + 6.00D; 59 and 313 leopard and non-leopard eyes with refractions in the range of 0D to + 3.00D; Leopard and non-leopard eyes with refractive error in the range of -3.000D to 0D were 248 and 840 eyes, respectively. Leopard and non-leopard-shaped eyes in the refractive error range of -6.00D to -3.00D were 155 and 217 eyes, respectively; and leopard and non-leopard eyes in the refractive error range of less than − 6.00D were 34 and 30 eyes, respectively (Fig. 3).
Fig. 3.
Comparison of the number of eyes in different refractive ranges between TF and non- TF
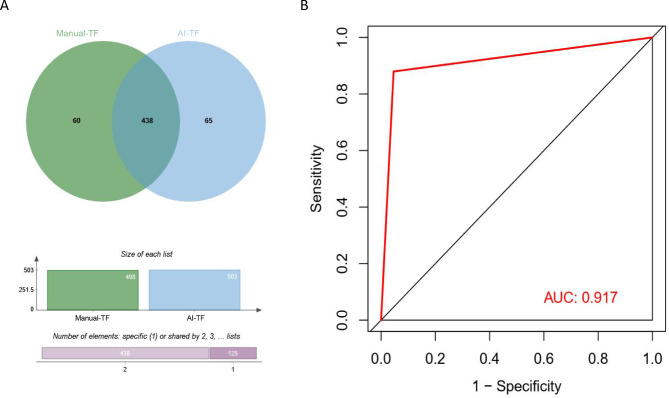
Among 1907 eyes, the manual analysis showed 498 and 1409 eyes with TF changes and non-TF changes, respectively. The AI analysis showed 503 and 1404 eyes with TF changes and non-TF changes, respectively. The results of manual analysis and AI analysis were in complete agreement in 1782 eyes, accounting for 93.4%, and there were differences in the analysis in 125 eyes, accounting for 6.6%. The diagnostic rates of manual analysis and AI analysis for TF changes were 26.1% and 26.4%, respectively. The results of the two modalities for TF interpretation were consistent for 438 eyes (Table 1; Fig. 4A). The sensitivity, specificity, total effective rate and area of the ROC curve (AUC) of the AI interpretation of TF in primary and secondary school students were 88.0%, 95.4%, 93.4% and 0.917, respectively (Fig. 4B). The kappa concordance test showed a high degree of consistency between the diagnostic results of the manual and AI analyses (κ = 0.831, P = 0.000).
Table 1.
Cross-tabulation of the results of manual and AI analysis of TF eyes in primary and secondary school students
| Manul AI | tessellated fundus | Non tessellated fundus | Total |
|---|---|---|---|
| tessellated fundus | 438 | 65 | 503 |
| Non tessellated fundus | 60 | 1344 | 1404 |
| Total | 498 | 1409 | 1907 |
AI, artificial intelligence
Fig. 4.
A, Wayne diagram showing comparison of overlap between manual and AI interpretation of the TF. B, ROC curve showed the accuracy of AI’s fundus diagnosis of leopard shape. TF; AUC, area under curve
Discussion
In this study, we evaluated the accuracy and feasibility of using AI technology to recognize TF in students aged 7–14 years during visual function screening. A retrospective design was employed, involving 1,907 students whose fundus photographs were analyzed both manually by experienced ophthalmologists and automatically by AI software. It demonstrated that AI has high sensitivity (88.0%) and specificity (95.4%) for recognizing TF, showing strong agreement with manual analysis. These findings highlight the potential of AI in early detection of pathological myopia among children, which is crucial for timely intervention and management. Adolescent myopia has become a serious public health problem in China, showing a rapid increase in incidence rate and constantly low age. Studies have shown that behavioral changes during COVID-19 isolation may have an impact on the progression of myopia [23, 24]. High myopia increases the risk of myopic choroidal neovascularization (mCNV), glaucoma, cataract, and retinal detachment [25, 26]. Children aged 7–14 years are in a sensitive and critical period of vision development, so early detection and intervention of myopia are of great significance. AI technology represented by Deep convolutional neural network (DCNN) has been widely applied to various retinopathy based on fundus color photography, such as diabetic retinopathy, age-related macular degeneration, glaucoma optic neuropathy, etc [10–17].
In the current study, we compared the mean axial length of the fundus of 1907 primary and secondary school students aged 7–14 years in Haikou City with TF and non-TF, and found that the mean axes of the TF were higher than those of the non-TF in each age group, and tended to be more flattened than those of the non-TF in the refractive power distribution, which suggests a connection between the leopard patterned fundus and the axes of the eye and refractive power. Moreover, the results of manual and AI analysis of fundus photography TF alterations showed a high degree of consistency with 93.4% complete agreement between manual and AI analysis. The reason for the difference in diagnosis may be due to the insufficient recognition of atypical diseases such as optic disc edema, increased cup-to-disc ratio, myopic arcuate spot, and others by AI technology, or the poor quality of fundus images with smaller pupils or refractive interstitial clouding. The sensitivity, specificity and AUC of AI analysis of TF in primary and secondary school students were 88.0%, 95.4% and 0.917, respectively, which demonstrated that AI technology has a high specificity for TF recognition in primary and secondary school students. This study demonstrated that artificial intelligence technology has high specificity and sensitivity for the identification of TF, and that it is feasible to apply artificial intelligence technology to the screening of visual function in primary and secondary school students, which can detect early myopia and TF changes in 7–14 years old children in China, and play a key role in the tracking of myopia changes and the corresponding complications at a later stage, and provide a scientific basis for the prevention and control of myopia.
With the advancement of technology, AI diagnosis will be more accurate in the future, and develop in the direction of integrating picture interpretation, etiology analysis, and treatment plan provision. Comprehensive AI analysis of the results, to carry out timely treatment, which is the significance of the rapid detection of AI. Although AI analysis is not perfect at this stage and needs to be further optimized, it is undeniable that AI analysis provides a new method for fundus screening of primary and secondary school students, which is a trend of future medical development, especially for areas with backward medical resources, it can effectively reduce the workload of ophthalmologists, and it has a significant value for the discovery and follow-up of the TF in primary and secondary school students.
This study has some limitations, firstly, the diagnosis of TF is subjective and lacks objective indicators. However, the two reviewers are experienced ophthalmologists in this field and their decisions were based on the same review standard. This maximizes the accuracy of the results. Secondly, the results of this study were limited to primary and secondary school students and were the results of the follow-up examinations of those who were in doubt in the initial screening of visual function. This may lead to a selection bias, resulting in a high prevalence rate of TF of 26.1%, which is not representative of the prevalence rate of leptomeningeal fund in the general population. In addition, corneal curvature, corneal thickness, OCT, and OCT angiography (OCTA) examinations can be added in the future to analyze their relationship with the prevalence of TF and to further validate the feasibility of artificial intelligence to analyze TF alterations in visual function screening.
Conclusion
AI analysis has high specificity and sensitivity for TF identification in students aged 7–14 years, and it is feasible to apply artificial intelligence to visual function screening in students aged 7–14 years.
Acknowledgements
Not applicable.
Abbreviations
- MMD
myopic macular degeneration
- DCNN
deep convolutional neural networks
- DR
diabetic retinopathy
- ROP
retinopathy of prematurity
- AMD
age-related macular degeneration
- DL
deep learning algorithms
- AUC
area of the ROC curve
- mCNV
myopic choroidal neovascularization
Author contributions
Meng-ying Guo, Yun-yan Zheng and Qing Xie contributed to the study conception and design. Material preparation, data collection and analysis were performed by Meng-ying Guo, Yun-yan Zheng and Qing Xie. The first draft of this manuscript was written by Meng-ying Guo. Meng-ying Guo,Qing Xie read and approved the final manuscript.
Funding
This study is supported by Key R&D Plan Projects in Hainan Province (ZDYF2020110).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This was a retrospective study approved by the Ethics Committee of Haikou People’s Hospital (approval number: 2020 − 232). Informed consent to all participates were obtained from their parent or legal guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meng-ying Guo and Yun-yan Zheng contributed equally to this work as Co-first author.
References
- 1.Modjtahedi BS, Ferris FL 3rd, Hunter DG, Fong DS. Public Health Burden and Potential Interventions for myopia. Ophthalmology. 2018;125(5):628–30. [DOI] [PubMed] [Google Scholar]
- 2.Grzybowski A, Kanclerz P, Tsubota K, Lanca C, Saw SM. A review on the epidemiology of myopia in school children worldwide. BMC Ophthalmol. 2020;20(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Resnikoff S, Jonas JB, Friedman D, He M, Jong M, Nichols JJ, Ohno-Matsui K, Smith EL III, Wildsoet CF, Taylor HR, et al. Myopia - A 21st Century Public Health Issue. Invest Ophthalmol Vis Sci. 2019;60(3):Mi–Mii. [DOI] [PMC free article] [PubMed]
- 4.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–42. [DOI] [PubMed] [Google Scholar]
- 5.Wang SK, Guo Y, Liao C, Chen Y, Su G, Zhang G, Zhang L, He M. Incidence of and factors Associated with myopia and high myopia in Chinese children, based on refraction without Cycloplegia. JAMA Ophthalmol. 2018;136(9):1017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohno-Matsui K, Kawasaki R, Jonas JB, Cheung CM, Saw SM, Verhoeven VJ, Klaver CC, Moriyama M, Shinohara K, Kawasaki Y, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159(5):877–e883877. [DOI] [PubMed] [Google Scholar]
- 7.Yan YN, Wang YX, Xu L, Xu J, Wei WB, Jonas JB. Fundus Tessellation: Prevalence and Associated factors: the Beijing Eye Study 2011. Ophthalmology. 2015;122(9):1873–80. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Foo LL, Wong CW, Li J, Hoang QV, Schmetterer L, Ting DSW, Ang M. Pathologic myopia: advances in imaging and the potential role of artificial intelligence. Br J Ophthalmol. 2023;107(5):600–6. [DOI] [PubMed] [Google Scholar]
- 9.Guo Y, Liu L, Zheng D, Duan J, Wang Y, Jonas JB, Tian F, Wang S, Sang Y, Zhang X, et al. Prevalence and associations of Fundus Tessellation among Junior Students from Greater Beijing. Invest Ophthalmol Vis Sci. 2019;60(12):4033–40. [DOI] [PubMed] [Google Scholar]
- 10.Ting DSW, Cheung CY, Lim G, Tan GSW, Quang ND, Gan A, Hamzah H, Garcia-Franco R, San Yeo IY, Lee SY, et al. Development and validation of a Deep Learning System for Diabetic Retinopathy and Related Eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318(22):2211–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voets M, Mollersen K, Bongo LA. Reproduction study using public data of: Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. PLoS ONE. 2019;14(6):e0217541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown JM, Campbell JP, Beers A, Chang K, Ostmo S, Chan RVP, Dy J, Erdogmus D, Ioannidis S, Kalpathy-Cramer J, et al. Automated diagnosis of plus Disease in Retinopathy of Prematurity using deep convolutional neural networks. JAMA Ophthalmol. 2018;136(7):803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao J, Luo Y, Liu L, Lao J, Shao Y, Zhang M, Zhang C, Sun M, Shen L. Automated diagnosis and quantitative analysis of plus disease in retinopathy of prematurity based on deep convolutional neural networks. Acta Ophthalmol. 2020;98(3):e339–45. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Gonzalo C, Sanchez-Gutierrez V, Hernandez-Martinez P, Contreras I, Lechanteur YT, Domanian A, van Ginneken B, Sanchez CI. Evaluation of a deep learning system for the joint automated detection of diabetic retinopathy and age-related macular degeneration. Acta Ophthalmol. 2020;98(4):368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Li L, Wormstone IM, Qiao C, Zhang C, Liu P, Li S, Wang H, Mou D, Pang R, et al. Development and validation of a Deep Learning System to Detect Glaucomatous Optic Neuropathy using Fundus photographs. JAMA Ophthalmol. 2019;137(12):1353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, He Y, Keel S, Meng W, Chang RT, He M. Efficacy of a deep learning system for detecting Glaucomatous Optic Neuropathy based on Color Fundus photographs. Ophthalmology. 2018;125(8):1199–206. [DOI] [PubMed] [Google Scholar]
- 17.Mirzania D, Thompson AC, Muir KW. Applications of deep learning in detection of glaucoma: a systematic review. Eur J Ophthalmol. 2021;31(4):1618–42. [DOI] [PubMed] [Google Scholar]
- 18.Du R, Ohno-Matsui KJD. Novel uses and challenges of artificial intelligence in diagnosing and managing eyes with high myopia and pathologic myopia. 2022, 12(5):1210. [DOI] [PMC free article] [PubMed]
- 19.Lu L, Ren P, Tang X, Yang M, Yuan M, Yu W, Huang J, Zhou E, Lu L, He QJFC et al. AI-model for identifying pathologic myopia based on deep learning algorithms of myopic maculopathy classification and plus lesion detection in fundus images. 2021, 9:719262. [DOI] [PMC free article] [PubMed]
- 20.Tan TE, Anees A, Chen C, Li S, Xu X, Li Z, Xiao Z, Yang Y, Lei X, Ang M, et al. Retinal photograph-based deep learning algorithms for myopia and a blockchain platform to facilitate artificial intelligence medical research: a retrospective multicohort study. Lancet Digit Health. 2021;3(5):e317–29. [DOI] [PubMed] [Google Scholar]
- 21.Lu L, Ren P, Tang X, Yang M, Yuan M, Yu W, Huang J, Zhou E, Lu L, He Q, et al. AI-Model for identifying pathologic myopia based on Deep Learning algorithms of myopic Maculopathy classification and plus lesion detection in Fundus images. Front Cell Dev Biol. 2021;9:719262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du R, Xie S, Fang Y, Igarashi-Yokoi T, Moriyama M, Ogata S, Tsunoda T, Kamatani T, Yamamoto S, Cheng CY, et al. Deep Learning Approach for Automated Detection of Myopic Maculopathy and pathologic myopia in Fundus images. Ophthalmol Retina. 2021;5(12):1235–44. [DOI] [PubMed] [Google Scholar]
- 23.Xu L, Ma Y, Yuan J, Zhang Y, Wang H, Zhang G, Tu C, Lu X, Li J, Xiong Y, et al. COVID-19 Quarantine reveals that behavioral changes have an effect on myopia progression. Ophthalmology. 2021;128(11):1652–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang P, Zhang B, Lin L, Chen R, Chen S, Zhao Y, Qu J. Comparison of myopic progression before, during, and after COVID-19 lockdown. Ophthalmology. 2021;128(11):1655–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohno-Matsui K, Ikuno Y, Lai TYY, Gemmy Cheung CM. Diagnosis and treatment guideline for myopic choroidal neovascularization due to pathologic myopia. Prog Retin Eye Res. 2018;63:92–106. [DOI] [PubMed] [Google Scholar]
- 26.Williams KM, Bertelsen G, Cumberland P, Wolfram C, Verhoeven VJ, Anastasopoulos E, Buitendijk GH, Cougnard-Gregoire A, Creuzot-Garcher C, Erke MG, et al. Increasing prevalence of myopia in Europe and the impact of education. Ophthalmology. 2015;122(7):1489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.