Abstract
Background: Radiation therapy is an integral component of treatment that can predispose to carotid artery stenosis (CAS) and increase the risk of cerebrovascular events for head and neck cancer survivors. The utility of screening for CAS with carotid ultrasound in asymptomatic head and neck cancer survivors is unclear.
Methods: In this prospective, cross-sectional pilot study, 60 patients who have no evidence of cancer at least 2 years from completion of RT will undergo screening carotid ultrasound to identify patients with high risk of cardiovascular events.
Results: Outcomes will include clinically significant CAS, carotid intima-media thickness, acceptability/feasibility of screening, barriers to care and preliminary data on changes to medical management because of screening. Correlative multi-omics analyses will examine biomarkers of CAS after radiation therapy.
Conclusion: The results of this study will provide valuable data on the prevalence of CAS and preliminary patient-centered data that will inform the design of a future large-scale, multi-site clinical trial.
Clinical Trial Registration: NCT05490875 (ClinicalTrials.gov)
Keywords: : carotid stenosis, carotid ultrasound, head and neck cancer, radiotherapy, survivorship
Plain Language Summary
Patients with head and neck cancer are often treated with radiation therapy. Radiation therapy can cause damage to the blood vessels in the neck. This damage can manifest as narrowing of the blood vessels like the carotid artery, which can lead to stroke. Currently, it is not clear if screening head and neck cancer survivors with ultrasound scans of the carotid arteries is feasible or acceptable to patients. This has also not been formally assessed using a prospective clinical trial. In this study, patients with a history of head and neck cancer who have no evidence of their cancer for at least 2 years since completion of their radiation therapy will be enrolled. They will undergo blood testing and a research ultrasound of the carotid arteries to check for narrowing and other findings that may signal a high risk of stroke or another cardiovascular event. Participants will complete surveys on their experience with the process and how likely they are to accept further screening or additional treatment if something is found. They will also complete surveys on their perception of their personal risk of stroke and barriers to care that would prevent them from getting screening ultrasounds. Patients will be followed for up to 6 months after the ultrasound to check for any changes in their medical care that occurred because of the screening ultrasound.
Plain language summary
Article highlights.
Carotid artery stenosis in head & neck cancer survivors treated with radiation therapy
Radiotherapy for head and neck (HN) cancer may increase the risk of accelerated atherosclerosis and late carotid artery stenosis (CAS) in 25% of patients, and screening of asymptomatic stenosis may be useful and cost-effective in specific high-risk populations.
If clinically significant stenosis is identified, risk reduction strategies such as optimal medical management with or without procedural intervention may reduce the risk of stroke.
Data supporting the use of carotid ultrasound (CUS) for HN cancer survivors are sparse, and no guideline currently recommends CUS screening for asymptomatic patients.
Carotid ultrasound surveillance for detection of asymptomatic carotid artery stenosis after head & neck radiotherapy
CUS allows for cardiovascular risk stratification using stenosis and intima-media thickness measurements.
Knowledge gaps with regard to the use of CUS screening in this population include patient acceptability, feasibility, and clinical utility.
Rates of change in medical management for screen-detected carotid stenosis in HN cancer survivors have not been assessed in a prospective study.
Pilot trial of carotid artery ultrasound in head & neck cancer survivors
In this single-institution trial, 60 patients with a history of HN cancer treated with radiotherapy will undergo screening CUS.
Patients will be evaluated for CAS (peak systolic velocity by Doppler ultrasonography), and intima-media thickness will be measured.
Outcomes include patient acceptability, feasibility, barriers to care and perceived stroke risk among participants. In patients with clinically significant stenosis, the rate of resultant medical interventions (e.g., new medication, additional imaging or intervention) will be determined.
Conclusion
This prospective study for CUS screening in HN cancer survivors will obtain novel preliminary data to better understand the utility and feasibility of a CUS screening program.
Preliminary results will inform future trials focused on the efficacy and cost-effectiveness of carotid artery ultrasound screening in this patient population.
Correlative analysis will elucidate novel biomarkers and mechanisms of post-radiotherapy CAS.
1. Introduction
1.1. Background & rationale
Head and neck (HN) cancer is a disease of several sites within the HN region, including the nasopharynx, oropharynx, oral cavity, larynx, hypopharynx, sinuses and skin. Over 66,000 patients are diagnosed in the United States with HN cancer each year [1]. Radiation therapy (RT) is a common method of treatment for HN cancers, either as the primary definitive treatment (with or without concurrent chemotherapy) or as adjuvant treatment following a curative-intent surgery. Due to the rich lymphatic drainage of the HN region, a substantial risk of regional lymph node involvement at diagnosis or subsequent recurrence (if untreated) warrants treatment to neck in most patients treated with HN RT [2,3]. Based on their anatomic and surgical definitions, neck lymph node regions include the carotid arteries, which are thus included in the RT target volume [4]. As a result, the carotid arteries are often exposed to a high dose of therapeutic ionizing radiation and are at risk for late-developing injury.
RT-induced carotid artery stenosis (CAS) is a significant issue that may lead to CVA or transient ischemic attack (TIA). This is particularly a problem given increases in cure rates over the decades as well as the substantial increase in human papillomavirus-associated oropharyngeal cancer, which carries an excellent long-term oncologic prognosis [5–7]. Despite this issue, strategies to mitigate the potentially catastrophic risks associated with RT-induced CAS are not well studied. The primary mechanism of carotid artery damage secondary to irradiation has not yet been precisely determined. RT-induced CAS is characterized by endothelial cell damage and subsequent malfunction that manifests as lipid and fibrin deposition, platelet aggregation, thickening of the endothelium and fibrosis of the vessel wall [8,9]. Damage to the small blood vessels that provide blood supply to the vessel wall itself (vasa vasorum) is thought to contribute to vessel wall necrosis and fibrosis [10]. IMT, an ultrasound-based measurement of the thickness of the two inner layers of the arterial wall, has been evaluated in multiple studies to quantify the extent of carotid damage/atherosclerotic burden. In patients treated with neck RT, the IMT is significantly greater than in normal controls [11,12]. Additionally, smoking and alcohol use are some of the most common risk factors for HN cancer that also act as independent risk factors of atherosclerotic disease/CAS in patients already at risk for RT-induced acceleration of atherosclerotic disease, likely secondary to the mechanisms described above [13,14].
A meta-analysis of CAS after RT identified clinically significant (>50%) CAS in 25% of patients [15]. This increased over time from 4% at 1 year to 12% at 2 years and 21% at 3 years, consistent with an expected time course of late-reacting RT-induced tissue damage. An observational study evaluated the carotid arteries of 4–20-year nasopharyngeal cancer survivors treated with RT as well as those with newly diagnosed disease that had not yet undergone RT [16]. The prevalence of significant internal or common CAS was 30% (78% for any stenosis) in post-RT patients compared with 0% (22%) for untreated patients. More consequential is the risk of stroke or TIA in this cohort, which approaches 5% [15,17,18]. A SEER analysis of HN cancer patients over the age of 65 treated with either definitive RT, surgery plus adjuvant RT, or surgery alone found a very high 10-year rate of cerebrovascular events (defined as stroke, carotid revascularization or stroke death) of 34%, compared with 25% after surgery and RT or 26% after surgery alone [19]. Predictive measures for this population do not exist to inform screening standards. Carotid IMT is a well-established measure of atherosclerotic disease that predicts adverse events such as cardiovascular events and coronary heart disease [20,21]. This measure may allow us to predict cerebrovascular events prior to the development of clinically relevant CAS and warrants prospective study.
Significant CAS can be defined as ≥50% narrowing of the vessel lumen and the risk of ipsilateral stroke increases with the degree of narrowing [22]. Multiple large clinical trials have investigated the comparative benefit of different interventions for asymptomatic CAS, but none have included a placebo or no-treatment group [23,24]. Thus, it is well-accepted that patients with asymptomatic carotid stenosis should undergo some form of therapy [25]. This applies to both the general population and patients with HN cancer. Treatment options for asymptomatic CAS include the following: lipid-lowering medications/statin therapy, antithrombotic therapies such as aspirin or other medications, active management of other risk factors (e.g., hypertension, hyperglycemia), smoking cessation, dietary modification, physical activity/exercise and weight reduction [26]. These interventions are generally accepted within the medical community as the standard of care for patients with CAS detected by clinical exam or screening ultrasound. Additionally, once identified, routine ultrasound imaging of the carotid arteries to monitor for stability/progression is usually recommended [27].
To prevent potentially catastrophic cerebrovascular events like CVA or TIA from occurring secondary to RT-induced CAS lesions, a screening program to identify and intervene upon high-risk arteries is warranted. Carotid ultrasound (CUS) is an available and simple technique that uses non-ionizing ultrasound imaging to evaluate for CAS [28]. Surveillance strategies are not mentioned in the American Cancer Society Head and Neck Cancer Survivorship Guideline (despite it specifically listing CAS and carotid obstruction as potential late effects), nor are they mentioned in the National Comprehensive Cancer Network Clinical Practice Guidelines for Head and Neck Cancer [29,30]. The Society for Vascular Surgery suggests that the risk of CAS in HN RT patients is likely sufficiently high to warrant screening, though limited data on prevalence and optimal intervention (stenting favored over carotid endarterectomy [CEA]) preclude the formulation of more specific screening guidelines [31]. This is primarily due to a lack of concrete evidence demonstrating that screening CUS in HN cancer survivors treated with RT changes management in a way that would meaningfully improve cerebrovascular outcomes and decrease the risk of CVA/TIA. Others concerns regarding the limited therapeutic options for screen-detected CAS call into question the benefit of screening in this population. However, optimal medical management and attention to other modifiable risk reduction is the standard of care for any patient with asymptomatic significant CAS, regardless of etiology or clinical background [32]. In a retrospective analysis of over 600 patients treated with curative RT for HN cancer, 54% of patients with screen-detected CAS received medical intervention [18]. Another concern is that interventional procedures carry inherently greater risks to the patient with an irradiated neck, possibly minimizing the effectiveness of screening for asymptomatic disease. However, 20% of patients with screen-detected CAS undergo a procedural intervention (CEA or stent) [18]. A meta-analysis of 27 studies comprising 533 patients who had received prior cervical irradiation found no significant difference in adverse events between CEA and carotid stenting with overall favorable cerebrovascular outcomes [33]. Similar rates of any cerebrovascular adverse event between CEA (3.5%) and carotid stenting (3.9%) were observed with a lower rate of cerebrovascular events in the CEA group (2.8 events per 100 person-years) versus carotid stenting (4.9 events per 100 person-years, p = 0.014).
For patients with post-RT CAS, intervention (medical management, surgical or intravascular revascularization procedures, or subsequent surveillance) is warranted and efficacious in reducing the risk of CVA/TIA. Data on the outcomes of implementation of a formal screening CUS program for HN cancer survivors treated with RT are limited. Further research is sorely needed to define the feasibility and acceptability of routine post-RT CUS in this patient population and to determine the optimal timing of initiation and frequency of CUS screening. These preliminary data will allow us to continue studying this important survivorship issue in this patient population that, despite carrying a higher prevalence of CAS, is under-screened compared with the general population.
1.2. Objectives
The primary objective is to determine the proportion of patients with clinically significant CAS (≥50% stenosis, defined by peak systolic flow velocity of 150 cm/s or higher on Doppler ultrasonography) among HN cancer survivors treated with HN radiotherapy. Clinically significant CAS >50% is a well-established marker of carotid artery disease that represents an indication to potentially change a patient's medical care through lifestyle modifications (e.g., diet, exercise and smoking cessation), antiplatelet medications, lipid-lowering therapy, antihypertensive medications, glucose-lowering therapies, subsequent follow-up imaging tests, or consideration of revascularization [34,35]. The rationale for this end point is to identify patients that would experience changes in their medical care (not limited to revascularization) based on the presence of asymptomatic CAS that may not have been detected without a screening CUS. Medical interventions including lifestyle modification and optimal medical management are accepted standards of care for patients with cardiovascular disease such as asymptomatic CAS and are widely recommended by multiple consensus guideline statements [35–39].
Secondary objectives are to determine the following: the IMT of the carotid arteries in HN cancer survivors, the proportion of patients with high-risk carotid IMT (at least 0.9 mm and/or a relative risk of 1.50 or greater based on matched population-based controls), the proportion of patients with carotid plaques (at least 2 mm in thickness), the risk of developing CAS based on clinical and HN cancer-related factors, and the feasibility of CUS screening in this patient population. We also aim to obtain preliminary data on the acceptability of CUS screening, potential barriers to undergoing CUS screening, and baseline stroke risk perception among HN cancer survivors treated with RT. Exploratory objectives are (1) to determine the proportion of actionable patients with abnormal CUS findings for which a risk-reduction intervention is recommended, scheduled/prescribed or initiated/completed within the 6 months after CUS and (2) to correlate carotid IMT measurements with RT dose to the carotid artery.
1.3. Trial design
This is a single-arm, non-randomized study to evaluate the efficacy, feasibility and acceptability of screening CUS to detect CAS in HN cancer survivors who were previously treated with RT. Patients will be identified through the electronic medical record and contacted to assess their interest in the study and to perform a screening questionnaire. After enrollment, a baseline cancer history form and will undergo a CUS for screening for CAS and for measurement of carotid IMT. After the CUS examination, patients will then be asked to complete surveys on the acceptability of CUS, preferences for the frequency and timing of screening CUS, barriers to care and patient-perceived risks of stroke and CAS. The results of the CUS will be made available to the patient and will be sent to the patient's primary care provider and oncology providers. If the patient does not have a primary care provider, referrals will be made, and the results provided accordingly. In a case where significant CAS is detected, a referral will be placed for a formal clinical CUS imaging procedure to characterize better the extent of CAS and direct further management per standard of care. Follow-up assessments by phone will occur at 3- and 6-months after CUS and will assess for changes to the patients' health or healthcare management (e.g., new physician visits or referrals, new medications, subsequent imaging and medical procedures) that were recommended (or, prescribed, scheduled, started or completed) in the interim as a result of the CUS findings.
2. Methods
2.1. Study setting
This is a prospective, cross-sectional study of patients with HN cancer treated with RT who have no evidence of disease at least 2 years from the completion of RT without any clinical symptoms or personal history of CAS, CVA or TIA. While study procedures and assessments will occur at a single National Cancer Institute-designated Comprehensive Cancer Center, patients are not required to have been treated with RT at the study site. Potentially eligible participants will be identified from two sites in the Southeast United States: an academic referral center and an affiliated community practice. Patients will be primarily identified through clinical visits and targeted communications to HN cancer survivors treated at the participating sites identified through review of the medical record. IRB-approved materials such as brochures and flyers are available for dissemination to the general public, as patients treated previously at other facilities are eligible.
2.2. Eligibility criteria
Eligibility and exclusion criteria for this study are summarized in Table 1. Patients must have a history of HN cancer treated with at least 45 Gy to at least one region of the neck. Patients must have no evidence of HN cancer at least 2 years since completion of RT. They must have no history of CVA or TIA as verified by the Questionnaire for Verifying Stroke-Free Status (QVSFS) and must have no known history of CAS. They must be able to understand and willing to sign an IRB-approved informed consent document. Patients will be excluded if they have a personal history of CAS, any prior CVA or TIA, have had a CEA or carotid stent placement, have undergone a prior CUS between the completion of RT and study registration, if their most recent RT was for the treatment of recurrent or second primary HN cancer, if they have a history of re-irradiation in the HN region, or if they have an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or higher.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria |
|---|
| • History of head and neck cancer treated with radiotherapy. Radiotherapy target volume(s) must have included at least one region of the neck to a total dose of at least 45 Gray (Gy). • At least 2 years since completion of radiotherapy with no evidence of disease at the time of last clinical follow-up. • Eligible by Screening Questionnaire • Ability to understand and the willingness to sign an IRB-approved informed consent document (either directly or via a legally authorized representative). |
| Exclusion criteria |
|---|
| • Personal history of any of the following: ○ Carotid artery stenosis on either side of the neck ○ Stroke (CVA) or transient ischemic attack (TIA) ○ Carotid endarterectomy ○ Carotid stent placement • Prior carotid artery ultrasound examination between completion of RT and registration. • Most recent radiotherapy treatment was for any recurrence of a prior head and neck cancer and/or treatment for a subsequent head and neck cancer after diagnosis and treatment of an initial head and neck cancer. • Any history of re-irradiation to the head and neck region. Re-irradiation is defined as a subsequent individual course of RT where the target overlaps a region of the head/neck that was previously targeted by the initial course of RT. • Eastern Cooperative Oncology Group Performance Status of 2 or greater. |
2.3. Interventions
The intervention is a bilateral carotid artery ultrasound that will be administered on an outpatient basis at a single site at the coordinating center. High-resolution ultrasound scans of the carotid arteries will be acquired using a GE Logiq 9 ultrasound system with an L9-3 MHz linear array transducer. Measurement of IMT will be performed following guidelines from the American Society of Echocardiography and in regions not containing plaque [40]. R-wave gated still frame images will be acquired of the distal common carotid artery (CCA), the bulb at the flow divider, and the proximal internal carotid artery far wall at three separate angles bilaterally (anterior, lateral and posterior). Maximum IMT at each site will be analyzed offline using semi-automated edge detection software. This ultrasound examination will take approximately 1 hour to complete. The patient will be given a Carotid IMT Screening Exam Report on the ultrasound result on the day of the intervention. The results of the ultrasound, as well as a form letter, will be sent to the patient's primary care provider, their oncology treatment team, and the study PI. If the patient does not have a primary care doctor, a referral will be recommended and scheduled if accepted. In the event an abnormality is detected on the study ultrasound, the following procedures will occur: (1) referral for complete clinical US for confirmation of findings, (2) an indication of the abnormal findings will be present on the form letter sent to the patient's primary care doctor, their oncology treatment team and the PI.
2.4. Outcomes
The primary outcome will be the proportion of patients undergoing ultrasound with at least 50% stenosis identified at any position. This will be defined as having a peak systolic flow velocity of 150 cm/s or higher on Doppler ultrasonography. Carotid IMT will be measured at three separate angles (anterior, lateral and posterior) at the level of the distal CCA, carotid bulb at the flow divider, and the proximal internal carotid artery. The proportion of patients with high-risk IMT measurements will be calculated and defined as at least one IMT measurement of 0.9 mm or greater or a high-risk of cardiovascular events (relative risk of 1.5 or greater) based on average far-wall percentile scores and comparison with patient data from 4 to 7 years of follow-up in the ARIC study [21]. The proportion of patients with carotid plaque of maximum thickness of 2 mm or greater will be calculated. The association between clinically significant CAS and known cardiovascular risk factors (gender, age, blood pressure, smoking history, known cardiovascular disease, atrial fibrillation, ventricular hypertrophy, antihypertensive use and radiation dose to the carotid arteries will be evaluated. The proportion of approached patients who enroll and receive the study CUS will be determined. The study will be considered feasible if 60% of responding subjects enroll and receive carotid US. If less than 40% of responding subjects enroll and receive the study carotid US, we will re-evaluate our methods for subsequent study. Measures of acceptability, barriers to care, and stroke risk perception will be assessed. Acceptability of the CUS intervention itself will be measured using the Acceptability of Intervention Measure [41]; acceptability of treatment (if clinically significant CAS were to be identified) and acceptability of screening frequency will be measured using parameters developed for the purposes of this study (Supplemental Appendix 1). Perception of stroke risk and level of concern regarding this risk will also be assessed using an adapted measure (Supplemental Appendix 2) [42]. Barriers to care with regard to screening CUS will be evaluated using a measure adapted from previous work from our group (Supplemental Appendix 3) [43,44].
Of patients identified to have clinically significant CAS on screening CUS, the proportion of patients who have any change in their medical care recommended, scheduled (prescribed, if medication) or completed (taken, if medication) as a result of the study ultrasound will be determined. Potential events that will be assessed include clinical diagnostic CUS, follow-up CUS screening, medical management (prescription or initiation) of a risk-reducing medication (i.e., anti-hypertensive medication, anti-hyperlipidemic medication, aspirin, anti-platelet agents and anticoagulation), carotid stent placement, CEA, stroke and/or TIA, or a healthcare visit with primary care (new or follow-up), cardiology, vascular surgery, neurology, neurosurgery or interventional radiology. The mean carotid dose (in Gy, continuous) will be assessed on each side of the neck for correlation with mean IMT (mean of the three angles) at the distal CCA, carotid bulb at the flow divider, and proximal internal carotid artery.
Blood will be collected on or around the time of CUS for additional correlative biomarker analyses. A standard lipid panel and C-reactive protein assay will be completed in our institutional clinical laboratory. Lipid values (such as low-density lipoprotein, high-density lipoprotein and triglycerides) and CRP levels will be examined and correlated with high-risk status defined by research CUS in patients who opt to provide a blood sample. Interactions between CUS findings and lipids at time of examination will be examined. Computed tomography of the neck (if available within the electronic medical record), both pre-treatment (at diagnosis) and post-treatment (follow-up exams) as well as the radiation planning CT sim will be analyzed. The visible carotid artery calcifications will be quantified and interrogated for associations between study outcomes and other factors. Blood inflammatory cytokine profiling and complementary mass spectrometry-based omics (proteomics, metabolomics and lipidomics) on peripheral blood mononuclear cells and plasma will be performed to identify biomarkers of high-risk cardiovascular status based on CAS, IMT, plaque presence as defined in the outcomes.
2.5. Participant timeline
The protocol timeline schema is displayed in Figure 1. Patients will be identified in clinic or by review of the electronic medical record or by physician referral for consideration during clinical visits. Identified patients will be screened in person or over the telephone. If screen-eligible, the patient will be registered. Baseline factors including age, sex as a biological variable, body mass index, ECOG performance status, medical history (including cardiovascular risk factors), and concurrent medications will be recorded, and the CUS will be performed. Immediately after the CUS is performed (or within 14 days), the patient will complete acceptability, stroke risk perception, and barriers to care surveys. Blood samples will be collected on the day of CUS or within 90 days after CUS. Follow-up assessments will occur at 3-months and 6-months post-CUS. If the RT plan is available, a carotid artery RT dose form will be completed after the patient completes the study to document the dose (in Gy) to both carotid arteries. For patients treated outside the study institution, efforts will be made to obtain the RT plan for the purposes of carotid artery RT dose calculation.
Figure 1.
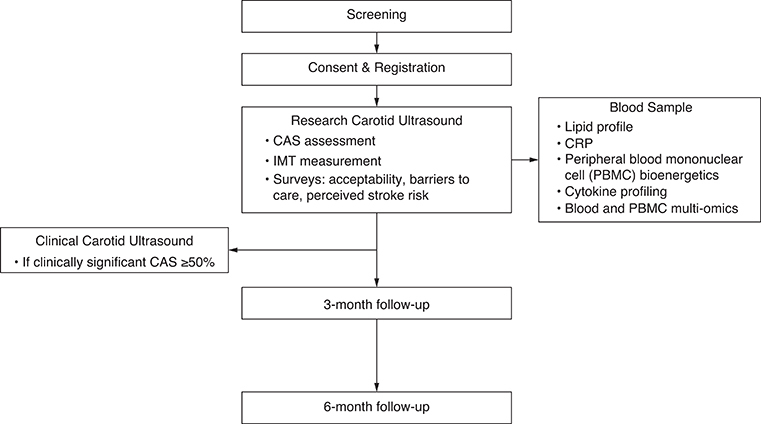
Protocol timeline.
3. Statistics
3.1. Planned sample size
The expected sample size is 60 patients. We anticipate that the rate of clinically significant CAS will be 20–25% in this patient population. If the rate is 25%, with a sample size of 60, the proportion with clinically significant CAS will be estimated to be within +/- 11.6% based on an exact 95% binomial confidence interval. The maximum half-width of an exact 95% confidence interval is 13.2%, so even if the rate of clinically significant CAS is as high as 50%, the proportion can still be estimated within +/- 13.2% with a sample size of 60. While the study is not formally powered to detect a difference in the proportion with clinically significant CAS between subgroups, there is moderate power to detect some clinically relevant differences with a sample size of 60. For example, there is at least 75% power to detect a difference between a subgroup with a 10% rate and one with a 35% rate when there are 30 patients in each group using a test with alpha = 0.1. The precision estimates for a sample size of 60 were calculated using PASS 16 and calculated for a two-sided confidence interval for one proportion using exact Clopper-Pearson method (PASS 16 Power Analysis and Sample Size Software, NCSS, LLC., Kaysville, Utah).
3.2. Study period
We expect to accrue approximately 4–5 patients per month for a total of 60 patients over approximately 1–2 years. The length of the study will be approximately 2 years. We anticipate accrual to span approximately 1 year, and the study will end after the last study follow-up for the last patient has been completed.
3.3. Analysis plan
The primary end point, the proportion of all patients undergoing ultrasound with 50% stenosis identified at any position (i.e., clinically significant CAS), will be estimated and reported along with an exact 95% confidence interval.
Mean, median, standard deviation and interquartile range will be used to describe IMT as a continuous variable. The proportion with at least one carotid IMT measurement of 0.9 mm or greater, the proportion with carotid plaque of maximum thickness 2 mm or greater and the proportion with high risk of cardiovascular events (relative risk 1.50 or greater) based on IMT measurements will be each be estimated and reported along with an exact 95% confidence interval. Chi-square or Fisher's exact tests will be used to evaluate associations between binary clinical characteristics and clinically significant CAS. The proportion of patients with clinically significant CAS may also be estimated and reported along with an exact 95% confidence interval within subgroups defined by clinical characteristics. Associations between continuous clinical characteristics and clinically significant CAS will be evaluated using t-tests or Wilcoxon rank-sum tests.
Feasibility will be evaluated based on the proportion of approached patients who enroll and receive the study carotid US. This proportion will be reported along with an exact 95% confidence interval. The study will be considered feasible if 60% of responding subjects enroll and receive carotid US. If less than 40% of responding subjects enroll and receive the study carotid US, we will re-evaluate our methods. Descriptive statistics will be used to characterize the acceptability of CUS (Supplemental Appendix 1), stroke risk perception (Supplemental Appendix 2), and barriers to getting a CUS (Supplemental Appendix 3).
4. Discussion
In the years to decades after treatment, HN cancer survivors experience high rates of carotid artery disease and stroke [18,45,46]. This risk, coupled with increasing survival rates driven by the excellent prognosis associated with human papillomavirus-associated oropharyngeal squamous cell carcinoma, warrants additional focus on the detection and management of late toxicities [5]. In the general population, the utility and cost effectiveness of CUS screening in asymptomatic patients increases with the prevalence of CAS [31]. The rate of clinically significant CAS identified in HN cancer survivors after RT increases with duration of follow-up and has been consistently reported to be above 20% [15,18,47]. This suggests a role for a screening exam to identify carotid disease early and initiate lifestyle modifications and medical management to reduce the subsequent long-term risk of cerebrovascular events.
Multiple prospective and retrospective studies have identified a high risk of CAS in post-RT HN cancer survivors [15]. Prospective, longitudinal studies have identified increases in CAS and carotid IMT after radiotherapy [48–50]. These studies have established RT-associated carotid artery injury as an important problem, but few studies have investigated potential solutions to this issue. Our prospective pilot study is unique in that the objectives are focused on understanding different methods to identify high-risk carotid artery disease (through multiple CAS and IMT end points) as well as elucidating optimal methods to approach a future trial of a screening program. This study is the first to our knowledge to collect feasibility, acceptability, barriers to care, and stroke risk perception data from HN cancer survivors in this context. To design a randomized trial testing the clinical impact and cost effectiveness of CUS screening in HN cancer survivors, an understanding of patients' perception of their own stroke risk, expected enrollment rates, willingness to adhere to trial activities, and barriers to completion of study activities are critical. The key secondary objectives in this study will directly inform decision-making regarding the feasibility and design of a future randomized trial.
Further, information regarding the acceptability of the intervention and likelihood of patients to adhere to CUS if it were to enter the standard of care will guide next steps. Considering the burdensome follow-up tasks often asked of patients with HN cancer, the unclear risk-benefit ratio and cost effectiveness of CUS screening, we must first understand the reasonableness of an additional test before embarking on large prospective, randomized trials. Another important and pragmatic end point of this study is the proportion of patients with abnormal CUS findings for which a risk-reduction intervention is recommended, scheduled/prescribed, or initiated/completed within the 6 months after CUS. This will provide additional data to better understand what changes (if any) in medical management follow the CUS, if a high-risk feature is identified.
Finally, planned correlative, exploratory analyses such as correlation of carotid IMT measurements with RT dose to the carotid artery, peripheral blood mononuclear cell bioenergetics, cytokine profiling and multi-omics analyses of patients with versus without high-risk CUS findings may elucidate additional mechanistic pathways or biomarkers of advanced carotid atherosclerotic disease after RT for HN cancer.
This study is limited by its sample size, single geographical site of accrual, and the lack of a pre-treatment baseline assessment. The purpose of this study is to test the feasibility and accumulate preliminary data to inform future trial design, and its statistical design was intended to provide moderate power for subgroup analyses while obtaining sufficient feasibility and acceptability data. Given the nature of the study, a multi-site trial to obtain data from a more generalizable population was not feasible. Within this limitation, the results produced by this study will be the first of their kind in the context of CUS screening of HN cancer survivors and will provide meaningful information with which a larger, multi-site clinical trial may be designed. The patient selection, while narrowed somewhat by inclusion criteria, was inclusive of all-comers who were interested in participating in a clinical trial. Thus, the findings regarding feasibility, acceptability and barriers to care must be interpreted with caution, as these results may differ from the population of patients not willing to participate or provide these data. All these limitations are inherent to a preliminary pilot study aimed to gain the understanding needed to design a future well-powered, comparative effectiveness study. To better understand the utility of CUS screening of asymptomatic HN cancer survivors treated with RT, a large, randomized, multi-site clinical trial will be needed to test the clinical impact and cost-effectiveness.
5. Conclusion
To better understand the clinical usefulness of CUS screening in HN cancer survivors treated with RT, we aim to assess the screening outcomes, acceptability and feasibility of CUS in a prospective clinical trial. For patients where CAS is identified, we aim to understand the frequency and type of changes in medical management that occur because of the screening CUS. Additional correlative analyses are planned to better understand the physiologic and biological processes associated with post-RT CAS and identify biomarkers of a high-risk population.
Supplementary Material
Acknowledgments
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive payment related to the development of the manuscript.
Funding Statement
The clinical study is supported and funded by the National Cancer Institute's Cancer Center Support Grant award number P30CA012197. The authors wish to acknowledge the support of the Wake Forest Baptist Comprehensive Cancer Center Bioinformatics Shared Resource and the Clinical Protocol and Data Management Shared Resource, supported by the National Cancer Institute's Cancer Center Support Grant award number P30CA012197. This work is also supported by the Wake Forest NCORP Research Base (UG1CA189824; Hughes, Dressler, Weaver, Lesser). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. This work is also supported by the Radiation Oncology Institute (ROI) through the ROI Publication Award (Hughes).
Supplemental material
Supplemental data for this article can be accessed at https://doi.org/10.1080/14796694.2024.2386927
Author contributions
RT Hughes: conception, design, drafting, revising, approval and agreement
AC Snavely: conception, design, drafting, revising, approval and agreement
EV Dressler: conception, design, drafting, revising, approval and agreement
CH Tegeler IV: design, drafting, revising, approval and agreement
CL Nightingale: design, drafting, revising, approval and agreement
CM Furdui: design, drafting, revising, approval and agreement
DR Soto: design, drafting, revising, approval and agreement
TC Register: design, drafting, revising, approval and agreement
KE Weaver: conception, design, drafting, revising, approval and agreement
GJ Lesser: conception, design, drafting, revising, approval and agreement
Financial disclosure
The clinical study is supported and funded by the National Cancer Institute's Cancer Center Support Grant award number P30CA012197. The authors wish to acknowledge the support of the Wake Forest Baptist Comprehensive Cancer Center Bioinformatics Shared Resource and the Clinical Protocol and Data Management Shared Resource, supported by the National Cancer Institute's Cancer Center Support Grant award number P30CA012197. This work is also supported by the Wake Forest NCORP Research Base (UG1CA189824; Hughes, Dressler, Weaver, Lesser). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. This work is also supported by the Radiation Oncology Institute (ROI) through the ROI Publication Award (Hughes).
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Ethical conduct of research
This study (WFBCCC 98322) was registered in clinicaltrials.gov (NCT05490875) and approved by the Institutional Review Board of Wake Forest University Health Sciences (IRB00087922). Written informed consent was obtained from all participants to participate in the study.
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest
- 1.American Cancer Society . Cancer Facts & Figures 2022. Atlanta: American Cancer Society; 2022. [Google Scholar]
- 2.Harrison LB, Sessions RB, Kies MS. Head and Neck Cancer: A Multidisciplinary Approach (4th Edition). Philadelphia, PA: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 3.Vorwerk H, Hess CF. Guidelines for delineation of lymphatic clinical target volumes for high conformal radiotherapy: head and neck region. Radiat Oncol. 2011;6:97. doi: 10.1186/1748-717X-6-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregoire V, Ang K, Budach W, et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. 2014;110(1):172–181. doi: 10.1016/j.radonc.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 5.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shenker RF, May NH, Waltonen JD, et al. Comparing outcomes for patients with human papillomavirus (HPV) type 16 versus other high-risk HPV types in oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2021;15(3):866–874. doi: 10.1007/s12105-021-01308-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist. 2010;15(9):994–1001. doi: 10.1634/theoncologist.2009-0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konings AW, Hardonk MJ, Wieringa RA, et al. Initial events in radiation-induced atheromatosis I. Activation of lysosomal enzymes. Strahlentherapie. 1975;150(4):444–448. [PubMed] [Google Scholar]
- 9.Fonkalsrud EW, Sanchez M, Zerubavel R, et al. Serial changes in arterial structure following radiation therapy. Surg Gynecol Obstet. 1977;145(3):395–400. [PubMed] [Google Scholar]
- 10.Murros KE, Toole JF. The effect of radiation on carotid arteries. A review article. Arch Neurol. 1989;46(4):449–455. doi: 10.1001/archneur.1989.00520400109029 [DOI] [PubMed] [Google Scholar]
- 11.Cheng SW, Ting AC, Lam LK, et al. Carotid stenosis after radiotherapy for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2000;126(4):517–521. doi: 10.1001/archotol.126.4.517 [DOI] [PubMed] [Google Scholar]
- 12.Cheng SW, Ting AC, Wu LL. Ultrasonic analysis of plaque characteristics and intimal-medial thickness in radiation-induced atherosclerotic carotid arteries. Eur J Vasc Endovasc Surg. 2002;24(6):499–504. doi: 10.1053/ejvs.2002.1752 [DOI] [PubMed] [Google Scholar]
- 13.Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489–501. doi: 10.4065/83.4.489 [DOI] [PubMed] [Google Scholar]
- 14.Ji R, Pan Y, Yan H, et al. Current smoking is associated with extracranial carotid atherosclerotic stenosis but not with intracranial large artery disease. BMC Neurol. 2017;17(1):120–120. doi: 10.1186/s12883-017-0873-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Texakalidis P, Giannopoulos S, Tsouknidas I, et al. Prevalence of carotid stenosis following radiotherapy for head and neck cancer: a systematic review and meta-analysis. Head Neck. 2020;42(5):1077–1088. doi: 10.1002/hed.26102 [DOI] [PubMed] [Google Scholar]; • Describes the prevalence of carotid stenosis in head and neck cancer patients after radiotherapy.
- 16.Lam WW, Leung SF, So NM, et al. Incidence of carotid stenosis in nasopharyngeal carcinoma patients after radiotherapy. Cancer. 2001;92(9):2357–2363. doi: 10.1002/1097-0142(20011101)92:9 [DOI] [PubMed] [Google Scholar]
- 17.Carpenter DJ, Mowery YM, Broadwater G, et al. The risk of carotid stenosis in head and neck cancer patients after radiation therapy. Oral Oncol. 2018;80:9–15. doi: 10.1016/j.oraloncology.2018.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carpenter DJ, Patel P, Niedzwiecki D, et al. Long-term risk of carotid stenosis and cerebrovascular disease after radiation therapy for head and neck cancer. Cancer. 2023;1–11. doi: 10.1002/cncr.35089 [DOI] [PubMed] [Google Scholar]; • Shows a retrospective institutional series of patients followed with routine CUS after head and neck radiotherapy outlines the rates of symptomatic, asymptomatic and composite CAS.
- 19.Smith GL, Smith BD, Buchholz TA, et al. Cerebrovascular disease risk in older head and neck cancer patients after radiotherapy. J Clin Oncol. 2008;26(31):5119–5125. doi: 10.1200/JCO.2008.16.6546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prati P, Tosetto A, Vanuzzo D, et al. Carotid intima media thickness and plaques can predict the occurrence of ischemic cerebrovascular events. Stroke. 2008;39(9):2470–2476. doi: 10.1161/STROKEAHA.107.511584 [DOI] [PubMed] [Google Scholar]
- 21.Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146(6):483–494. doi: 10.1093/oxfordjournals.aje.a009302 [DOI] [PubMed] [Google Scholar]
- 22.Howard DPJ, Gaziano L, Rothwell PM. Risk of stroke in relation to degree of asymptomatic carotid stenosis: a population-based cohort study, systematic review, and meta-analysis. Lancet Neurol. 2021;20(3):193–202. doi: 10.1016/S1474-4422(20)30484-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenfield K, Matsumura JS, Chaturvedi S, et al. Randomized trial of stent versus surgery for asymptomatic carotid stenosis. N Engl J Med. 2016;374(11):1011–1020. doi: 10.1056/NEJMoa1515706 [DOI] [PubMed] [Google Scholar]
- 24.Walker MD, Marler JR, Goldstein M, et al. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273(18):1421–1428. doi: 10.1001/jama.1995.03520420037035 [DOI] [PubMed] [Google Scholar]
- 25.Abbott AL, Paraskevas KI, Kakkos SK, et al. Systematic review of guidelines for the management of asymptomatic and symptomatic carotid stenosis. Stroke. 2015;46(11):3288–3301. doi: 10.1161/STROKEAHA.115.003390 [DOI] [PubMed] [Google Scholar]
- 26.Mullen MT, Jim J. Management of asymptomatic extracranial carotid atherosclerotic disease. 2023. [cited 12/20/2023]. In: UpToDate [Internet]. Wolters Kluwer, [cited 12/20/2023]. [Google Scholar]
- 27.Mohler ER 3rd, Gornik HL, Gerhard-Herman M, et al. ACCF/ACR/AIUM/ASE/ASN/ICAVL/SCAI/SCCT/SIR/SVM/SVS 2012 appropriate use criteria for peripheral vascular ultrasound and physiological testing part I: arterial ultrasound and physiological testing: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American College of Radiology, American Institute of Ultrasound in Medicine, American Society of Echocardiography, American Society of Nephrology, Intersocietal Commission for the Accreditation of Vascular Laboratories, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Interventional Radiology, Society for Vascular Medicine, and Society for Vascular Surgery. J Vasc Surg. 2012;56(1):e17–e51. doi: 10.1177/1358863X12452197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qureshi AI, Alexandrov AV, Tegeler CH, et al. Guidelines for screening of extracranial carotid artery disease: a statement for healthcare professionals from the multidisciplinary practice guidelines committee of the American Society of Neuroimaging; Cosponsored by the Society of Vascular and Interventional Neurology. J Neuroimaging. 2007;17(1):19–47. doi: 10.1111/j.1552-6569.2006.00085.x [DOI] [PubMed] [Google Scholar]
- 29.Cohen EEW, LaMonte SJ, Erb NL, et al. American Cancer Society Head and Neck Cancer Survivorship Care Guideline. CA Cancer J Clin. 2016;66(3):203–239. doi: 10.3322/caac.21343 [DOI] [PubMed] [Google Scholar]
- 30.National Comprehensive Cancer Network . Head and Neck Cancers (Version 3.2024). Accessed April 4, 2024. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- 31.Ricotta JJ, Aburahma A, Ascher E, et al. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54(3):e1–e31. doi: 10.1016/j.jvs.2011.07.031 [DOI] [PubMed] [Google Scholar]
- 32.Messas E, Goudot G, Halliday A, et al. Management of carotid stenosis for primary and secondary prevention of stroke: state-of-the-art 2020: a critical review. Eur Heart J Suppl. 2020;22(Suppl. M):M35–M42. doi: 10.1093/eurheartj/suaa162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fokkema M, den Hartog AG, Bots ML, et al. Stenting versus surgery in patients with carotid stenosis after previous cervical radiation therapy: systematic review and meta-analysis. Stroke. 2012;43(3):793–801. doi: 10.1161/STROKEAHA.111.633743 [DOI] [PubMed] [Google Scholar]; • A meta-analysis of 27 studies comprising 533 patients who had received prior cervical irradiation found no significant difference in adverse events between CEA and carotid stenting with overall favorable cerebrovascular outcomes.
- 34.Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis-society of radiologists in ultrasound consensus conference. Radiology. 2003;229(2):340–346. doi: 10.1148/radiol.2292030516 [DOI] [PubMed] [Google Scholar]
- 35.Hackam DG. Optimal medical management of asymptomatic carotid stenosis. Stroke. 2021;52(6):2191–2198. doi: 10.1161/STROKEAHA.120.033994 [DOI] [PubMed] [Google Scholar]; • Provides a comprehensive review of current management practices for patients that are identified by CUS.
- 36.Naylor R, Rantner B, Ancetti S, et al. Editor's Choice – European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur J Vasc Endovasc Surg. 2023;65(1):7–111. [DOI] [PubMed] [Google Scholar]
- 37.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1046–e1081. doi: 10.1161/CIR.0000000000000699 [DOI] [PubMed] [Google Scholar]
- 38.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138(17):e426–e483. doi: 10.1161/CIR.0000000000000597 [DOI] [PubMed] [Google Scholar]
- 39.Wein T, Lindsay MP, Côté R, et al. Canadian stroke best practice recommendations: secondary prevention of stroke, sixth edition practice guidelines, update 2017. Int J Stroke. 2018;13(4):420–443. doi: 10.1177/1747493017743062 [DOI] [PubMed] [Google Scholar]
- 40.Johri AM, Nambi V, Naqvi TZ, et al. Recommendations for the assessment of carotid arterial plaque by ultrasound for the characterization of atherosclerosis and evaluation of cardiovascular risk: from the American Society of Echocardiography. J Am Soc Echocardiogr. 2020;33(8):917–933. doi: 10.1016/j.echo.2020.04.021 [DOI] [PubMed] [Google Scholar]
- 41.Weiner BJ, Lewis CC, Stanick C, et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci. 2017;12(1):108. doi: 10.1186/s13012-017-0635-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dearborn JL, McCullough LD. Perception of risk and knowledge of risk factors in women at high risk for stroke. Stroke. 2009;40(4):1181–1186. doi: 10.1161/STROKEAHA.108.543272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nightingale CL, McLouth LE, Levine BJ, et al. Selected Findings from a Randomized Pilot Trial to Test the Feasibility and Acceptability of the Caregiver Oncology Needs Evaluation Tool for Lung Cancer Caregivers. San Diego, CA: American Society of Preventive Oncology 47th Annual Meeting; 2023. [Google Scholar]
- 44.Nightingale C, Sterba KR, Levine B, et al. Feasibility and acceptability of a multi-modality self-management intervention for head and neck cancer caregivers: a pilot randomized trial. Integrative Cancer Ther. 2022;21:15347354221098984. doi: 10.1177/15347354221098984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun L, Brody R, Candelieri D, et al. Association between up-front surgery and risk of stroke in US Veterans with oropharyngeal carcinoma. JAMA Otolaryngol Head & Neck Surg. 2022;148(8):740–747. doi: 10.1001/jamaoto.2022.1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yip PL, Zheng H, Cheo T, et al. Stroke risk in survivors of head and neck cancer. JAMA Network Open. 2024;7(2):e2354947–e2354947. doi: 10.1001/jamanetworkopen.2023.54947 [DOI] [PMC free article] [PubMed] [Google Scholar]; • Identifies high rates of stroke among HN cancer survivors compared to age-standardized general population, particularly in patients treated with primary radiotherapy.
- 47.Steele SR, Martin MJ, Mullenix PS, et al. Focused high-risk population screening for carotid arterial stenosis after radiation therapy for head and neck cancer. Am J Surg. 2004;187(5):594–598. doi: 10.1016/j.jvs.2004.09.009 [DOI] [PubMed] [Google Scholar]
- 48.Akhavan A, Farghadani M, Emami H, et al. Effects of neck radiation on result of doppler sonography of carotid arteries in head and neck cancer patients. J Stroke Cerebrovasc Dis. 2021;30(4):105607. doi: 10.1016/j.jstrokecerebrovasdis.2021.105607 [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto Y, Okawa M, Suzuki K, et al. Continuous and early progression of carotid intima-media thickness after radiotherapy for head and neck cancer: 5-year prospective observational study. Cerebrovasc Dis. 2023;52(5):543–551. doi: 10.1159/000528622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muzaffar K, Collins SL, Labropoulos N, et al. A prospective study of the effects of irradiation on the carotid artery. Laryngoscope. 2000;110(11):1811–1814. doi: 10.1097/00005537-200011000-00007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


