Abstract
Chimeric Antigen Receptor T (CAR-T) cell therapy has significantly advanced in treating B-cell acute lymphoblastic leukemia (B-ALL) and has shown efficacy in managing relapsed B-ALL after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Donor-derived CAR-T cell offer both high efficacy and rapid response. Although promising results exist, current research lacks definitive evidence of long-term survival benefits for patients treated with donor-derived CAR-T therapy. We report the long-term survival of 32 patients with post-transplant relapsed B-ALL treated with donor-derived CD19 CAR-T cell, achieving either complete Remission (CR) or CR with incomplete peripheral blood recovery (CRi). The median follow-up was 42 months, with 2-year overall survival (OS) and event-free survival (EFS) rates of 56.25% and 50.0%, respectively. The 5-year OS and EFS rates were 53.13% and 46.88%, with no new long-term adverse events observed. These findings demonstrate good long-term safety, supporting donor-derived CAR-T cell as a recommended treatment option for relapsed B-ALL patients post-transplantation.
Trial registration: https://www.chictr.org.cn/showproj.html?proj=14315.
Registration number: ChiCTR-OOC-16008447.
Keywords: Donor-derived CD19 CAR-T, Allo-HSCT, Relapsed, Long-term survival
To the editor:
Significant advances have been made in the treatment of B-cell acute lymphoblastic leukemia (B-ALL) with chimeric antigen receptor (CAR) T-cell therapy [1–4]. Using allogeneic (donor)-derived CAR-T cell carries a risk of severe graft-versus-host disease (GVHD), potentially leading to fatal outcomes. However, donor-derived CAR-T cell poses minimal GVHD risk and is a viable option for treating relapsed B-ALL after allogeneic hematopoietic stem cell transplantation (allo-HSCT) [5, 6]. Most patients who achieve complete remission (CR) after autologous CAR-T cell therapy experience relapse, making bridge transplantation a crucial intervention. Although a second transplantation offers benefits, it is linked with high treatment-related mortality and low survival rates. Its necessity remains uncertain for relapsed CD19-positive B-ALL patients who achieved CR after receiving donor-derived CD19 CAR-T cell post-allo-HSCT. In our previous report, relapsed CD19-positive B-ALL patients received donor-derived CD19 CAR-T cell after allo-HSCT and achieved CR without requiring a second transplantation. These patients showed a high probability of achieving one-year overall survival (OS) and event-free survival (EFS) [7, 8]. In this study, we updated the long-term survival outcomes for the patients who achieved CR after donor CAR-T cell treatment without the second transplantation.
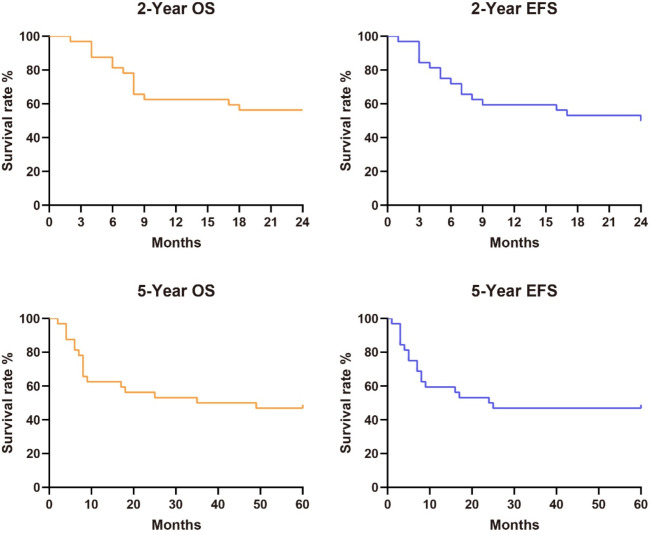
A total of 32 relapsed patients with CD19-positive B-ALL after allo-HSCT who received donor-derived CD19 CAR-T cell and achieved CR were included in the study. Patient baseline characteristics are summarized in Table 1. The study period ranged from October 2015 to March 2019, with the last follow-up on April 1, 2024. The cohort consisted of 19 male and 13 female patients, with a median age of 24 years (range, 4 to 60). The median follow-up period was 42 months (range, 1 to 91). For safety analysis, we did not observe any new long-term toxicities. The duration of EFS was calculated from the time of CR, no response, relapse or death, whichever occurred first, was regarded as the event. The OS was calculated from the time of CR until death. The 2-year OS and EFS were 56.25% and 50.0%, respectively. The 5-year OS and EFS were 53.13% and 46.88%, respectively (Fig. 1).
Table 1.
Characteristics of evaluated patients (N = 32), MSD-HSCT matched sibling donor hematopoietic stem cell transplantation, HID-HSCT haploidentical hematopoietic stem cell transplantation, MRD minimal residual disease, Ph philadelphia chromosome, GVHD graft-versus-host disease, CRS Cytokine Release Syndrome, CRES CAR-T cell relevant encephalopathy syndrome, FA fludarabine, CTX cyclophosphamide
| Donor CD19 CAR-T | |
|---|---|
| Age(year, median, range) | 24(4–60) |
| Sex(%) | |
| Male | 22(68.7) |
| Female | 10(31.3) |
| HSCT(%) | |
| MSD-HSCT | 15(46.9) |
| HID-HSCT | 17(53.1) |
| Bone marrow blasts pre infusion | |
| 0.01%-(MRD-positive) | 11(34.4) |
| 5–50% | 14(43.8) |
| >50% | 7(21.8) |
| Karyotype or genetic abnormalities(%) | |
| normal | 15(46.9) |
| Ph | 8(25.0) |
| others | 9(28.1) |
| Co-stimulatory molecular(%) | |
| CD28 | 14(43.8) |
| 41-BB | 18(56.2) |
| Transplant conditioning regimen(%) | |
| FA/CTX | 24(75.0) |
| others | 8(25.0) |
| CAR-T cell Dose (×106/Kg, median, range) | 1.79(0.04-12.0) |
| < 1 | 2(6.2) |
| 1–2 | 21(65.6) |
| >2 | 9(28.2) |
| GVHD(%) | |
| YES | 2(6.3) |
| NO | 30(93.7) |
| CRS(%) | |
| NO | 4(12.5) |
| grade 1–2 | 22(68.8) |
| grade 3–4 | 6(18.7) |
| CRES(%) | |
| NO | 27(84.4) |
| grade 1–2 | 2(6.3) |
| grade 3–4 | 3(9.3) |
Fig. 1.
Long-term survival after CD19 donor-derived CAR-T cell treatment of relapsed B-ALL patients after allogeneic hematopoietic stem cell transplant achieved complete remission without a second transplantation. (A) The 2-year OS. (B) The 2-year EFS. (C) The 5-year OS. (D) The 5-year EFS. Notes: EFS: Event-free survival, OS: Overall survival
These results surpass those of relapse patients who underwent a second transplantation or donor lymphocyte infusion without receiving CAR-T cell therapy. The favorable long-term survival demonstrated by donor-derived CD19 CAR-T cell indicates that this treatment is effective for relapsed patients following allo-HSCT. While our long-term data provides valuable insights into the efficacy of donor-derived CAR-T cell therapy after allo-HSCT, the potential benefits of a second transplantation in this setting may still require further validation through multicenter, prospective, and randomized controlled trials. Relapse occurred in the majority of patients during the initial years following CAR-T cell therapy, particularly within the first six months, despite the use of healthy T cells as carriers [9]. Therefore, it is critical to develop early detection methods for preventing or identifying the cause of relapse following CAR-T cell therapy [10, 11].
The administration of donor-derived CD19 CAR-T cell in relapsed CD19-positive B-ALL patients post-allo-HSCT has shown promising long-term survival outcomes. The study highlights the potential of donor-derived CAR-T cell as a safer and more effective treatment option compared to traditional second transplantation or donor lymphocyte infusion. However, the necessity and efficacy of a second transplantation for achieving CR in relapsed patients remain uncertain and warrant further investigation through robust clinical trials.
Acknowledgements
We would like to express our gratitude to all the patients and their families. We appreciate the efforts of the clinical staff at all participating centers for their dedication and hard work.
Abbreviations
- CAR-T
Chimeric antigen receptor T
- B-ALL
B-cell acute lymphoblastic leukemia
- Allo-HSCT
Allogeneic hematopoietic stem cell transplantation
- GVHD
Graft versus host disease
- CR
Complete remission
- OS
Overall survival
- EFS
Event-free survival
- CD
Cluster of differentiation
Author contributions
CZ and XW contributed in making the table, figure and writing the draft manuscript. HY, YW, ZY, JZ, TY, AL, ZW, YM, LG, LG PK and JL contributed in collecting clinical data. EJ and XZ designed and revised the manuscript. All authors read and approved the final manuscript.
Funding
National Natural Science Foundation of China (Grant No. 82341201, 82370181), the National Key R&D Program of China (Grant No. 2022YFA1103300, 2022YFA1103304), and the Special Project for Talent Construction in Xinqiao Hospital (Grant No. 2022XKRC001, 2022YQB004).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Studies involving human participants were reviewed and approved by the Ethics Committee of Xinqiao Hospital of Army Medical University.
Patient consent statement
All patients provided written informed consent.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cheng Zhang and Xiaoqi Wang contributed equally to this work.
Contributor Information
Erlie Jiang, Email: Jiangerlie@163.com.
Xi Zhang, Email: zhangxxi@sina.com, Email: zhangxi@tmmu.edu.cn.
References
- 1.Zhang C, He J, Liu L, Wang J, Wang S, Liu L, et al. Novel CD19 chimeric antigen receptor T cells manufactured next-day for acute lymphoblastic leukemia. Blood Cancer J. 2022;12:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang R, Wang X, Zhang X. Unity brings strength: combination of CAR-T cell therapy and HSCT. Cancer Lett. 2022;549:215721. [DOI] [PubMed] [Google Scholar]
- 3.Huang R, Li X, He Y, Zhu W, Gao L, Liu Y, et al. Recent advances in CAR-T cell engineering. J Hematol Oncol. 2020;13:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiesa R, Georgiadis C, Syed F, Zhan H, Etuk A, Gkazi SA, et al. Base-edited CAR7 T cells for relapsed T-Cell Acute Lymphoblastic Leukemia. N Engl J Med. 2023;389:899–910. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C, Ma Y-Y, Liu J, Liu Y, Gao L, Gao L, et al. Preventive infusion of donor-derived CAR-T cells after haploidentical transplantation: two cases report. Med (Baltim). 2019;98:e16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan X, Wang X-Q, Zhang C, Zhao X-L, Yao H, Chen G, et al. Donor-derived CD19 CAR-T cells versus Chemotherapy Plus Donor Lymphocyte infusion for treatment of recurrent CD19-positive B-ALL after allogeneic hematopoietic stem cell transplantation. Curr Med Sci. 2023;43:733–40. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C, Wang X-Q, Zhang R-L, Liu F, Wang Y, Yan Z-L, et al. Donor-derived CD19 CAR-T cell therapy of relapse of CD19-positive B-ALL post allotransplant. Leukemia. 2021;35:1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo Y, Gao L, Liu J, Yang L, Wang L, Lai X, et al. Donor-derived Anti-CD19 CAR T cells GC007g for relapsed or refractory B-cell acute lymphoblastic leukemia after allogeneic HSCT: a phase 1 trial. EClinicalMedicine. 2024;67:102377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cappell KM, Kochenderfer JN. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. 2023;20:359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Huang R, Wang Z, Xiong J, Wang X, Zhang X. Facing challenges with hope: universal immune cells for hematologic malignancies. Cancer Biol Med. 2023;20:229–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, An L, Huang R, Xiong J, Yang H, Wang X, et al. Strategies to enhance CAR-T persistence. Biomark Res. 2022;10:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.