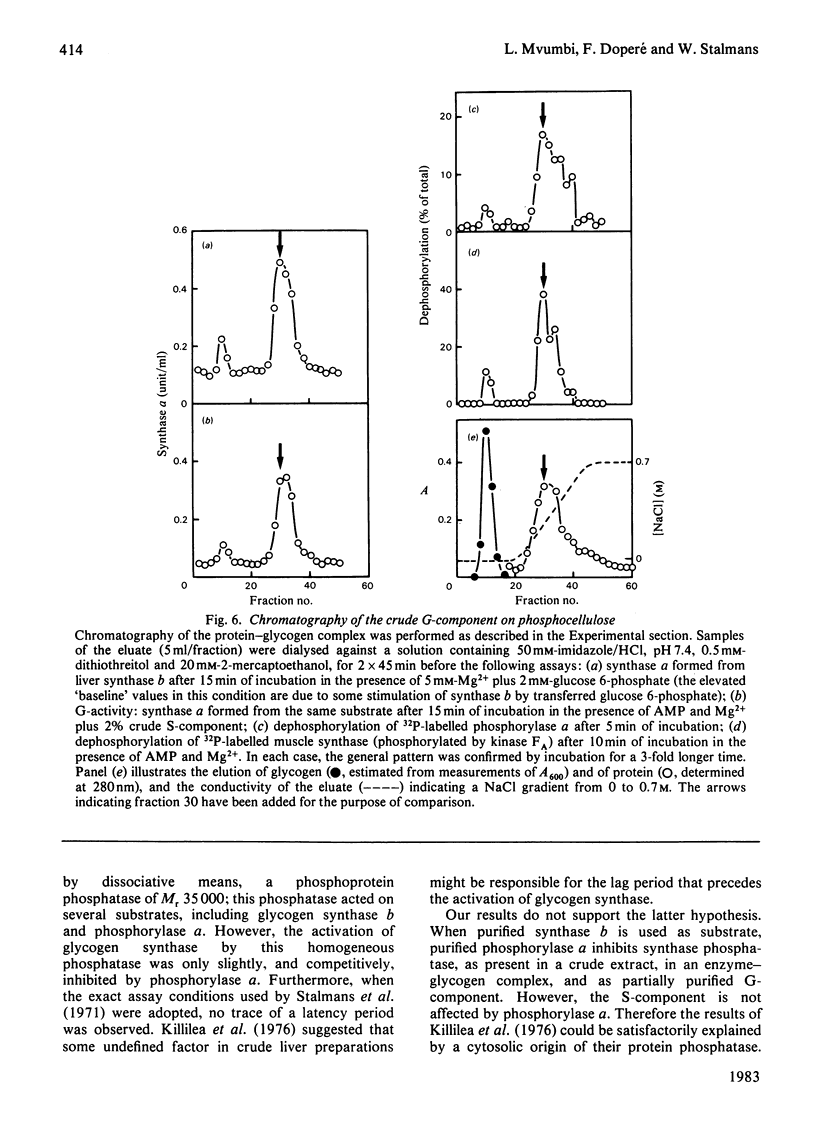
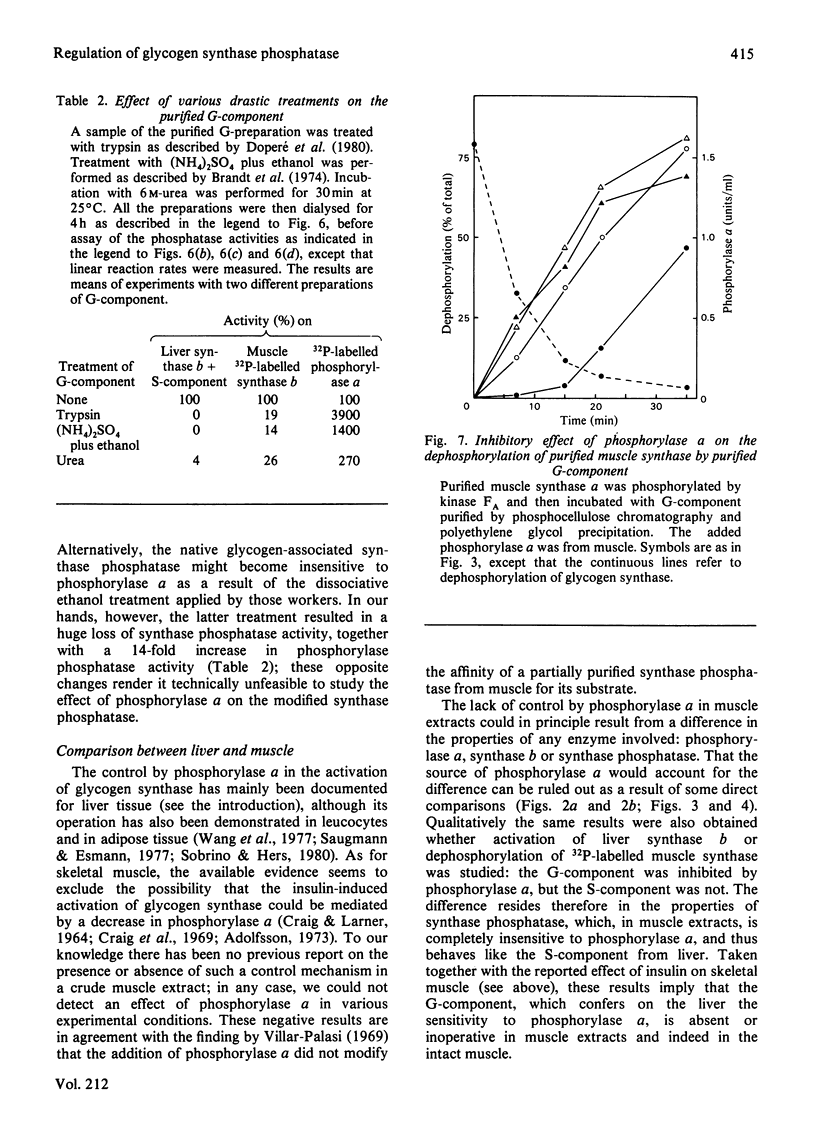
Abstract
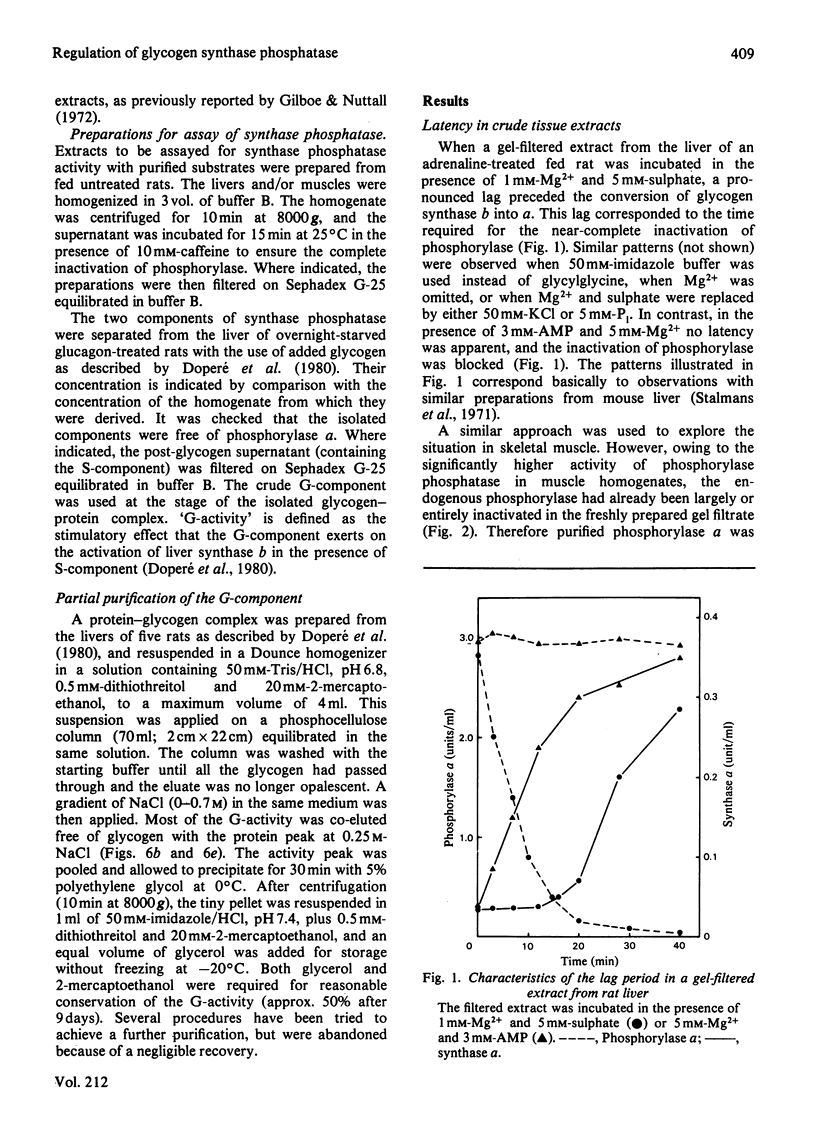
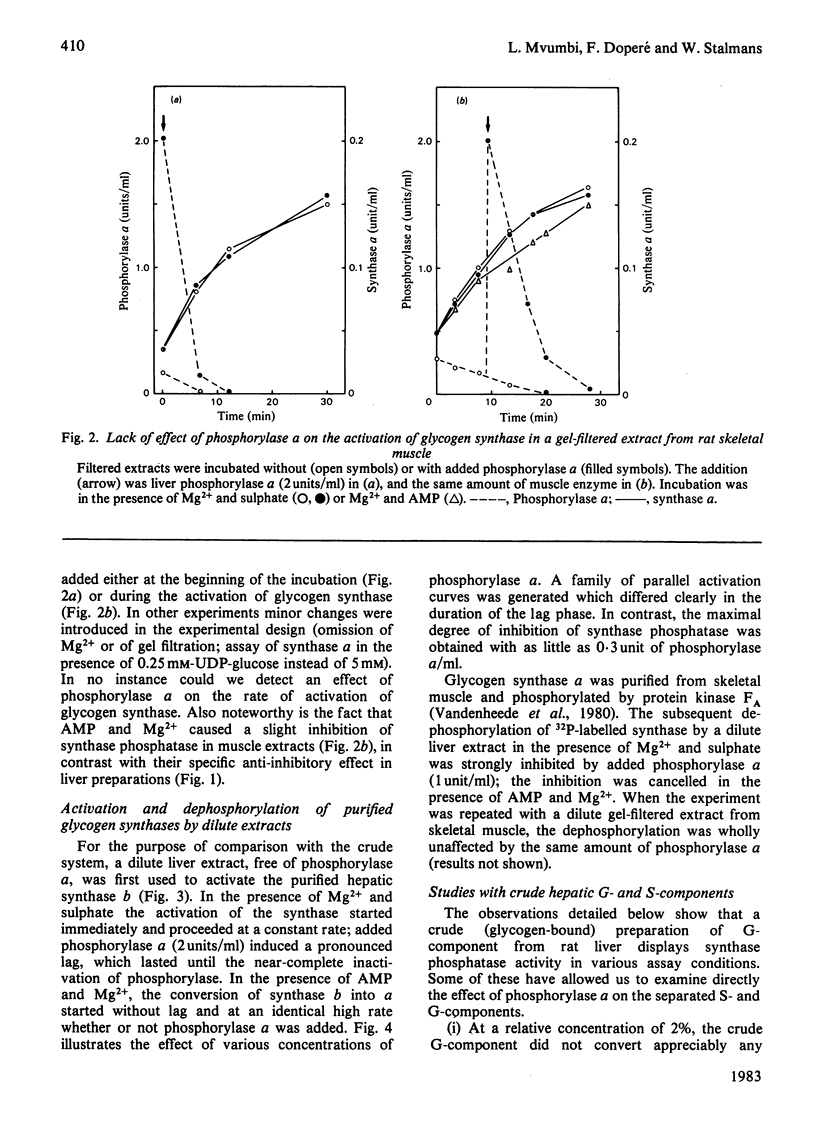
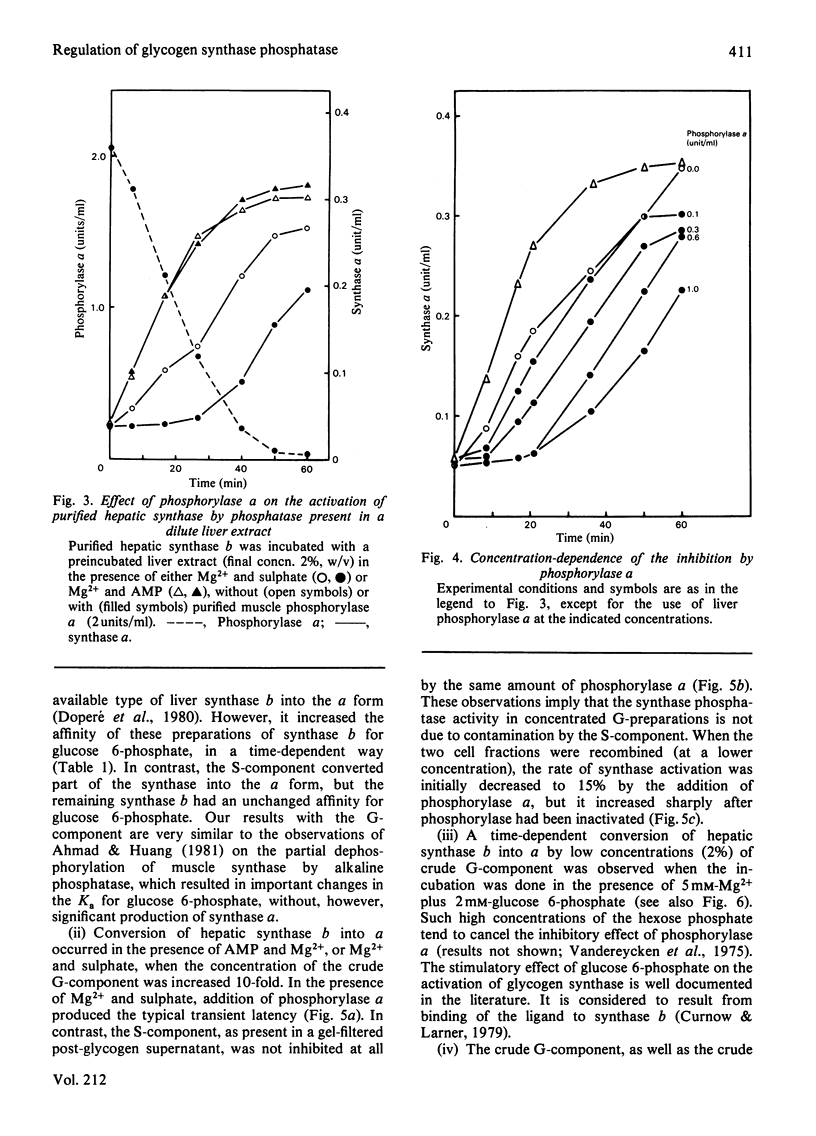
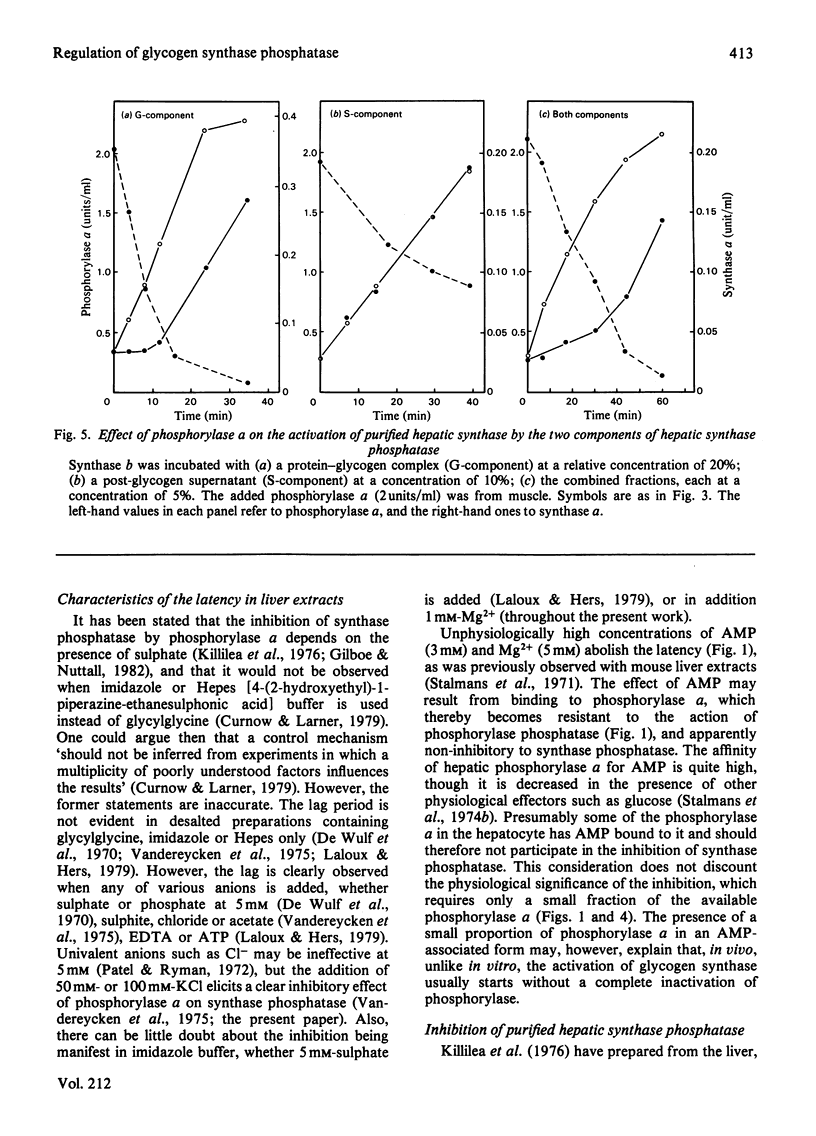
The activity of glycogen synthase phosphatase in rat liver stems from the co-operation of two proteins, a cytosolic S-component and a glycogen-bound G-component. It is shown that both components possess synthase phosphatase activity. The G-component was partially purified from the enzyme-glycogen complex. Dissociative treatments, which increase the activity of phosphorylase phosphatase manyfold, substantially decrease the synthase phosphatase activity of the purified G-component. The specific inhibition of glycogen synthase phosphatase by phosphorylase a, originally observed in crude liver extracts, was investigated with purified liver synthase b and purified phosphorylase a. Synthase phosphatase is strongly inhibited, whether present in a dilute liver extract, in an isolated enzyme-glycogen complex, or as G-component purified therefrom. In contrast, the cytosolic S-component is insensitive to phosphorylase a. The activation of glycogen synthase in crude extracts of skeletal muscle is not affected by phosphorylase a from muscle or liver. Consequently we have studied the dephosphorylation of purified muscle glycogen synthase, previously phosphorylated with any of three protein kinases. Phosphorylase a strongly inhibits the dephosphorylation by the hepatic G-component, but not by the hepatic S-component or by a muscle extract. These observations show that the inhibitory effect of phosphorylase a on the activation of glycogen synthase depends on the type of synthase phosphatase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolfesson S. Glycogen synthesis in rat diaphragm in vivo: a biphasic effect of insulin on glycogen synthetase enzyme. Acta Physiol Scand. 1973 Apr;87(4):465–473. doi: 10.1111/j.1748-1716.1973.tb05413.x. [DOI] [PubMed] [Google Scholar]
- Ahmad Z., Huang K. P. Dephosphorylation of rabbit skeletal muscle glycogen synthase (phosphorylated by cyclic AMP-independent synthase kinase 1) by phosphatases. J Biol Chem. 1981 Jan 25;256(2):757–760. [PubMed] [Google Scholar]
- Brandt H., Killilea S. D., Lee E. Y. Activation of phosphorylase phosphatase by a novel procedure: evidence for a regulatory mechanism involving the release of a catalytic subunit from enxyme-inhibitor complex(es) of higher molecular weight. Biochem Biophys Res Commun. 1974 Nov 27;61(2):598–604. doi: 10.1016/0006-291x(74)90999-1. [DOI] [PubMed] [Google Scholar]
- CRAIG J. W., LARNER J. INFLUENCE OF EPINEPHRINE AND INSULIN ON URIDINE DIPHOSPHATE GLUCOSE-ALPHA-GLUCAN TRANSFERASE AND PHOSPHORYLASE IN MUSCLE. Nature. 1964 Jun 6;202:971–973. doi: 10.1038/202971a0. [DOI] [PubMed] [Google Scholar]
- Craig J. W., Rall T. W., Larner J. The influence of insulin and epinephrine on adenosine 3',5'-phosphate and glycogen transferase in muscle. Biochim Biophys Acta. 1969 Apr 1;177(2):213–219. doi: 10.1016/0304-4165(69)90130-5. [DOI] [PubMed] [Google Scholar]
- De Wulf H., Stalmans W., Hers H. G. The effect of glucose and of a treatment by glucocorticoids on the activation in vitro of liver glycogen synthetase. Eur J Biochem. 1970 Jul;15(1):1–8. doi: 10.1111/j.1432-1033.1970.tb00967.x. [DOI] [PubMed] [Google Scholar]
- Devos P., Hers H. G. Glycogen metabolism in the liver of the foetal rat. Biochem J. 1974 May;140(2):331–340. doi: 10.1042/bj1400331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doperé F., Stalmans W. Release and activation of phosphorylase phosphatase upon rupture of organelles from rat liver. Biochem Biophys Res Commun. 1982 Jan 29;104(2):443–450. doi: 10.1016/0006-291x(82)90657-x. [DOI] [PubMed] [Google Scholar]
- Doperé F., Vanstapel F., Stalmans W. Glycogen-synthase phosphatase activity in rat liver. Two protein components and their requirement for the activation of different types of substrate. Eur J Biochem. 1980 Feb;104(1):137–146. doi: 10.1111/j.1432-1033.1980.tb04409.x. [DOI] [PubMed] [Google Scholar]
- Embi N., Rylatt D. B., Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980 Jun;107(2):519–527. [PubMed] [Google Scholar]
- Gilbert M., Bourbon J. Effects of acute variation of fetal glycemia on glycogen storage and on glycogen synthase and phosphorylase activities in the liver of the rat fetus. Diabetes. 1980 Apr;29(4):266–271. doi: 10.2337/diab.29.4.266. [DOI] [PubMed] [Google Scholar]
- Gilboe D. P., Nuttall F. Q. The role of ATP and glucose 6-phosphate in the regulation of glycogen synthetase D phosphatase. Biochem Biophys Res Commun. 1972 Aug 21;48(4):898–906. doi: 10.1016/0006-291x(72)90693-6. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Itarte E., Singh T. J., Akatsuka A. Phosphorylation of glycogen synthase by cyclic AMP-independent casein kinase-2 from rabbit skeletal muscle. J Biol Chem. 1982 Mar 25;257(6):3236–3242. [PubMed] [Google Scholar]
- Hue L., Bontemps F., Hers H. The effects of glucose and of potassium ions on the interconversion of the two forms of glycogen phosphorylase and of glycogen synthetase in isolated rat liver preparations. Biochem J. 1975 Oct;152(1):105–114. doi: 10.1042/bj1520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itarte E., Mor A., Pena J. M., Salavert A., Cussó R., Guinovart J. J. Cyclic AMP-independent glycogen synthase kinase from rat liver. FEBS Lett. 1979 May 15;101(2):347–350. doi: 10.1016/0014-5793(79)81041-8. [DOI] [PubMed] [Google Scholar]
- Katz J., Golden S., Wals P. A. Glycogen synthesis by rat hepatocytes. Biochem J. 1979 May 15;180(2):389–402. doi: 10.1042/bj1800389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K., Tamura S., Hiraga A., Tsuiki S. Glycogen synthase phosphatase of rat liver. Its separation from phosphorylase phosphatase on DE-52 columns. Biochem Biophys Res Commun. 1977 Mar 7;75(1):29–37. doi: 10.1016/0006-291x(77)91284-0. [DOI] [PubMed] [Google Scholar]
- Killilea S. D., Brandt H., Lee E. Y., Whelan W. J. Evidence for the coordinate control of activity of liver glycogen synthase and phosphorylase by a single protein phosphatase. J Biol Chem. 1976 Apr 25;251(8):2363–2368. [PubMed] [Google Scholar]
- Laloux M., Hers H. G. The role of phosphorylase in the inhibitory effect of EDTA and ATP on liver glycogen synthase phosphatase. Biochem Biophys Res Commun. 1979 Feb 14;86(3):762–768. doi: 10.1016/0006-291x(79)91778-9. [DOI] [PubMed] [Google Scholar]
- Laloux M., Stalmans W., Hers H. G. Native and latent forms of liver phosphorylase phosphatase. The non-identity of native phosphorylase phosphatase and synthase phosphatase. Eur J Biochem. 1978 Dec 1;92(1):15–24. doi: 10.1111/j.1432-1033.1978.tb12718.x. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., Silberman S. R., Ganapathi M. K., Petrović S., Paris H. The phosphoprotein phosphatases: properties of the enzymes involved in the regulation of glycogen metabolism. Adv Cyclic Nucleotide Res. 1980;13:95–131. [PubMed] [Google Scholar]
- Nimmo H. G., Proud C. G., Cohen P. The purification and properties of rabbit skeletal muscle glycogen synthase. Eur J Biochem. 1976 Sep;68(1):21–30. doi: 10.1111/j.1432-1033.1976.tb10761.x. [DOI] [PubMed] [Google Scholar]
- Postle A. D., Bloxham D. P. The use of tritiated water to measure absolute rates of hepatic glycogen synthesis. Biochem J. 1980 Oct 15;192(1):65–73. doi: 10.1042/bj1920065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugmann P., Esmann V. Glycogen metabolism: the integrated cellular response to a bi-directional metabolic stimulus. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1520–1527. doi: 10.1016/0006-291x(77)90615-5. [DOI] [PubMed] [Google Scholar]
- Sobrino F., Hers H. G. The inactivation of phosphorylase and activation of glycogen synthase in the adipose tissue. Eur J Biochem. 1980 Aug;109(1):239–246. doi: 10.1111/j.1432-1033.1980.tb04789.x. [DOI] [PubMed] [Google Scholar]
- Stalmans W., De Wulf H., Hue L., Hers H. G. The sequential inactivation of glycogen phosphorylase and activation of glycogen synthetase in liver after the administration of glucose to mice and rats. The mechanism of the hepatic threshold to glucose. Eur J Biochem. 1974 Jan 3;41(1):127–134. doi: 10.1111/j.1432-1033.1974.tb03252.x. [DOI] [PubMed] [Google Scholar]
- Stalmans W., Gevers G. The catalytic activity of phosphorylase b in the liver. With a note on the assay in the glycogenolytic direction. Biochem J. 1981 Nov 15;200(2):327–336. doi: 10.1042/bj2000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalmans W., Hers H. G. The stimulation of liver phosphorylase b by AMP, fluoride and sulfate. A technical note on the specific determination of the a and b forms of liver glycogen phosphorylase. Eur J Biochem. 1975 Jun;54(2):341–350. doi: 10.1111/j.1432-1033.1975.tb04144.x. [DOI] [PubMed] [Google Scholar]
- Stalmans W., Laloux M., Hers H. G. The interaction of liver phosphorylase a with glucose and AMP. Eur J Biochem. 1974 Nov 15;49(2):415–427. doi: 10.1111/j.1432-1033.1974.tb03847.x. [DOI] [PubMed] [Google Scholar]
- Stalmans W. The role of the liver in the homeostasis of blood glucose. Curr Top Cell Regul. 1976;11:51–97. doi: 10.1016/b978-0-12-152811-9.50009-2. [DOI] [PubMed] [Google Scholar]
- Stalmans W., de Wulf H., Hers H. G. The control of liver glycogen synthetase phosphatase by phosphorylase. Eur J Biochem. 1971 Feb;18(4):582–587. doi: 10.1111/j.1432-1033.1971.tb01279.x. [DOI] [PubMed] [Google Scholar]
- Tan A. W., Nuttall F. Q. Evidence for the non-identity of proteins having synthase phosphatase, phosphorylase phosphatase and histone phosphatase activity in rat liver. Biochim Biophys Acta. 1978 Jan 12;522(1):139–150. doi: 10.1016/0005-2744(78)90330-3. [DOI] [PubMed] [Google Scholar]
- Vandenheede J. R., Yang S. D., Goris J., Merlevede W. ATP x Mg-dependent protein phosphatase from rabbit skeletal muscle. II. Purification of the activating factor and its characterization as a bifunctional protein also displaying synthase kinase activity. J Biol Chem. 1980 Dec 25;255(24):11768–11774. [PubMed] [Google Scholar]
- Vandereycken G., Keppens S., De Wulf H. Ionic requirements for the inhibition of liver glycogen synthetase phosphatase by phosphorylase a. Arch Int Physiol Biochim. 1975 Dec;83(5):1013–1014. [PubMed] [Google Scholar]
- Vanstapel F., Doperé F., Stalmans W. The role of glycogen synthase phosphatase in the glucocorticoid-induced deposition of glycogen in foetal rat liver. Biochem J. 1980 Nov 15;192(2):607–612. doi: 10.1042/bj1920607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar-Palasi C. Oligo- and polysaccharide inhibition of muscle transferase D phosphatase. Ann N Y Acad Sci. 1969 Oct 14;166(2):719–730. doi: 10.1111/j.1749-6632.1969.tb46429.x. [DOI] [PubMed] [Google Scholar]
- Wang P., Bantle G., Sorensen N. B. Effect of metabolites and phosphorylase on the D to I conversion of glycogen synthase from human polymorphonuclear leukocytes. Biochim Biophys Acta. 1977 Feb 28;496(2):436–447. doi: 10.1016/0304-4165(77)90326-9. [DOI] [PubMed] [Google Scholar]
- Yang S. D., Vandenheede J. R., Goris J., Merlevede W. ATP x Mg-dependent protein phosphatase from rabbit skeletal muscle. I. Purification of the enzyme and its regulation by the interaction with an activating protein factor. J Biol Chem. 1980 Dec 25;255(24):11759–11767. [PubMed] [Google Scholar]


