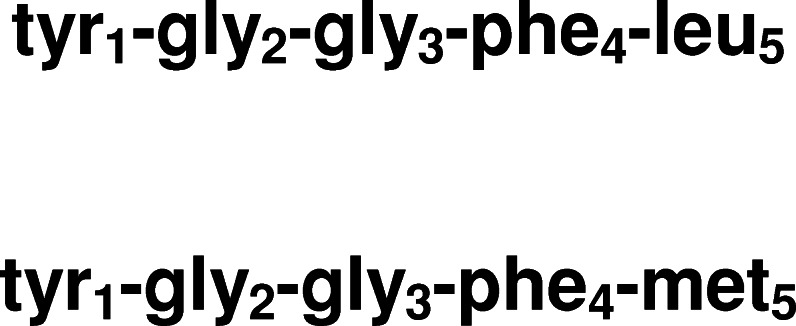Abstract
1. In addition to his many fine contributions in furthering our understanding of the neurochemical action of ecosanoids, catchelomines, steroids, anandamines, cannabinoids, endorphins, and the many modifications made to these neural factors, twenty years ago Julius Axelrod published a noteworthy paper concerning the nature of neuropeptides and their potential for multiple neurophysiological effects (Redgate et al., 1986).
2. In that report, Axelrod and coworkers described the neurological actions of the then recently discovered leucine- and methionine-enkephalins, and their biological functions which were novel, atypical, and in possession of neurological effects that were significantly “much more than additive.”
3. In this short communication I would like to expand on this observation concerning the “additive effects” contained within the amino acid sequence of the atypical neurotransmitter peptides leucine- and methonine-enkephalin.
KEY WORDS: biological complexity, dopaminergic, enkephalinergic, glycinergic, leucine-enkepahlin, methionine-enkephalin, neural peptides, opiate receptors
INTRODUCTION
The enkephalins, endogenous brain pentapeptides with opiate-like activities, were first isolated from pig brain by Hughes et al. (1975). They were described by their discoverers as ‘neuromodulators with highly transient action and rapid inactivation’, with activities mediated through specific and non-specific deactivating peptidases (Hughes et al., 1975). In keeping with Axelrod's ideas that the actions of these pentapeptides may be “much more than additive,” the hypothesis is advanced here that the transient, short-lived initial activity of the neuropeptides leucine-enkephalin (leu-enk) and methionine-enkephalin (met-enk) may be temporally overshadowed by the accessory actions of their individual post-degradation constituent amino acids, namely tyrosine, glycines (of which there are two), phenylalanine and at the carboxyl-terminal, the residues leucine or methionione (Fig. 1). Many references to the early original works describing the actions of these atypical neurotransmitters are included in this communication. It is speculated here that the evolution-driven selection of the amino acids used within these neuropeptides may represent part of a novel, intrinsic signaling system specifically tailored to the transmission of neural information within dopaminergic, glycinergic and enkephalinergic pathways.
Fig. 1.
Pentapeptide structures of leucine- and methionine-enkephalin.
THE NATURE, SEQUENCE AND CONFORMATION OF ENKEPHALIN
Since their initial discovery some 30 years ago, ongoing neuropeptide research has suggested a remarkable endogenous complexity to the enkephalin pentapeptide signal, supporting the idea that the overall neurophysiological action of the enkephalins appears to be one of multiple neural signaling function. If one considers residue replacement at each of the five positions of the enkephalins with any of the twenty-plus amino acids commonly found in proteins, rare amino acids or R-group modified residues, many millions of derivatives are possible. Since their discovery, systematic modification of the pentapeptide sequence has been yielding novel and interesting euphoriants, sedatives, hypnotics, antitussives and analgesic agents (reviewed by Snyder, 2004). The component amino acids in both leu-enk and met-enk, and especially in the tyrosine and glycine residues, ultimately have the potential to perform several ancillary, non-enkephalinergic, nervous system functions (Fig. 1). Concerning enkepahlin structure, Tyr1 cannot be replaced by any other amino acid residue, and chemical modification or stereo-chemical reorientation at this position results in a large loss of activity (Simantov and Snyder, 1976). The aminopeptidase sensitive amino terminal tyr1 residue is the immediate precursor of L-dihydroxyphenylalanine (L-Dopa), and ultimately, of both dopamine and norepinephrine. Glycine is a versatile non-essential uncharged polar amino acid which easily transverses the blood-brain barrier and two of these simplest of the amino acids occupy the second and third position in the enkephalin molecule. Gly2 is an absolute requirement and any side chain modification or substitution inhibits enkephalin activity (Salvadori and Temussi, 2004). Gly3 is also an absolute requirement at the central position within the pentapeptide (Simantov and Snyder, 1976). The presence of a butyl R group here may prevent formation of proper configuration essential for neurochemical function, since steric flexibility is probably essential to induce a conformational fit with enkephalin's opioid receptor (Bradbury et al., 1976; Snyder and Childers, 1979). Phe4 can be replaced by tryptophan or parachloro-phenylalanine (L isomer only) with no ensuing loss of activity. Some replacements at this position appear to exhibit enhanced enkephalin potency; an aromatic hydrophobic side chain is preferred, however, any replacement at this fourth site with tyrosine results in a lowered activity, presumably because of the exposed hydroxyl group. In general, the further away one moves from tyr1, the less specific is the absolute requirement for a certain amino acid. Leucine or methionine at position 5 defines leu-enk or met-enk specificity, and position five can be replaced by any of a large number of residues, although a hydrophobic amino acid such as alanine, valine, isoleucine, phenylalanine or tryptophan is preferred. The existence of a fifth amino acid at position five is also essential for enkephalin activity, and can be of either the D or L configuration, and optimal activity in the bound peptide is retained (Simantov and Snyder, 1976). Through nuclear magnetic resonance studies, theoretical calculation and model building, Bradbury et al. (1976), Schiller (1977) and Gorin and Marshall (1977) originally suggested a β-bend conformation at positions gly3 and phe4, allowing enkephalin to readily assume a configuration which mimics that of the rigid fused ring of the opiates. Both molecular species have terminal hydroxyl functions and tertiary amine groups which most likely are of necessity to receptor binding, and are presumably under the steric influence of the opiate receptor (Salvadori and Temussi, 2004). In this respect, residues situated around this putative β-kink might be expected to be integral in the performance of this pentapeptide (Bradbury et al., 1976; Snyder and Childers et al., 1979). This becomes apparent when synthetically modifying enkephalin molecules that yield several extremely potent experimental analgesics (Janecka et al., 2004).
ENKEPHALIN METABOLISM
Enkephalins typically have a turnover rate measured in seconds to minutes, both in vivo and in vitro (Hughes et al., 1975; Simantov and Snyder, 1976), and this stability is related to the age of the cell system under study (McGeer and McGeer, 1978; Sokolov et al., 2004). One major initial catabolic step involves hydrolysis and cleavage of the tyr1-gly2 bond by a specific membrane-associated amino-peptidase (Hambrook et al., 1976; Dupont, 1977). Thus, aminopeptidase catalyzed hydrolysis is itself largely responsible for the short duration of enkephalin action. Other membrane bound peptidases have been described and like all peptides, enkephalin can be acted upon by numerous non-specific peptidases. In 1978, Schwartz's group, and others, reported a membrane-associated enzyme which cleaves enkephalin between gly3 and phe4 generating the tyr1-gly2-gly3 fragment (Malfroy et al., 1978). Named ‘enkephalinase A,’ the enzyme had affinity for its substrate in the nanomolar range and displayed a membrane topographical variability like those for opiate receptors. Snyder's group, in 1979, discovered two additional subspecies of this enkephalinase as well as an enkephalinase B which hydrolyzes the molecule between the gly2-gly3 and another membrane-associated aminopeptidase that cleaved the terminal tyrosine (Childers et al., 1979; Snyder and Childers, 1979). Interestingly, all enkephalinases have similarly high affinities for the substrate, so it seems highly probable that each of the four peptide bonds in enkephalin have roughly equal susceptibility to rapid degradation into their constituent amino acid, assuming an open configuration is maintained. Non-specific aminopeptidases and carboxypeptidase remove the amino and carboxyl terminals of enkephalin while other endogenous endo-peptidases such as cathepsin C and angiotensin—converting enzyme can cleave the innermost peptide linkages. Taken together, these data show that enkephalin degradation is rapid, specific, and at the site of catabolism and termination of the enkephalin signal constituent free amino acids are released. The precise role and mode of action of the enzyme system responsible for these cleavages can therefore be expected to have a major effect on the pharmacology and biochemistry of the enkephalins. It is noteworthy that the residues most essential to enkephalin function are also either neurotransmitters themselves (glycine) or immediate precursors of L-Dopa, dopamine and norepinephrine (tyrosine and phenylalanine). The variable fifth amino acid leucine (leu5) or methionine (met5) has not yet been assigned any neurotransmitter candidacy. Again, this suggests that enkephalin is polyfunctional in that, in its intact state it elicits binding to the same sites in the brain as morphine and other opiates, and its degradation products have a potential for follow-up accessory functions by reacting as signaling entities themselves, or as the immediate precursors to inhibitory or metabotropic neurotransmitters.
OVERLAPPING NEURAL FUNCTION AND CO-LOCALIZATION OF DOPAMINERGIC, GLYCINERGIC AND ENKEPHALINERGIC PATHWAYS
Dopamine
The neurophysiological effects of any neurotransmitter can be best understood by considering the major anatomical pathways where they and their anabolic and catabolic enzymes co-exist. These can best be experimentally determined via a process termed immunohistochemical mapping (reviewed by Snyder, 2004). Dopamine exerts a profound influence over a wide variety of neurophysiological functions ‘without being crucial to any one of them’ (Hiroi et al., 2002; Ikemoto, 2002). Dopamine's neuroactive effects are most likely metabotropic (second messenger or cyclic AMP modulated) which ultimately alter membrane permeability (Hiroi et al., 2002). The catecholamine family, comprised of dopamine, noradrenaline, adrenaline, and others, are formed from the nonessential dietary amino acid tyrosine by the sequence of reactions L-tyrosine → L-dihydroxyphenylalanine (L-Dopa) → dopamine → L-noradrenaline → L-adrenaline (Roques 2000; Ikemoto, 2002). Tyrosine can be formed from the essential amino acid phenylalanine via a hydroxylation, and tyrosine in the basal ganglia can be converted directly to dopamine by tyrosine hydroxylase, a soluble enzyme within the synaptosome, and also by a widely distributed decarboxylase nonspecific for L-Dopa. The presence of dopamine in large amounts in localized areas of the brain, unaccompanied by comparative amounts of noradrenalin or adrenalin, suggests specific neuronal roles for dopamine (Hiroi et al., 2002; Ikemoto, 2002). Using histochemistry, pharmacological manipulations, immunohistochemistry, enzyme histochemistry, autoradiography, electron microscopy and axoplasmic flow techniques, three principle dopaminergic pathways have been shown to occur in the central nervous system. Dopaminergic neuronal systems emanate from a series of cell bodies in the brain stem, one of the most prominent dopaminergic pathways being the nigrostriatal system (Hiroi et al., 2002). The lightly myelinated axons from this system ultimately fan out through the globus pallidus to enter the caudate and putamen. Dopamine terminals can enter into the amygdaloid nucleus and seem to be an extension of the terminals of the caudate nucleus and putamen (Ungerstedt, 1971). Axons from the meso-limbic meso-cortical pathway innervate limbic structures, the olfactory tubercle and amygdala. Thirdly, the tuberoinfundibular dopamine system has cell bodies located within the arcuate nucleus of the hypothalamus and neurons from here can innervate the external layer of the medial eminance. There are also some interstitial neurons in the hypothalamus which may have possible connections to ascending axons into the diencephalon, particularly the thalamus. Therefore, all three major dopaminergic pathways in the brain i.e. the nigrostriatal, the meso-cortical, meso-limbic, and the tuberoinfundibular system relate respectively to extrapyramidal emotional, behavioral, and neuroendocrine physiology, pathways enriched in enkephalinergic neurons.
Glycine
The uneven distribution of glycinergic neurons in the central nervous system, particularly in the diencephalon, and within the dorsal and ventral grey of the spinal cord, and the presence of a high affinity uptake system originally suggested evidence for glycine possessing a neurotransmitter role (Iversen et al., 1973; Snyder, 2004). As the simplest dietary amino acid, glycine has numerous metabolic functions, is synthesized, and broken down by, several different independent enzymatic routes, and this versatile amino acid may end up in a large variety of anabolites, such as polypeptides, purine nucleotides and acetyl Co-A. Glycine hyperpolarizes the membrane, lowers the membrane resistance, and increases chloride impermeability, strongly supporting a role for glycine as an inhibitory neurotransmitter, especially in the spinal cord and brainstem. It is largely absent in the central or cerebellar cortical neurons. Based on neurophysiological studies using iontophoresed glycine, Tebecis (1973), originally suggested that glycine may be an inhibitory neurotransmitter for reticulospinal neurons in the medullar reticular formation, which provide a major input into the intralaminar nuclei. The globus pallidus projects upon intralaminar nuclei with afferent connections correlating to, and overlapping with, enkephalinergic pathways.
Enkephalins and “Additive Effects”
As for other neurotransmitter pathways, the localization and mapping of enkephalin containing, and releasing, neurons, and enkephalin signaling centers throughout the brain have been largely accomplished through the technique of fluorescent immunohistochemical mapping (Roques, 2000; Snyder, 2004). Enkephalins are contained within specific neuronal systems localized to brain anatomical regions in which they play prominent roles in processing sensory information, with their overall action being one of inhibition or depression (Hiroi et al., 2002; Snyder, 2004). Enkephalins may also serve as modulators of synaptic activity in ways other than by functioning as neurotransmitters (Ungerstedt, 1971; Roques, 2000; Snyder, 2004). Both the substantia gelatinosa in the spinal cord and lower brain stem, and the periaqueductal grey matter are enriched in enkephalin containing neurons. The substantia gelatinosa, a narrow band in the grey matter of the spinal cord where incoming sensory nerves terminate, serves as an integration center for pain perception (Hughes et al., 1975; Snyder and Childers, 1979; Janecka et al., 2004). Electrical stimulation of the periaqueductal grey produces analgesia in rats and enkephalins released here function to inhibit the release of excitatory neurotransmitter signals from sensory nerves, particularly those carrying information about pain (Snyder, 2004). The amygdaloid nuclei, a prominent group of structures within the limbic system, long implicated in regulating behavior and emotional control, further contain high densities of enkephalinergic neurons and these enkephalin tracts in the limbic system may help explain the euphoric effects of opiates (Childers et al., 1979; Simantov and Snyder, 1976; Snyder, 2004). The presence of high concentrations of enkephalin in the hypothalamus suggests a role in regulating the release of releasing factors, and hence a neuroendocrine modulatory function (Childers et al., 1979; Redgate et al., 1986; Dores et al., 2002). However, by far the highest concentrations of these pentapeptides are found in the globus pallidus, a primary motor nucleus, suggesting that enkephalins somehow moderate motor activity (Hiroi et al., 2002). Most immunohistochemical staining in the globus pallidus appears to be on nerve endings from cell bodies located in the caudate-putamen, in close conjunction with dopaminergic pathways (Childers et al., 1979; Hiroi et al., 2002). The localization of enkephalin in the intestinal tract has also been well documented as apparently having a putative role in the peristalsis and anti-diarrhetic action (Coupar, 1995). Interestingly, large quantities of dopamine are found in the duodenum of certain ruminant animals where it seems to be correlated with special types of mast cell (von Dorsche et al., 1970; Snyder, 2004).
To summarize, major enkephalin pathways in the brain involve the extrapyramidal system, including motor pathways controlled by the basal ganglia, the limbic system that governs emotional and behavioral control, and the hypothalamic-neuroendocrine axis. The apparent overlap of localization within the central nervous system of dopaminergic, glycinergic, and enkephalinergic pathways is speculated to be of neurophysiological signifigance, especially in light of the relatively short half-life of the enkephalins and the immediate precursor-product relationship between tyrosine and dopamine, and glycinergic signaling (Ungerstedt, 1971; Redgate et al., 1986; Hiroi et al., 2002). This may also have important consequences for the neurophysiological effects of inhibition and excitation that can be simultaneously mediated by modulatory neuropeptides, which possess intrinsic neuroactivity in their degradation products (Fig. 1.; Snyder, 2004). In a complex system of signal integration, inhibition of inhibitory neurons can lead to a dis-inhibition of excitation of the system as a whole, while inhibition of excitatory neurons would lead to a more selective inhibition of the system. Inhibition of excitatory neurons that can act on coincident inhibitory neurons may lead to dis-inhibition, or, excitation and further modes of complex signal integration. Indeed, the stereochemistry and amino acid sequence of any neuropeptide and its transient position in a catabolic, biosynthetic or signaling pathway, has definitive importance and intimate relevance with respect to its overall function within that system. When, for example, neuropeptides are enzymatically degraded, some precursors may be retained, and reutilized, while other residues may enter local metabolic pools, that continue to turn over, or move into, adjacent metabolic or neural signaling pathways. Considering the complexity of the mammalian brain, with its unprecedented powers of signal integration, and the intricacies of molecular mechanism and architecture, the whole is immeasurably greater than the sum of its component parts. At integrative signaling levels, both neural peptides and their individual amino acids as modulators of the brain function may have additive roles, whose neural activities could be greater than, or, have a corroborative interrelation with, the intact neuropeptide. Importantly, the amino acid components of enkephalin, when compared to the action of the intact pentapeptide, often have contrasting signaling roles (Snyder, 2004). There is also reason to believe that other neural peptides, whose constituent amino acids have been selected and tested over the evolutionary process of natural selection, may possess similar multiplicity in signaling function. Hence, these peptides do not transmit information as a single directed neurophysiological signal, but rather anatomically and temporally modulate their signaling information as they are degraded into their component amino acids.
ACKNOWLEDGMENTS
Thanks are extended to Aileen I. Pogue for the assembling and proofreading of this manuscript. This work was supported in part by NIA AG18031.
REFERENCES
- Bradbury, A. F., Smyth, D. G., and Snell, C. R. (1976). Biosynthetic origin and receptor conformation of methionine enkephalin. Nature260:165–166. [DOI] [PubMed] [Google Scholar]
- Childers, S. R., Creese, I., Snowman, A. M., and Synder, S.H. (1979). Opiate receptor binding affected differentially by opiates and opioid peptides. Eur. J. Pharmacol.55:11–18. [DOI] [PubMed] [Google Scholar]
- Coupar, I. M. (1995). The peristaltic reflex in the rat ileum: Evidence for functional mu- and delta-opiate receptors. J. Pharm. Pharmacol.. 47:643–646. [DOI] [PubMed] [Google Scholar]
- Dores, R. M., Lecaude, S., Bauer, D., and Danielson, P. B. (2002). Analyzing the evolution of the opioid/orphanin gene family. Mass Spectrom. Rev.21:220–243. [DOI] [PubMed] [Google Scholar]
- Hambrook, J. M., Morgan, B. A., Rance, M. J., and Smith, C. F. (1976). Mode of deactivation of the enkephalins by rat and human plasma and rat brain homogenates. Nature262:782–783. [DOI] [PubMed] [Google Scholar]
- Hiroi, N., Martin, A. B., Grande, C., Alberti, I., Rivera, A., and Moratalla, R. (2002). Molecular dissection of dopamine receptor signaling. J. Chem. Neuroanat.23:237–242. [DOI] [PubMed] [Google Scholar]
- Hughes, J., Smith, T. W., Kosterlitz, H. W., Fothergill, L. A., Morgan, B. A., and Morris, H. R. (1975). Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature258:577–580. [DOI] [PubMed] [Google Scholar]
- Ikemoto, K. (2002). Human striatal D-neurons and their significance. Nihon Shinkei Seishin Yakurigaku Zasshi.22:131–135. [PubMed] [Google Scholar]
- Iversen, L. L., Kelly, J. S., Minchin, M., Schon, F., and Snodgrass, S. R. (1973). Role of amino acids and peptides in synaptic transmission. Brain Res.62:567–576. [DOI] [PubMed] [Google Scholar]
- Janecka, A., Fichna, J., and Janecki, T. (2004). Opioid receptors and their ligands. Curr. Top. Med. Chem.4:1–17. [DOI] [PubMed] [Google Scholar]
- Malfroy, B., Swerts, J. P., Guyon, A., Roques, B. P., and Schwartz J. C. (1978). High-affinity enkephalin-degrading peptidase in brain is increased after morphine. Nature276:523–526. [DOI] [PubMed] [Google Scholar]
- McGeer, P. L., and McGeer, E. G. (1978). Aging and neurotransmitter systems. Adv. Exp. Med. Biol.113:41–57. [DOI] [PubMed] [Google Scholar]
- Redgate, E. S., Deupree, J. D., and Axelrod, J. (1986). Interaction of neuropeptides and biogenic amines on cyclic adenosine monophosphate accumulation in hypothalamic nuclei. Brain Res.365:61–69. [DOI] [PubMed] [Google Scholar]
- Roques, B. P. (2000). Novel approaches to targeting neuropeptide systems. Trends Pharmacol Sci.21:475–483. [DOI] [PubMed] [Google Scholar]
- Simantov, R., and Snyder, S. H. (1976). Morphine-like peptides in mammalian brain: isolation, structure elucidation, and interactions with the opiate receptor. Proc. Natl. Acad. Sci. USA.73:2515–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadori, S., and Temussi, P. A. (2004). Antagonism in opioid peptides: the role of conformation. Curr. Top. Med. Chem.4:145–157. [DOI] [PubMed] [Google Scholar]
- Sokolov, O. Y., Kurasova, O. B., Kost, N. V., Gabaeva, M. V., Korneeva, E. V., Mikheeva, I. G., and Zozulya, A. A. (2004). Half-life of leu-enkephalin in the serum of infants of the first year of life on different types of feeding. Bull. Exp. Biol. Med.137:342–344. [DOI] [PubMed] [Google Scholar]
- Snyder, S. H. (2004). Opiate receptors and beyond: 30 years of neural signaling research. Neuropharmacology47:274–285. [DOI] [PubMed] [Google Scholar]
- Snyder, S. H., and Childers, S. R. (1979). Opiate receptors and opioid peptides. Annu. Rev. Neurosci.2:35–64. [DOI] [PubMed] [Google Scholar]
- Tebecis, A. K. (1973). Transmitters and reticulospinal neurons. Exp. Neurol.40:297–308. [DOI] [PubMed] [Google Scholar]
- Ungerstedt, U. (1971). Stereotaxic mapping of the monoamine pathways in the rat brain. Acta. Physiol. Scand. Suppl.367:1–48. [DOI] [PubMed] [Google Scholar]
- Vogel, Z., and Altstein, M. (1980). Inactivation of enkephalin by brain enzymes. Prog. Biochem. Pharmacol.16:49–59. [PubMed] [Google Scholar]
- von Dorsche, H. H., Fehrmann, P., and Sulzmann, R. (1970). The mast cell as a unicellular endocrine gland. Acta Anat (Basel)77:560–569. [PubMed] [Google Scholar]