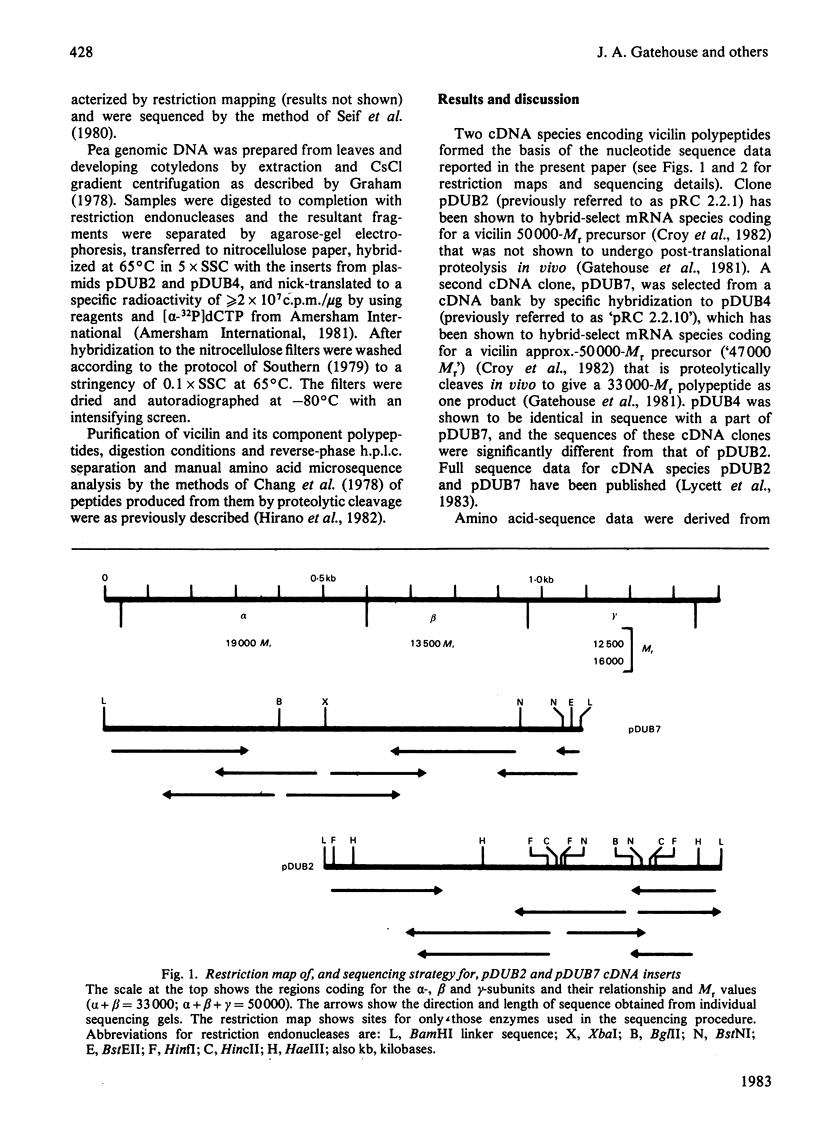
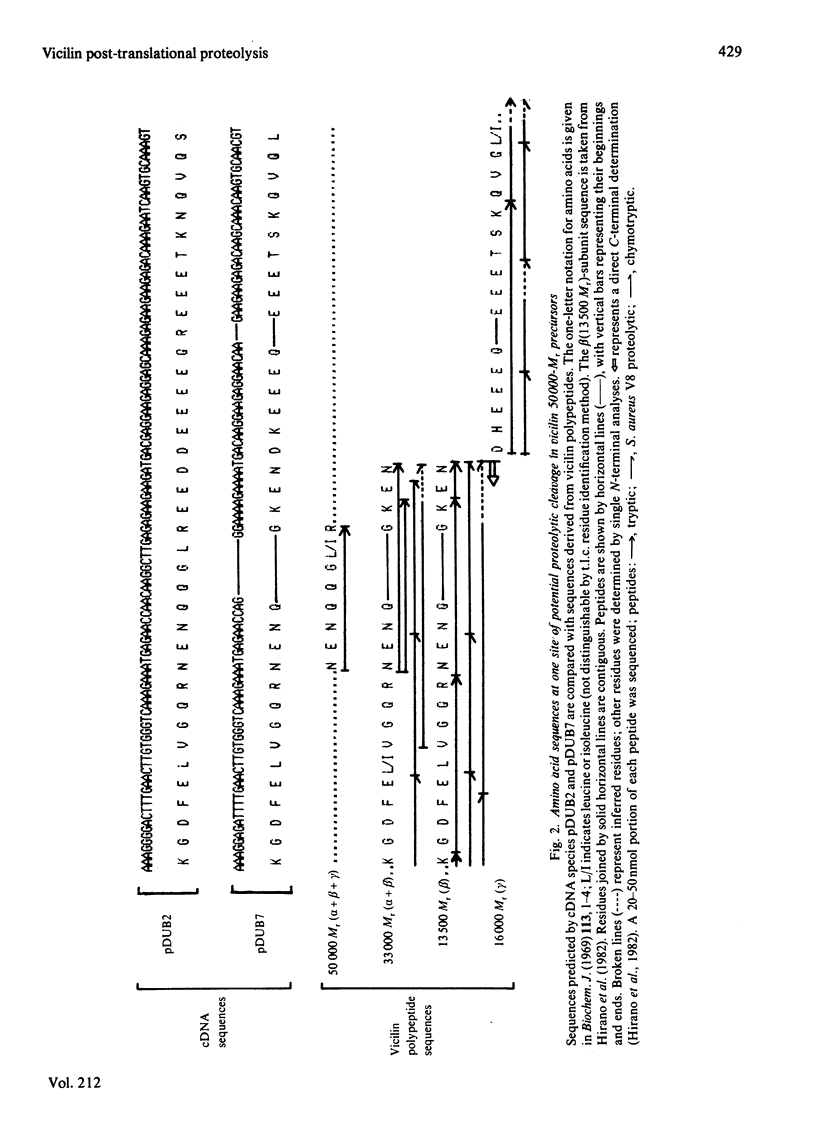
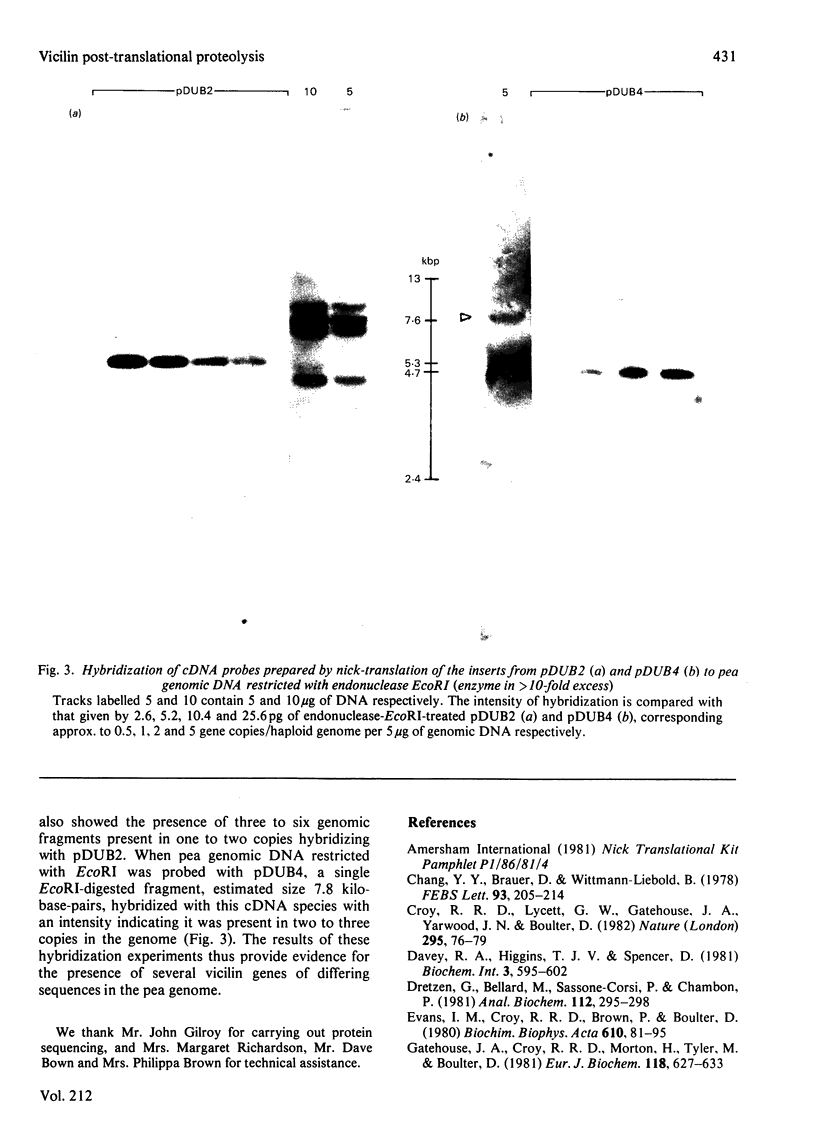
Abstract
Amino acid sequence data from vicilin of pea (Pisum sativum L.) were compared with predicted sequences from complementary DNA species. The sites of potential post-translational proteolytic cleavage of vicilin precursor polypeptides were located in polar regions of the polypeptide, at acidic or amide residues. Proteolysis did not take place in precursors containing a functionally distinct sequence: neutral residue-hydrophobic residue-basic residue at the cleavage site. Differences between the genomic sequences encoding vicilin thus specify proteolytic cleavage of vicilin precursor polypeptides.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans I. M., Croy R. R., Brown P., Boulter D. Synthesis of complementary DNAs to partially purified mRNAs coding for the storage proteins of Pisum sativum (L). Biochim Biophys Acta. 1980 Nov 14;610(1):81–95. doi: 10.1016/0005-2787(80)90058-1. [DOI] [PubMed] [Google Scholar]
- Gatehouse J. A., Croy R. R., Morton H., Tyler M., Boulter D. Characterisation and subunit structures of the vicilin storage proteins of pea (Pisum sativum L.). Eur J Biochem. 1981 Sep 1;118(3):627–633. doi: 10.1111/j.1432-1033.1981.tb05565.x. [DOI] [PubMed] [Google Scholar]
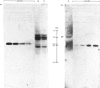
- Gatehouse J. A., Lycett G. W., Croy R. R., Boulter D. The post-translational proteolysis of the subunits of vicilin from pea (Pisum sativum L.). Biochem J. 1982 Dec 1;207(3):629–632. doi: 10.1042/bj2070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. E. The isolation of high molecular weight DNA from whole organisms or large tissue masses. Anal Biochem. 1978 Apr;85(2):609–613. doi: 10.1016/0003-2697(78)90262-2. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Wallis J. Colony hybridization. Methods Enzymol. 1979;68:379–389. doi: 10.1016/0076-6879(79)68027-8. [DOI] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. A rapid enzymatic DNA sequencing technique: determination of sequence alterations in early simian virus 40 temperature sensitive and deletion mutants. Nucleic Acids Res. 1980 May 24;8(10):2225–2240. doi: 10.1093/nar/8.10.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Quinn P. S., Chan S. J., Marsh J., Tager H. S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci. 1980;343:1–16. doi: 10.1111/j.1749-6632.1980.tb47238.x. [DOI] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]