Abstract
Background
Rezafungin is an echinocandin approved in the US and EU to treat candidaemia and/or invasive candidiasis. This post-hoc, pooled analysis of the Phase 2 STRIVE and Phase 3 ReSTORE trials assessed rezafungin versus caspofungin in patients with candidaemia and/or invasive candidiasis (IC) in the intensive care unit (ICU) at randomisation.
Methods
STRIVE and ReSTORE were randomised double-blind trials in adults with systemic signs and mycological confirmation of candidaemia and/or IC in blood or a normally sterile site ≤ 96 h before randomisation. Data were pooled for patients in the ICU at randomisation who received intravenous rezafungin (400 mg loading dose then 200 mg once weekly) or caspofungin (70 mg loading dose then 50 mg once daily) for ≤ 4 weeks. Outcomes were Day 30 all-cause mortality (primary outcome), Day 5 and 14 mycological eradication, time to negative blood culture, mortality attributable to candidaemia/invasive candidiasis, safety, and pharmacokinetics.
Results
Of 294 patients in STRIVE/ReSTORE, 113 were in the ICU at randomisation (rezafungin n = 46; caspofungin n = 67). At baseline, ~ 30% of patients in each group had impaired renal function and/or an Acute Physiologic Assessment and Chronic Health Evaluation II score ≥ 20. One patient (in the caspofungin group) was neutropenic at baseline. Day 30 all-cause mortality was 34.8% for rezafungin versus 25.4% for caspofungin. Day 5 and 14 mycological eradication was 78.3% and 71.7% for rezafungin versus 59.7% and 65.7% for caspofungin, respectively. Median time to negative blood culture was 18 (interquartile range, 12.6–43.0) versus 38 (interquartile range, 15.9–211.3) h for rezafungin versus caspofungin (stratified log-rank P = 0.001; nominal, not adjusted for multiplicity). Candidaemia/IC-attributable deaths occurred in two rezafungin patients versus one caspofungin patient. Safety profiles were similar between groups. Overall, 17.4% (rezafungin) versus 29.9% (caspofungin) of patients discontinued due to treatment-emergent adverse events. Rezafungin exposure following the initial 400-mg dose was comparable between patients in the ICU at randomisation (n = 50) and non-ICU patients (n = 117).
Conclusions
Rezafungin was well tolerated and efficacious in critically ill, mainly non-neutropenic patients with candidaemia and/or IC. This analysis provides additional insights into the efficacy and safety of rezafungin in the ICU population.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-024-05117-5.
Keywords: Candidaemia, Intensive care unit, Invasive candidiasis, Rezafungin
Background
Critically ill patients are at risk of candidaemia and invasive candidiasis (IC), with one-third to half of all candidaemia cases occurring in the intensive care unit (ICU) [1, 2]. Outcomes are poor, with an estimated crude mortality rate of 40‒55% in the ICU for IC [2].
Echinocandins are established first-line treatment for candidaemia and IC, with azoles an acceptable alternative in selected patients [3, 4]. However, timely initiation of appropriate antifungal therapy is essential; delay or inadequate treatment can increase mortality [5, 6]. Furthermore, the increasing prominence of non-albicans Candida species and growing challenge of azole and echinocandin resistance, especially with respect to C. glabrata and C. auris, are concerns [2]. Within the ICU, antifungal-resistant isolates spread between patients; outbreaks of azole-resistant C. parapsilosis with similar genotypes [7] and intra-hospital spread of azole-resistant C. glabrata have been reported [8]. The likelihood of achieving effective exposures with current dosing regimens of first-generation echinocandins (anidulafungin, caspofungin, and micafungin) is also unclear, especially regarding resistant Candida species [9]. New agents and/or dosing strategies are therefore needed for critically ill patients with candidaemia and/or IC.
The broad-spectrum echinocandin rezafungin was developed to treat candidaemia and IC and prevent invasive fungal disease caused by Candida, Aspergillus, and Pneumocystis species in patients undergoing allogeneic blood/bone marrow transplantation [10–12]. Rezafungin’s long half-life (~ 5–6 days) and high front-loaded dosing [13] permit extended-interval dosing and high plasma drug concentrations early in therapy [13, 14] owing to the concentration-dependent efficacy of echinocandins [13, 15, 16].
Rezafungin once weekly (QW) was approved by the US Food and Drug Administration (FDA) in March 2023 for the treatment of candidaemia and IC [17], and by the European Medicines Agency in December 2023 to treat IC in adults [18, 19]. In the Phase 2 STRIVE study, rezafungin was well tolerated and showed signs of efficacy in candidaemia and/or IC [10]. Non-inferiority of QW rezafungin versus once-daily (QD) caspofungin was subsequently demonstrated for the primary outcomes of Day 14 global cure and Day 30 all-cause mortality (ACM) in the Phase 3 ReSTORE trial [11]. Both trials suggested that rezafungin was associated with early efficacy benefits for time to negative blood culture (TTNBC) and Day 5 outcomes, including global (ReSTORE) or overall (STRIVE) cure and mycological eradication rates [10, 11]. Rezafungin and caspofungin had comparable safety profiles in both trials [10, 11]. A pooled analysis of STRIVE and ReSTORE confirmed these findings, and suggested a potentially faster TTNBC, especially in patients with a positive blood culture close to randomisation [20]. This post-hoc, pooled, patient-level analysis of STRIVE and ReSTORE investigated the efficacy and safety of rezafungin compared with caspofungin in the subgroup of patients with candidaemia and/or IC who were in the ICU at randomisation.
Methods
Study design, patients, and treatment
Methodology and primary data from STRIVE (ClinicalTrials.gov: NCT02734862) [10] and ReSTORE (NCT03667690) [11] have been reported previously. Both were multicentre, prospective, randomised, double-blind, double-dummy trials comparing QW intravenous rezafungin versus QD intravenous caspofungin in adults aged ≥ 18 years with systemic signs and mycological confirmation of candidaemia and/or IC in blood or a normally sterile site within 96 h before randomisation (as defined in Supplementary Material 1). Patients who had received a systemic antifungal agent to treat candidaemia (given for > 48 h) within 4 days prior to randomisation were excluded from both trials, except if the treatment was one for which any Candida species isolated in culture was not susceptible.
In this post-hoc, patient-level analysis, data were pooled for patients in the ICU at randomisation who received similar dosing regimens of rezafungin and caspofungin in STRIVE and ReSTORE. STRIVE had two rezafungin dosing schemes; the regimen that aligned with the one used in ReSTORE is the focus of this analysis. Patients received rezafungin QW (400 mg on Day 1, 200 mg on Day 8, with an optional 200 mg dose on Day 15). A 200 mg rezafungin dose on Day 22 was optional in ReSTORE and for those with IC in STRIVE. Caspofungin was administered QD (70 mg on Day 1, then 50 mg/day for ≥ 2 and up to 28 days [with dose adjustment for drug–drug interactions or patient weight according to the label and at the investigator’s discretion]). There was an optional step-down to oral fluconazole (caspofungin groups) or oral placebo (to maintain study blinding for rezafungin groups) if step-down criteria were met [10, 11]. ReSTORE and STRIVE protocols recommended removal of central venous catheters (CVCs) within 48 h after candidaemia diagnosis, aligned with Infectious Diseases Society of America (IDSA) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines [3, 4].
Outcomes
The primary outcome in this analysis was Day 30 ACM (FDA-mandated joint primary outcome in ReSTORE [11]). Secondary outcomes were mycological response (eradication) on Days 5 and 14 within patients with a positive baseline blood culture. The exploratory outcome was TTNBC within those with a positive baseline blood culture. The proportions of patients with positive baseline blood cultures who had negative blood cultures at 24 and 48 h were also assessed. Definitions are included in Supplementary Material 1. Mortality attributable to candidaemia and/or IC (patients who died with systemic signs or symptoms attributable to candidaemia and/or IC, assessed by investigators in STRIVE and by the data review committee [DRC] in ReSTORE) at Day 30 in a blinded manner, Day 14 global/overall response, duration of ICU/hospital stay, and administration of vasopressors within the ICU from Day 1 onwards were post-hoc analyses. Definitions of Day 14 global cure (ReSTORE) and overall response (STRIVE) were deemed sufficiently similar to be combined for this analysis (global cure: clinical cure, radiological cure, and mycological eradication determined by a blinded DRC [11]; overall response was programmatically derived as resolution of clinical signs of candidaemia/IC and mycological eradication [10]).
Safety was determined by treatment-emergent adverse events (TEAEs: AEs occurring during or after study drug administration until follow-up visit), study drug-related TEAEs, serious TEAEs and serious drug-related TEAEs, vital signs, and laboratory tests. AEs were coded using Medical Dictionary for Regulatory Activities (MedDRA) version 23.0 and stratified by protocol-specified severity grades (mild/moderate/severe).
An exploratory analysis of the pharmacokinetic parameters of rezafungin following the first 400-mg dose (area under the plasma concentration‒time curve from 0 to 168 h [AUC0–168 h], and maximum and minimum plasma concentrations [Cmax and Cmin]) in patients in the ICU at randomisation and non-ICU patients (i.e., all other patients in the pharmacokinetic analysis population who received the initial 400-mg dose of rezafungin who were not in the ICU at randomisation), was also performed.
Data analyses
Outcomes were evaluated in patients with candidaemia and/or IC who were in the ICU at randomisation. Data are reported for the modified intent-to-treat (mITT; patients with documented Candida infection within 96 h before randomisation who received ≥ 1 study drug dose) and safety populations (patients who received ≥ 1 study drug dose), respectively. Pharmacokinetic parameters were assessed in the pharmacokinetic analysis population, defined as all patients who received rezafungin with at least one plasma sample for pharmacokinetic analysis. As a post-hoc analysis, outcomes were summarised using descriptive statistics (because the analysis was not powered for formal comparisons), with associated two-sided 95% confidence intervals (CIs) provided for the primary and secondary outcome measures. Two-sided 95% CIs for the weighted (by study and Parts A and B of STRIVE) treatment difference estimate in response rates (rezafungin minus caspofungin) were calculated using stratified (by study and Parts A and B of STRIVE) Miettinen–Nurminen methodology. Kaplan–Meier methods were used to estimate median TTNBC. Statistical difference was assessed nominally for TTNBC using the stratified log-rank test (not adjusted for multiplicity) and exploratory, nominal P values are provided.
Results
Patient disposition
Of the 294 patients with candidaemia and/or IC included in STRIVE and ReSTORE, 113 were in the ICU at randomisation and comprised the mITT and safety populations for this analysis. Overall, 46 and 67 critically ill patients were treated with rezafungin and caspofungin, respectively (Supplementary Material 1: Fig. S1).
Demographics and baseline characteristics
Baseline patient demographics and characteristics were generally similar between the rezafungin and caspofungin groups (Table 1). Approximately 30% of patients in each group had impaired renal function (baseline creatinine clearance < 50 mL/min) and/or Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) II score ≥ 20; > 78% of patients in each group had a catheter at screening. Fewer patients were receiving mechanical ventilation in the rezafungin (34.8%; 16/46) than the caspofungin (49.3%; 33/67) group. Other common risk factors for candidaemia/IC included broad-spectrum antibiotic therapy (rezafungin 78.3%; caspofungin 67.2%), major surgery (rezafungin 30.4%; caspofungin 41.8%), and total parenteral nutrition (rezafungin 23.9%; caspofungin 32.8%). One patient (in the caspofungin group) was neutropenic at baseline. The most common Candida species isolated at baseline was C. albicans (rezafungin 41.3%; caspofungin 35.8%) (Supplementary Material 1: Table S1). Isolated Candida species were comparable between groups, except for C. parapsilosis (rezafungin 8.7%; caspofungin 26.9%) and C. glabrata (rezafungin 37.0%; caspofungin 16.4%).
Table 1.
Demographics and baseline characteristics (mITT population in the ICU at randomisation)
| Characteristic | Rezafungin 400/200 mg (n = 46) |
Caspofungin 70/50 mg (n = 67) |
|---|---|---|
| Age, mean years ± SD (range) | 61.6 ± 11.6 (34–81) | 61.5 ± 14.1 (21–87) |
| Female, n (%) | 11 (23.9) | 23 (34.3) |
| BMI, kg/m2, mean (SD) | 27.5 (7.6) | 25.2 (5.7) |
| Race, n (%)a | ||
| Asian | 7 (16.7) | 16 (24.2) |
| Black or African American | 5 (11.9) | 3 (4.5) |
| White | 30 (71.4) | 46 (69.7) |
| Other | 0 (0) | 1 (1.5) |
| Geographical region, n (%) | ||
| Asia–Pacific (excluding China/Taiwan) | 6 (13.0) | 11 (16.4) |
| China/Taiwan | 1 (2.2) | 2 (3.0) |
| Europe/Israel/Turkey | 23 (50.0) | 39 (58.2) |
| North/South America | 16 (34.8) | 15 (22.4) |
| Initial diagnosis, n (%) | ||
| Candidaemia only | 34 (73.9) | 50 (74.6) |
| Invasive candidiasis | 12 (26.1) | 17 (25.4) |
| Infection location, n (%)a | ||
| Catheterb | 18 (39.1) | 31 (50.0) |
| Percutaneous | 18 (39.1) | 18 (29.0) |
| Intra-abdominalc | 7 (15.2) | 10 (16.1) |
| Other | 3 (6.5) | 3 (4.8) |
| Baseline creatinine clearance, n (%)a | ||
| < 50 mL/min | 16 (34.8) | 17 (28.8) |
| ≥ 50 mL/min | 30 (65.2) | 42 (71.2) |
| Baseline absolute neutrophil count, n (%)a | ||
| < 500 cells/µL | 0 | 1 (1.5) |
| ≥ 500 cells/µL | 46 (100) | 64 (98.5) |
| Dialysis within last 3 days—yes, n (%) | 7 (15.2) | 4 (6.0) |
| Baseline APACHE II scorea | ||
| Median (range) | 16 (4–40) | 16 (0–37) |
| < 20, n (%) | 28 (63.6) | 47 (70.1) |
| ≥ 20, n (%) | 16 (36.4) | 20 (29.9) |
| Prior antifungal use, n (%) | 34 (73.9) | 45 (67.2) |
| Triazole | 10 (21.7) | 21 (31.3) |
| Echinocandin | 21 (45.7) | 18 (26.9) |
| Polyene | 4 (8.7) | 11 (16.4) |
| Duration of prior antifungal use, mean days ± SD (range)d | 1.9 ± 0.8 (1–4) | 3.6 ± 7.6 (1–49) |
| Mechanically ventilated—yes, n (%) | 16 (34.8) | 33 (49.3) |
| Central venous catheter at screening—yes, n (%) | 36 (78.3) | 54 (80.6) |
| Catheter removed within 48 h after diagnosis, n/N (%) | 3/36 (8.3) | 14/54 (25.9) |
APACHE Acute Physiologic Assessment and Chronic Health Evaluation, BMI body mass index, h hours, ICU intensive care unit, mITT modified intent-to-treat, SD standard deviation
aData were missing for race in 4 patients in the rezafungin group and 1 patient in the caspofungin group. Data were missing for infection location in 5 patients in the caspofungin group. Data were missing for creatinine clearance in 8 patients in the caspofungin group. Data were missing for absolute neutrophil count in 2 patients in the caspofungin group. Data were missing for APACHE II score in 2 patients in the rezafungin group
bSite of infection in 1 patient in the caspofungin group was catheter tip
cIncluding infections of the peritoneal space
dIf a patient had received ≥ 1 prior antifungal agent, the length of use was summed
Mean time from first positive blood sample to first dose of randomised treatment was 69.8 h (standard deviation [SD]: 20.6) versus 63.3 (SD: 25.6) in the rezafungin (n = 36) versus caspofungin (n = 54) groups, respectively. Mean time from first positive blood sample to first antifungal treatment for candidaemia/IC was 62.4 h (SD: 24.0) versus 54.9 (SD: 27.7), respectively.
Following intravenous therapy, 19.6% (9/46) of patients in the rezafungin group and 22.4% (15/67) of those in the caspofungin group switched to oral stepdown therapy.
Efficacy of rezafungin compared with caspofungin
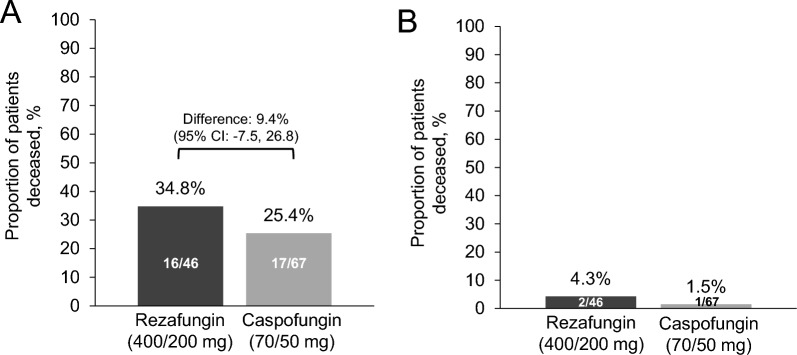
ACM rates at Day 30 were 34.8% (16/46) and 25.4% (17/67) for the rezafungin and caspofungin groups, respectively (treatment difference 9.4% [95% CI − 7.5, 26.8]) (Fig. 1A). There were 14 confirmed deaths at Day 30 (i.e., excluding patients with unknown survival status) in the rezafungin group and 15 in the caspofungin group. Of these, two (14.3%, 2/14) and one (6.6%, 1/15) were attributable to candidaemia and/or IC in the rezafungin and caspofungin groups, respectively. Deaths attributable to candidaemia and/or IC at Day 30 were 4.3% (2/46) and 1.5% (1/67) for the rezafungin and caspofungin groups, respectively (Fig. 1B).
Fig. 1.
Day-30 mortality. A Day-30 all-cause mortality (mITT population); B Deaths attributable to candidaemia and/or IC (Per DRC evaluation in ReSTORE; per investigator evaluation in STRIVE). CI confidence interval, DRC Data Review Committee, IC invasive candidiasis, mITT modified intent-to-treat
Mycological eradication on Days 5 and 14 was 78.3% (36/46) and 71.7% (33/46) in the rezafungin group versus 59.7% (40/67) and 65.7% (44/67), respectively, in the caspofungin group (treatment difference 18.6% [95% CI 0.9, 34.4] at Day 5 and 6.1% [95% CI −11.8, 22.7] at Day 14) (Fig. 2).
Fig. 2.
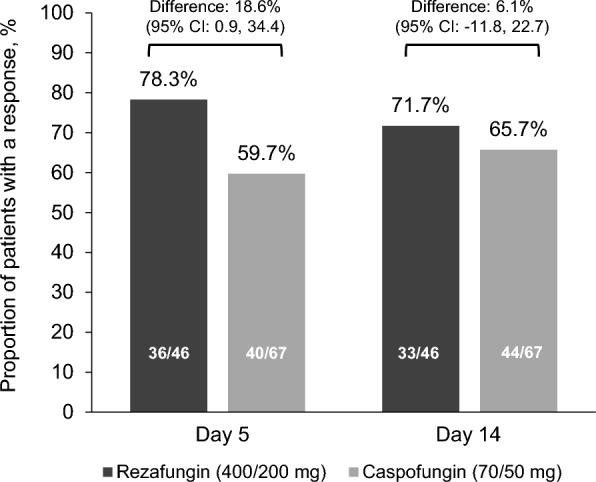
Mycological eradication at Day 5 and Day 14 (mITT population). CI confidence interval, mITT modified intent-to-treat
Median TTNBC was 18 h (interquartile range, 12.6–43.0) with rezafungin versus 38 h (interquartile range, 15.9–211.3) with caspofungin (stratified log-rank P = 0.001; nominal, not adjusted for multiplicity). The proportions of patients with a negative blood culture at 24 and 48 h in the rezafungin group were 62.9% (22/35) and 77.1% (27/35) versus 41.2% (21/51) and 54.9% (28/51), respectively, in the caspofungin group (Fig. 3).
Fig. 3.
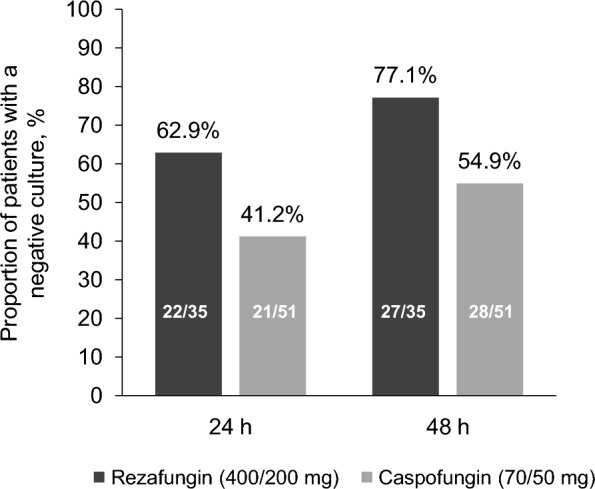
Negative blood culture. Proportion of patients with negative blood culture at 24 and 48 h (mITT population). mITT modified intent-to-treat
Global cure/overall response at Day 14 was achieved in 63.0% (29/46) and 56.7% (38/67) of those in the rezafungin and caspofungin groups, respectively (treatment difference: 8.3% [95% CI −10.5, 25.4]).
Other treatment outcomes
Mean number of days in the ICU was 17.5 (SD: 16.8) versus 23.0 (SD: 19.5) for patients in the rezafungin versus caspofungin groups, respectively. Mean number of days in hospital was 32.2 (SD: 19.1) versus 37.0 (SD: 17.4), respectively.
More patients received vasopressors within the ICU from Day 1 onwards in the rezafungin (52.2%; 24/46) than the caspofungin (40.3%; 27/67) group. The 24 patients in the rezafungin group received a mean of 8.0 (SD: 8.0) days of vasopressor treatment and had a mean of 12.9 (SD: 15.7) vasopressor-free days within the ICU. The 27 patients in the caspofungin group received a mean of 11.4 (SD: 13.9) days of vasopressor treatment and had a mean of 13.8 (SD: 14.9) vasopressor-free days within the ICU.
Of patients who had a CVC at screening, 8.3% (3/36) in the rezafungin group and 25.9% (14/54) in the caspofungin group had it removed within 48 h after diagnosis.
Safety
Rezafungin and caspofungin had comparable safety profiles (Table 2). The most common TEAEs (≥ 10% of patients in either group) were anaemia, diarrhoea, septic shock, vomiting, hypokalaemia, and sepsis. Overall, 17.4% (8/46) and 29.9% (20/67) of patients in the rezafungin and caspofungin groups, respectively, discontinued the study/drug due to TEAEs. Serious TEAEs reported in ≥ 5% of patients in either group were septic shock, multiple organ dysfunction syndrome, pneumonia, and respiratory failure. The only study drug-related TEAE occurring in > 1 patient in either group was increased gamma-glutamyl transferase (caspofungin: 2/67 [3.0%]). Study drug-related serious TEAEs occurred in 2.2% (1/46) of patients receiving rezafungin (infusion-related reaction) and 6.0% (4/67) receiving caspofungin (ventricular tachycardia, rectal haemorrhage, hypertransaminasaemia, and anaphylactic shock; all n = 1). Study drug-related TEAEs led to study discontinuation in no patients in the rezafungin group and two in the caspofungin group (ventricular tachycardia and anaphylactic shock). Laboratory parameters in the pooled ICU subgroup are shown in Supplementary Material 1: Table S2.
Table 2.
Summary of safety (safety population)
| n (%)a | Rezafungin 400/200 mg (n = 46) |
Caspofungin 70/50 mg (n = 67) |
|---|---|---|
| ≥ 1 TEAE | 41 (89.1) | 54 (80.6) |
| ≥ 1 serious TEAE | 28 (60.9) | 38 (56.7) |
| ≥ 1 study drug-related TEAE | 4 (8.7) | 11 (16.4) |
| ≥ 1 study drug-related serious TEAE | 1 (2.2) | 4 (6.0) |
| ≥ 1 TEAE leading to study/drug discontinuation | 8 (17.4) | 20 (29.9) |
| ≥ 1 study drug-related TEAE leading to study/drug discontinuation | 0 (0) | 2 (3.0) |
|
Most commonly occurring TEAEs (≥ 5% of either pooled treatment group) [event severity (n): mild/moderate/severe]b,c | ||
| Blood and lymphatic system disorders | ||
| Anaemia | 7 (15.2) [2/4/1] | 7 (10.4) [3/4/0] |
| Cardiac disorders | ||
| Atrial fibrillation | 3 (6.5) [1/2/0] | 3 (4.5) [1/2/0] |
| Bradycardia | 1 (2.2) [0/1/0] | 5 (7.5) [2/3/0] |
| Gastrointestinal disorders | ||
| Diarrhoea | 7 (15.2) [6/1/0] | 7 (10.4) [4/3/0] |
| Vomiting | 7 (15.2) [4/4/0] | 3 (4.5) [2/1/0] |
| Abdominal pain | 4 (8.7) [1/2/1] | 4 (6.0) [3/0/1] |
| Nausea | 4 (8.7) [0/4/0] | 4 (6.0) [1/3/0] |
| Constipation | 2 (4.3) [2/0/0] | 4 (6.0) [3/1/0] |
| General disorders and administration site conditions | ||
| Multiple organ dysfunction syndrome | 3 (6.5) [0/0/3] | 3 (4.5) [0/0/3] |
| Peripheral oedema | 3 (6.5) [3/0/0] | 2 (3.0) [1/1/0] |
| Pyrexia | 2 (4.3) [1/1/0] | 6 (9.0) [5/1/0] |
| Infections and infestations | ||
| Septic shock | 6 (13.0) [0/1/5] | 7 (10.4) [0/0/7] |
| Sepsis | 5 (10.9) [3/2/1] | 3 (4.5) [0/0/3] |
| Pneumonia | 3 (6.5) [0/0/3] | 2 (3.0) [1/1/0] |
| Abdominal abscess | 1 (2.2) [0/1/0] | 4 (6.0) [0/0/4] |
| Metabolism and nutrition disorders | ||
| Hypokalaemia | 4 (8.7) [3/1/0] | 10 (14.9) [8/2/0] |
| Fluid overload | 4 (8.7) [0/3/1] | 2 (3.0) [1/1/0] |
| Hyperkalaemia | 2 (4.3) [2/0/0] | 5 (7.5) [2/2/1] |
| Acidosis | 1 (2.2) [1/1/0] | 4 (6.0) [2/2/0] |
| Psychiatric disorders | ||
| Insomnia | 4 (8.7) [3/1/0] | 2 (3.0) [1/1/0] |
| Renal and urinary disorders | ||
| Acute kidney injury | 1 (2.2) [1/1/0] | 4 (6.0) [1/1/2] |
| Respiratory, thoracic, and mediastinal disorders | ||
| Dyspnoea | 0 (0) [0/0/0] | 4 (6.0) [1/2/1] |
| Pleural effusion | 0 (0) [0/0/0] | 4 (6.0) [1/3/0] |
| Pneumothorax | 0 (0) [0/0/0] | 4 (6.0) [1/3/0] |
| Respiratory failure | 0 (0) [0/0/0] | 4 (6.0) [0/0/4] |
| Skin and subcutaneous tissue disorders | ||
| Decubitus ulcer | 3 (6.5) [2/1/0] | 5 (7.5) [2/2/1] |
| Vascular disorders | ||
| Hypotension | 3 (6.5) [2/1/0] | 6 (9.0) [3/3/1] |
| Hypertension | 3 (6.5) [2/1/0] | 3 (4.5) [2/0/1] |
| Deep vein thrombosis | 1 (2.2) [1/0/0] | 4 (6.0) [1/3/0] |
TEAE treatment-emergent adverse event
aPatients with multiple events in the same system organ class or preferred term are counted only once (highest severity of TEAE reported)
bFor the number of events with mild/moderate/severe severity, it was possible for patients to experience an adverse event at ≥ 1 severity; therefore, the number of events could be more than the number of patients with events
cAs TEAEs were graded differently in STRIVE and ReSTORE, here they are listed by severity
Pharmacokinetics
Rezafungin exposure measures (AUC0–168 h, Cmax and Cmin) following the initial 400-mg dose were comparable between patients in the ICU at randomisation (n = 50) and non-ICU patients (n = 117) (Supplementary Material 1: Table S3).
Discussion
This post-hoc, patient-level analysis of STRIVE and ReSTORE data assessed rezafungin versus caspofungin in patients receiving care in an ICU. Our results provide further support for potential early treatment benefits with the front-loaded, QW rezafungin dosing regimen, as demonstrated through an indication of reduced TTNBC versus QD caspofungin treatment and numerically higher mycological eradication rates on Day 5. Day 30 ACM was numerically higher with rezafungin versus caspofungin, but candidaemia/IC-attributable mortality appeared to be similar between treatments. Rezafungin was generally well tolerated, with a comparable safety profile to caspofungin.
These data are broadly in agreement with primary results from the STRIVE and ReSTORE trials [10, 11] and a pooled overall analysis of these trials [20], in which rezafungin was non-inferior to caspofungin for primary efficacy outcomes, with secondary and exploratory analyses suggesting a potential early treatment benefit for rezafungin [10, 11, 20]. It should be noted that as ICU status was not a stratification factor in the primary trials, our pooled analysis had greater imbalances in patient characteristics between groups in terms of gender, race, geographical distribution, and APACHE II score, as compared with the previous pooled analysis of the whole population [20].
Interestingly, the approximately 20-h difference in median TTNBC between treatments was greater in the present subgroup of critically ill patients than the approximately 3-h treatment difference in the individual trials [10, 11]. Furthermore, both mean time from first positive blood sample to any antifungal treatment for candidaemia/IC and to the first dose of randomised treatment were longer for patients who received rezafungin versus those who received caspofungin in our analysis, which is consistent with the data reported for the ReSTORE trial [11]. However, TTNBC may have been confounded by many factors, such as differences between treatment groups in baseline Candida species (namely C. parapsilosis), comorbidities and the proportion of patients with catheter removal within 48 h after diagnosis. A multivariate analysis would be required to validate these findings in this small study population. There was a similar trend in favour of rezafungin in the proportions of patients achieving NBCs at 24 and 48 h in the ICU versus the overall population, although treatment differences between the groups were slightly greater in patients in the ICU versus the overall population [20].
As expected, Day 30 ACM rates were higher in this subgroup of critically ill patients than those in the pooled overall analysis [20]. Day 30 ACM rates were numerically lower with caspofungin versus rezafungin, despite a higher proportion of patients who received mechanical ventilation in the caspofungin group. However, 95% CIs for Day 30 ACM rates overlapped between the two treatment groups and there was a greater number of deaths not attributable to candidaemia in the rezafungin group. The number of deaths attributed to candidaemia/IC was similar between groups (rezafungin n = 2, 14.3% of confirmed deaths; caspofungin n = 1, 6.6% of confirmed deaths). Like TTNBC, ACM may have been affected by Candida- and non-Candida-related factors, for example, baseline Candida species, comorbidities (e.g., higher proportion of patients on dialysis in the rezafungin versus caspofungin group), and catheter management. The safety profile of rezafungin versus caspofungin in patients in the ICU was in line with previous reports from the primary and pooled analyses [10, 11, 20].
Rezafungin's early treatment benefits – high rates of early mycological eradication and TTNBC – are likely a result of its differentiated pharmacokinetic profile compared with caspofungin. The low clearance and long half-life of rezafungin enables a QW dosing regimen that results in higher front-loaded exposure versus caspofungin QD, and higher plasma drug concentrations early on in treatment [13]. These characteristics, along with overall cure and TTNBC results from STRIVE and ReSTORE [10, 11, 20], support the potential benefits of infrequent, front-loaded dosing of drugs that exhibit concentration-dependent antimicrobial killing [13] and the potential for rapid infection clearance [14, 20]. In contrast, exposure to first-generation echinocandin drugs at clinical doses may not always be sufficient to treat infections due to the increased minimum inhibitory concentrations of some Candida species, compounded by pharmacokinetic variability seen in critically ill patients [21–23]; this could result in reduced efficacy of these drugs in this population. The high plasma drug concentrations achieved early in rezafungin therapy may help reduce the development of antimicrobial resistance [24], which is particularly relevant for critically ill patients in the ICU. Indeed, the exploratory pharmacokinetic analysis of ICU versus non-ICU patients suggests that rezafungin exposure may be comparable in these two groups of patients. This observation is important, as exposure to other echinocandins, such as caspofungin, may be influenced by altered drug distribution and clearance in ICU patients [21]. These results suggest that rezafungin dose adjustment may not be required in ICU patients. However, further prospective data are required to understand rezafungin pharmacokinetics in ICU subpopulations, such as those undergoing extracorporeal membrane oxygenation.
The strengths and limitations of this analysis need to be acknowledged. Firstly, neither STRIVE nor ReSTORE were designed as ICU studies; most patients were treated in hospital wards, with only approximately one-third being in the ICU. Consequently, important data for ICU studies, for example Sequential Organ Failure Assessment (SOFA) score and Simplified Acute Physiology Score (SAPS) 3, were not available. Rates of catheter removal within 48 h were also lower than expected, as removal within this time frame was only recommended and not mandatory in both studies. Furthermore, this post-hoc analysis was not preplanned or powered to detect significant differences between treatment groups; patients in the ICU at randomisation were not a predefined subgroup of STRIVE or ReSTORE. It is therefore possible that the results observed were due to chance. Pooling data from the two trials is a strength, as this has generated a more robust dataset, although the sample size remains small and, as such, the clinical relevance of numerical differences between efficacy outcomes of rezafungin versus caspofungin groups cannot be determined. It should be noted that certain efficacy endpoints were defined differently in STRIVE and ReSTORE (e.g., global cure and overall response at Day 14), although these endpoints were deemed sufficiently similar to be combined for the purpose of this analysis.
Conclusions
This post-hoc, patient-level, pooled analysis of the STRIVE and ReSTORE data indicates that rezafungin is efficacious and well tolerated in critically ill, mainly non-neutropenic patients with candidaemia and/or IC. Our findings provide additional insights into rezafungin efficacy and safety and indicate the applicability of the overall trial results to this critical care population.
Supplementary Information
Acknowledgements
We thank all participants and investigators involved in the studies. Medical writing support (including development of a draft outline and subsequent drafts in consultation with the authors, assembling tables and figures, collating author comments, copyediting, fact checking and referencing) was provided by Rachel Hubbard, MSc, and Caroline Greenwood, BSc (Hons), of Aspire Scientific (Bollington, UK), and funded by Mundipharma.
Abbreviations
- ACM
All-cause mortality
- AE
Adverse event
- APACHE
Acute Physiologic Assessment and Chronic Health Evaluation
- AUC0–168h
Area under the plasma concentration‒time curve from 0 to 168 h
- CI
Confidence interval
- Cmax
Maximum plasma concentration
- Cmin
Minimum plasma concentration
- CVC
Central venous catheter
- DRC
Data review committee
- ESCMID
European Society of Clinical Microbiology and Infectious Diseases
- FDA
Food and Drug Administration
- IC
Invasive candidiasis
- ICU
Intensive care unit
- IDSA
Infectious Diseases Society of America
- mITT
Modified intent-to-treat
- QD
Once daily
- QW
Once weekly
- SAPS
Simplified Acute Physiology Score
- SD
Standard deviation
- SOFA
Sequential Organ Failure Assessment
- TEAE
Treatment-emergent adverse event
- TTNBC
Time to negative blood culture
Author contributions
All authors contributed to the analysis or interpretation of the data, reviewed and critically revised the manuscript, approved the final draft, and are accountable for the accuracy and integrity of this work. GRT, JV, BJK, and PGP contributed to the design of the study. MG, NM, and SN contributed to the data collection.
Funding
The analyses described in this manuscript were funded by Mundipharma (Cambridge, UK). STRIVE was funded by Cidara Therapeutics, Inc (San Diego, CA, USA). ReSTORE was funded by Cidara Therapeutics, Inc (San Diego, CA, USA) and Mundipharma (Cambridge, UK). Employees of the funding sources were involved in the study design; the collection, analysis, and interpretation of data; and in the writing of the report. All authors were provided full access to all study data and had the final responsibility for the decision to submit the publication.
Availability of data and materials
Access to the respective study protocols and anonymised data can be requested by contacting Enquiries@napp.co.uk. Each request will be reviewed by the sponsor for scientific merit.
Declarations
Ethics approval and consent to participate
STRIVE and ReSTORE were conducted in accordance with current regulations, the International Conference on Harmonisation Good Clinical Practice, and the Declaration of Helsinki. Ethics committees or institutional review boards at participating sites approved the protocol and all amendments. All patients, or their legally authorised representatives, provided written informed consent.
Consent for publication
Not applicable.
Competing interests
PMH reports grants or contracts from Baxter, Cytosorbents, and Pfizer; consulting fees from Baxter, Cytosorbents, and Pfizer; honoraria from Baxter, and Cytosorbents; and support for attending meetings from Mundipharma, and Pfizer, outside of the submitted work. MG reports consulting fees and/or payment for speaking for Biotest, Pfizer, MSD, Gilead, Estor, Viatris, AOP Pharma, Fresenius, and Orion. MK reports funding from the Barnes-Jewish Hospital Foundation. OAC reports grants or contracts from BMBF, Cidara, EU-DG RTD (101037867), F2G, Gilead, Medpace, MSD, Mundipharma, Octapharma, Pfizer, Scynexis; consulting fees from AbbVie, AiCuris, Biocon, Cidara, Gilead, IQVIA, Janssen, Matinas, Medpace, Menarini, Moderna, Molecular Partners, MSG-ERC, Noxxon, Octapharma, Pfizer, PSI, Scynexis, and Seres; honoraria for lectures from Abbott, AbbVie, Al-Jazeera Pharmaceuticals/Hikma, Gilead, Grupo Biotoscana/United Medical/Knight, Medscape, MedUpdate, Merck/MSD, Noscendo, Pfizer, Shionogi, and streamedup!; payment for expert testimony from Cidara; participation on a data safety monitoring board or advisory board from Boston Strategic Partners, Cidara, IQVIA, Janssen, Medpace, PSI, Pulmocide, Shionogi, and the Prime Meridian Group; and has stocks or stock options in CoRe Consulting and EasyRadiology. GRT reports grants and consulting fees from Amplyx, Astellas, Cidara, F2G, and Manye; grants from Merck; and data safety monitoring board membership for Pfizer. MB reports consulting fees and payment or honoraria for lectures, presentations, speaker bureaux, manuscript writing, or educational events from MSD, Pfizer, Menarini, Shionogi, Angelini, and Gilead; and data safety monitoring board or advisory board participation for Cidara and Mundipharma. AS reports grants from Pfizer and Gilead Sciences; and honoraria for lectures and advisory boards from Pfizer, MSD, Shionogi, Angelini, and Menarini. HH reports no conflicts of interest. JV reports grants from Cidara, F2G, Scynexis, and Amplyx; consulting fees from Cidara and F2G; payment or honoraria for lectures, presentations, speaker bureaux, manuscript writing, or educational events from Melinta; and data safety monitoring board or advisory board participation for F2G. BJK served on the independent data review committee for Cidara. PGP reports grants from and data review committee membership for Cidara and Melinta; grants from Astellas, Scynexis, and Merck; consulting fees from Cidara and Melinta; and advisory board membership for F2G, Matinas and TFF Pharma. NM is an employee of Mundipharma Research Ltd. TS is an employee and shareholder of Cidara Therapeutics. JP reports no conflicts of interest. SN reports honoraria for lectures and advisory boards from Pfizer, MSD, bioMérieux, Fisher and Paykel, Mundipharma, and Medtronic.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018;4:18026. [DOI] [PubMed] [Google Scholar]
- 2.Logan C, Martin-Loeches I, Bicanic T. Invasive candidiasis in critical care: challenges and future directions. Intensive Care Med. 2020;46(11):2001–14. [DOI] [PubMed] [Google Scholar]
- 3.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18(Suppl 7):19–37. [DOI] [PubMed] [Google Scholar]
- 4.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49(9):3640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis. 2012;54(12):1739–46. [DOI] [PubMed] [Google Scholar]
- 7.Daneshnia F, de Almeida Junior JN, Ilkit M, Lombardi L, Perry AM, Gao M, et al. Worldwide emergence of fluconazole-resistant Candida parapsilosis: current framework and future research roadmap. Lancet Microbe. 2023;4(6):e470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goemaere B, Lagrou K, Spriet I, Hendrickx M, Becker P. Clonal spread of Candida glabrata bloodstream isolates and fluconazole resistance affected by prolonged exposure: a 12-year single-center study in Belgium. Antimicrob Agents Chemother. 2018;62(8):e00591-e618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bader JC, Bhavnani SM, Andes DR, Ambrose PG. We can do better: a fresh look at echinocandin dosing. J Antimicrob Chemother. 2018;73(3):831. [DOI] [PubMed] [Google Scholar]
- 10.Thompson GR III, Soriano A, Skoutelis A, Vazquez JA, Honore PM, Horcajada JP, et al. Rezafungin versus caspofungin in a phase 2, randomized, double-blind study for the treatment of candidemia and invasive candidiasis: the STRIVE trial. Clin Infect Dis. 2021;73(11):e3647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson GR III, Soriano A, Cornely OA, Kullberg BJ, Kollef M, Vazquez J, et al. Rezafungin versus caspofungin for treatment of candidaemia and invasive candidiasis (ReSTORE): a multicentre, double-blind, double-dummy, randomised phase 3 trial. Lancet. 2023;401(10370):49–59. [DOI] [PubMed] [Google Scholar]
- 12.Clinical Trials.gov. Study of rezafungin compared to standard antimicrobial regimen for prevention of invasive fungal diseases in adults undergoing allogeneic blood and marrow transplantation (ReSPECT) [NCT04368559]. 2023. https://classic.clinicaltrials.gov/ct2/show/NCT04368559. Accessed 26 June 2023.
- 13.Sandison T, Ong V, Lee J, Thye D. Safety and pharmacokinetics of CD101 IV, a novel echinocandin, in healthy adults. Antimicrob Agents Chemother. 2017;61(2):e01627-e1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Perez WB, Jiménez-Ortigosa C, Hough G, Locke JB, Ong V, et al. CD101: a novel long-acting echinocandin. Cell Microbiol. 2016;18(9):1308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pound MW, Townsend ML, Drew RH. Echinocandin pharmacodynamics: review and clinical implications. J Antimicrob Chemother. 2010;65(6):1108–18. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Prideaux B, Nagasaki Y, Lee MH, Chen PY, Blanc L, et al. Unraveling drug penetration of echinocandin antifungals at the site of infection in an intra-abdominal abscess model. Antimicrob Agents Chemother. 2017;61(10):e01009-e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallagher A. FDA approves rezafungin injection for the treatment of candidemia, invasive candidiasis. 2023. https://www.pharmacytimes.com/view/fda-approves-rezafungin-injection-for-the-treatment-of-candidemia-invasive-candidiasis. Accessed 1 Jun 2023.
- 18.European Medicines Agency. Rezzayo (rezafungin) Summary of Product Characteristics. 2024. https://www.ema.europa.eu/en/documents/product-information/rezzayo-epar-product-information_en.pdf. Accessed 1 Feb 2024.
- 19.Mundipharma. Press release: European approval of REZZAYO® (rezafungin) for the treatment of Invasive Candidiasis in adults. 2023. https://www.mundipharma.com/mundipharma-announces-european-approval-of-rezzayo. Accessed 2 Jan 2024.
- 20.Thompson GR III, Soriano A, Honore PM, Bassetti M, Cornely OA, Kollef M, et al. Efficacy and safety of rezafungin and caspofungin in candidaemia and invasive candidiasis: pooled data from two prospective randomised controlled trials. Lancet Infect Dis. 2024;24(3):319–28. [DOI] [PubMed] [Google Scholar]
- 21.van der Elst KC, Veringa A, Zijlstra JG, Beishuizen A, Klont R, Brummelhuis-Visser P, et al. Low caspofungin exposure in patients in intensive care units. Antimicrob Agents Chemother. 2017;61(2):e01582-e1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boonstra JM, van der Elst KC, Veringa A, Jongedijk EM, Brüggemann RJ, Koster RA, et al. Pharmacokinetic properties of micafungin in critically ill patients diagnosed with invasive candidiasis. Antimicrob Agents Chemother. 2017;61(12):e01398-e1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pea F, Lewis RE. Overview of antifungal dosing in invasive candidiasis. J Antimicrob Chemother. 2018;73(suppl_1):i33–43. [DOI] [PubMed] [Google Scholar]
- 24.Locke JB, Almaguer AL, Zuill DE, Bartizal K. Characterization of in vitro resistance development to the novel echinocandin CD101 in Candida species. Antimicrob Agents Chemother. 2016;60(10):6100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to the respective study protocols and anonymised data can be requested by contacting Enquiries@napp.co.uk. Each request will be reviewed by the sponsor for scientific merit.